Extraction of Antioxidants from Brown Macroalgae Fucus spiralis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Experimental Results
2.2. Response Surface Methodology and Statistical Analysis
2.2.1. Vortex-Assisted Extraction
2.2.2. Extraction with Ultra-Turrax® Homogenizer
2.2.3. Ultrasonic Bath-Assisted Extraction
2.2.4. Ultrasonic Probe-Assisted Extraction
2.2.5. High-Pressure-Assisted Extraction
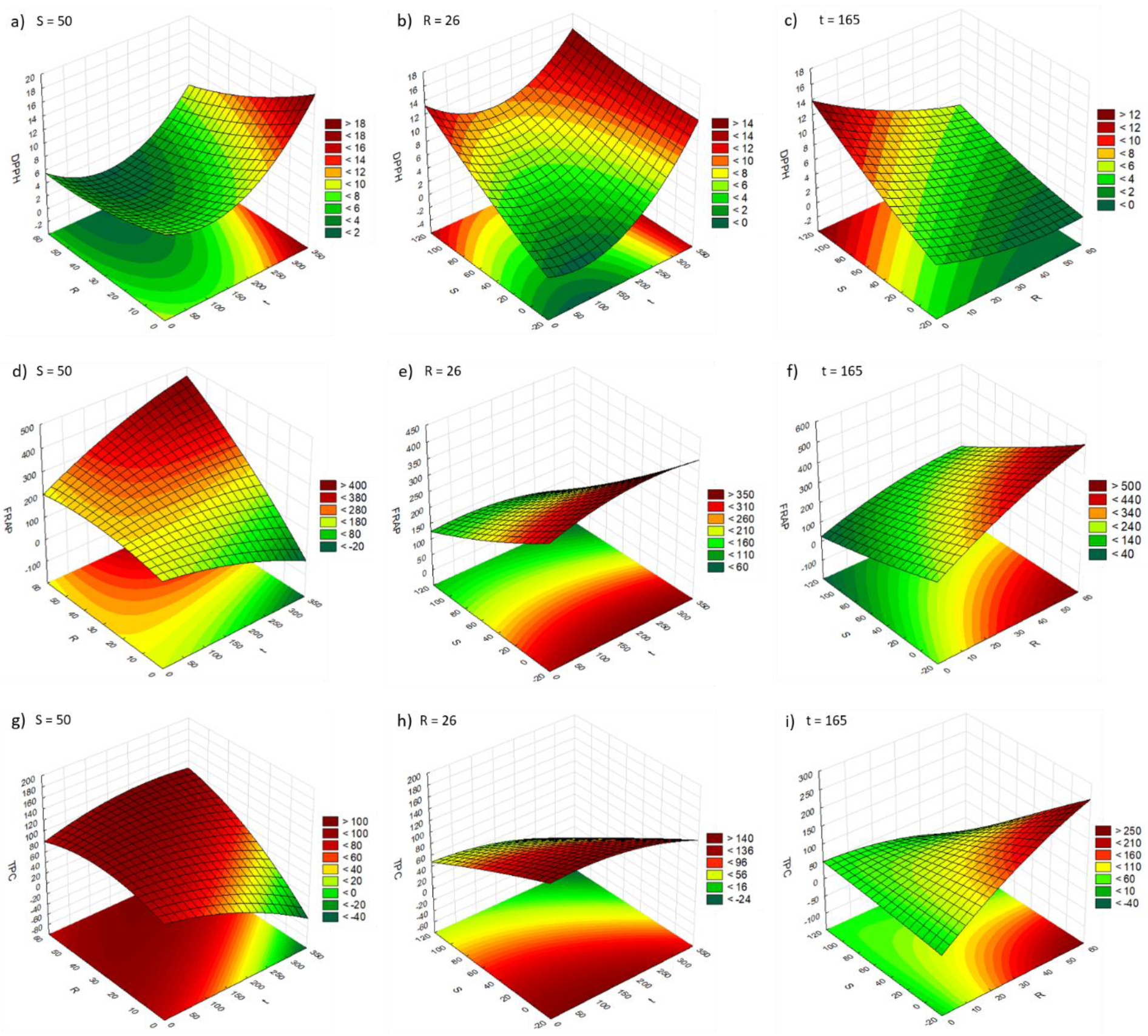
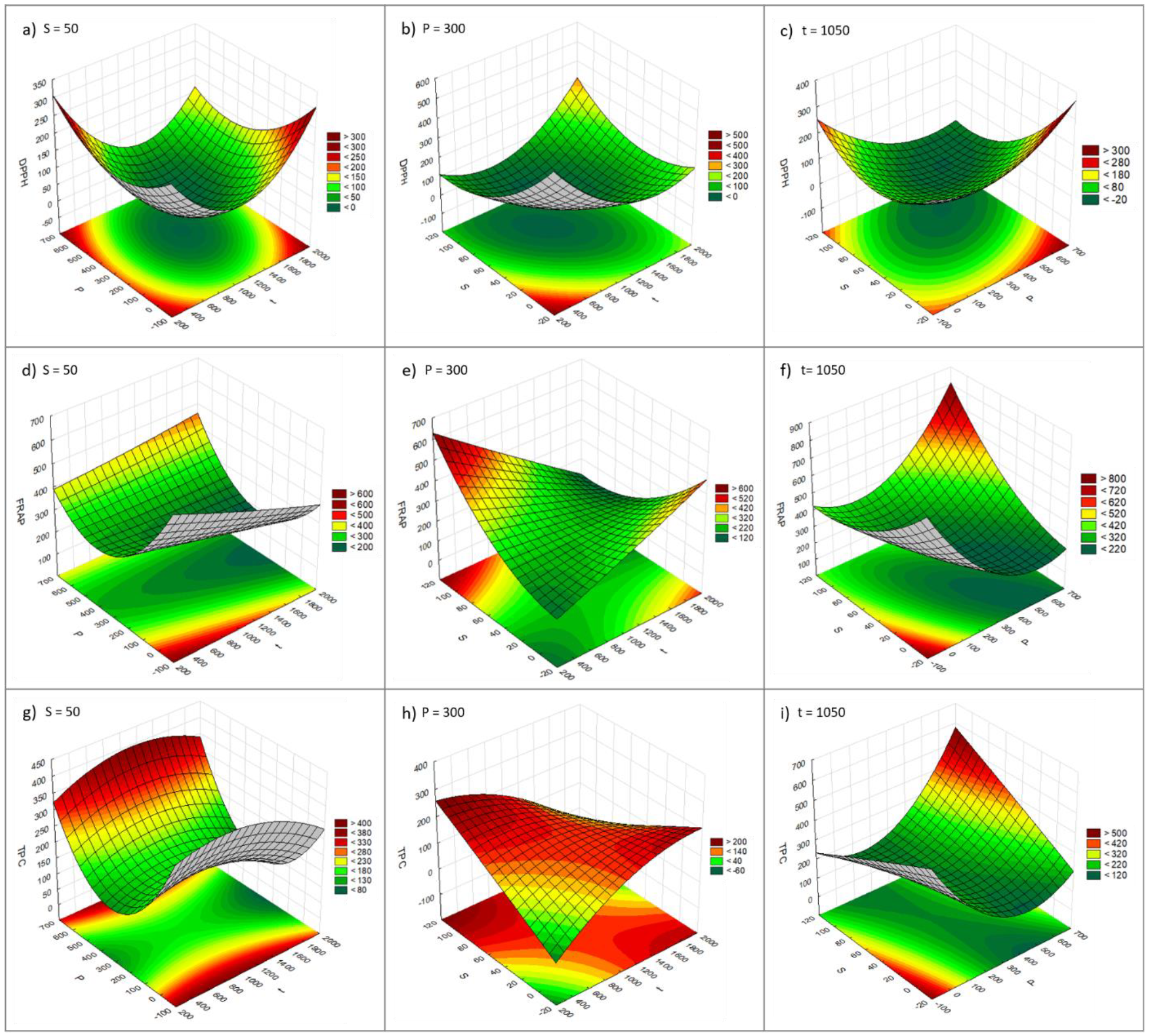
2.3. Effect of t, R, S, and P on the Phenolic Content and Antioxidant Capacity of the Extracts
2.4. Selection of the Best Conditions
3. Materials and Methods
3.1. Biomass
3.2. Chemicals
3.3. Extraction Procedures
3.3.1. Ultrasound-Assisted Extraction
3.3.2. Vortex Extraction
3.3.3. Extraction with Ultra-Turrax® Homogenizer
3.3.4. High-Pressure-Assisted Extraction
3.4. TPC—Total Phenolic Content
3.5. DPPH Radical Scavenging Activity
3.6. FRAP—Ferric Reducing Antioxidant Power
3.7. Variable Selection and Design of Experiment
3.8. Mathematical Modeling and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Hassan, H.A.; Awasthi, M.K.; Gajendran, B.; Sharma, M.; Ji, M.-K.; Salama, E.-S. The recent progress on the bioactive compounds from algal biomass for human health applications. Food Biosci. 2023, 51, 102267. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Fakhri, S.; Farzaei, M.H. Chapter 6.1—Antioxidants effects in health: The bright and the dark sides. In Antioxidants Effects in Health; Nabavi, S.M., Silva, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 819–836. ISBN 9780128190968. [Google Scholar]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef]
- Shanab, S.M.; Shalaby, E.A. Algal Chemical Compounds; Lambert Academic Publishing: Saarbrucken, Germany, 2016. [Google Scholar]
- Cikos, A.-M.; Jokic, S.; Subaric, D.; Jerkovic, I. Overview on the Application of Modern Methods for the Extraction of Bioactive Compounds from Marine Macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef]
