Progress in the Regulation of Immune Cells in the Tumor Microenvironment by Bioactive Compounds of Traditional Chinese Medicine
Abstract
:1. Introduction
2. Bioactive Compounds in Traditional Chinese Medicine
3. Regulation of Macrophages by the Bioactive Compounds in Traditional Chinese Medicine
4. Regulation of Dendritic Cells by the Bioactive Compounds in Traditional Chinese Medicine
5. Regulation of Natural Killer Cells by the Bioactive Compounds in Traditional Chinese Medicine
6. Regulation of T-Cells by the Bioactive Compounds in Traditional Chinese Medicine
7. Regulation of Immune Checkpoints by Bioactive Compounds in Traditional Chinese Medicine
8. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Guo, X.; Han, F.; He, Z.; Wang, Y. Emerging role of natural products in cancer immunotherapy. Acta Pharm. Sin. B 2022, 12, 1163–1185. [Google Scholar] [CrossRef] [PubMed]
- Couzin-Frankel, J. Cancer immunotherapy. Science 2013, 342, 1432–1433. [Google Scholar] [CrossRef] [PubMed]
- Artis, D.; Spits, H. The biology of innate lymphoid cells. Nature 2015, 517, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Yost, K.E.; Satpathy, A.T.; Wells, D.K.; Qi, Y.; Wang, C.; Kageyama, R.; McNamara, K.L.; Granja, J.M.; Sarin, K.Y.; Brown, R.A.; et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat. Med. 2019, 25, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.; Simon, M. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef] [PubMed]
- Knorr, D.A.; Bachanova, V.; Verneris, M.R.; Miller, J.S. Clinical utility of natural killer cells in cancer therapy and transplantation. Semin. Immunol. 2014, 26, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, X.; Huang, L. Macrophage-mediated tumor cell phagocytosis: Opportunity for nanomedicine intervention. Adv. Funct. Mater. 2021, 31, 2006220. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Barnoud, C.; Cenerenti, M.; Sun, M.; Caffa, I.; Kizil, B.; Bill, R.; Liu, Y.; Pick, R.; Garnier, L.; et al. Dendritic cells direct circadian anti-tumour immune responses. Nature 2023, 614, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Archilla-Ortega, A.; Domuro, C.; Martin-Liberal, J.; Muñoz, P. Blockade of novel immune checkpoints and new therapeutic combinations to boost antitumor immunity. J. Exp. Clin. Cancer Res. 2022, 41, 62. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Wang, L. Using Traditional Chinese Medicine to treat hepatocellular carcinoma by targeting tumor immunity. Evid. Based Complement. Altern. Med. 2020, 2020, 9843486. [Google Scholar] [CrossRef] [PubMed]
- Finck, A.; Gill, S.I.; June, C.H. Cancer immunotherapy comes of age and looks for maturity. Nat. Commun. 2020, 11, 3325. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, S.; Xin, Y. Advances in tumor immunotherapy tesearch. Chin. J. Clin. Pharmacol. Ther. 2016, 21, 1074–1080. [Google Scholar]
- He, J.; Yin, P.; Xu, K. Effect and molecular mechanisms of traditional Chinese medicine on tumor targeting tumor-associated macrophages. Drug Des. Dev. Ther. 2020, 14, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yin, S.; Lu, J.; Zhou, S.; Shao, Y.; Bao, X.; Wang, T.; Qiu, Y.; Yu, H. Tumor microenvironment: A prospective target of natural alkaloids for cancer treatment. Cancer Cell Int. 2021, 21, 386. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Pan, J.; Wang, J.; Pei, Y.; Zhou, R. Current research status of alkaloids against breast cancer. J. Physiol. Investig. 2022, 65, 12. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Li, M.; Tian, F.; Yu, F.; Zhao, W. An overview of antitumour activity of polysaccharides. Molecules 2022, 27, 8083. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, G. Antitumor activity of polysaccharides: An overview. Curr. Drug Targets 2018, 19, 89–96. [Google Scholar] [CrossRef]
