Cosmetic Products with Potential Photoprotective Effects Based on Natural Compounds Extracted from Waste of the Winemaking Industry
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction of Polyphenolic Compounds from Grape Marc
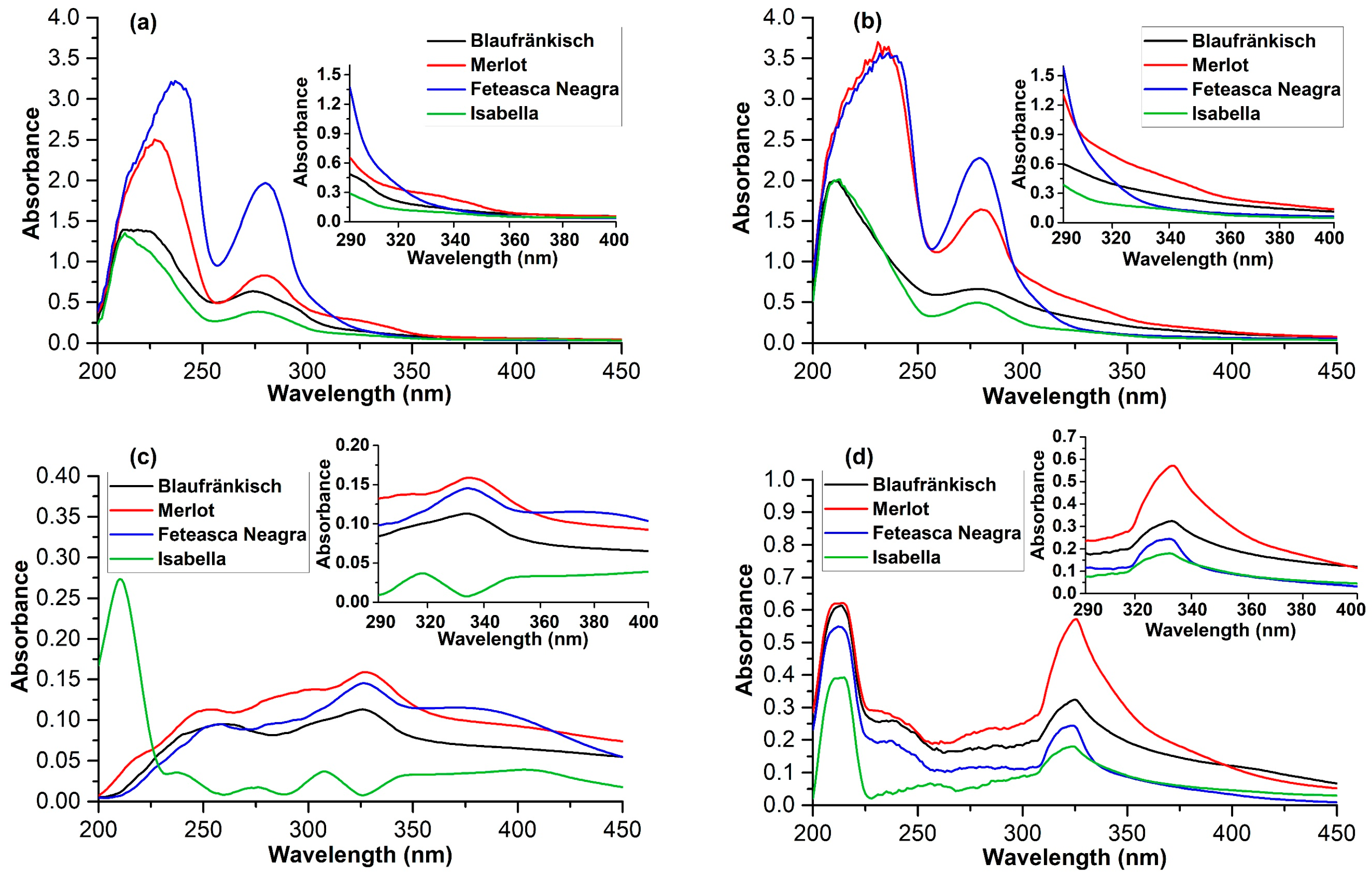
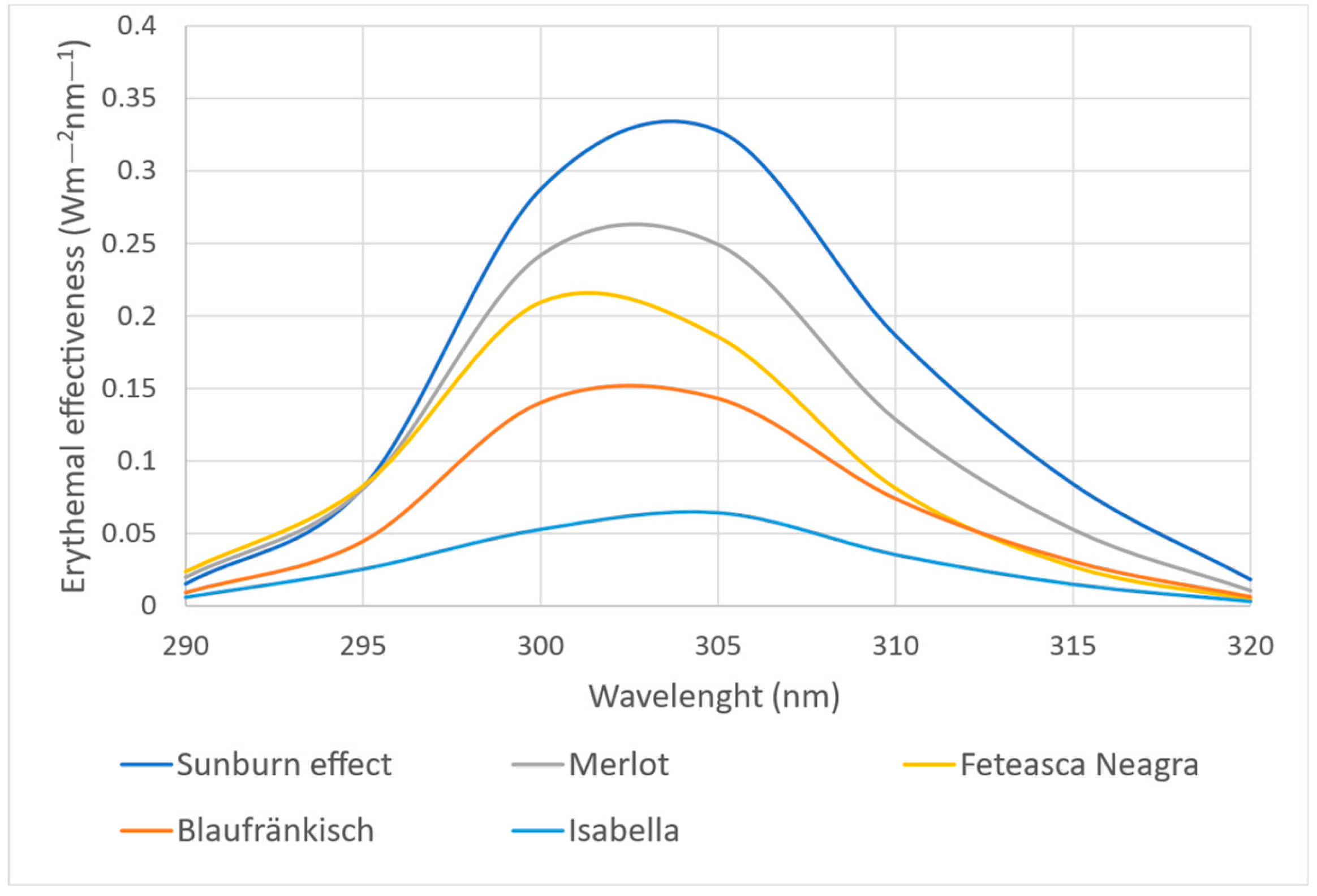
2.2. Spectrophotometric Analysis of the Photoprotective Effect of Grape Marc Extracts
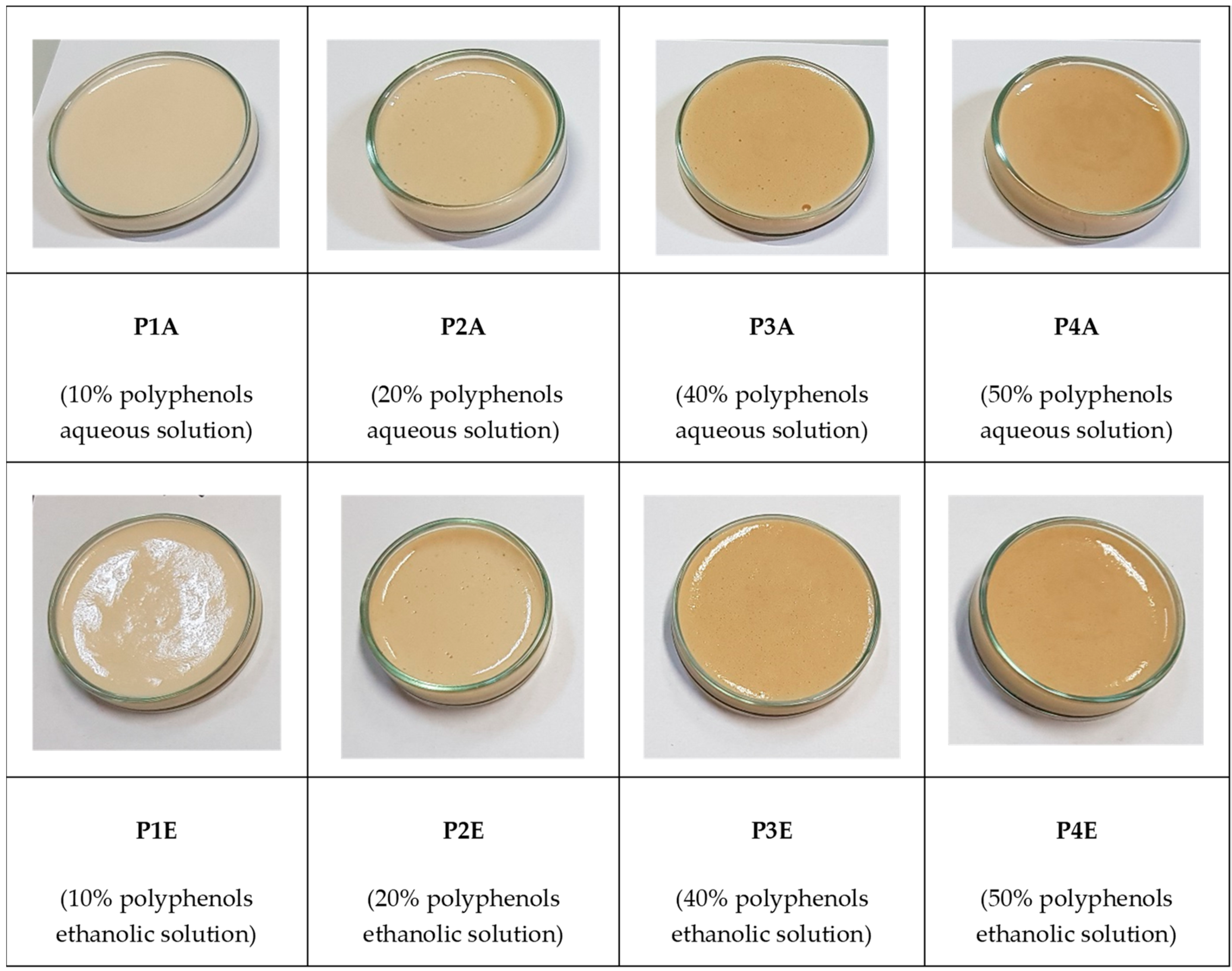
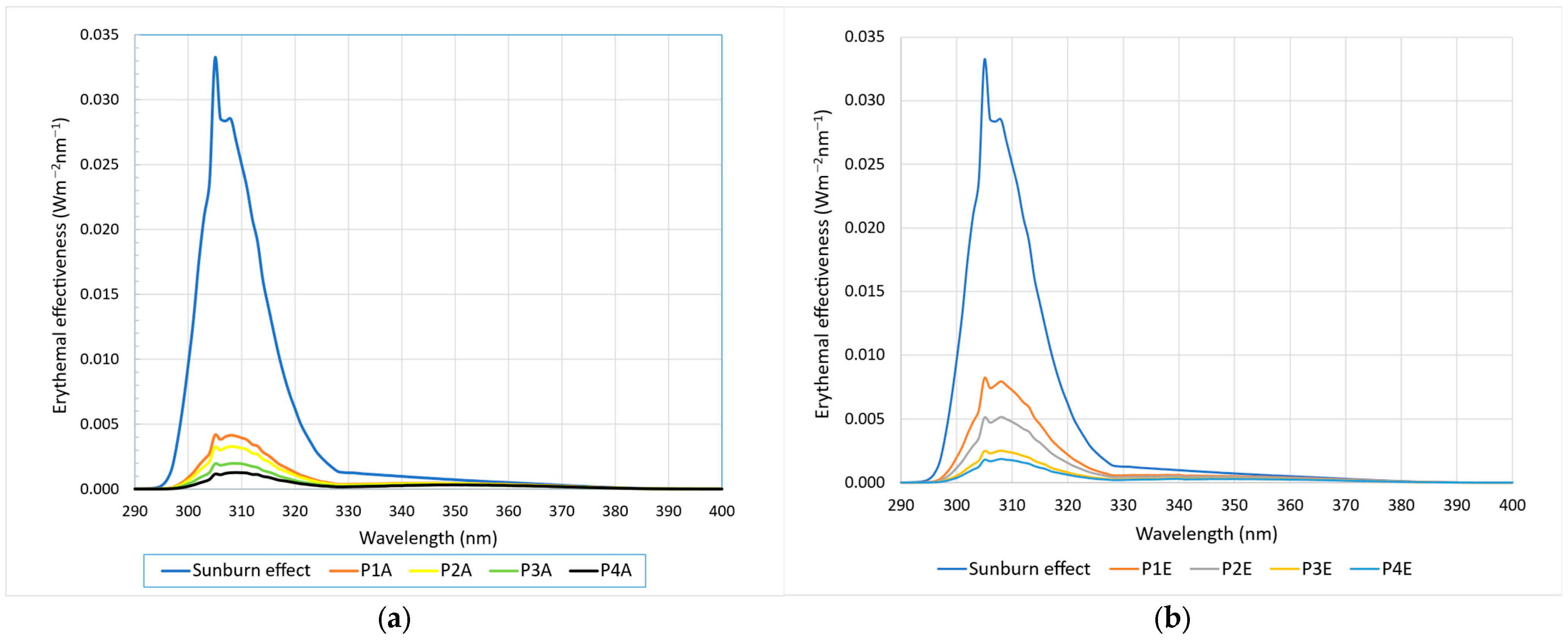
2.3. Obtaining Sun Protection Creams and In Vitro Analysis of Their Photoprotective Effects
2.4. Study Limitations and Development Directions
3. Materials and Methods
3.1. Materials
3.2. Extraction of Polyphenols from Grape Marc
3.3. Determination of Total Polyphenolics OD280 Index
3.4. Determination of Total Phenolic Content
3.5. Determination of Antioxidant Activity
3.6. Spectrophotometric Analysis of the Photoprotective Effect of Grape Marc Extracts
3.7. Obtaining Lotions with a Photoprotective Effect Based on Grape Marc Extracts
3.8. Analysis of the Photoprotective Effect of Polyphenol Extracts Containing Lotions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dale Wilson, B.; Moon, S.; Armstrong, F. Comprehensive review of ultraviolet radiation and the current status on sunscreens. J. Clin. Aesthet. Dermatol. 2012, 5, 18–23. [Google Scholar] [PubMed]
- Gragnani, A.; Mac Cornick, S.; Chominski, V.; De Noronha, S.M.R.; De Noronha, S.A.A.C.; Ferreira, L.M. Review of Major Theories of Skin Aging. Adv. Aging Res. 2014, 3, 265–284. [Google Scholar] [CrossRef]
- Lucas, R.M.; Norval, M.; Neale, R.E.; Young, A.; De Gruijl, F.R.; Takizawa, Y.; Van Der Leun, J.C. The consequences for human health of stratospheric ozone depletion in association with other environmental factors. Photochem. Photobiol. Sci. 2015, 14, 53–87. [Google Scholar] [CrossRef] [PubMed]