- Tziveleka, L.-A.; Tammam, M.A.; Tzakou, O.; Roussis, V.; Ioannou, E. Metabolites with Antioxidant Activity from Marine Macroalgae. Antioxidants 2021, 10, 1431. [Google Scholar] [CrossRef]
- Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar. Drugs 2021, 19, 484. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Hernandez, J.E.; Escobedo-Avellaneda, Z.; Iqbal, H.M.N.; Welti-Chanes, J. State-of-the-Art Extraction Methodologies for Bioactive Compounds from Algal Biome to Meet Bio-Economy Challenges and Opportunities. Molecules 2018, 23, 2953. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M.; Mohammad, S.; Ketut, S.I. Rahmatang Environmental-Friendly Extraction Methods to produce Bioactive Compounds in Seaweed. Res. J. Chem. Environ. 2023, 27, 114. [Google Scholar]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and Drawbacks of Ultrasound-Assisted Extraction for the Recovery of Bioactive Compounds from Marine Algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef]
- Xi, J. Ultrahigh pressure extraction of bioactive compounds from plants-A review. Crit. Rev. Food. Sci. Nutr. 2017, 57, 1097. [Google Scholar] [CrossRef]
- Pereira, T.; Barroso, S.; Mendes, S.; Amaral, R.A.; Dias, J.R.; Baptista, T.; Saraiva, J.A.; Alves, N.M.; Gil, M.M. Optimization of phycobiliprotein pigments extraction from red algae Gracilaria gracilis for substitution of synthetic food colorants. Food Chem. 2020, 321, 126688. [Google Scholar] [CrossRef] [PubMed]
- Francisco, J.; Horta, A.; Pedrosa, R.; Afonso, C.; Cardoso, C.; Bandarra, N.M.; Gil, M.M. Bioaccessibility of Antioxidants and Fatty Acids from Fucus Spiralis. Foods 2020, 9, 440. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.; Martins, A.; Silva, J.; Alves, C.; Pinteus, S.; Alves, J.; Teodoro, F.; Ribeiro, H.M.; Gonçalves, L.; Petrovski, Ž.; et al. Highlighting the Biological Potential of the Brown Seaweed Fucus spiralis for Skin Applications. Antioxidants 2020, 9, 611. [Google Scholar] [CrossRef] [PubMed]
