Discovery of Nine Dipeptidyl Peptidase-4 Inhibitors from Coptis chinensis Using Virtual Screening, Bioactivity Evaluation, and Binding Studies
Abstract
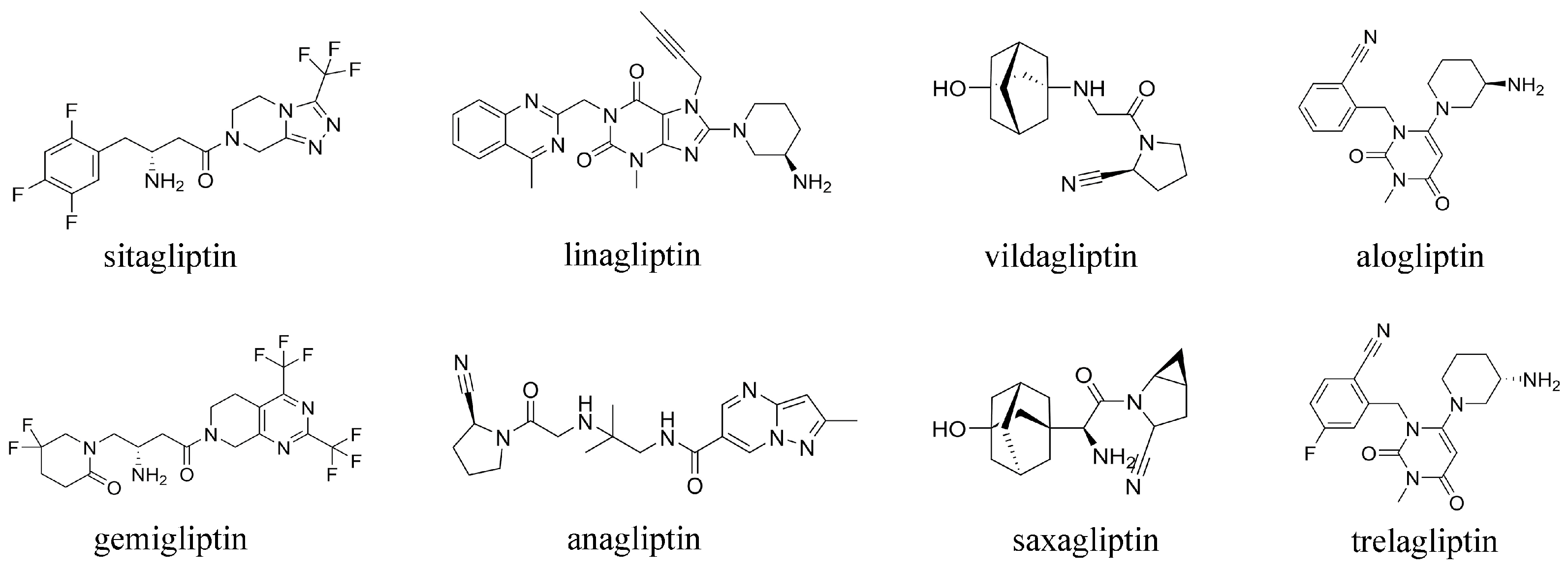
1. Introduction
2. Results
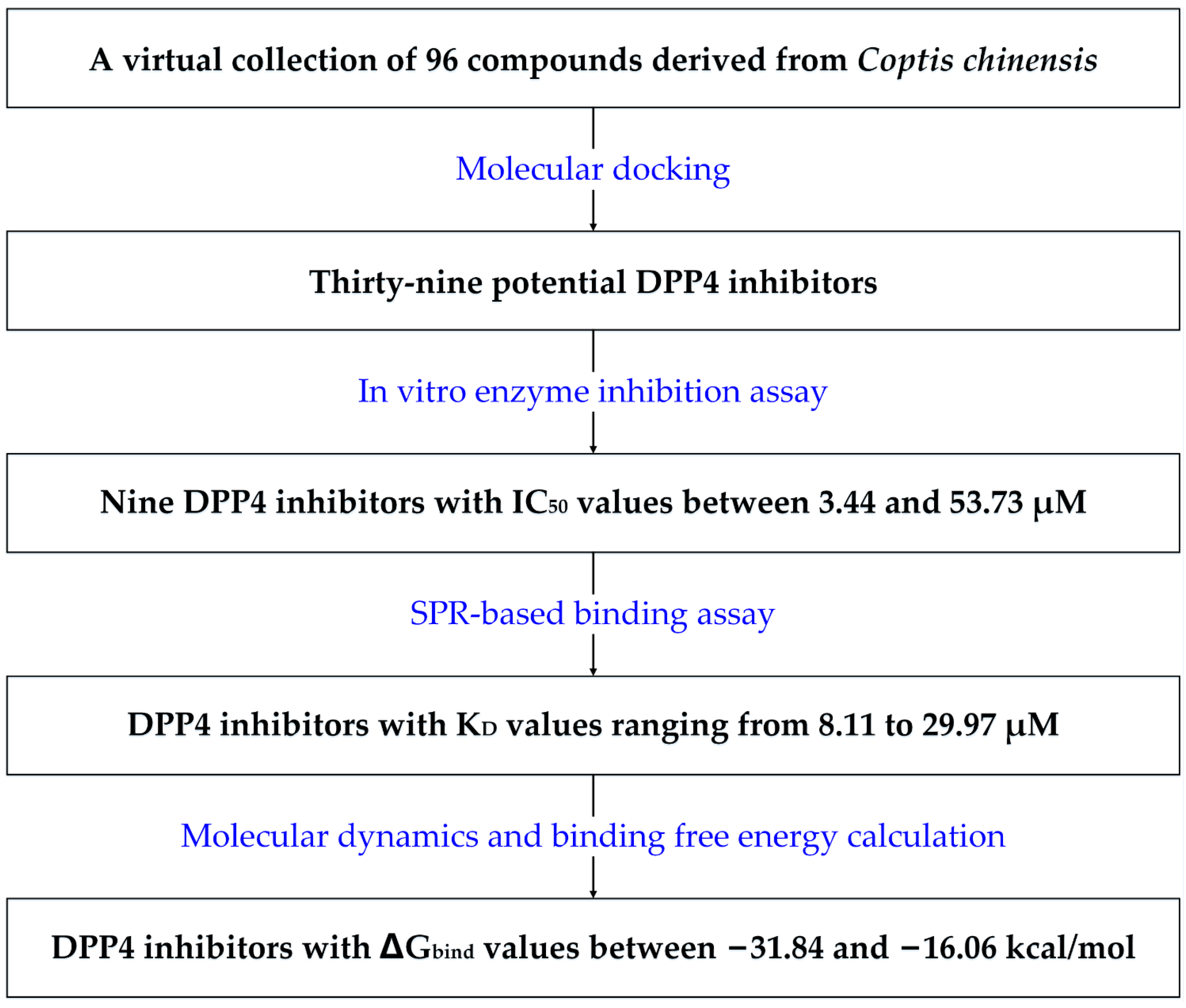
2.1. Potential DPP-4 Inhibitors Identified through Molecular Docking
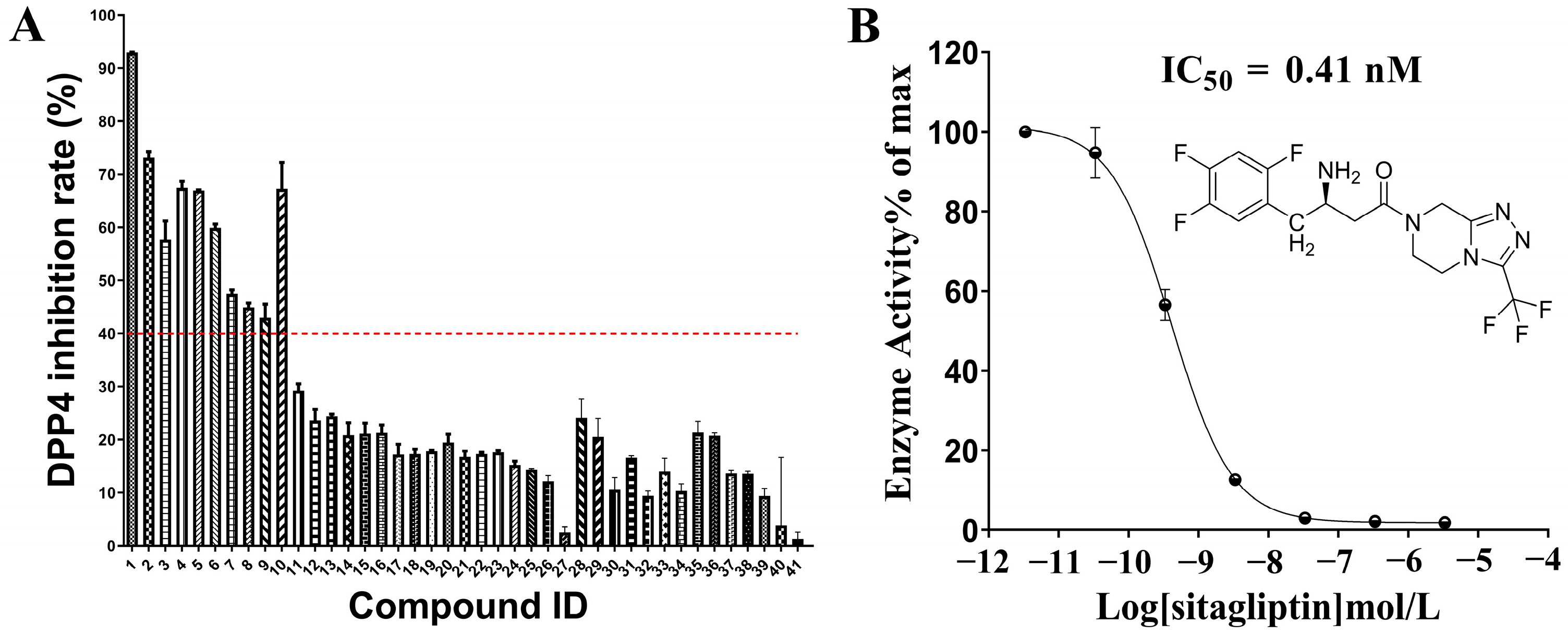
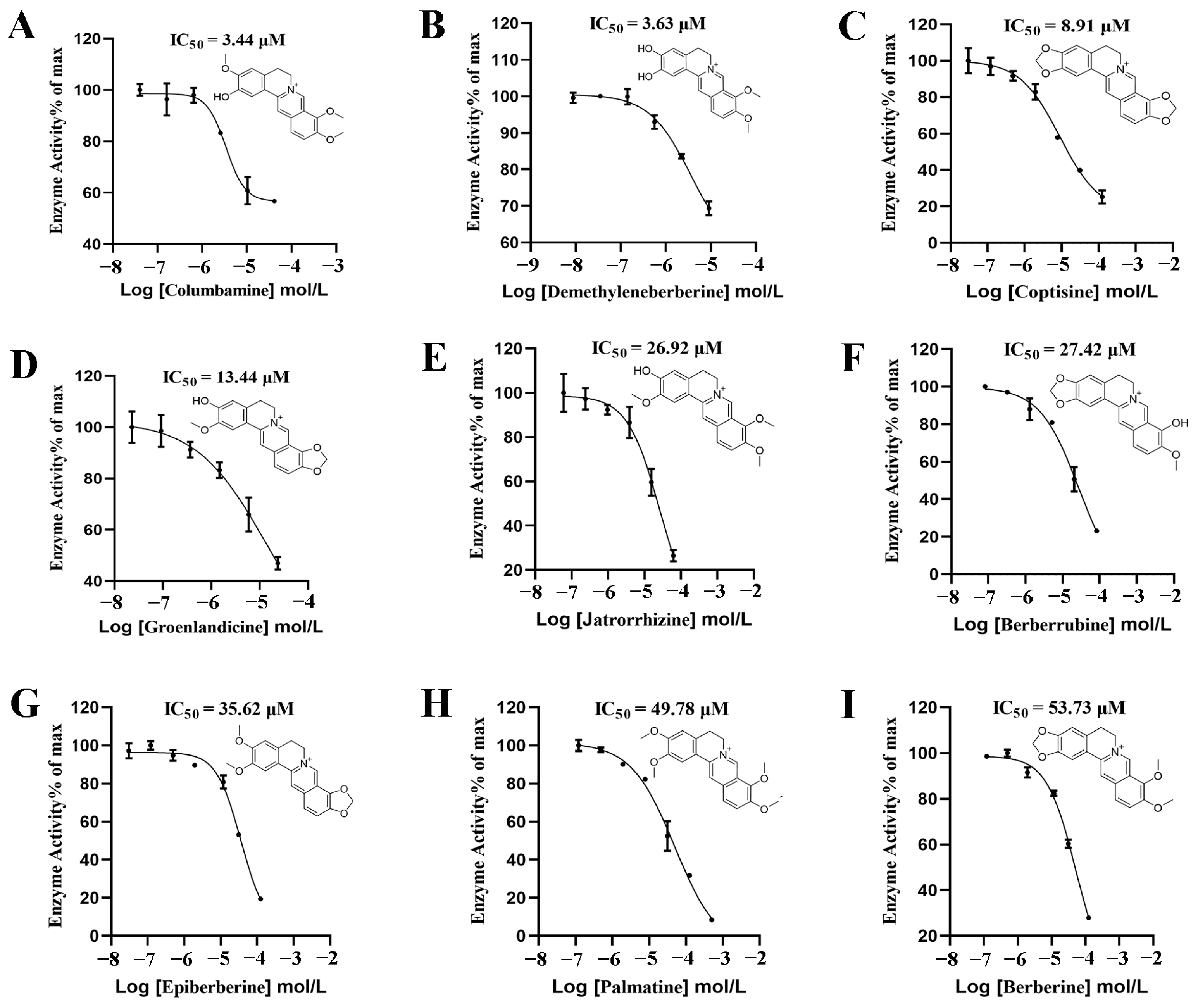
2.2. In Vitro Evaluation of DPP-4 Inhibitory Activity
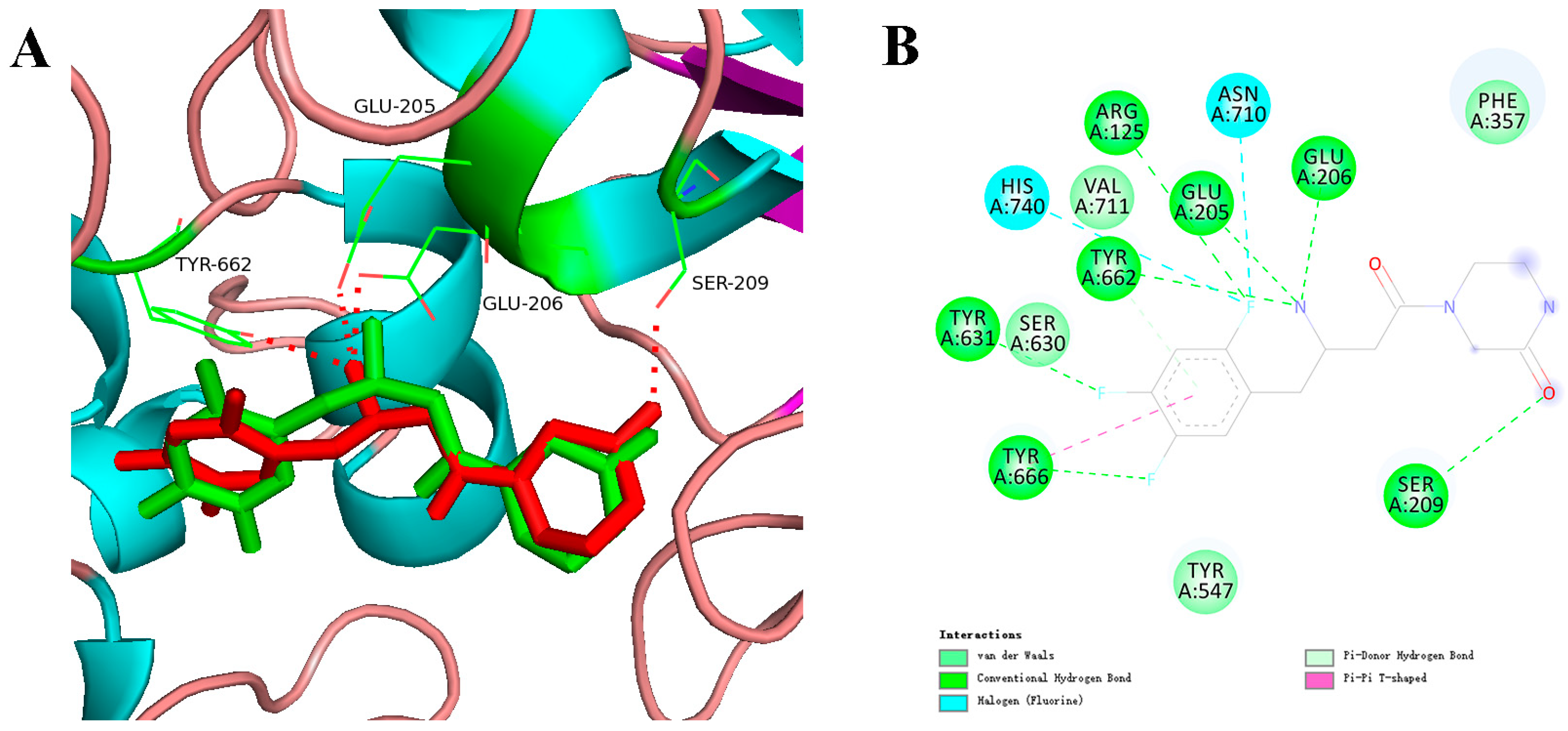
2.3. Interaction Analysis-Based Molecular Docking
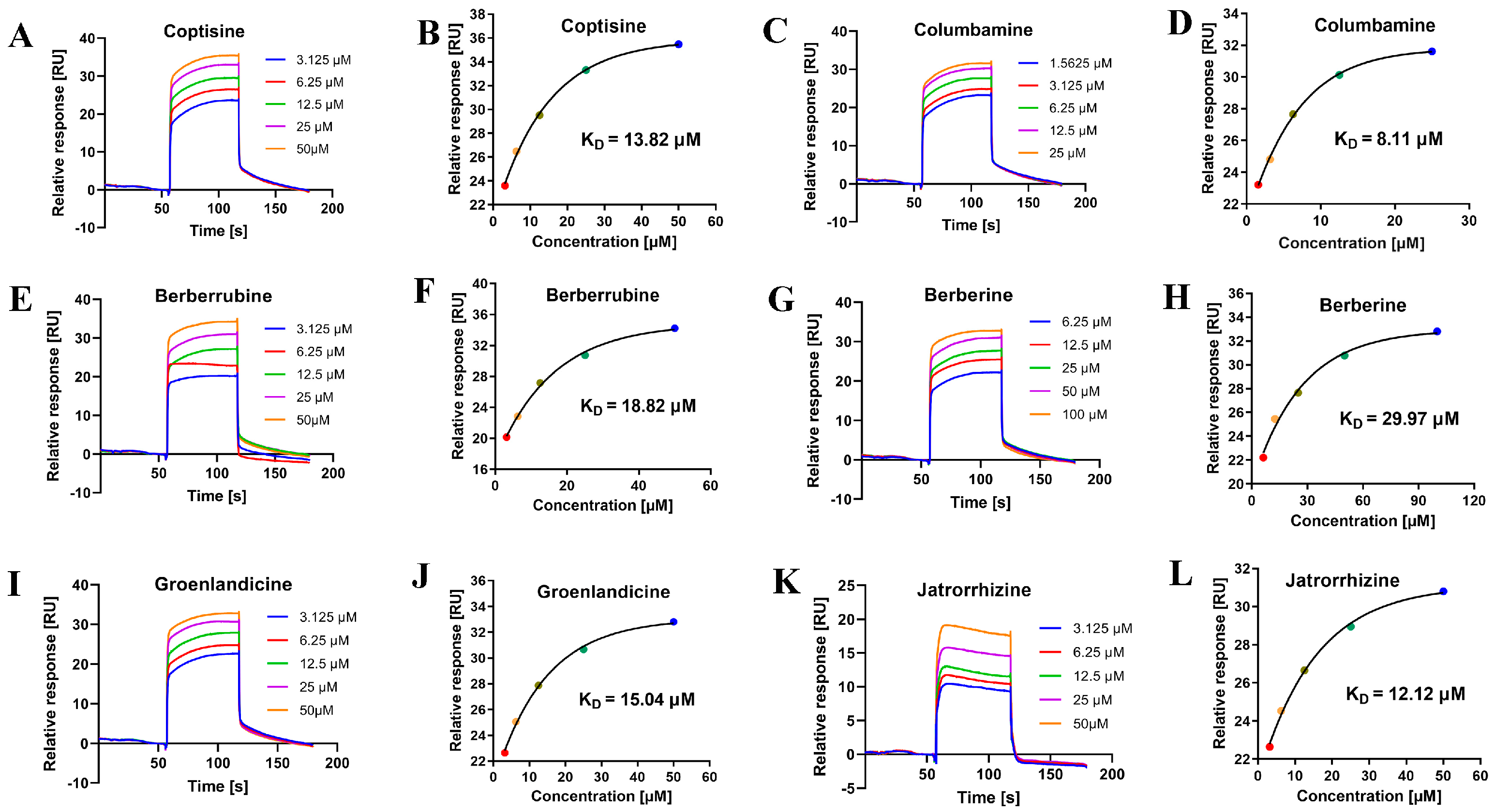
2.4. Binding Analysis Based on SPR
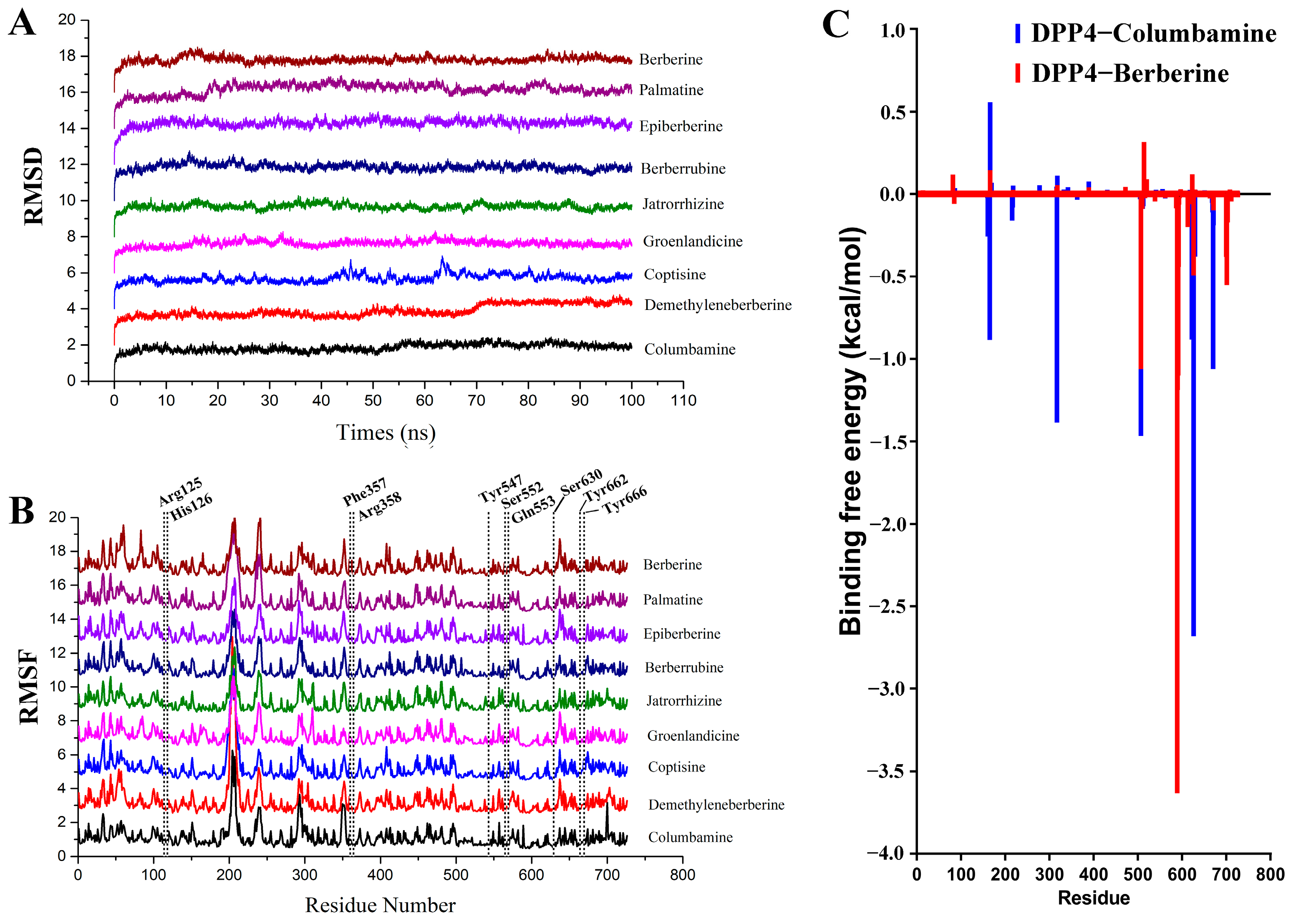
2.5. Molecular Dynamics Simulation
3. Discussion
4. Materials and Methods
4.1. Molecular Docking-Based Virtual Screening
4.2. DPP-4 Inhibition Assay
4.3. SPR-Based Binding Assay
4.4. Molecular Dynamics and Binding Free Energy Calculation
4.5. Statistical Method
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niebrzydowska-Tatus, M.; Pelech, A.; Bien, K.; Mekler, J.; Santiago, M.; Kimber-Trojnar, Z.; Trojnar, M. Association of dpp-4 concentrations with the occurrence of gestational diabetes mellitus and excessive gestational weight gain. Int. J. Mol. Sci. 2024, 25, 1829. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, E.; Wexler, D.J.; Kim, S.C.; Paik, J.M.; Alt, E.; Patorno, E. Comparing effectiveness and safety of sglt2 inhibitors vs dpp-4 inhibitors in patients with type 2 diabetes and varying baseline hba1c levels. JAMA Intern. Med. 2023, 183, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, Z.; Hang, J.; Liu, J.; Guo, F.; Ding, Y.; Li, M.; Nie, Q.; Lin, J.; Zhuo, Y.; et al. Microbial-host-isozyme analyses reveal microbial dpp4 as a potential antidiabetic target. Science 2023, 381, eadd5787. [Google Scholar] [CrossRef] [PubMed]