- Xiao, G.; Shao, X.; Zhu, D.; Yu, B. Chemical synthesis of marine saponins. Nat. Prod. Rep. 2019, 36, 769–787. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, T.; Fong, C.M.V.; Chen, X.; Chen, X.; Wang, Y.; Huang, M.; Lu, J. Saponins from Chinese Medicines as Anticancer Agents. Molecules 2016, 21, 1326. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.; Fang, X.; Yang, J.; Yao, Y.; Nandakumar, K.S.; Salem, M.L.; Cheng, K. Recent research on flavonoids and their biomedical applications. Curr. Med. Chem. 2021, 28, 1042–1066. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Ahmad, J.; Ahamad, J.; Kundu, S.; Goel, A.; Mishra, A. Flavonoids as promising anticancer therapeutics: Contemporary research, nanoantioxidant potential, and future scope. Phytother. Res. 2023, 37, 5159–5192. [Google Scholar] [CrossRef]
- Min, L.; Wang, H.; Qi, H. Astragaloside IV inhibits the progression of liver cancer by modulating macrophage polarization through the TLR4/NF-κB/STAT3 signaling pathway. Am. J. Transl. Res. 2022, 14, 1551–1566. [Google Scholar]
- Xu, F.; Cui, W.; Wei, Y.; Cui, J.; Qiu, J.; Hu, L.; Gong, W.; Dong, J.; Liu, B. Astragaloside IV inhibits lung cancer progression and metastasis by modulating macrophage polarization through AMPK signaling. J. Exp. Clin. Cancer Res. 2018, 37, 207. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, N.; Zhu, W.; Wu, J.; Yang, X.; Teng, W.; Tian, J.; Fang, Z.; Luo, Y.; Chen, M.; et al. Modulation the crosstalk between tumor-associated macrophages and non-small cell lung cancer to inhibit tumor migration and invasion by ginsenoside Rh2. BMC Cancer 2018, 18, 579. [Google Scholar] [CrossRef] [PubMed]
- Takei, M.; Tachikawa, E.; Umeyama, A. Dendritic cells promoted by Ginseng Saponins drive a potent Th1 polarization. Biomark. Insights 2008, 3, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Ru, Q.; Chen, L.; Ma, B.; Li, C. Stereospecificity of ginsenoside Rg3 in the promotion of cellular immunity in hepatoma H22-bearing mice. J. Food Sci. 2014, 79, H1430–H1435. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Zhang, J.; Zhou, C.; Li, Y.; Duan, W.; Zhang, B.; Wang, M.; Fang, J. 20(S)-Ginsenoside Rh2 displays efficacy against T-cell acute lymphoblastic leukemia through the PI3K/Akt/mTOR signal pathway. J. Ginseng Res. 2020, 44, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, J.; Ge, S.; Chen, C.; Li, S.; Wu, X.; Feng, X.; Wang, Y.; Cai, D. Saikosaponin A Inhibits Breast Cancer by Regulating Th1/Th2 Balance. Front. Pharmacol. 2019, 10, 624. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, X.; Yu, S.; Huang, S.; Ding, Y.; Chen, W.; Wang, A.; Lu, Y. Role and mechanism of ginsenoside Rg3 in regulating the immune checkpoint PD-L1 to inhibit the proliferation of lung cancer Lewis cells. Chin. Tradit. Herbal. Drugs 2019, 50, 166–171. [Google Scholar]
- Qu, X.; Zeng, Y.; Yao, L. Inhibitory effect of Astragaloside down-regulation of PD-1 and PD-L1 expression on invasion and migration of cervical cancer Hela cells. Immunol. J. 2018, 34, 850–855. [Google Scholar]
- Liu, C.; Zhang, X.; Tan, Q.; Xu, W.; Zhou, C.; Luo, M.; Li, X.; Huang, R.; Zeng, X. NF-κB pathways are involved in M1 polarization of RAW 264.7 macrophage by polyporus polysaccharide in the tumor microenvironment. PLoS ONE 2017, 12, e0188317. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, X.; Lai, G.; Li, J.; Jia, W.; Cao, Y.; Xu, W.; Tan, Q.; Zhou, C.; Luo, M.; et al. Mechanisms of macrophage immunomodulatory activity induced by a new polysaccharide isolated from Polyporus umbellatus (Pers.) fries. Front. Chem. 2020, 8, 581. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; He, D.; Zhang, S.; Chen, H.; Zhao, J.; Li, X.; Zeng, X. Homogeneous Polyporus Polysaccharide inhibit bladder cancer by resetting tumor-associated macrophages toward M1 through NF-κB/NLRP3 signaling. Front. Immunol. 2022, 13, 839460. [Google Scholar] [CrossRef] [PubMed]