- Almeida, B.; Barroso, S.; Ferreira, A.S.D.; Adão, P.; Mendes, S.; Gil, M.M. Seasonal Evaluation of Phlorotannin-Enriched Extracts from Brown Macroalgae Fucus spiralis. Molecules 2021, 26, 4287. [Google Scholar] [CrossRef]
- Khuri, A.I. Response surface methodology and its applications in agricultural and food sciences. Biom. Biostat. Int. J. 2017, 5, 155. [Google Scholar] [CrossRef]
- Peinado, I.; Girón, J.; Koutsidis, G.; Ames, J.M. Chemical composition, antioxidant activity and sensory evaluation of five different species of brown edible seaweeds. Food Res. Int. 2014, 66, 36–44. [Google Scholar] [CrossRef]
- Farvin, K.H.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef]
- Bittkau, K.S.; Neupane, S.; Alban, S. Initial evaluation of six different algae species as source for crude bioactive fucoidans. Algal Res. 2020, 45, 101759. [Google Scholar] [CrossRef]
- Kim, S.-K. Springer Handbook of Marine Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Rastogi, R.; Madamwar, D.; Pandey, A. Algal Green Chemistry: Recent Progress in Biotechnology; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Tierney, M.S.; Smyth, T.J.; Rai, D.K.; Soler-Vila, A.; Croft, A.K.; Brunton, N. Enrichment of polyphenol contents and antioxidant activities of Irish brown macroalgae using food-friendly techniques based on polarity and molecular size. Food Chem. 2013, 139, 753. [Google Scholar] [CrossRef]
- Dang, T.T.; Vuong, Q.V.; Schreider, M.J.; Bowyer, M.C.; Altena, I.A.V.; Scarlett, C.J. Optimization of ultrasound-assited extraction conditions for phenolic content and antioxidant activities of the alga Hormosira banksia using response surface methodology. J. Appl. Phycol. 2017, 29, 3161–3173. [Google Scholar] [CrossRef]
- Hassan, I.H.; Pham, H.N.T.; Nguyen, T.H. Optimization of ultrasound-assisted extraction conditions for phenolics, antioxidant, and tyrosinase inhibitory activities of Vietnamese brown seaweed (Padina australis). J. Food Process. Preserv. 2021, 45, e15386. [Google Scholar] [CrossRef]