- Falcone, D.; Richters, R.J.H.; Uzunbajakava, N.E.; van Erp, P.E.J. Risk factors associated with sensitive skin and potenţial role of lifestyle habits: A cross-sectional study. Clin. Exp. Dermatol. 2017, 42, 656–658. [Google Scholar] [CrossRef] [PubMed]
- Modenese, A.; Bisegna, F.; Borra, M.; Grandi, C.; Gugliermetti, F.; Militello, A.; Gobba, F. Outdoor work and solar radiation exposure: Evaluation method for epidemiological studies. Med. Pr. 2016, 67, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.M.; Murphy, G.; Ralph, N.; O’Gorman, S.M. Polypharmacy and sun exposure: Implications for mitochondrial DNA deletions in skin. J. Photochem. Photobiol. B 2017, 173, 397–403. [Google Scholar] [CrossRef]
- Wong, Q.Y.A.; Chew, F.T. Defining skin aging and its risk factors: A systematic review and meta-analysis. Sci Rep. 2021, 11, 22075. [Google Scholar] [CrossRef] [PubMed]
- Drucker, A.M.; Rosen, C.F. Drug-induced photosensitivity. Drug Saf. 2011, 34, 821–837. [Google Scholar] [CrossRef] [PubMed]
- Scheuer, C. Melatonin for prevention of erythema and oxidative stress in response to ultraviolet radiation. Dan. Med. J. 2017, 64, B5358. [Google Scholar]
- Raymond-Lezman, J.R.; Riskin, S.I. Benefits and Risks of Sun Exposure to Maintain Adequate Vitamin D Levels. Cureus 2023, 15, e38578. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Rae, V.; Bruins-Slot, W.; Van den Berg, J.-W.; Taylor, J.R.; Streilein, J.W. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J. Invest Dermatol. 1990, 95, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Braun, H.A.; Adler, C.H.; Goodman, M.; Yeung, H. Sunburn frequency and risk and protective factors: A cross-sectional survey. Dermatol. Online J. 2021, 27, 13030/qt6qn7k2gp. [Google Scholar] [CrossRef] [PubMed]