- Bamodu, O.A.; Kuo, K.; Wang, C.; Huang, W.; Wu, A.T.H.; Tsai, J.; Lee, K.; Yeh, C.; Wang, L. Astragalus polysaccharides (PG2) enhances the M1 polarization of macrophages, functional maturation of dendritic cells, and T cell-mediated anticancer immune responses in patients with lung cancer. Nutrients 2019, 11, 2264. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Lai, T.H.; Chyan, Y.J.; Yin, S.Y.; Chen, Y.H.; Wei, W.C.; Yang, N.-S. Specific medicinal plant polysaccharides effectively enhance the potency of a DC-based vaccine against mouse mammary tumor metastasis. PLoS ONE 2015, 10, e0122374. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Lin, Z.; Duan, X.; Lu, J.; Ge, Z.; Song, Y.; Li, X.; Li, M.; Xing, E.; Yang, N.; et al. Stronger cytotoxicity in CTLs with granzyme B and porforin was induced by Ganoderma lucidum polysaccharides acting on B16F10 cells. Biomed. Prev. Nutr. 2012, 2, 113–118. [Google Scholar] [CrossRef]
- Li, A.; Shuai, X.; Jia, Z.; Li, H.; Liang, X.; Su, D.; Guo, W. Ganoderma lucidum polysaccharide extract inhibits hepatocellular carcinoma growth by downregulating regulatory T cells accumulation and function by inducing microRNA-125b. J. Transl. Med. 2015, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, X.; Liu, B.; Xu, L.; Zhao, H.; Lu, A. Down-regulation of Treg cells and up-regulation of TH1/TH2 cytokine ratio were induced by polysaccharide from Radix Glycyrrhizae in H22 hepatocarcinoma bearing mice. Molecules 2011, 16, 8343–8352. [Google Scholar] [CrossRef]
- Ayeka, P.; Bian, Y.; Githaiga, P.; Zhao, Y. The immunomodulatory activities of licorice polysaccharides (Glycyrrhiza uralensis Fisch.) in CT 26 tumor-bearing mice. BMC Complement. Altern. Med. 2017, 17, 536. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, Q.; Xiao, J.; Zhang, X.; Han, X.; Shi, X.; Hu, J.; Li, L.; Qian, X. Cancer cell membrane-coated Gambogic Acid nanoparticles for effective anticancer vaccination by activating dendritic cells. Int. J. Nanomed. 2023, 18, 2261–2273. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Kuang, X.; Yin, M.; Liu, X.; Liu, Y.; Deng, H. Hyperoside exerts anti-non-small cell lung cancer effects by down-regulating PD-L1 expression study. Acta Pharm. Sin. 2021, 56, 2817–2824. [Google Scholar]
- Peng, F.; Xiong, L.; Peng, C. (-)-Sativan inhibits tumor development and regulates miR-200c/PD-L1 in triple negative breast cancer cells. Front. Pharmacol. 2020, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Lin, J.; Yang, Y.; Tao, L.; Li, Y.; Liu, Z.; Zhao, Q.; Diao, A. Quercetin inhibiting the PD-1/PD-L1 interaction for immune-enhancing cancer chemopreventive agent. Phytother. Res. 2021, 35, 6441–6451. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, M.; Liu, T.; Li, J.; Sun, S.; Chen, J.; Liu, Z.; Zhang, Z.; Zhang, L. Quercetin-ferrum nanoparticles enhance photothermal therapy by modulating the tumor immunosuppressive microenvironment. Acta Biomater. 2022, 154, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wang, W.; Xu, C.; Xie, Y.; Wang, X.; Zhang, Y.; Huang, J.; Huang, M.; Xie, C.; Liu, P.; et al. Luteolin and its derivative apigenin suppress the inducible PD-L1 expression to improve anti-tumor immunity in KRAS-mutant lung cancer. Cancer Lett. 2021, 515, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Sun, R.; Xiao, W.; Feng, J.; Zhen, C.; Xu, X.; Tian, Z. Type two cytokines predominance of human lung cancer and its reverse by Traditional Chinese Medicine TTMP. Mol. Immunol. 2004, 1, 8. [Google Scholar]
