Enhancing Photocatalytic Activities for Sustainable Hydrogen Evolution on Structurally Matched CuInS2/ZnIn2S4 Heterojunctions
Abstract
:1. Introduction
2. Results and Discussion
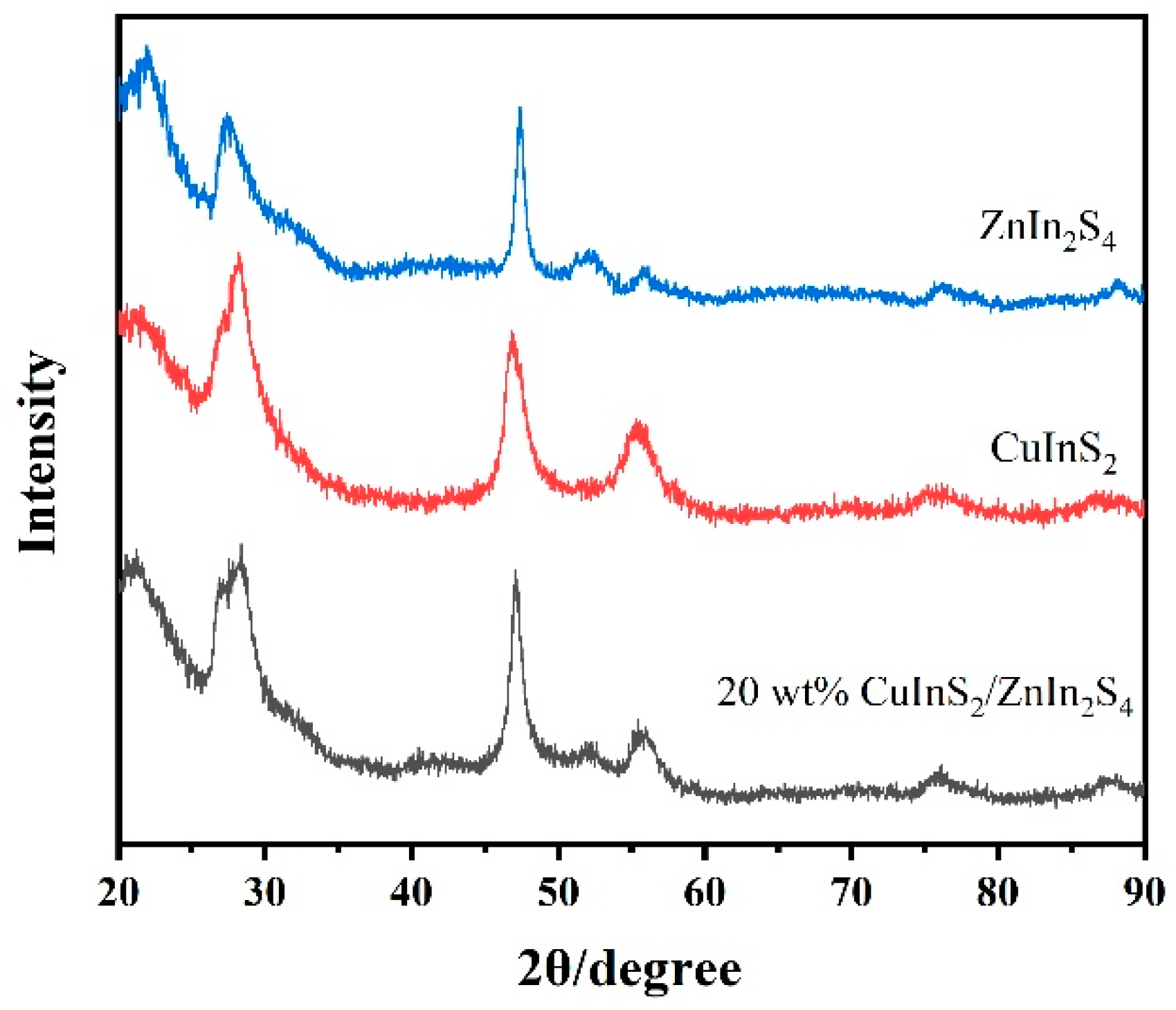
2.1. X-ray Diffraction (XRD)
2.2. Scanning Electron Microscopy (SEM)
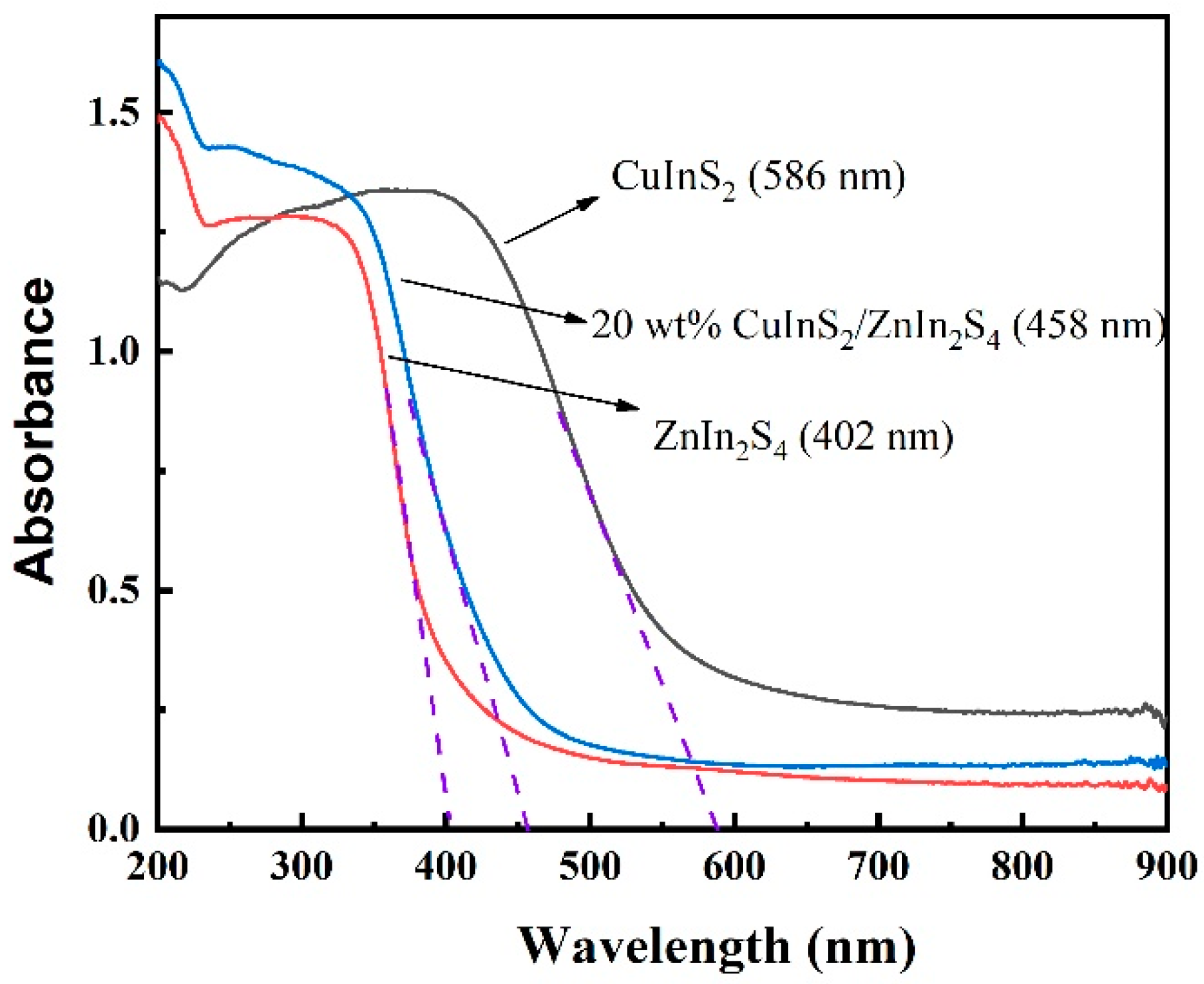
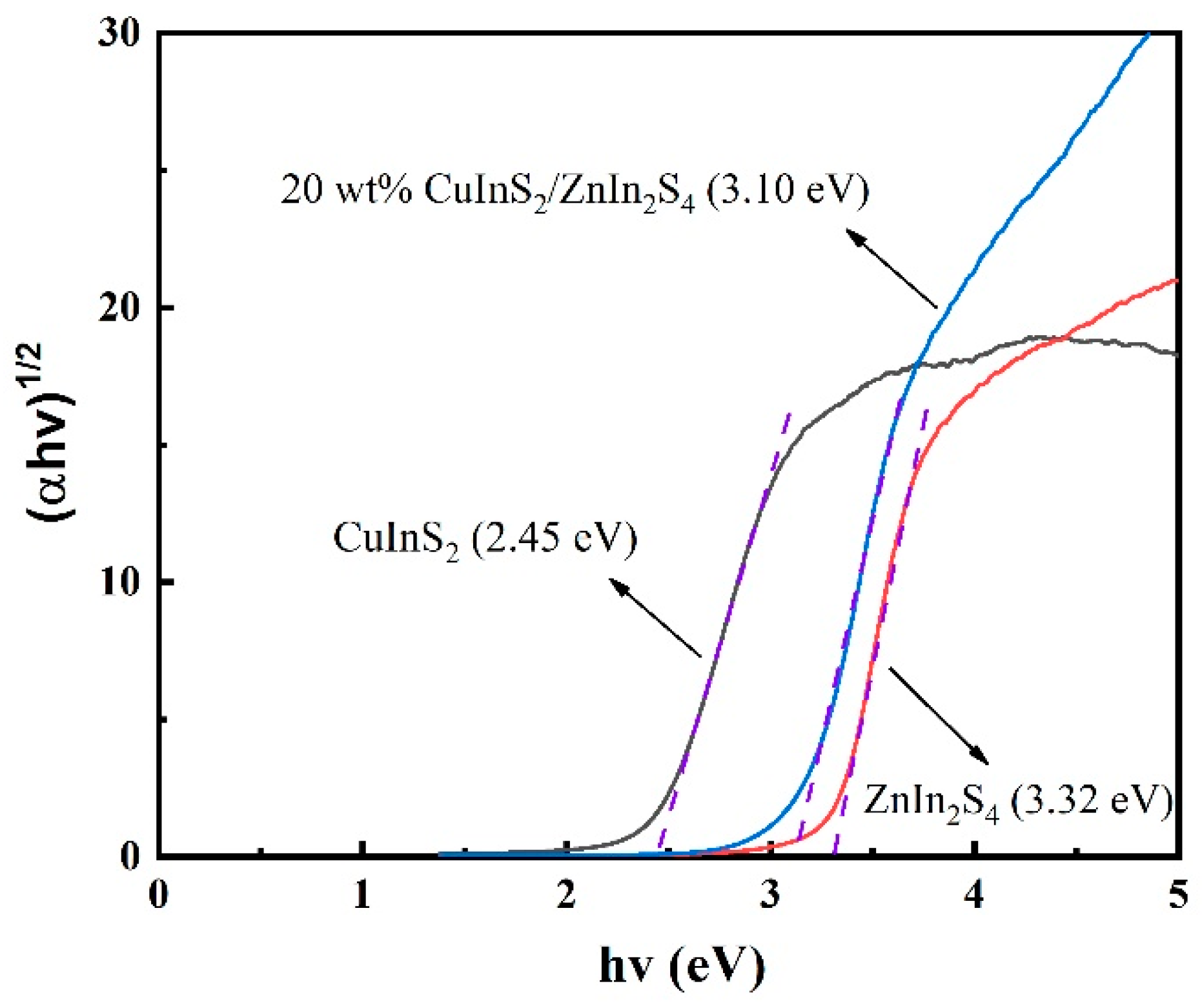
2.3. UV–Vis Diffuse Reflection Spectroscopy (DRS)
2.4. Transient Photocurrent Response (TPR) and Electrochemical Impedance Spectroscopy (EIS)
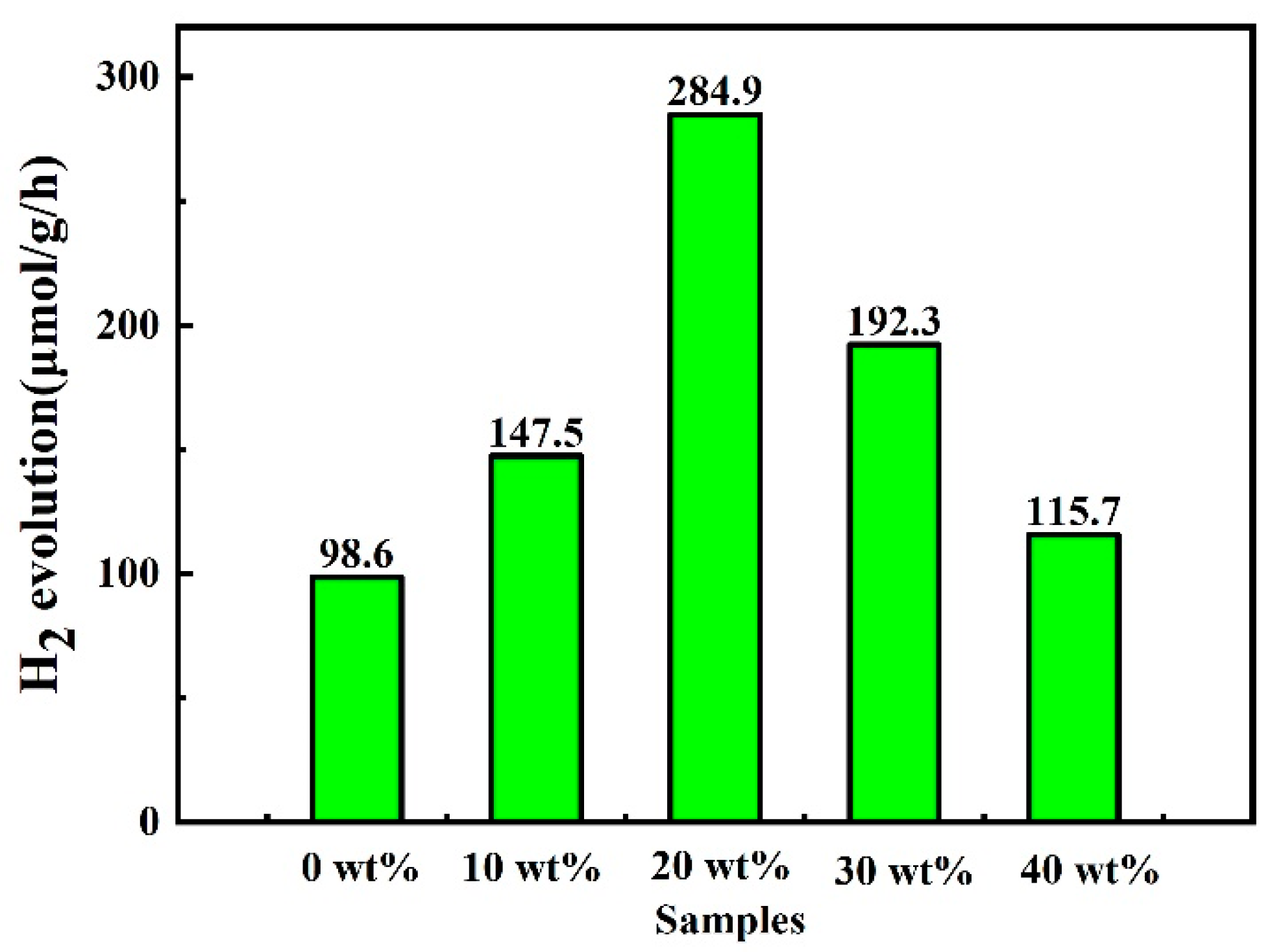
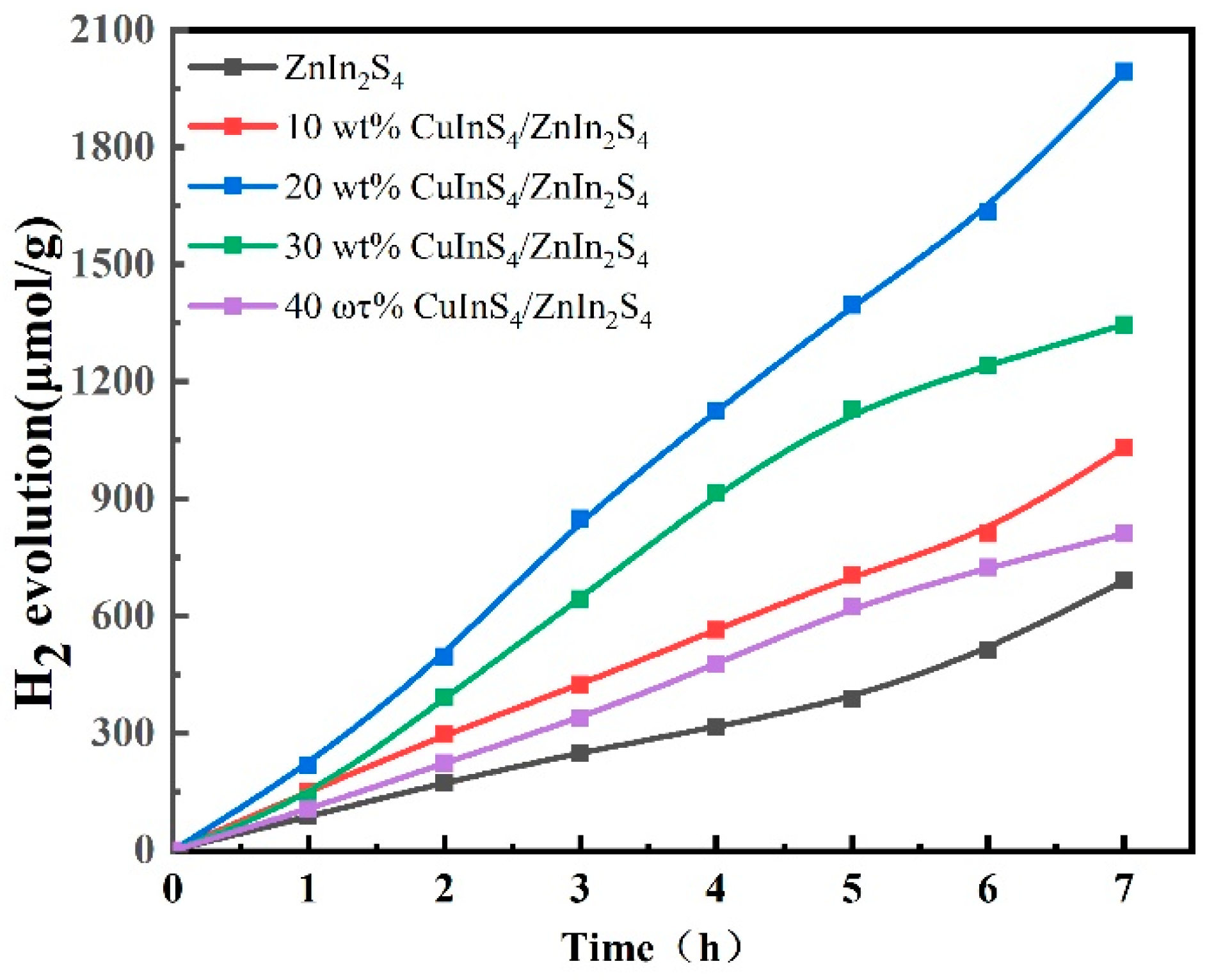
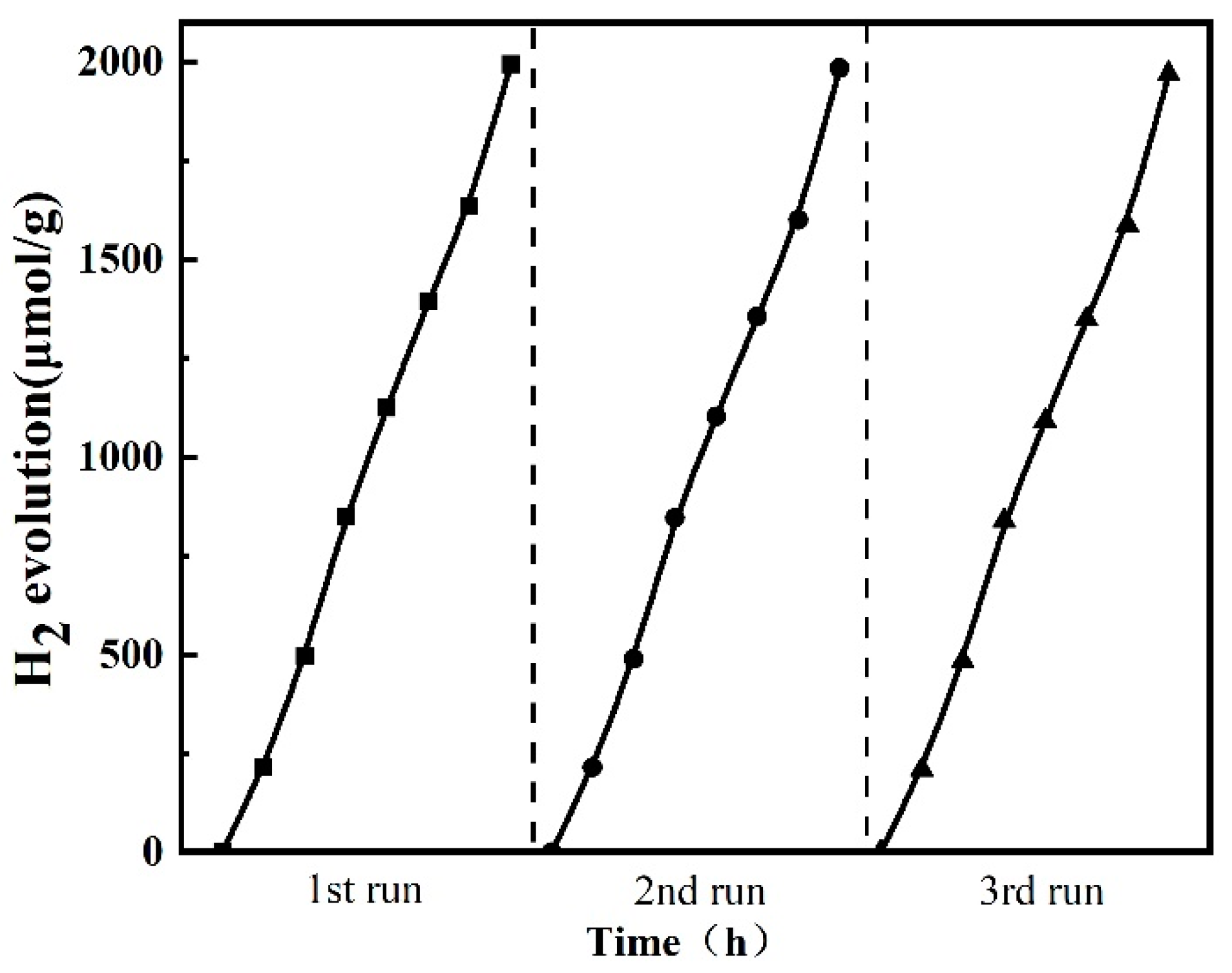
2.5. Photocatalytic Performance for Hydrogen Evolution Reaction (HER)
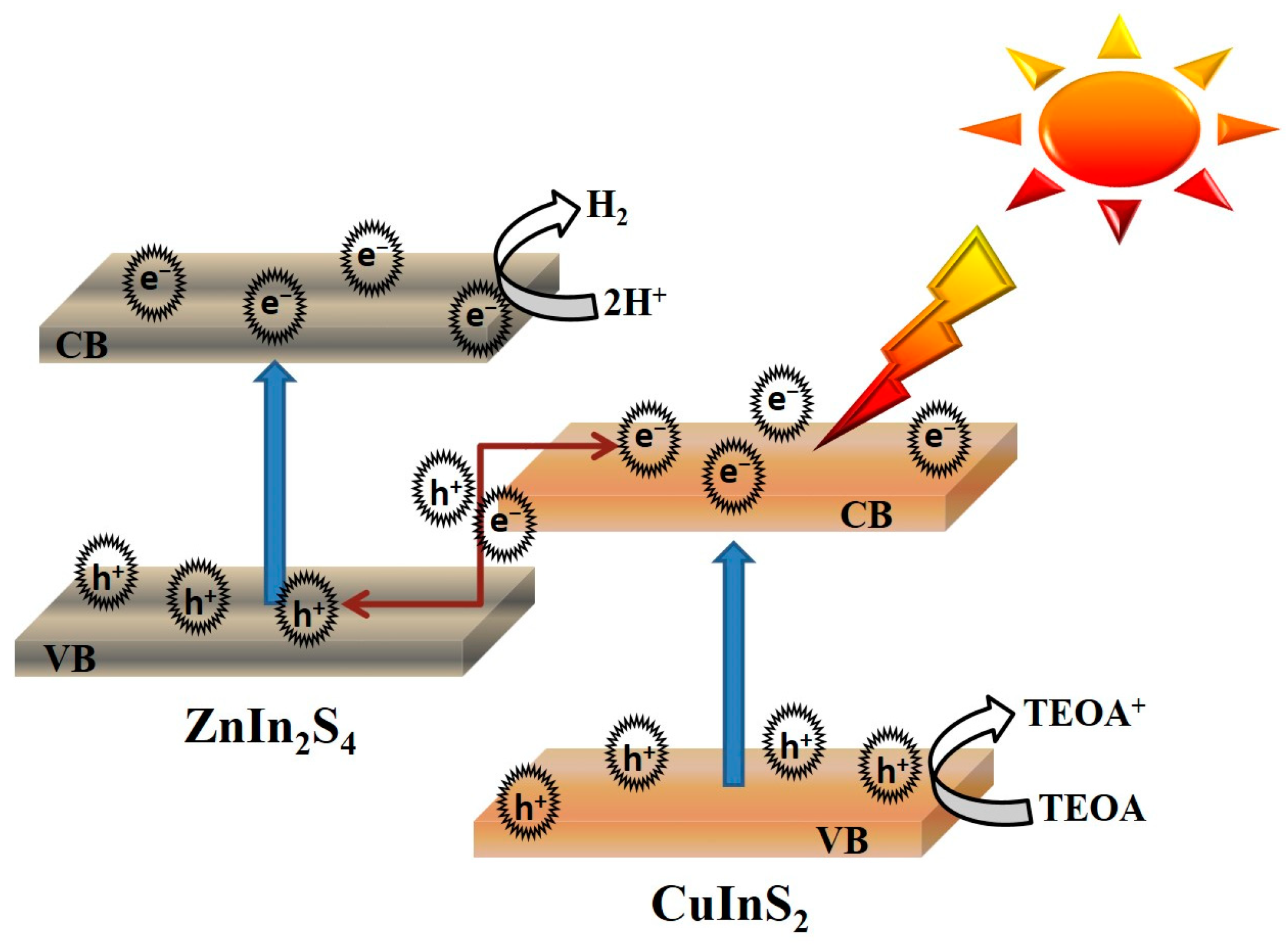
2.6. Photocatalytic HER Mechanism
3. Experimental Section
3.1. Photocatalyst Preparation
3.1.1. Preparation of CuInS2 and ZnIn2S4
3.1.2. Preparation of CuInS2/ZnIn2S4
3.2. Characterization of Photocatalysts
3.3. Photocatalytic HER Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, Z.; Yang, K.; Yu, C.; Lu, K.; Huang, W.; Xu, L.; Zou, L.; Wang, S.; Chen, Z.; Hu, J.; et al. Steering Unit Cell Dipole and Internal Electric Field by Highly Dispersed Er atoms Embedded into NiO for Efficient CO2 Photoreduction. Adv. Funct. Mater. 2022, 32, 2111999. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, G.; Jia, S.; Xie, H.; Kang, Z.; Chen, W.; Cui, M.; Wang, B.; Wang, B.; Chen, X.; et al. Intrinsic carbon defects induced nickel phosphate/carbon photocatalyst for high performance bacterial disinfection. Chem. Eng. J. 2022, 438, 135624. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Y.; Meng, S.; Wang, Z.; Wang, P.; Wang, Z.; Qiu, C.; Chen, S.; Weng, B.; Zheng, Y.-M. Metal Sulfide S-Scheme Homojunction for Photocatalytic Selective Phenylcarbinol Oxidation. Adv. Sci. 2024, 11, 202400099. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Shen, J.; Xie, Y.; Qiu, C.; Zhang, Z.; Long, J.; Lin, H.; Wang, X. Metallic Pt and PtO2 Dual-Cocatalyst-Loaded Binary Composite RGO-CNx for the Photocatalytic Production of Hydrogen and Hydrogen Peroxide. ACS Sustain. Chem. Eng. 2021, 9, 6380–6389. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Liang, J.; Zhou, Y.; Tong, Y.; Wang, Y.; Wang, X. Freestanding single layers of non-layered material γ-Ga2O3 as an efficient photocatalyst for overall water splitting. J. Mater. Chem. A 2017, 5, 9702–9708. [Google Scholar] [CrossRef]
- Villa, K.; Galán-Mascarós, J.R.; López, N.; Palomares, E. Photocatalytic water splitting: Advantages and challenges. Sustain. Energy Fuels 2021, 5, 4560–4569. [Google Scholar] [CrossRef]
- Nishiyama, H.; Yamada, T.; Nakabayashi, M.; Maehara, Y.; Yamaguchi, M.; Kuromiya, Y.; Nagatsuma, Y.; Tokudome, H.; Akiyama, S.; Watanabe, T.; et al. Photocatalytic solar hydrogen production from water on a 100 m2 scale. Nature 2021, 598, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Huang, K.; Zhang, Y.; Jin, Y.; Chen, Y.; Chen, F.; Zhang, X. Photodynamically Functionalized CoP/MoS2 Nanocomposites with Antibacterial Activity. ACS Appl. Nano Mater. 2024, 7, 3260–3268. [Google Scholar] [CrossRef]