- Zhuge, F.; Ni, Y.; Nagashimada, M.; Nagata, N.; Xu, L.; Mukaida, N.; Kaneko, S.; Ota, T. Dpp-4 inhibition by linagliptin attenuates obesity-related inflammation and insulin resistance by regulating m1/m2 macrophage polarization. Diabetes 2016, 65, 2966–2979. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.K.; Nguyen, T.; Yi, J.M.; Kim, G.S.; Yun, H.R.; Kim, H.K.; Won, J.C. Evogliptin, a dpp-4 inhibitor, prevents diabetic cardiomyopathy by alleviating cardiac lipotoxicity in db/db mice. Exp. Mol. Med. 2023, 55, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Jun, D.W.; Kim, H.Y.; Lee, S.M.; Yoon, E.L.; Hwang, J.; Park, J.H.; Lee, H.; Kim, W.; Kim, H. Discovery of dipeptidyl peptidase-4 inhibitor specific biomarker in non-alcoholic fatty liver disease mouse models using modified basket trial. Clin. Mol. Hepatol. 2022, 28, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Stensen, S.; Gasbjerg, L.S.; Rosenkilde, M.M.; Vilsboll, T.; Holst, J.J.; Hartmann, B.; Christensen, M.B.; Knop, F.K. Endogenous glucose-dependent insulinotropic polypeptide contributes to sitagliptin-mediated improvement in beta-cell function in patients with type 2 diabetes. Diabetes 2022, 71, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Almagthali, A.G.; Alkhaldi, E.H.; Alzahrani, A.S.; Alghamdi, A.K.; Alghamdi, W.Y.; Kabel, A.M. Dipeptidyl peptidase-4 inhibitors: Anti-diabetic drugs with potential effects on cancer. Diabetes Metab. Syndr.-Clin. Res. Rev. 2019, 13, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Lyseng-Williamson, K.A. Sitagliptin. Drugs 2007, 67, 587–597. [Google Scholar] [CrossRef]
- Henness, S.; Keam, S.J. Vildagliptin. Drugs 2006, 66, 1989–2001, 2002–2004. [Google Scholar] [CrossRef]
- Garnock-Jones, K.P. Saxagliptin/dapagliflozin: A review in type 2 diabetes mellitus. Drugs 2017, 77, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M. Alogliptin: A review of its use in patients with type 2 diabetes mellitus. Drugs 2015, 75, 777–796. [Google Scholar] [CrossRef] [PubMed]
- Laffel, L.M.; Danne, T.; Klingensmith, G.J.; Tamborlane, W.V.; Willi, S.; Zeitler, P.; Neubacher, D.; Marquard, J. Efficacy and safety of the sglt2 inhibitor empagliflozin versus placebo and the dpp-4 inhibitor linagliptin versus placebo in young people with type 2 diabetes (dinamo): A multicentre, randomised, double-blind, parallel group, phase 3 trial. Lancet Diabetes Endocrinol. 