- Liu, Y.; Liu, X.; Zhang, N.; Yin, M.; Dong, J.; Zeng, Q.; Mao, G.; Song, D.; Liu, L.; Deng, H. Berberine diminishes cancer cell PD-L1 expression and facilitates antitumor immunity via inhibiting the deubiquitination activity of CSN5. Acta Pharm. Sin. B 2020, 10, 2299–2312. [Google Scholar] [CrossRef]
- Jiang, Z.; Huang, J.; Xie, Y.; Zhang, Y.; Chang, C.; Lai, H.; Wang, W.; Yao, X.; Fan, X.; Wu, Q.; et al. Evodiamine suppresses non-small cell lung cancer by elevating CD8+ T cells and downregulating the MUC1-C/PD-L1 axis. J. Exp. Clin. Cancer Res. 2020, 39, 249. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.A.; Li, M.O. Ontogeny of tumor-associated macrophages and its implication in cancer regulation. Trends Cancer 2016, 2, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Orekhov, A.; Orekhova, V.; Nikiforov, N.; Myasoedova, V.; Grechko, A.; Romanenko, E.; Zhang, D.; Chistiakov, D. Monocyte differentiation and macrophage polarization. Vessel. Plus 2019, 3, 10. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Chen, Y.; Liang, C.-L.; Liu, H.; Qiu, F.; Dai, Z. Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomed. Pharmacother. 2020, 121, 109570. [Google Scholar] [CrossRef] [PubMed]
- Italiani, P.; Boraschi, D. From monocytes to M1/M2 macrophages: Phenotypical vs. functional differentiation. Front. Immunol. 2014, 5, 116283. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Jin, Z.; Yuan, Y.; Liu, R.; Xu, T.; Wei, H.; Xu, X.; He, S.; Chen, S.; Shi, Z.; et al. New mechanisms of tumor-associated macrophages on promoting tumor progression: Recent research advances and potential targets for tumor immunotherapy. J. Immunol. Res. 2016, 2016, 9720912. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Min, J.; Zeng, R.; Liu, H.; Li, H.; Zhang, C.; Zheng, A.; Lin, J.; Liu, X.; Wu, M. Self-assembled traditional Chinese nanomedicine modulating tumor immunosuppressive microenvironment for colorectal cancer immunotherapy. Theranostics 2022, 12, 6088–6105. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Li, Y.; Wang, Y.; Zhang, Y.; Chen, J.; Liu, W.; Tang, J.; Yue, F.; Yang, J. Dihydroartemisinin inhibits Lewis Lung carcinoma progression by inducing macrophages M1 polarization via AKT/mTOR pathway. Int. Immunopharmacol. 2022, 103, 108427. [Google Scholar] [CrossRef] [PubMed]
- Yen, J.; Huang, W.; Lin, S.; Huang, Y.; Chio, W.; Tsay, G.; Hung, M.; Huang, S.-T. Metabolic remodeling in tumor-associated macrophages contributing to antitumor activity of cryptotanshinone by regulating TRAF6-ASK1 axis. Mol. Ther. Oncolytics 2022, 26, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wei, Q.; Lv, Y.; Weng, L.; Huang, H.; Wei, Q.; Li, M.; Mao, Y.; Hua, D.; Cai, X.; et al. Ginseng-derived nanoparticles potentiate immune checkpoint antibody efficacy by reprogramming the cold tumor microenvironment. Mol. Ther. 2022, 30, 327–340. [Google Scholar] [CrossRef]
- Zhou, Y.; Slone, N.; Chrisikos, T.; Kyrysyuk, O.; Babcock, R.; Medik, Y.; Li, H.; Kleinerman, E.; Watowich, S. Vaccine efficacy against primary and metastatic cancer with in vitro-generated CD103+ conventional dendritic cells. J. Immunother. Cancer 2020, 8, e000474. [Google Scholar] [CrossRef] [PubMed]