- Song, H.; Luo, S.; Huang, H.; Deng, B.; Ye, J. Solar-Driven Hydrogen Production: Recent Advances, Challenges, and Future Perspectives. ACS Energy Lett. 2022, 7, 1043–1065. [Google Scholar] [CrossRef]
- Guo, S.; Li, X.; Li, J.; Wei, B. Boosting photocatalytic hydrogen production from water by photothermally induced biphase systems. Nat. Commun. 2021, 12, 1343. [Google Scholar] [CrossRef]
- Zhuang, H.; Lin, l.; Xu, M.; Xu, W.; Liu, X. Construction of g-C3N4-based photoelectrodes towards photoelectrochemical water splitting: A review. J. Alloys Compd. 2023, 969, 172302. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, M.; Yang, K.; Yu, C.; Mu, P.; Yu, Z.; Lu, K.; Huang, W.; Dai, W. Photocatalytic H2O2 production and removal of Cr (VI) via a novel Lu3NbO7: Yb, Ho/CQDs/AgInS2/In2S3 heterostructure with broad spectral response. J. Hazard. Mater. 2022, 423, 127172. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Yuan, C.; Cui, Y.; Dong, Y.; Chen, D.; Ge, H.; Ke, J. Construction of zinc-indium-sulfide/indium oxide step-scheme junction catalyst for enhanced photocatalytic activities of pollutant degradation and hydrogen generation. Sep. Purif. Technol. 2021, 266, 118545. [Google Scholar] [CrossRef]