- Fu, C.W.F.; Ho, C.W.; Yong, W.T.L.; Abas, F.; Tan, T.B.; Tan, C.P. Extraction of phenolic antioxidants from four selected seaweeds obtained from Sabah. Int. Food Res. J. 2016, 23, 2363–2369. [Google Scholar]
- Heffernan, N.; Smyth, T.J.; FitzGerald, R.J.; Soler-Vila, A.; Brunton, N. Antioxidant activity and phenolic content of pressurized liquid and solid-liquid extracts from Irish origin macroalgae. Int. J. Food Sci. Technol. 2014, 49, 1765–1772. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phospomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]

| Independent Variables | Vortex | Ultra-Turrax® Homogenizer | Ultrasonic Bath | Ultrasonic Probe | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Run | t (s) | R (g) | S (%) | DPPH | FRAP | TPC | DPPH | FRAP | TPC | DPPH | FRAP | TPC | DPPH | FRAP | TPC |
| 1 | 85 | 12 | 20 | 2.19 ± 0.19 | 265.06 ± 3.08 | 100.46 ± 2.86 | 13.25 ± 0.06 | 620.74 ± 52.80 | 189.90 ± 16.99 | 2.25 ± 0.01 | 265.10 ± 37.67 | 290.25 ± 11.27 | 3.73 ± 0.31 | 321.27 ± 24.83 | 291.17 ± 15.26 |
| 2 | 85 | 12 | 80 | 7.51 ± 0.14 | 157.30 ± 27.06 | 61.16 ± 4.39 | 20.24 ± 2.82 | 858.11 ± 119.49 | 110.05 ± 20.63 | 49.25 ± 8.65 | 157.15 ± 6.44 | 66.72 ± 1.45 | 33.18 ± 5.40 | 215.36 ± 10.27 | 173.29 ± 24.34 |
| 3 | 85 | 40 | 20 | 2.33 ± 0.16 | 331.37 ± 19.11 | 155.63 ± 9.13 | 11.27 ± 0.73 | 835.03 ± 44.70 | 154.89 ± 19.32 | 11.81 ± 1.27 | 331.53 ± 15.04 | 259.84 ± 19.60 | 6.07 ± 0.87 | 412.24 ± 60.41 | 474.16 ± 33.46 |
| 4 | 85 | 40 | 80 | 5.47 ± 1.07 | 227.70 ± 21.80 | 65.86 ± 1.46 | 8.64 ± 1.46 | 945.63 ± 134.87 | 157.02 ± 4.18 | 5.59 ± 0.43 | 227.67 ± 26.39 | 170.66 ± 20.99 | 8.21 ± 0.22 | 233.05 ± 29.32 | 213.30 ± 23.12 |
| 5 | 245 | 12 | 20 | 4.13 ± 0.00 | 208.01 ± 5.30 | 71.70 ± 11.45 | 9.77 ± 1.87 | 971.09 ± 138.90 | 185.60 ± 3.61 | 6.47 ± 1.13 | 207.95 ± 9.92 | 177.32 ± 9.56 | 6.43 ± 0.69 | 399.73 ± 64.34 | 179.60 ± 17.44 |