- Sabado, R.L.; Balan, S.; Bhardwaj, N. Dendritic cell-based immunotherapy. Cell Res. 2017, 27, 74–95. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.-M.; Park, H.-B.; Jin, J.-O. Polysaccharide from Astragalus membranaceus promotes the activation of human peripheral blood and mouse spleen dendritic cells. Chin. J. Nat. Med. 2021, 19, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Han, Z.; Trivett, A.; Lin, H.; Hannifin, S.; Yang, D.; Oppenheim, J. Cryptotanshinone has curative dual anti-proliferative and immunotherapeutic effects on mouse Lewis lung carcinoma. Cancer Immunol. Immunother. 2019, 68, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chiang, C.; Tsai, M.; Hseu, R.; Shu, W.; Chuang, C.; Sun, Y.; Chang, Y.; Lin, J.; Chen, C.; et al. Two-sided effect of Cordyceps sinensis on dendritic cells in different physiological stages. J. Leukoc. Biol. 2009, 85, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Bi, S.; Guo, T.; Sun, D.; Zou, Y.; Wang, L.; Song, L.; Chu, D.; Liao, A.; Song, X.; et al. Nano co-delivery of Plumbagin and Dihydrotanshinone I reverses immunosuppressive TME of liver cancer. J. Control. Release 2022, 348, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Zhang, Q.; Wang, S.; Gao, Y.; Chen, X.; Zhao, Y.; Qian, X. Gambogic acid impairs tumor angiogenesis by targeting YAP/STAT3 signaling axis. Phytother. Res. 2019, 33, 1579–1591. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhu, H. Advances in the study of NK cells in tumor immunotherapy. Chin. J. Immunol. 2023, 39, 1318–1325. [Google Scholar]
- Wu, X.; Yang, H.; Chen, X.; Gao, J.; Duan, Y.; Wei, D.; Zhang, J.; Ge, K.; Liang, X.-J.; Huang, Y.; et al. Nano-herb medicine and PDT induced synergistic immunotherapy for colon cancer treatment. Biomaterials 2021, 269, 120654. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C.; Piao, X.; Song, W.; Wang, W.; Bai, J.; Wang, B. Experimental studies on the killing of lung cancer A549 cells by Emodin through modulation of in vitro expanded NK cells. J. Liaodong Univ. (Nat. Sci.) 2019, 26, 102–106. [Google Scholar]
- Lee, J.; Lee, S.; Lim, K. ZPDC glycoprotein (24kDa) induces apoptosis and enhances activity of NK cells in N-nitrosodiethylamine-injected Balb/c. Cell. Immunol. 2014, 289, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, J.; Lu, X.; Chen, F.; Zhou, Z.; Wang, T.; Zhu, S.; Fei, S. The enhanced effect of lupeol on the destruction of gastric cancer cells by NK cells. Int. Immunopharmacol. 2013, 16, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Ogawara, H.; Hiroshi, H. Th1/Th2 cells in patients with multiple myeloma. Hematology 2004, 9, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Sakaguchi, S. Regulatory T cells in immune surveillance and treatment of cancer. Semin. Cancer Biol. 2006, 16, 115–123. [Google Scholar] [CrossRef]
- Karpisheh, V.; Mousavi, S.M.; Naghavi Sheykholeslami, P.; Fathi, M.; Mohammadpour Saray, M.; Aghebati-Maleki, L.; Jafari, R.; Majidi Zolbanin, N.; Jadidi-Niaragh, F. The role of regulatory T cells in the pathogenesis and treatment of prostate cancer. Life Sci. 2021, 284, 119132. [Google Scholar] [CrossRef] [PubMed]
- Golubovskaya, V.; Wu, L. Different subsets of T cells, memory, effector functions, and CAR-T immunotherapy. Cancers 2016, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Ostroumov, D.; Fekete-Drimusz, N.; Saborowski, M.; Kühnel, F.; Woller, N. CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell. Mol. Life Sci. 2018, 75, 689–713. [Google Scholar] [CrossRef] [PubMed]