2023, 11, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yang, H.; Kim, J.; Lee, Y.; Kim, S.H.; Do, I.G.; Park, C.Y. Gemigliptin, a dpp4 inhibitor, ameliorates nonalcoholic steatohepatitis through amp-activated protein kinase-independent and ulk1-mediated autophagy. Mol. Metab. 2023, 78, 101806. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, L.; Xing, M.; Xu, X.; Wei, J.; Wang, J.; Kang, W. Trelagliptin succinate: Dpp-4 inhibitor to improve insulin resistance in adipocytes. Biomed. Pharmacother. 2020, 125, 109952. [Google Scholar] [CrossRef]
- Wu, M.Z.; Chandramouli, C.; Wong, P.F.; Chan, Y.H.; Li, H.L.; Yu, S.Y.; Tse, Y.K.; Ren, Q.W.; Yu, S.Y.; Tse, H.F.; et al. Risk of sepsis and pneumonia in patients initiated on sglt2 inhibitors and dpp-4 inhibitors. Diabetes Metab. 2022, 48, 101367. [Google Scholar] [CrossRef] [PubMed]
- Fralick, M.; Colacci, M.; Thiruchelvam, D.; Gomes, T.; Redelmeier, D.A. Sodium-glucose co-transporter-2 inhibitors versus dipeptidyl peptidase-4 inhibitors and the risk of heart failure: A nationwide cohort study of older adults with diabetes mellitus. Diabetes Obes. Metab. 2021, 23, 950–960. [Google Scholar] [CrossRef]
- Zannad, F.; Rossignol, P. Dipeptidyl peptidase-4 inhibitors and the risk of heart failure. Circulation 2019, 139, 362–365. [Google Scholar] [CrossRef]
- Lepelley, M.; Khouri, C.; Lacroix, C.; Bouillet, L. Angiotensin-converting enzyme and dipeptidyl peptidase-4 inhibitor-induced angioedema: A disproportionality analysis of the who pharmacovigilance database. J. Allergy Clin. Immunol. 2020, 8, 2406–2408. [Google Scholar] [CrossRef]
- Ohyama, K.; Shindo, J.; Takahashi, T.; Takeuchi, H.; Hori, Y. Pharmacovigilance study of the association between dipeptidyl peptidase-4 inhibitors and angioedema using the fda adverse event reporting system (faers). Sci. Rep. 2022, 12, 13122. [Google Scholar] [CrossRef]
- Kim, N.H.; Choi, J.; Kim, N.H.; Choi, K.M.; Baik, S.H.; Lee, J.; Kim, S.G. Dipeptidyl peptidase-4 inhibitor use and risk of diabetic retinopathy: A population-based study. Diabetes Metab. 2018, 44, 361–367. [Google Scholar] [CrossRef]