| 6 | 245 | 12 | 80 | 6.43 ± 0.39 | 119.42 ± 8.93 | 44.07 ± 7.22 | 28.34 ± 4.74 | 502.16 ± 80.13 | 120.16 ± 19.94 | 39.19 ± 2.14 | 119.20 ± 8.69 | 106.57 ± 17.86 | 20.06 ± 3.85 | 302.36 ± 59.94 | 269.45 ± 46.08 |
| 7 | 245 | 40 | 20 | 1.89 ± 0.25 | 419.45 ± 19.25 | 176.26 ± 6.87 | 7.14 ± 0.92 | 850.46 ± 24.06 | 185.11 ± 12.43 | 12.06 ± 0.00 | 419.76 ± 29.59 | 154.22 ± 27.72 | 5.57 ± 1.07 | 442.01 ± 48.25 | 408.05 ± 20.69 |
| 8 | 245 | 40 | 80 | 4.13 ± 0.48 | 253.09 ± 8.01 | 63.43 ± 9.06 | 9.10 ± 0.45 | 1143.08 ± 101.74 | 157.23 ± 4.28 | 4.91 ± 0.87 | 253.10 ± 0.52 | 170.47 ± 19.73 | 13.86 ± 1.90 | 181.79 ± 20.03 | 94.78 ± 16.12 |
| 9 | 30 | 26 | 50 | 3.78 ± 0.06 | 218.84 ± 1.35 | 108.49 ± 8.79 | 23.67 ± 2.51 | 1188.81 ± 185.00 | 121.22 ± 9.63 | 46.60 ± 0.42 | 218.79 ± 28.17 | 197.55 ± 13.50 | 6.65 ± 0.46 | 263.77 ± 38.71 | 211.72 ± 27.07 |
| 10 | 300 | 26 | 50 | 16.00 ± 0.28 | 200.15 ± 9.36 | 25.04 ± 4.46 | 12.70 ± 1.63 | 820.07 ± 33.70 | 116.01 ± 17.99 | 15.90 ± 2.90 | 200.08 ± 1.08 | 400.12 ± 32.14 | 7.14 ± 0.81 | 352.05 ± 59.94 | 220.83 ± 36.78 |
| 11 | 165 | 2 | 50 | 11.90 ± 0.71 | 96.51 ± 5.96 | 36.48 ± 6.04 | 20.96 ± 3.77 | 791.74 ± 51.39 | 135.98 ± 12.87 | 20.15 ± 1.87 | 96.25 ± 6.58 | 71.03 ± 11.33 | 18.46 ± 3.44 | 154.91 ± 29.34 | 181.25 ± 29.90 |
| 12 | 165 | 50 | 50 | 2.68 ± 0.30 | 316.70 ± 20.01 | 86.01 ± 11.85 | 10.55 ± 1.95 | 620.53 ± 86.74 | 123.95 ± 16.66 | 4.83 ± 0.00 | 316.83 ± 44.00 | 321.31 ± 19.63 | 12.99 ± 2.18 | 371.83 ± 65.08 | 435.08 ± 62.97 |
| 13 | 165 | 26 | 0 | 3.78 ± 0.06 | 325.37 ± 47.01 | 95.77 ± 7.41 | 3.67 ± 0.30 | 1011.33 ± 142.54 | 249.93 ± 38.65 | 2.65 ± 0.13 | 299.70 ± 5.22 | 171.79 ± 7.93 | 2.79 ± 0.43 | 517.85 ± 4.53 | 262.35 ± 1.22 |
| 14 | 165 | 26 | 100 | 9.97 ± 0.47 | 139.97 ± 24.35 | 53.91 ± 6.79 | 16.89 ± 1.80 | 718.92 ± 97.12 | 119.92 ± 10.17 | 103.55 ± 14.95 | 139.79 ± 7.64 | 140.76 ± 13.92 | 11.14 ± 0.28 | 215.79 ± 39.54 | 269.59 ± 12.20 |