- Lin, M.; Chen, H.; Zhang, Z.; Wang, X. Engineering interface structures for heterojunction photocatalysts. Phys. Chem. Chem. Phys. 2023, 25, 4388–4407. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guan, B.Y.; Lou, X.W.D. Construction of ZnIn2S4–In2O3 Hierarchical Tubular Heterostructures for Efficient CO2 Photoreduction. J. Am. Chem. Soc. 2018, 140, 5037–5040. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Chen, Z.; Lu, J.; Sun, X.; Wang, Z.; Yan, Y.; Guo, F.; Chen, L.; Wang, G. Construction of ZrC@ZnIn2S4 core–shell heterostructures for boosted near-infrared-light driven photothermal-assisted photocatalytic H2 evolution. Chem. Eng. J. 2023, 474, 145690. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, G.; Xie, L.; Wu, P.; Liu, H.; Wang, J.; Xie, Y.; Chen, J.; Lu, C.-Z. Promoting photocatalytic H2 evolution through interfacial charge separation on the direct Z-scheme ZnIn2S4/ZrO2 heterojunction. Int. J. Hydrog. Energy 2023, 48, 32782–32796. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, D.; Su, P.; Yao, X.; Liu, J.; Pu, X.; Li, H.; Cai, P. In-situ preparation of mossy tile-like ZnIn2S4/Cu2MoS4 S-scheme heterojunction for efficient photocatalytic H2 evolution under visible light. J. Colloid Interface Sci. 2023, 650, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Que, L.; Lu, L.; Xu, Y.; Xu, X.; Li, H.; Cao, J.; Zhu, M.; Li, C.; Pan, J. The ZnIn2S4/CdS hollow core-shell nanoheterostructure towards enhanced visible light photocatalytic H2 evolution via bimetallic synergism. Int. J. Hydrog. Energy 2023, 48, 4708–4718. [Google Scholar] [CrossRef]