- Ceriello, A.; De Nigris, V.; Iijima, H.; Matsui, T.; Gouda, M. The unique pharmacological and pharmacokinetic profile of teneligliptin: Implications for clinical practice. Drugs 2019, 79, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Golightly, L.K.; Drayna, C.C.; Mcdermott, M.T. Comparative clinical pharmacokinetics of dipeptidyl peptidase-4 inhibitors. Clin. Pharmacokinet. 2012, 51, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, H.; Runge, F.; Held, H.D. Excretion of the dipeptidyl peptidase-4 inhibitor linagliptin in rats is primarily by biliary excretion and p-gp-mediated efflux. Eur. J. Pharm. Sci. 2012, 45, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, Q.; Li, Y.; Li, P.; Wang, M.; Wang, T.; Wang, C.; Wang, T.; Zhao, B. Research progress on traditional chinese medicine syndromes of diabetes mellitus. Biomed. Pharmacother. 2020, 121, 109565. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Jin, D.; Bao, Q.; Ding, Q.; Zhang, H.; Gao, Z.; Song, J.; Lian, F.; Tong, X. Evidence and potential mechanisms of traditional chinese medicine for the treatment of type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes. Metab. 2019, 21, 1801–1816. [Google Scholar] [CrossRef]
- Lyu, Y.; Lin, L.; Xie, Y.; Li, D.; Xiao, M.; Zhang, Y.; Cheung, S.; Shaw, P.C.; Yang, X.; Chan, P.; et al. Blood-glucose-lowering effect of coptidis rhizoma extracts from different origins via gut microbiota modulation in db/db mice. Front. Pharmacol. 2021, 12, 684358. [Google Scholar] [CrossRef] [PubMed]
- Ran, Q.; Wang, J.; Wang, L.; Zeng, H.R.; Yang, X.B.; Huang, Q.W. Rhizoma coptidis as a potential treatment agent for type 2 diabetes mellitus and the underlying mechanisms: A review. Front. Pharmacol. 2019, 10, 805. [Google Scholar] [CrossRef]