| 15 (C) | 165 | 26 | 50 | 4.18 ± 0.68 | 248.31 ± 0.09 | 80.83 ± 9.05 | 15.88 ± 2.01 | 798.40 ± 69.88 | 149.39 ± 21.45 | 6.15 ± 1.11 | 248.31 ± 36.77 | 162.89 ± 20.28 | 5.14 ± 0.75 | 463.98 ± 76.80 | 165.33 ± 17.81 |
| 16 (C) | 165 | 26 | 50 | 3.75 ± 0.30 | 250.27 ± 31.50 | 106.26 ± 20.60 | 21.28 ± 2.82 | 568.89 ± 82.02 | 101.53 ± 3.89 | 10.09 ± 1.59 | 250.28 ± 10.94 | 163.02 ± 16.34 | 8.41 ± 0.06 | 286.66 ± 48.55 | 460.03 ± 62.87 |
| Independent Variables | High Pressure | |||||
|---|---|---|---|---|---|---|
| Run | t (s) | P (MPa) | S (%) | DPPH | FRAP | TPC |
| 1 | 604 | 122 | 20 | 212.83 ± 82.17 | 324.92 ± 17.87 | 190.66 ± 0.77 |
| 2 | 1496 | 122 | 20 | 163.45 ± 0.35 | 329.35 ± 26.56 | 190.62 ± 2.02 |
| 3 | 604 | 479 | 20 | 300.00 ± 0.00 | 178.07 ± 20.43 | 127.89 ± 0.83 |
| 4 | 1496 | 479 | 20 | 64.31 ± 10.80 | 229.72 ± 43.05 | 190.42 ± 1.38 |
| 5 | 604 | 122 | 80 | 143.97 ± 41.10 | 399.01 ± 37.12 | 193.86 ± 1.22 |
| 6 | 1496 | 122 | 80 | 54.77 ± 6.34 | 202.42 ± 16.09 | 111.05 ± 4.47 |
| 7 | 604 | 479 | 80 | 14.24 ± 1.09 | 409.50 ± 7.42 | 300.04 ± 5.14 |
| 8 | 1496 | 479 | 80 | 34.50 ± 2.18 | 305.96 ± 17.05 | 205.55 ± 1.21 |
| 9 | 300 | 300 | 50 | 53.36 ± 13.71 | 243.42 ± 0.88 | 103.45 ± 2.80 |
| 10 | 1800 | 300 | 50 | 78.01 ± 30.04 | 206.25 ± 18.56 | 118.88 ± 1.63 |
| 11 | 1050 | 0 | 50 | 4.39 ± 0.43 | 391.56 ± 32.04 | 331.42 ± 1.49 |
| 12 | 1050 | 600 | 50 | 4.06 ± 1.68 | 312.01 ± 46.85 | 246.45 ± 1.66 |
| 13 | 1050 | 300 | 0 | 12.28 4.06 | 272.19 ± 27.18 | 162.99 ± 1.20 |
| 14 | 1050 | 300 | 100 | 10.83 ± 2.31 | 280.76 ± 14.22 | 144.92 ± 0.99 |
| 15 (C) | 1050 | 300 | 50 | 11.11 ± 5.04 | 186.15 ± 17.83 | 105.59 ± 1.09 |
| 16 (C) | 1050 | 300 | 50 | 11.09 ± 0.81 | 300.31 ± 19.27 | 190.37 ± 0.86 |
| 17 (C) | 1050 | 300 | 50 | 6.46 ± 3.75 | 141.46 ± 10.30 | 82.16 ± 1.04 |
| 18 (C) | 1050 | 300 | 50 | 4.06 ± 0.57 | 281.04 ± 8.25 | 170.54 ± 1.32 |
| 19 (C) | 1050 | 300 | 50 | 4.80 ± 0.50 | 262.40 ± 4.64 | 223.85 ± 2.07 |