- Shen, J.; Liu, X.; Zhong, Y.; Qiu, C.; Xu, H.; He, H.; Li, H.; Lin, J.; Zhang, Z.; Wang, X. Photocatalytic Overall Water Splitting Reaction Feature on Photodeposited NixP/γ-Ga2O3 Nanosheets. ACS Appl. Energy Mater. 2023, 6, 4317–4326. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, L.; Liu, L.; Li, H.; Meng, S.; Ye, X.; Chen, S. In situ photodeposition of MoSx on CdS nanorods as a highly efficient cocatalyst for photocatalytic hydrogen production. J. Mater. Chem. A 2017, 5, 15287–15293. [Google Scholar] [CrossRef]
- Lin, J.; Hu, J.; Qiu, C.; Huang, H.; Chen, L.; Xie, Y.; Zhang, Z.; Lin, H.; Wang, X. In situ hydrothermal etching fabrication of CaTiO3 on TiO2 nanosheets with heterojunction effects to enhance CO2 adsorption and photocatalytic reduction. Catal. Sci. Technol. 2019, 9, 336–346. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, X.; Song, Y.; Liu, Y.; Wu, D.; Li, J.; Liu, W.; Fu, L.; Shen, Y.; Tian, X. CuInS2-based photocatalysts for photocatalytic hydrogen evolution via water splitting. Int. J. Hydrog. Energy 2023, 48, 3791–3806. [Google Scholar] [CrossRef]
- Gunawan; Adi Wijaya, R.; Suseno, A.; Lusiana, R.A.; Septina, W.; Harada, T. Synthesis of CuInS2 thin film photocathode with variation of sulfurization sources and Pt-In2S3 modification for photoelectrochemical water splitting. J. Electroanal. Chem. 2023, 945, 117683. [Google Scholar] [CrossRef]
- Luo, J.; Lin, Z.; Zhao, Y.; Jiang, S.; Song, S. The embedded CuInS2 into hollow-concave carbon nitride for photocatalytic H2O splitting into H2 with S-scheme principle. Chin. J. Catal. 2020, 41, 122–130. [Google Scholar] [CrossRef]