- Vantourout, P.; Hayday, A. Six-of-the-best: Unique contributions of γδ T cells to immunology. Nat. Rev. Immunol. 2013, 13, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Sutton, C.E.; Lalor, S.J.; Sweeney, C.M.; Brereton, C.F.; Lavelle, E.C.; Mills, K.H.G. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity 2009, 31, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Yang, B.; Chen, J.; Wang, S.; Zhang, W.; Guo, Y.; Han, Y.; Li, H.; Dang, Y.; Yuan, Y.; et al. Gallic acid induces T-helper-1-like Treg cells and strengthens immune checkpoint blockade efficacy. J. Immunother. Cancer 2022, 10, e004037. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Park, S.-M.; Lee, M.; Ha, I.J.; Jeong, M. The Sesquiterpene Lactone-Rich Fraction of Inula helenium L. enhances the antitumor effect of anti-PD-1 antibody in colorectal cancer: Integrative phytochemical, transcriptomic, and experimental analyses. Cancers 2023, 15, 653. [Google Scholar] [CrossRef] [PubMed]
- Kan, X.; Zhang, W.; You, R.; Niu, Y.; Guo, J.; Xue, J. Scutellaria barbata D. Don extract inhibits the tumor growth through down-regulating of Treg cells and manipulating Th1/Th17 immune response in hepatoma H22-bearing mice. BMC Complement. Altern. Med. 2017, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, W.; Yu, S.; Zhao, S. Current status of resistance studies to PD-1/PD-L1 blockade therapy in colorectal cancer. Chin. J. Color. Dis. (Electron. Ed.) 2021, 10, 205–210. [Google Scholar]
- Andrews, L.P.; Yano, H.; Vignali, D.A.A. Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: Breakthroughs or backups. Nat. Immunol. 2019, 20, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Hong, Y.; Weng, S.; Guo, P.; Li, B.; Zhang, Y.; Yu, C.; Wang, S.; Mo, P. Traditional Chinese Medicine Pien-Tze-Huang inhibits colorectal cancer growth and immune evasion by reducing β-catenin transcriptional activity and PD-L1 expression. Front. Pharmacol. 2022, 13, 828440. [Google Scholar] [CrossRef]
- Ren, T.; Bai, X.; Yang, M.; Xu, N.; Guo, X.; Qin, L.; Huang, Z.; Zhong, Q.; Huang, Y.; Lin, W.; et al. Gambogic acid suppresses nasopharyngeal carcinoma via rewiring molecular network of cancer malignancy and immunosurveillance. Biomed. Pharmacother. 2022, 150, 113012. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Xu, K.; Niu, L.; Lin, L. Astragalus polysaccharide (APS) attenuated PD-L1-mediated immunosuppression via the miR-133a-3p/MSN axis in HCC. Pharm. Biol. 2022, 60, 1710–1720. [Google Scholar] [CrossRef]
- Wan, N.; Jiang, C.; Gao, M.; Zhang, J.; Yin, Z.; Pan, K. Paeoniflorin regulates the JAK/STAT3 pathway to interfere with PD-L1 expression in HepG2 cells. J. China Pharm. Univ. 2019, 50, 213–221. [Google Scholar]
- Qiu, N.; Liu, Y.; Liu, Q.; Chen, Y.; Shen, L.; Hu, M.; Zhou, X.; Shen, Y.; Gao, J.; Huang, L. Celastrol nanoemulsion induces immunogenicity and downregulates PD-L1 to boost abscopal effect in melanoma therapy. Biomaterials 2021, 269, 120604. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, Z.; Xu, C.; Meng, W.; Liu, P.; Zhang, Y.; Xie, C.; Xu, J.; Xie, Y.; Liang, T.; et al. Andrographolide suppresses non-small-cell lung cancer progression through induction of autophagy and antitumor immune response. Pharmacol. Res. 2022, 179, 106198. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Sun, G.; Qi, X.; Li, D.; Zhang, P.; Wu, J. Intervention of Caesalpinina sappan, Caesalpinina sappan and Radix Astragali on CD4+ CD25+ regulatory T cells and related regulatory molecules in hormonal mice. Chin. J. Basic. Med. Tradit. Chin. Med. 2010, 16, 384–386. [Google Scholar]
- Casey, S.; Amedei, A.; Aquilano, K.; Azmi, A.; Benencia, F.; Bhakta, D.; Bilsland, A.; Boosani, C.; Chen, S.; Ciriolo, M.; et al. Cancer prevention and therapy through the modulation of the tumor microenvironment. Semin. Cancer Biol. 2015, 35, S199–S223. [Google Scholar] [CrossRef] [PubMed]
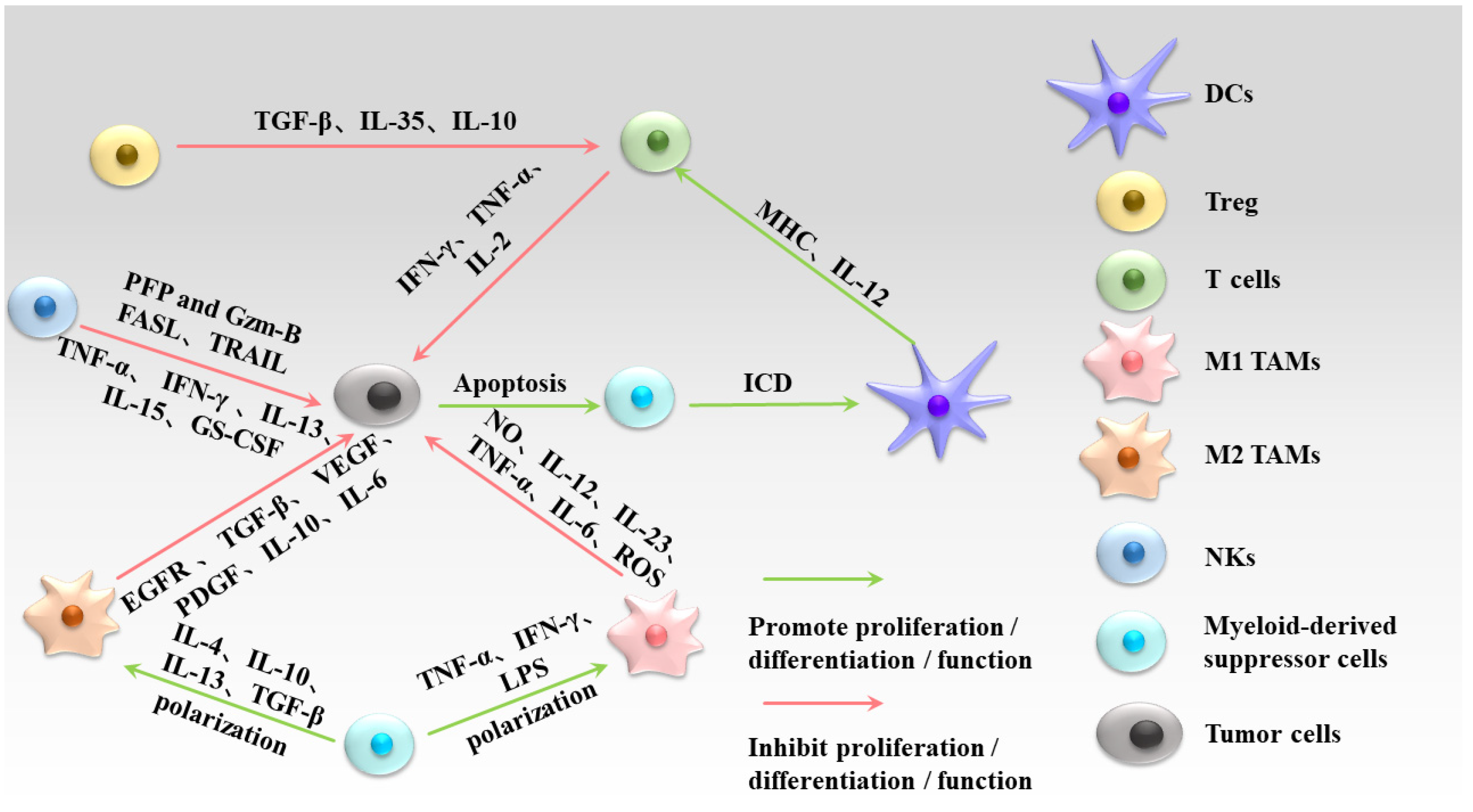
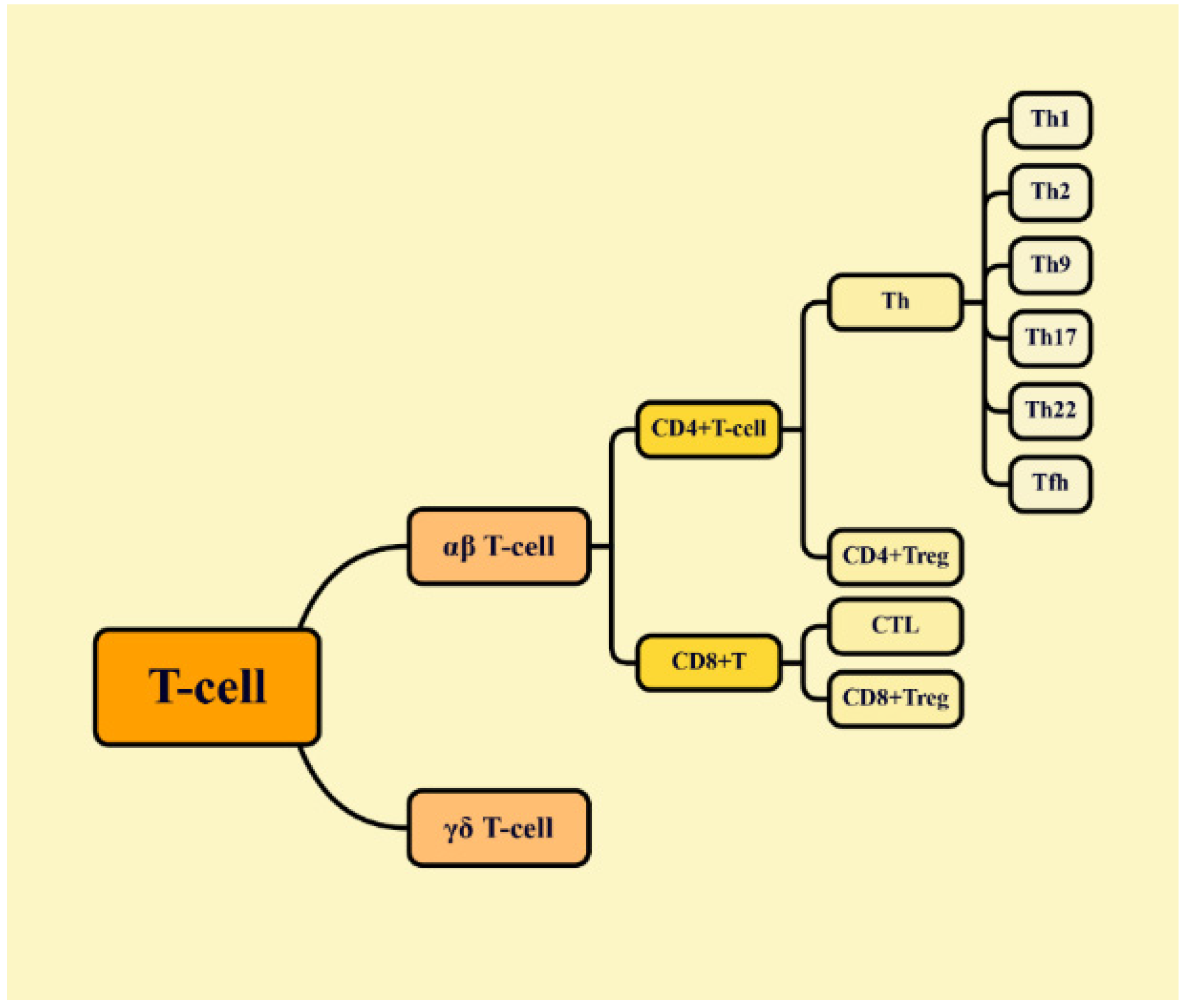