- Holman, D.M.; Ding, H.; Guy, G.; Watson, M.; Hartman, A.M.; Perna, F.M. Prevalence of sun protection use and sunburn and association of demographic and behaviorial characteristics with sunburn among US adults. JAMA Dermatol. 2018, 154, 561–568. [Google Scholar] [CrossRef]
- Sola, Y.; Lorente, J. Contribution of UVA irradiance to the erythema and photoaging effects in solar and sunbed exposures. J. Photochem. Photobiol. B 2015, 143, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Addor, F.A.S. Beyond photoaging: Additional factors involved in the process of skin aging. Clin Cosmet Investig Dermatol. 2018, 11, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, Z. Skin of colour: Characteristics and disease. J. Pak. Med Assoc. 2017, 67, 292–299. [Google Scholar] [PubMed]
- Sivamani, R.K.; Crane, L.A.; Dellavalle, R.P. The benefits and risks of ultraviolet tanning and its alternatives: The role of prudent sun exposure. Dermatol. Clin. 2009, 27, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Hearing, V.J. The Protective Role of Melanin Against UV Damage in Human Skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, L.T.N.; Van Tran, V.; Moon, J.-Y.; Chae, M.; Park, D.; Lee, Y.-C. Recent Trends of Sunscreen Cosmetic: An Update Review. Cosmetics 2019, 6, 64. [Google Scholar] [CrossRef]
- Fischer, A.H.; Wang, T.S.; Yenokyan, G.; Kang, S. Sunburn and sun- protective behaviours among adults with and without previous non- melanoma skin cancer (NMSC): A population-based study. J. Am. Acad. Dermatol. 2016, 75, 371–379. [Google Scholar] [CrossRef]