- Chae, S.Y.; Yoon, N.; Park, E.D.; Joo, O.S. Surface modification of CuInS2 photocathodes with ruthenium co-catalysts for efficient solar water splitting. Appl. Surf. Sci. 2023, 612, 155856. [Google Scholar] [CrossRef]
- Matoba, K.; Matsuda, Y.; Takahashi, M.; Sakata, Y.; Zhang, J.; Higashimoto, S. Fabrication of Pt/In2S3/CuInS2 thin film as stable photoelectrode for water splitting under solar light irradiation. Catal. Today 2021, 375, 87–93. [Google Scholar] [CrossRef]
- Guan, Z.; Pan, J.; Li, Q.; Li, G.; Yang, J. Boosting Visible-Light Photocatalytic Hydrogen Evolution with an Efficient CuInS2/ZnIn2S4 2D/2D Heterojunction. ACS Sustain. Chem. Eng. 2019, 7, 7736–7742. [Google Scholar] [CrossRef]
- Guo, X.; Peng, Y.; Liu, G.; Xie, G.; Guo, Y.; Zhang, Y.; Yu, J. An Efficient ZnIn2S4@CuInS2 Core–Shell p–n Heterojunction to Boost Visible-Light Photocatalytic Hydrogen Evolution. J. Phys. Chem. C 2020, 124, 5934–5943. [Google Scholar] [CrossRef]
- Lu, J.; Zhou, B.; Zhang, X.; Zhao, X.; Liu, X.; Wu, S.; Yang, D.-P. Oyster shell-derived CuFe2O4-Hap nanocomposite for healthy houses: Bacterial and formaldehyde elimination. Chem. Eng. J. 2023, 477, 147054. [Google Scholar] [CrossRef]
- Tsao, C.; Fang, M.; Hsu, Y. Modulation of interfacial charge dynamics of semiconductor heterostructures for advanced photocatalytic applications. Coord. Chem. Rev. 2021, 438, 213876. [Google Scholar] [CrossRef]