| 20 (C) | 1050 | 300 | 50 | 3.57 ± 0.58 | 184.79 ± 19.45 | 168.55 ± 1.73 |
| Extraction Method | Response Variable | Independent Variables with Significant Effects | Equation | R2 | R2 adj. |
|---|---|---|---|---|---|
| Vortex | DPPH | t(L,+); R(L,−); S(L,+) | ns | 0.523 | 0.000 |
| FRAP | t(Q,−); R(L,+;Q,−); S(L,−) t(L) by R(L) (+); R(L) by S(L) (−) | YFRAP = 235.285 + 3.571XR − 1.192XS − 0.001Xt2 − 0.036XR2 + 0.023XtXR − 0.022XRXS (Equation (1)) | 0.957 | 0.892 | |
| TPC | ns | ns | 0.762 | 0.405 | |
| Ultra-Turrax® homogenizer | DPPH | ns | ns | 0.899 | 0.747 |
| FRAP | ns | ns | 0.466 | 0.000 | |
| TPC | ns | ns | 0.857 | 0.642 | |
| Ultrasonic bath | DPPH | S(L,+;Q,+) | ns | 0.739 | 0.348 |
| FRAP | R(L,+;Q,+); S(L,−) t(L) by R(L) (+); R(L) by S(L) (−) | YFRAP = 227.603 + 3.306XR − 0.031XR2 + 0.023XtXR − 0.022XRXS (Equation (2)) | 0.942 | 0.855 | |
| TPC | t(L,+;Q,+); R(L,+;Q,+); S(L,−;Q,−) t(L) by R(L) (−); t(L) by S(L) (+); R(L) by S(L) (+) | YTPC = 428.734 − 2.349Xt − 0.195XR − 3.484XS + 0.006Xt2 + 0.005XR2 − 0.015XS2 − 0.004XtXR + 0.014XtXS + 0.065XRXS (Equation (3)) | 0.614 | 0.034 | |
| Ultrasonic probe | DPPH | ns | ns | 0.831 | 0.578 |
| FRAP | ns | ns | 0.830 | 0.575 | |
| TPC | ns | ns | 0.602 | 0.005 | |
| High pressure | DPPH | t(L,−;Q,+); P(L,−;Q,+); S(L,−;Q,+) t(L) by P(L) (−); t(L) by S(L) (+); P(L) by S(L) (−) | YDPPH = 501.12 − 0.55Xt − 0.14XP − 4.84XS + 0.001XP2 + 0.025XS2 + 0.002XtXS − 0.003XPXS (Equation (4)) | 0.520 | 0.087 |
| FRAP | P(Q,+) | YFRAP = 494.823 − 1.640XP + 0.002XP2 (Equation (5)) | 0.775 | 0.573 | |
| TPC | P(Q,+) | YTPC = 207.798 − 1.286XP + 0.001XP2 (Equation (6)) | 0.705 | 0.440 |
| Extraction Method | Optimal Conditions | Predicted | ||||
|---|---|---|---|---|---|---|
| t (s) | R (g) | S (%) | DPPH | FRAP | TPC | |
| Vortex | 165 | 50 | 25 | 1.36 | ||
| 300 | 50 | 25 | 454.64 | |||
| 300 | 50 | 0 | 193.86 | |||
| 165 | 50 | 0 | 0.51 | 457.62 | 197.35 | |