- Azim, S.A.; Bainvoll, L.; Vecerek, N.; DeLeo, V.A.; Adler, B.L. Sunscreens Part 1: Mechanisms and Efficacy. J. Am. Acad. Dermatol. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.L.; Lim, H.W. A review of inorganic UV filters zinc oxide and titanium dioxide. Photodermatol. Photoimmunol. Photomed. 2019, 35, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.S.; Hsia, A.; Miller, J.D.; Hanneman, K.; Scull, H.; Cooper, K.D.; Baron, E. Non-sunscreen photoprotection: Antioxidants add value to a sunscreen. JID Symp. Proc. 2009, 14, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Eberlein-König, B.; Ring, J. Relevance of vitamins C and E in cutaneous photoprotection. J. Cosmet. Dermatol. 2005, 4, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Freitas, J.V.; Junqueira, H.C.; Martins, W.K.; Baptista, M.S.; Gaspar, L.R. Antioxidant role on the protection of melanocytes against visible light-induced photodamage. Free Rad. Biol. Med. 2019, 131, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.; Osterwalder, U.; Wang, S.Q.; Burnett, M.; Lim, H.W. Photoprotection: Part II. Sunscreen: Development, efficacy, and controversies. J. Am. Acad. Dermatol. 2013, 69, 867.e1–867.e14. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Mohammed, Y.; Prow, T.W. Advances and controversies in studying sunscreen delivery and toxicity. Adv. Drug Deliv. Rev. 2020, 153, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, Y.H.; Holmes, A.; Haridass, I.N.; Sanchez, W.Y.; Studier, H.; Grice, J.E.; Benson, H.A.; Roberts, M.S. Support for the safe use of zinc oxide nanoparticle sunscreens: Lack of skin penetration or cellular toxicity after repeated application in volunteers. J. Invest Dermatol. 2019, 139, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Fastelli, P.; Renzi, M. Exposure of key marine species to sunscreens: Changing ecotoxicity as a possible indirect effect of global warming. Mar. Pollut. Bull. 2019, 149, 110517. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Marcellini, F.; Nepote, E.; Damiani, E.; Danovaro, R. Impact of inorganic UV filters contained in sunscreen products on tropical stony corals (Acropora spp.). Sci. Total Environ. 2018, 637–638, 1279–1285. [Google Scholar] [CrossRef]