- Huang, W.Y.; Dong, H. Coptidis rhizoma-contained traditional formulae for insomnia: A potential to prevent diabetes? Chin. J. Integr. Med. 2018, 24, 785–788. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, C.; Chen, M.; Zou, J.; Zhang, Z.; Cui, X.; Jiang, S.; Shang, E.; Qian, D.; Duan, J. Scutellariae radix and coptidis rhizoma ameliorate glycolipid metabolism of type 2 diabetic rats by modulating gut microbiota and its metabolites. Appl. Microbiol. Biotechnol. 2020, 104, 303–317. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, Y.; Ren, H.; Wang, S.; Zhong, H.; Zhao, X.; Ma, J.; Gu, X.; Xue, Y.; Huang, S.; et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the premote study). Nat. Commun. 2020, 11, 5015. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ren, H.; Zhong, H.; Zhao, X.; Li, C.; Ma, J.; Gu, X.; Xue, Y.; Huang, S.; Yang, J.; et al. Combined berberine and probiotic treatment as an effective regimen for improving postprandial hyperlipidemia in type 2 diabetes patients: A double blinded placebo controlled randomized study. Gut Microbes 2022, 14, 2003176. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Dong, H.; Guo, Y.; Gong, M.; Xia, Q.; Lu, F.; Wang, D. Multi-target regulation of intestinal microbiota by berberine to improve type 2 diabetes mellitus. Front. Endocrinol. 2022, 13, 1074348. [Google Scholar] [CrossRef]
- Naik, S.; Deora, N.; Pal, S.K.; Ahmed, M.Z.; Alqahtani, A.S.; Shukla, P.K.; Venkatraman, K.; Kumar, S. Purification, biochemical characterization, and dpp-iv and alpha-amylase inhibitory activity of berberine from cardiospermum halicacabum. J. Mol. Recognit. 2022, 35, e2983. [Google Scholar] [CrossRef] [PubMed]
- Al-Masri, I.M.; Mohammad, M.K.; Tahaa, M.O. Inhibition of dipeptidyl peptidase iv (dpp iv) is one of the mechanisms explaining the hypoglycemic effect of berberine. J. Enzym. Inhib. Med. Chem. 2009, 24, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; He, K.; Zhu, J.; Li, X.; Ye, X. The anti-hyperglycemia effects of rhizoma coptidis alkaloids: A systematic review of modern pharmacological studies of the traditional herbal medicine. Fitoterapia 2019, 134, 210–220. [Google Scholar] [CrossRef]