- Mamiyev, Z.; Balayeva, N.O. Metal Sulfide Photocatalysts for Hydrogen Generation: A Review of Recent Advances. Catalysts 2022, 12, 1316. [Google Scholar] [CrossRef]
- Xiao, L.; Yuan, C.; Chen, P.; Liu, Y.; Sheng, J.; Zhang, S.; Dong, F.; Sun, Y. Cu–S Bonds as an Atomic-Level Transfer Channel to Achieve Photocatalytic CO2 Reduction to CO on Cu-Substituted ZnIn2S4. ACS Sustain. Chem. Eng. 2022, 10, 11902–11912. [Google Scholar] [CrossRef]
- Wang, Z.; Qi, Z.; Fan, X.; Leung, D.Y.C.; Long, J.; Zhang, Z.; Miao, T.; Meng, S.; Chen, S.; Fu, X. Intimately Contacted Ni2P on CdS Nanorods for Highly Efficient Photocatalytic H2 Evolution: New Phosphidation Route and the Interfacial Separation Mechanism of Charge Carriers. Appl. Catal. B Environ. 2021, 281, 119443. [Google Scholar] [CrossRef]
- Pu, Y.; Lin, W.; Hsu, Y. Modulation of charge carrier dynamics of NaxH2−xTi3O7-Au-Cu2O Z-scheme nanoheterostructures through size effect. Appl. Catal. B Environ. 2015, 163, 343–351. [Google Scholar] [CrossRef]
- Li, J.; Tsao, C.; Fang, M.; Chen, C.; Liu, C.; Hsu, Y. TiO2-Au-Cu2O Photocath odes: Au-Mediated Z-Scheme Charge Transfer for Efficient Solar-Driven Photoelectrochemical Reduction. ACS Appl. Nano Mater. 2018, 1, 6843–6853. [Google Scholar] [CrossRef]
- Cavdar, O.; Malankowska, A.; Amgar, D.; Mazierski, P.; Łuczak, J.; Lisowski, W.; Zaleska-Medynska, A. Remarkable visible-light induced hydrogen generation with ZnIn2S4 microspheres/CuInS2 quantum dots photocatalytic system. Int. J. Hydrog. Energy 2021, 46, 486–498. [Google Scholar] [CrossRef]
