Enzymatic Carboxylation of Resorcinol in Aqueous Triethanolamine at Elevated CO2 Pressure
Abstract
:1. Introduction
2. Results and Discussion
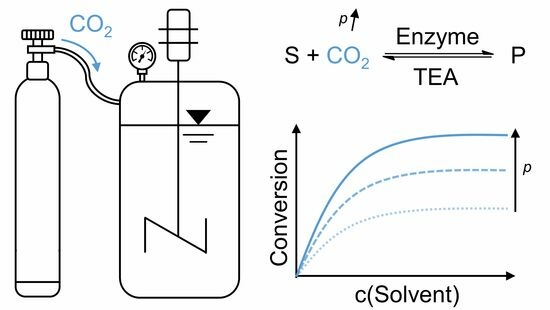
2.1. Carboxylation at Elevated CO2 Pressure
2.2. Precipitation at Elevated CO2 Pressure
3. Materials and Methods
3.1. General
3.2. Production of His-Tagged 2,6-Dihydroxybenzoic Acid Decarboxylase
3.3. Enzymatic Reactions at Elevated CO2 Pressure
3.4. Enzymatic Reactions at Elevated CO2 Pressure with TBA-Br
3.5. Analytics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sato, M.; Sakurai, N.; Suzuki, H.; Shibata, D.; Kino, K. Enzymatic carboxylation of hydroxystilbenes by the γ-resorcylic acid decarboxylase from Rhizobium radiobacter WU-0108 under reverse reaction conditions. J. Mol. Catal. B Enzym. 2015, 122, 348–352. [Google Scholar] [CrossRef]
- Kirimura, K.; Ishii, Y. Enzymatic Kolbe–Schmitt Reaction for the Syntheses of Value-Added Compounds. In Future Directions in Biocatalysis, 2nd ed.; Matsuda, T., Ed.; Elsevier: Amsterdam, The Netherlands; Cambridge, MA, USA, 2017; pp. 135–147. ISBN 9780444637437. [Google Scholar]
- Payer, S.E.; Faber, K.; Glueck, S.M. Non-Oxidative Enzymatic (De)Carboxylation of (Hetero)Aromatics and Acrylic Acid Derivatives. Adv. Synth. Catal. 2019, 361, 2402–2420. [Google Scholar] [CrossRef] [PubMed]
- Aleku, G.A.; Roberts, G.W.; Titchiner, G.R.; Leys, D. Synthetic Enzyme-Catalyzed CO2 Fixation Reactions. ChemSusChem 2021, 14, 1781–1804. [Google Scholar] [CrossRef] [PubMed]
- Lide, D.R. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data, 88th ed.; CRC: Boca Raton, FL, USA; London, UK, 2008; ISBN 0849304881. [Google Scholar]
- Pesci, L.; Glueck, S.M.; Gurikov, P.; Smirnova, I.; Faber, K.; Liese, A. Biocatalytic carboxylation of phenol derivatives: Kinetics and thermodynamics of the biological Kolbe-Schmitt synthesis: Kinetics and thermodynamics of the biological Kolbe-Schmitt synthesis. FEBS J. 2015, 282, 1334–1345. [Google Scholar] [CrossRef]
- Wuensch, C.; Schmidt, N.; Gross, J.; Grischek, B.; Glueck, S.M.; Faber, K. Pushing the equilibrium of regio-complementary carboxylation of phenols and hydroxystyrene derivatives. J. Biotechnol. 2013, 168, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Pesci, L.; Gurikov, P.; Liese, A.; Kara, S. Amine-Mediated Enzymatic Carboxylation of Phenols Using CO2 as Substrate Increases Equilibrium Conversions and Reaction Rates. Biotechnol. J. 2017, 12, 1700332. [Google Scholar] [CrossRef] [PubMed]
- Ohde, D.; Thomas, B.; Matthes, S.; Tanaka, S.; Bubenheim, P.; Terasaka, K.; Schlüter, M.; Liese, A. Microbubble enhanced mass transfer efficiency of CO2 capture utilizing aqueous triethanolamine for enzymatic resorcinol carboxylation. RSC Adv. 2021, 11, 4087–4096. [Google Scholar] [CrossRef]
- Ohde, D.; Thomas, B.; Bubenheim, P.; Liese, A. Enhanced CO2 fixation in the biocatalytic carboxylation of resorcinol: Utilization of amines for amine scrubbing and in situ product precipitation. Biochem. Eng. J. 2021, 166, 107825. [Google Scholar] [CrossRef]
- Fan, Y.; Feng, J.; Yang, M.; Tan, X.; Fan, H.; Guo, M.; Wang, B.; Xue, S. CO2 (aq) concentration–dependent CO2 fixation via carboxylation by decarboxylase. Green Chem. 2021, 23, 4403–4409. [Google Scholar] [CrossRef]