- Wang, K.; Feng, X.; Chai, L.; Cao, S.; Qiu, F. The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab. Rev. 2017, 49, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.L.; Jia, W.H.; Zhang, L.; Xu, C.Y.; Chen, X.; Yin, L.; Wang, N.Q.; Fang, L.H.; Qiang, G.F.; Yang, X.Y.; et al. Glucose consumption assay discovers coptisine with beneficial effect on diabetic mice. Eur. J. Pharmacol. 2019, 859, 172523. [Google Scholar] [CrossRef]
- Yang, T.C.; Chao, H.F.; Shi, L.S.; Chang, T.C.; Lin, H.C.; Chang, W.L. Alkaloids from coptis chinensis root promote glucose uptake in c2c12 myotubes. Fitoterapia 2014, 93, 239–244. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, Y.; Li, H.; Li, Y.; Wang, N.; Zhang, W.; Ma, B. Palmatine ameliorates high fat diet induced impaired glucose tolerance. Biol. Res. 2020, 53, 39. [Google Scholar] [CrossRef]
- Ekeuku, S.O.; Pang, K.L.; Chin, K.Y. Palmatine as an agent against metabolic syndrome and its related complications: A review. Drug Des. Dev. Ther. 2020, 14, 4963–4974. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Tong, J.; Zhou, G.; Mo, Q.; He, J.; Wang, Y. Coptis chinensis inflorescence ameliorates hyperglycaemia in 3t3-l1 preadipocyte and streptozotocin-induced diabetic mice. J. Funct. Food. 2016, 21, 455–462. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Wu, X.; Gao, Y.; Guo, J.; Tian, Y.; Lin, Z.; Wang, X. Discovery of natural 15-lox small molecule inhibitors from chinese herbal medicine using virtual screening, biological evaluation and molecular dynamics studies. Bioorganic Chem. 2021, 115, 105197. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Liu, Q.; Ai, Z.; Zhang, Y.; Xiang, Y.; Qiao, Y. Discovery of dual eta/etb receptor antagonists from traditional chinese herbs through in silico and in vitro screening. Int. J. Mol. Sci. 2016, 17, 389. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Kim, M.K.; Kim, H.D.; Kim, H.J.; Kim, J.W.; Lee, J.O.; Kim, C.W.; Kim, E.E. Unique binding mode of evogliptin with human dipeptidyl peptidase iv. Biochem. Biophys. Res. Commun. 2017, 494, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, J.; Ning, Z.; Wu, X. Discovery of a natural syk inhibitor from chinese medicine through a docking-based virtual screening and biological assay study. Molecules 2018, 23, 3114. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.G.; Wu, F.; Wang, Y.J.; Fu, Z.; Wang, Y.; Feng, Y.; Liang, S. Research on bitter components from coptis chinensis based on electronic tongue. Zhongguo Zhong Yao Za Zhi 2014, 39, 3326–3329. [Google Scholar] [PubMed]
- Zhong, F.; Shen, C.; Qi, L.; Ma, Y. A multi-level strategy based on metabolic and molecular genetic approaches for the characterization of different coptis medicines using hplc-uv and rad-seq techniques. Molecules 2018, 23, 3090. [Google Scholar] [CrossRef] [PubMed]
- Long, C.Y.; Yang, Y.; Huang, S.X.; Zhang, L.; Yang, Y.; Guo, Y.L. Changing regularity of alkaloids from coptis chinensis before and after processing based on hr-ms-database. Chin. Tradit. Herb. Drugs 2018, 53, 5972–5979. [Google Scholar]
- Qi, L.; Ma, Y.; Zhong, F.; Shen, C. Comprehensive quality assessment for rhizoma coptidis based on quantitative and qualitative metabolic profiles using high performance liquid chromatography, fourier transform near-infrared and fourier transform mid-infrared combined with multivariate statistical analysis. J. Pharm. Biomed. Anal. 2018, 161, 436–443. [Google Scholar] [CrossRef]
- Krishna, R.; Herman, G.; Wagner, J.A. Accelerating drug development using biomarkers: A case study with sitagliptin, a novel dpp4 inhibitor for type 2 diabetes. Aaps J. 2008, 10, 401–409. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schnapp, G.; Klein, T.; Hoevels, Y.; Bakker, R.A.; Nar, H. Comparative analysis of binding kinetics and thermodynamics of dipeptidyl peptidase-4 inhibitors and their relationship to structure. J. Med. Chem. 2016, 59, 7466–7477. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H. Gaussian~16 Revision c. 01 (2016); Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Case, D.A.; Belfon, K.; Ben-Shalom, I.; Brozell, S.R.; Cerutti, D.; Cheatham, T.; Cruzeiro, V.W.D.; Darden, T.; Duke, R.E.; Giambasu, G. Amber 2020. 2020. Available online: https://ambermd.org/CiteAmber.php (accessed on 16 January 2024).