- Sendra, M.; Sánchez-Quiles, D.; Blasco, J.; Moreno-Garrido, I.; Lubián, L.M.M.; Pérez-García, S.; Tovar-Sánchez, A. Effects of TiO2 nanoparticles and sunscreens on coastal marine microalgae: Ultraviolet radiation is key variable for toxicity assessment. Environ. Int. 2017, 98, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, M.; Schiavo, S.; Rametta, G.; Miglietta, M.L.; Manzo, S. Different sizes of ZnO diversely affected the cytogenesis of the sea urchin Paracentrotus lividus. Sci. Total Environ. 2017, 607, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Arbeloa, E.M.; Bertolotti, S.G.; Churio, M.S. Photophysics and reductive quenching reactivity of gadusol in solution. Photochem. Photobiol. Sci. 2011, 10, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Ignasiak, M.T.; Houée-Levin, C.; Kciuk, G.; Marciniak, B.; Pedzinski, T. A reevaluation of the photolytic properties of 2-hydroxybenzophenone-based UV sunscreens: Are chemical sunscreens inoffensive? ChemPhysChem 2015, 16, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Glaser, K.S.; Tomecki, K.J. Sunscreens in the United States: Current status and future outlook. Adv. Exp. Med. Biol. 2020, 1268, 355–379. [Google Scholar] [PubMed]
- Holt, E.L.; Rodrigues, N.D.N.; Cebrián, J.; Stavros, V.G. Determining the photostability of avobenzone in sunscreen formulation models using ultrafast spectroscopy. Phys. Chem. Chem. Phys. 2021, 23, 24439–24448. [Google Scholar] [CrossRef] [PubMed]
- Abiola, T.T.; Whittock, A.L.; Stavros, V.G. Unravelling the Photoprotective Mechanisms of Nature-Inspired Ultraviolet Filters Using Ultrafast Spectroscopy. Molecules 2020, 25, 3945. [Google Scholar] [CrossRef] [PubMed]