- Meyer, L.-E.; Plasch, K.; Kragl, U.; von Langermann, J. Adsorbent-Based Downstream-Processing of the Decarboxylase-Based Synthesis of 2,6-Dihydroxy-4-methylbenzoic Acid. Org. Process Res. Dev. 2018, 22, 963–970. [Google Scholar] [CrossRef]
- Pesci, L. Biocatalytic (De)carboxylation of Phenolic Compounds: Fundamentals and Applications. Ph.D. Dissertation, TUHH Universitätsbibliothek, Hamburg, Germany, 2017. [Google Scholar]
- Martin, J.; Eisoldt, L.; Skerra, A. Fixation of gaseous CO2 by reversing a decarboxylase for the biocatalytic synthesis of the essential amino acid l-methionine. Nat. Catal. 2018, 1, 555–561. [Google Scholar] [CrossRef]
- Ren, J.; Yao, P.; Yu, S.; Dong, W.; Chen, Q.; Feng, J.; Wu, Q.; Zhu, D. An Unprecedented Effective Enzymatic Carboxylation of Phenols. ACS Catal. 2016, 6, 564–567. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, J.; Yao, P.; Gong, R.; Wang, M.; Wu, Q.; Zhu, D. Biochemical characterization and substrate profiling of a reversible 2,3-dihydroxybenzoic acid decarboxylase for biocatalytic Kolbe-Schmitt reaction. Enzym. Microb. Technol. 2018, 113, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Jou, F.-Y.; Otto, F.D.; Mather, A.E. Equilibria of H2S and CO2 in triethanolamine solutions. Can. J. Chem. Eng. 1985, 63, 122–125. [Google Scholar] [CrossRef]
- Kent, R.L.; Elsenberg, B. Better Data for Amine Treating. Hydrocarb. Process. 1976, 55, 87–90. [Google Scholar]
- Yoshida, T.; Hayakawa, Y.; Matsui, T.; Nagasawa, T. Purification and characterization of 2,6-dihydroxybenzoate decarboxylase reversibly catalyzing nonoxidative decarboxylation. Arch. Microbiol. 2004, 181, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Kilmartin, J.V.; Rossi-Bernardi, L. Inhibition of CO2 combination and reduction of the Bohr effect in haemoglobin chemically modified at its alpha-amino groups. Nature 1969, 222, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Kilmartin, J.V.; Rossi-Bernardi, L. The binding of carbon dioxide by horse haemoglobin. Biochem. J. 1971, 124, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Kilmartin, J.V.; Rossi-Bernardi, L. Interaction of hemoglobin with hydrogen ions, carbon dioxide, and organic phosphates. Physiol. Rev. 1973, 53, 836–890. [Google Scholar] [CrossRef]
- Andrews, T.J.; Badger, M.R.; Lorimer, G.H. Factors affecting interconversion between kinetic forms of ribulose diphosphate carboxylase-oxygenase from spinach. Arch. Biochem. Biophys. 1975, 171, 93–103. [Google Scholar] [CrossRef]
- Cleland, W.W.; Andrews, T.J.; Gutteridge, S.; Hartman, F.C.; Lorimer, G.H. Mechanism of Rubisco: The Carbamate as General Base. Chem. Rev. 1998, 98, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Lorimer, G.H.; Badger, M.R.; Andrews, T.J. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry 1976, 15, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Lorimer, G.H.; Miziorko, H.M. Carbamate formation on the epsilon-amino group of a lysyl residue as the basis for the activation of ribulosebisphosphate carboxylase by CO2 and Mg2+. Biochemistry 1980, 19, 5321–5328. [Google Scholar] [CrossRef] [PubMed]