- Pathak, A.K.; Bandyopadhyay, T. Temperature induced dynamical transition of biomolecules in polarizable and nonpolarizable tip3p water. J. Chem. Theory Comput. 2019, 15, 2706–2718. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Wang, W.; Ye, W.; Ji, D.; Luo, R.; Chen, H.F. Ff14idps force field improving the conformation sampling of intrinsically disordered proteins. Chem. Biol. Drug Des. 2017, 89, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Liu, R.; Martins, D.O.V.; Vazquez-Montelongo, E.A.; Henderson, J.A.; Shen, J. Gpu-accelerated all-atom particle-mesh ewald continuous constant ph molecular dynamics in amber. J. Chem. Theory Comput. 2022, 18, 7510–7527. [Google Scholar] [CrossRef]
- Genheden, S.; Ryde, U. Comparison of end-point continuum-solvation methods for the calculation of protein-ligand binding free energies. Proteins 2012, 80, 1326–1342. [Google Scholar] [CrossRef]
- Wu, F.; Hou, X.; Luo, H.; Zhou, M.; Zhang, W.; Ding, Z. Exploring the selectivity of pi3kα and mtor inhibitors by 3d-qsar, molecular dynamics simulations and mm/gbsa binding free energy decomposition. Medchemcomm 2013, 4, 1482. [Google Scholar] [CrossRef]

| Energy Component | Average | Std. Dev. | Std. Err. of Mean | |
|---|---|---|---|---|
| DPP-4– Columbamine | ΔEvdw | −35.93 | 2.65 | 0.08 |
| ∆Eele | −282.02 | 8.30 | 0.26 | |
| ∆EGB | 291.16 | 7.77 | 0.25 | |
| ∆ESURF | −5.05 | 0.16 | 0.00 | |
| ∆Ggas | −317.95 | 8.30 | 0.26 | |
| ∆Gsol | 286.11 | 7.77 | 0.25 | |
| ∆Gbind | −31.84 | 2.54 | 0.08 | |
| DPP-4– Demethyleneberberine | ΔEvdw | −23.95 | 5.13 | 0.16 |
| ∆Eele | −277.99 | 17.25 | 0.55 | |
| ∆EGB | 276.92 | 16.17 | 0.51 | |
| ∆ESURF | −3.70 | 0.41 | 0.01 | |
| ∆Ggas | −301.94 | 14.42 | 0.46 | |
| ∆Gsol | 273.21 | 16.45 | 0.52 | |
| ∆Gbind | −28.73 | 5.45 | 0.17 | |
| DPP-4– Coptisine | ΔEvdw | −28.79 | 2.24 | 0.07 |
| ∆Eele | −216.09 | 8.99 | 0.28 | |
| ∆EGB | 229.81 | 8.74 | 0.28 | |
| ∆ESURF | −2.90 | 0.15 | 0.00 | |
| ∆Ggas | −244.88 | 9.13 | 0.29 | |
| ∆Gsol | 226.91 | 8.70 | 0.28 | |
| ∆Gbind | −17.98 | 2.15 | 0.07 | |
| DPP-4– Groenlandicine | ΔEvdw | −23.60 | 3.52 | 0.11 |
| ∆Eele | −283.96 | 10.39 | 0.33 | |
| ∆EGB | 291.75 | 10.14 | 0.32 | |
| ∆ESURF | −3.61 | 0.33 | 0.01 | |
| ∆Ggas | −307.57 | 11.24 | 0.36 | |
| ∆Gsol | 288.15 | 9.98 | 0.32 | |
| ∆Gbind | −19.42 | 3.45 | 0.11 | |
| DPP-4– Jatrorrhizine | ΔEvdw | −32.43 | 2.76 | 0.09 |
| ∆Eele | −228.65 | 8.00 | 0.25 | |
| ∆EGB | 238.54 | 7.04 | 0.22 | |
| ∆ESURF | −4.42 | 0.19 | 0.01 | |
| ∆Ggas | −261.09 | 8.05 | 0.25 | |
| ∆Gsol | 234.11 | 7.03 | 0.22 | |
| ∆Gbind | −26.97 | 2.89 | 0.09 | |
| DPP-4– Berberrubine | ΔEvdw | −32.80 | 3.32 | 0.11 |
| ∆Eele | −289.40 | 10.88 | 0.34 | |
| ∆EGB | 300.01 | 8.61 | 0.27 | |
| ∆ESURF | −4.55 | 0.23 | 0.01 | |
| ∆Ggas | −322.21 | 10.99 | 0.35 | |
| ∆Gsol | 295.45 | 8.58 | 0.27 | |
| ∆Gbind | −26.75 | 4.77 | 0.15 | |
| DPP-4– Epiberberine | ΔEvdw | −30.50 | 2.68 | 0.08 |
| ∆Eele | −241.47 | 8.29 | 0.26 | |
| ∆EGB | 260.01 | 8.56 | 0.27 | |
| ∆ESURF | −4.10 | 0.38 | 0.01 | |
| ∆Ggas | −271.98 | 9.41 | 0.30 | |
| ∆Gsol | 255.92 | 8.38 | 0.26 | |
| ∆Gbind | −16.06 | 2.56 | 0.08 | |
| DPP-4– Palmatine | ΔEvdw | −33.91 | 2.33 | 0.07 |
| ∆Eele | −255.44 | 7.37 | 0.23 | |
| ∆EGB | 269.54 | 7.02 | 0.22 | |
| ∆ESURF | −4.27 | 0.24 | 0.01 | |
| ∆Ggas | −289.35 | 7.45 | 0.24 | |
| ∆Gsol | 265.27 | 6.98 | 0.22 | |
| ∆Gbind | −24.08 | 2.58 | 0.08 | |
| DPP-4– Berberine | ΔEvdw | −30.70 | 2.31 | 0.07 |
| ∆Eele | −204.52 | 12.53 | 0.40 | |
| ∆EGB | 218.71 | 12.96 | 0.41 | |
| ∆ESURF | −3.20 | 0.36 | 0.01 | |
| ∆Ggas | −235.23 | 12.68 | 0.40 | |
| ∆Gsol | 215.51 | 12.82 | 0.41 | |
| ∆Gbind | −19.72 | 2.40 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Ma, R.; Ma, Y.; Zhao, L.; Wang, L.; Fang, Y.; Zhang, Y.; Wu, X.; Wang, X. Discovery of Nine Dipeptidyl Peptidase-4 Inhibitors from Coptis chinensis Using Virtual Screening, Bioactivity Evaluation, and Binding Studies. Molecules 2024, 29, 2304. https://doi.org/10.3390/molecules29102304
Zhao Z, Ma R, Ma Y, Zhao L, Wang L, Fang Y, Zhang Y, Wu X, Wang X. Discovery of Nine Dipeptidyl Peptidase-4 Inhibitors from Coptis chinensis Using Virtual Screening, Bioactivity Evaluation, and Binding Studies. Molecules. 2024; 29(10):2304. https://doi.org/10.3390/molecules29102304
Chicago/Turabian StyleZhao, Zixi, Ruonan Ma, Yuqing Ma, Liqiang Zhao, Lele Wang, Yuzhen Fang, Yuxin Zhang, Xia Wu, and Xing Wang. 2024. "Discovery of Nine Dipeptidyl Peptidase-4 Inhibitors from Coptis chinensis Using Virtual Screening, Bioactivity Evaluation, and Binding Studies" Molecules 29, no. 10: 2304. https://doi.org/10.3390/molecules29102304
APA StyleZhao, Z., Ma, R., Ma, Y., Zhao, L., Wang, L., Fang, Y., Zhang, Y., Wu, X., & Wang, X. (2024). Discovery of Nine Dipeptidyl Peptidase-4 Inhibitors from Coptis chinensis Using Virtual Screening, Bioactivity Evaluation, and Binding Studies. Molecules, 29(10), 2304. https://doi.org/10.3390/molecules29102304