- Holt, E.L.; Krokidi, K.M.; Turner, M.A.P.; Mishra, P.; Zwier, T.S.; Rodrigues, N.D.N.; Stavros, V.G. Insights into the photoprotection mechanism of the UV filter homosalate. Phys. Chem. Chem. Phys. 2020, 22, 15509–15519. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.D.N.; Stavros, V.G. From fundamental science to product: A bottom-up approach to sunscreen development. Sci. Prog. 2018, 101, 8–31. [Google Scholar] [CrossRef]
- Ruszkiewicz, J.A.; Pinkas, A.; Ferrer, B.; Peres, T.V.; Tsatsakis, A.; Aschner, M. Neurotoxic effect of active ingredients in sunscreen products, a contemporary review. Toxicol Rep. 2017, 4, 245–259. [Google Scholar] [CrossRef]
- Shetty, P.K.; Venuvanka, V.; Jagani, H.V.; Chethan, G.H.; Ligade, V.S. Development and evaluation of sunscreen creams containing morin-encapsulated nanoparticles for enhanced UV radiation protection and antioxidant activity. Int. J. Nanomed. 2015, 10, 6477–6491. [Google Scholar]
- Elmets, C.A.; Singh, D.; Tubesing, K.; Matsui, M.; Katiyar, S.; Mukhtar, H. Cutaneous photoprotection from ultraviolet injury by green tea polyphenols. J. Am. Acad. Dermatol. 2001, 44, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Feng, C.; Ruan, Z. Inhibitory effect of a genistein derivative on pigmentation of guinea pig skin. RSC Adv. 2017, 7, 7914–7919. [Google Scholar] [CrossRef]
- Radice, M.; Manfredini, S.; Ziosi, P.; Dissette, V.; Buso, P.; Fallacara, A.; Vertuani, S. Herbal extracts, lichens and biomolecules as natural photo-protection alternatives to synthetic UV filters. A systematic review. Fitoterapia 2016, 114, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, S. Do the polyphenolic compounds from natural products can protect the skin from ultraviolet rays? Results Chem. 2022, 4, 100428. [Google Scholar] [CrossRef]