| Ultra-Turrax® homogenizer | 300 | 2 | 100 | 33.20 | ||
| 300 | 50 | 100 | 1246.87 | |||
| 300 | 14 | 0 | 267.53 | |||
| 165 | 26 | 50 | 18.68 | 686.38 | 124.32 | |
| Ultrasonic bath | 300 | 50 | 75 | 2.14 | ||
| 300 | 50 | 25 | 455.44 | |||
| 300 | 50 | 100 | 431.29 | |||
| 165 | 26 | 50 | 9.79 | 245.63 | 167.38 | |
| Ultrasonic probe | 30 | 14 | 100 | 35.42 | ||
| 165 | 50 | 0 | 534.31 | |||
| 165 | 50 | 25 | 517.81 | |||
| 165 | 26 | 50 | 6.57 | 374.88 | 312.44 | |
| t (s) | P (MPa) | S (%) | DPPH | FRAP | TPC | |
| High pressure | 1050 | 600 | 100 | 0 | ||
| 1800 | 600 | 100 | 431.12 | |||
| 1425 | 600 | 100 | 341.19 | |||
| 1050 | 600 | 100 | 0 | 540.66 | 391.06 | |
| Optimal Conditions | Predicted | Experimental | ||||||
|---|---|---|---|---|---|---|---|---|
| t (s) | R (g) | S (%) | DPPH | FRAP | TPC | DPPH | FRAP | TPC |
| 300 | 50 | 25 | 6.82 | 454.64 | 147.98 | 2.42 ± 0.35 | 324.54 ± 15.17 | 141.92 ± 13.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horta, A.; Duarte, A.M.; Barroso, S.; Pinto, F.R.; Mendes, S.; Lima, V.; Saraiva, J.A.; Gil, M.M. Extraction of Antioxidants from Brown Macroalgae Fucus spiralis. Molecules 2024, 29, 2271. https://doi.org/10.3390/molecules29102271
Horta A, Duarte AM, Barroso S, Pinto FR, Mendes S, Lima V, Saraiva JA, Gil MM. Extraction of Antioxidants from Brown Macroalgae Fucus spiralis. Molecules. 2024; 29(10):2271. https://doi.org/10.3390/molecules29102271
Chicago/Turabian StyleHorta, André, Ana M. Duarte, Sónia Barroso, Filipa R. Pinto, Susana Mendes, Vasco Lima, Jorge A. Saraiva, and Maria M. Gil. 2024. "Extraction of Antioxidants from Brown Macroalgae Fucus spiralis" Molecules 29, no. 10: 2271. https://doi.org/10.3390/molecules29102271
APA StyleHorta, A., Duarte, A. M., Barroso, S., Pinto, F. R., Mendes, S., Lima, V., Saraiva, J. A., & Gil, M. M. (2024). Extraction of Antioxidants from Brown Macroalgae Fucus spiralis. Molecules, 29(10), 2271. https://doi.org/10.3390/molecules29102271