- Xiang, X.; Zhang, M.; Huang, Q.; Mao, Y.; Jia, J.; Zeng, X.; Dong, Y.; Liao, J.; Chen, X.; Yao, X.; et al. Construction of S-scheme CuInS2/ZnIn2S4 heterostructures for enhanced photocatalytic activity towards Cr(VI) removal and antibiotics degradation. Chemosphere 2024, 352, 41351. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Liao, B.; Shen, J.; Ke, J.; Zhang, R.; Wang, Y.; Niu, Y. Enhancing Photocatalytic Activities for Sustainable Hydrogen Evolution on Structurally Matched CuInS2/ZnIn2S4 Heterojunctions. Molecules 2024, 29, 2447. https://doi.org/10.3390/molecules29112447
Li F, Liao B, Shen J, Ke J, Zhang R, Wang Y, Niu Y. Enhancing Photocatalytic Activities for Sustainable Hydrogen Evolution on Structurally Matched CuInS2/ZnIn2S4 Heterojunctions. Molecules. 2024; 29(11):2447. https://doi.org/10.3390/molecules29112447
Chicago/Turabian StyleLi, Fuying, Boiyee Liao, Jinni Shen, Junni Ke, Rongxin Zhang, Yueqi Wang, and Yu Niu. 2024. "Enhancing Photocatalytic Activities for Sustainable Hydrogen Evolution on Structurally Matched CuInS2/ZnIn2S4 Heterojunctions" Molecules 29, no. 11: 2447. https://doi.org/10.3390/molecules29112447
APA StyleLi, F., Liao, B., Shen, J., Ke, J., Zhang, R., Wang, Y., & Niu, Y. (2024). Enhancing Photocatalytic Activities for Sustainable Hydrogen Evolution on Structurally Matched CuInS2/ZnIn2S4 Heterojunctions. Molecules, 29(11), 2447. https://doi.org/10.3390/molecules29112447