| Compound | Model | Adjustment Mode | References |
|---|---|---|---|
| Saponins | HCC liver cancer (in vivo); Lewis lung cancer (in vivo) | TAMs | [23,24,25] |
| H22 liver cancer (in vivo); Jurkat leukemia mice (in vivo); breast cancer (in vivo) | T-cells | [26,27,28,29] | |
| Lewis lung cancer cells (in vitro); cervical cancer Hela cells (in vitro) | PD-L1 | [30,31] | |
| Polysaccharides | BBN bladder cancer (in vivo); Lewis lung cancer (in vivo) | TAMs | [32,33,34,35] |
| 4T1 breast cancer (in vivo) | DCs | [36] | |
| B16F10 melanoma (in vivo); liver cancer (in vivo); CT26 colorectal cancer (in vivo) | T-cells | [37,38,39,40] | |
| Flavonoids | CT26 colorectal cancer (in vivo); | DCs | [41] |
| breast cancer cells (in vitro); Lewis lung cancer (in vivo); NSCLC mice (in vivo) | PD-L1 | [42,43,44,45,46] | |
| Alkaloids | Lung cancer patients (in vivo) | T-cells | [47] |
| Lewis lung cancer (in vivo) | PD-L1 | [48,49] |
| Form | Compound | Model | Mechanisms of Action | References |
|---|---|---|---|---|
| Saponins | Astragaloside IV | Huh-7 nude mice with hepatocellular carcinoma (in vivo) | Inhibition of M2 polarization through the TLR4/NF-κB/STAT3 signaling pathway inhibits tumor proliferation, invasion and migration | [23] |
| Astragaloside IV | Lewis lung cancer mice (in vivo) | Blocks macrophage M2 polarization via the AMPK signaling pathway | [24] | |
| Ginsenoside | Lewis lung cancer mice (in vivo) | Reduces expression of the M2 macrophage markers CD206 and VEGF in vivo | [25] | |
| Polysaccharides | Poria mushroom polysaccharide | BBN bladder cancer rats (in vivo) | Activates macrophages through the TLR4/NF-κB signaling pathway | [32,33,34] |
| Astragalus polysaccharide PG2 | Lewis lung cancer mice (in vivo) | Dose-dependently enhances M1 polarization and down-regulates IL-4-/IL-13-induced M2 polarization | [35] |
| Form | Compound | Model | Mechanisms of Action | References |
|---|---|---|---|---|
| Polysaccharides | Radix Astragalus Radix Conopsis | 4T1 breast cancer mice (in vivo) | Stimulates DCs to express higher levels of CD80 and CD86; increases the infiltration of CD8+ T cell in tumors | [36] |
| Flavonoids | Gambogic acid | CT26 colorectal cancer mice (in vivo) | Stimulates maturation of DCs | [41] |
| Terpenes | Cryptotanshinone | Lewis lung cancer mice (in vivo) | Induces maturation of DCs in a MyD88-dependent manner | [62] |
| Dihydrotanshinone | HCC liver cancer mice (in vivo) | Generates ROS effectively, enhances plumbagin-mediated ICD and promotes DC maturation | [64] | |
| Quinones | Plumbagin | HCC liver cancer mice (in vivo) | Induces ICD to stimulate maturation of DCs | [64] |
| Form | Compound | Model | Mechanisms of Action | References |
|---|---|---|---|---|
| Anthraquinones | Emodin | A549 lung cancer cells (in vitro) | Enhances the killing effect of NKs on A549 by affecting the equilibrium state of signaling by NKs | [68] |
| Plant- extracted glycoproteins | ZPDC glycoprotein | Diethylnitrosamine (DEN)-induced hepatocellular carcinoma in mice (in vivo) | Secretes perforin and granzyme B and activates NKs | [69] |
| Terpenes | Lupeol | Gastric cancer HGC27 cells (in vitro) | Enhances the proliferative capacity and cytotoxicity of NKs against gastric cancer cells | [70] |
| Form | Compound | Model | Mechanisms of Action | References |
|---|---|---|---|---|
| Saponins | Ginsenosides | Human monocyte-derived DCs (in vitro) | Promotes the conversion of naive T cells to Th1 cells through DCs | [26] |
| Ginsenoside Rg3 | H22 liver cancer mice (in vivo) | Increases IFN-γ and IL-2 production | [27] | |
| Ginsenoside Rh2 | Jurkat leukemia mice (in vivo) | May inhibit cell growth through the PI3K/Akt/mTOR pathway | [28] | |