- Blake, L.I.; Cann, M.J. Carbon Dioxide and the Carbamate Post-Translational Modification. Front. Mol. Biosci. 2022, 9, 825706. [Google Scholar] [CrossRef]
- Linthwaite, V.L.; Janus, J.M.; Brown, A.P.; Wong-Pascua, D.; O’Donoghue, A.C.; Porter, A.; Treumann, A.; Hodgson, D.R.W.; Cann, M.J. The identification of carbon dioxide mediated protein post-translational modifications. Nat. Commun. 2018, 9, 3092. [Google Scholar] [CrossRef]
- Wimmer, Z.; Zarevúcka, M. A review on the effects of supercritical carbon dioxide on enzyme activity. Int. J. Mol. Sci. 2010, 11, 233–253. [Google Scholar] [CrossRef]
- Wang, W.; Rao, L.; Wu, X.; Wang, Y.; Zhao, L.; Liao, X. Supercritical Carbon Dioxide Applications in Food Processing. Food Eng. Rev. 2021, 13, 570–591. [Google Scholar] [CrossRef]
- Silva, E.K.; Meireles, M.A.A.; Saldaña, M.D. Supercritical carbon dioxide technology: A promising technique for the non-thermal processing of freshly fruit and vegetable juices. Trends Food Sci. Technol. 2020, 97, 381–390. [Google Scholar] [CrossRef]
- Balaban, M.O.; Arreola, A.G.; Marshall, M.; Peplow, A.; Wei, C.I.; Cornell, J. Inactivation of Pectinesterase in Orange Juice by Supercritical Carbon Dioxide. J. Food Sci. 1991, 56, 743–746. [Google Scholar] [CrossRef]
- Park, S.-J.; Lee, J.-I.; Park, J. Effects of a Combined Process of High-Pressure Carbon Dioxide and High Hydrostatic Pressure on the Quality of Carrot Juice. J. Food Sci. 2002, 67, 1827–1834. [Google Scholar] [CrossRef]
- Habulin, M.; Knez, Ž. Activity and stability of lipases from different sources in supercritical carbon dioxide and near-critical propane. J. Chem. Technol. Biotechnol. 2001, 76, 1260–1266. [Google Scholar] [CrossRef]
- Perumal, M.; Jayaraman, D.; Balraj, A. Experimental studies on CO2 absorption and solvent recovery in aqueous blends of monoethanolamine and tetrabutylammonium hydroxide. Chemosphere 2021, 276, 130159. [Google Scholar] [CrossRef] [PubMed]
- Bertani, G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951, 62, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Ohde, D.; Thomas, B.; Matthes, S.; Percin, Z.; Engelmann, C.; Bubenheim, P.; Terasaka, K.; Schlüter, M.; Liese, A. Fine Bubble-based CO2 Capture Mediated by Triethanolamine Coupled to Whole Cell Biotransformation. Chem. Ing. Tech. 2019, 91, 1822–1826. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohde, D.; Thomas, B.; Bubenheim, P.; Liese, A. Enzymatic Carboxylation of Resorcinol in Aqueous Triethanolamine at Elevated CO2 Pressure. Molecules 2024, 29, 25. https://doi.org/10.3390/molecules29010025
Ohde D, Thomas B, Bubenheim P, Liese A. Enzymatic Carboxylation of Resorcinol in Aqueous Triethanolamine at Elevated CO2 Pressure. Molecules. 2024; 29(1):25. https://doi.org/10.3390/molecules29010025
Chicago/Turabian StyleOhde, Daniel, Benjamin Thomas, Paul Bubenheim, and Andreas Liese. 2024. "Enzymatic Carboxylation of Resorcinol in Aqueous Triethanolamine at Elevated CO2 Pressure" Molecules 29, no. 1: 25. https://doi.org/10.3390/molecules29010025
APA StyleOhde, D., Thomas, B., Bubenheim, P., & Liese, A. (2024). Enzymatic Carboxylation of Resorcinol in Aqueous Triethanolamine at Elevated CO2 Pressure. Molecules, 29(1), 25. https://doi.org/10.3390/molecules29010025