| Saikosaponin A | Dimethyl-benthrathracene (DMBA)-induced breast cancer in rats (in vivo) | Transforms Th2 cells into Th1 cells | [29] | |
| Polysaccharides | Ganoderma lucidum | B16F10 melanoma mice; HCC liver cancer mice | Induces the production of granzyme B and perforin to enhance the cytotoxicity of CTLS to melanoma cells; Inhibits Treg cells action by up-regulating miR-125b, leading to suppression of Notch1 signaling pathway and FoxP3 expression | [37,38] |
| Licorice polysaccharide | H22 liver cancer mice; CT26 colorectal cancer mice | Activates CD4+ and CD8+ T cells and increases Th1/Th2 | [39,40] | |
| Alkaloids | Ligustrazine | Lung cancer patients (pre-clinical patients) | Reduces the expression of Th2 cytokines | [47] |
| Terpenoids | Elecampane | MC38 colorectal cancer mice | Increases the proportion of CD8+ T cells and M1 macrophages in the tumor microenvironment | [79] |
| Form | Compound | Model | Mechanisms of Action | References |
|---|---|---|---|---|
| Saponins | Ginsenoside Rg3 | Lewis lung cancer cells (in vitro) | Suppresses PD-L1 in LLC through the PI3K/Akt/mTOR pathway | [30] |
| Astragaloside IV | Cervical cancer Hela cells (in vitro) | Inhibits PD-1 and PD-L1 expression through the P38 signaling pathway | [31] | |
| Flavonoids | Hyperoside | MC38 colorectal cancer mice (in vivo) | Down-regulates PD-L1 and CD47 expression by degradation of c-Myc | [42] |
| (-)-Sativan (SA) | Breast cancer cells (in vitro) | Inhibits PD-L1 expression by up-regulation of miR-200c | [43] | |
| Quercetin | Lewis lung cancer mice (in vivo) | Suppresses PD-L1 through the JAK2/STAT3 pathway | [44,45] | |
| Apigenin and lignans | NSCLC mice (in vivo) | Inhibits KRAS mutant lung cancer proliferation and down-regulates IFN-γ-induced PD-L1 expression | [46] | |
| Alkaloids | Berberine | Lewis lung cancer mice (in vivo) | Specific binding to CSN5 leads to PD-L1 degradation | [48] |
| Evodiamine | Lewis lung cancer mice (in vivo) | Increases CD8+ T-cell activity and down-regulates MUC1-C/PD-L1 | [49] | |
| Terpenoids | Paeoniflorin | HepG2 cells (in vitro) | Suppresses PD-L1 in HepG2 cells through the JAK/STAT3 pathway | [86] |
| Celastrol | B16F10 melanoma mice (in vivo) | Down-regulates PD-L1 expression in tumor cells via the NF-κB pathway | [87] | |
| Andrographolide | Lewis lung cancer mice and NSCLC mice (in vivo) | Oxidatively inhibits STAT3 phosphorylation and p62 accumulation and regulates selective autophagic degradation of PD-L1 | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Fan, W.; Zhao, Y.; Liu, M.; Hu, L.; Zhang, W. Progress in the Regulation of Immune Cells in the Tumor Microenvironment by Bioactive Compounds of Traditional Chinese Medicine. Molecules 2024, 29, 2374. https://doi.org/10.3390/molecules29102374
Chen Y, Fan W, Zhao Y, Liu M, Hu L, Zhang W. Progress in the Regulation of Immune Cells in the Tumor Microenvironment by Bioactive Compounds of Traditional Chinese Medicine. Molecules. 2024; 29(10):2374. https://doi.org/10.3390/molecules29102374
Chicago/Turabian StyleChen, Yuqian, Wenshuang Fan, Yanyan Zhao, Meijun Liu, Linlin Hu, and Weifen Zhang. 2024. "Progress in the Regulation of Immune Cells in the Tumor Microenvironment by Bioactive Compounds of Traditional Chinese Medicine" Molecules 29, no. 10: 2374. https://doi.org/10.3390/molecules29102374
APA StyleChen, Y., Fan, W., Zhao, Y., Liu, M., Hu, L., & Zhang, W. (2024). Progress in the Regulation of Immune Cells in the Tumor Microenvironment by Bioactive Compounds of Traditional Chinese Medicine. Molecules, 29(10), 2374. https://doi.org/10.3390/molecules29102374