- Saewan, N.; Jimtaisong, A. Photoprotection of natural flavonoids. J. Appl. Pharm. Sci. 2013, 3, 129–141. [Google Scholar]
- Williams, S.; Tamburic, S.; Lally, C. Eating chocolate can significantly protect the skin from UV light. J. Cosmet. Dermatol. 2009, 8, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Mansur, M.C.P.R.; Leitão, S.G.; Cerqueira-Coutinho, C.; Vermelho, A.B.; Silva, R.S.; Presgrave, O.A.; Leitão, Á.A.; Leitão, G.G.; Ricci-Júnior, E.; Santos, E.P. In vitro and in vivo evaluation of efficacy and safety of photoprotective formulations containing antioxidant extracts. Rev. Bras. Farm. 2016, 26, 251–258. [Google Scholar] [CrossRef]
- Camouse, M.M.; Domingo, D.S.; Swain, F.R.; Conrad, E.P.; Matsui, M.S.; Maes, D.; Declercq, L.; Cooper, K.D.; Stevens, S.R.; Baron, E.D. Topical application of green and white tea extracts provides protection from solar-simulated ultraviolet light in human skin. Exp. Dermatol. 2009, 18, 522–526. [Google Scholar] [CrossRef]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Vitis vinifera (Grape)-Derived Ingredients as Used in Cosmetics. Int. J. Toxicol. 2014, 33, 48S–83S. [Google Scholar] [CrossRef]
- Ky, I.; Teissedre, P.-L. Characterisation of Mediterranean Grape Pomace Seed and Skin Extracts: Polyphenolic Content and Antioxidant Activity. Molecules 2015, 20, 2190–2207. [Google Scholar] [CrossRef] [PubMed]
- Hubner, A.; Sobreira, F.; Neto, A.V.; Pinto, C.A.S.D.O.; Dario, M.F.; Díaz, I.E.C.; Lourenço, F.R.; Rosado, C.; Baby, A.R.; Bacchi, E.M. The Synergistic Behavior of Antioxidant Phenolic Compounds Obtained from Winemaking Waste’s Valorization, Increased the Efficacy of a Sunscreen System. Antioxidants 2019, 8, 530. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Burton, S.; Kim, C.; Sismour, E.N. Phenolic compounds, antioxidant, and antibacterial properties of pomace extracts from four Virginia-grown grape varieties. Food Sci. Nutr. 2016, 4, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.L.; Falqué, E.; Domínguez, H. Relevance of Natural Phenolics from Grape and Derivative Products in the Formulation of Cosmetics. Cosmetics 2015, 2, 259–276. [Google Scholar] [CrossRef]
- Chambers, P.J.; Pretorius, I.S. Fermenting knowledge: The history of winemaking, science and yeast research. Sci. Soc. Ser. Food Sci. 2010, 11, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Pullammanappallil, P. Value Added Products From Vineyard Wastes—A Review. In Proceedings of the ORBIT, Perth, WA, Canada, 30 April–2 May 2003; pp. 108–118. [Google Scholar]
- Georgiev, V.; Ananga, A.; Tsolova, V. Recent advances and uses of grape flavonoids as nutraceuticals. Nutrients 2014, 6, 391–415. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.A.; Saucier, C.; Glories, Y. Grape and Wine Phenolics: History and Perspective. Am. J. Enol. Vitic. 2006, 57, 239–247. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Major Flavonoids in Grape Seeds and Skins: Antioxidant Capacity of Catechin, Epicatechin, and Gallic Acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Jara-Palacios, M.J.; Hernanz, D.; Escudero-Gilete, M.L.; Heredia, F.J. The Use of Grape Seed Byproducts Rich in Flavonoids to Improve the Antioxidant Potential of Red Wines. Molecules 2016, 21, 1526. [Google Scholar] [CrossRef]
- Sudhahar, V.; Balasubramanian, V. Sun production factor (SPF) determination of marketed sunscreen formulation by In-Vitro method using UV-VIS spectrophotometer. Arch. Appl. Sci. Res. 2013, 5, 119–122. [Google Scholar]
- Moyal D, Alard V, Bertin C, Boyer F, Brown MW, Kolbe L, Matts P, Pissavini M, The revised COLIPA in Vitro UVA method. Int. J. Cosmet. Sci. 2013, 35, 35–40.
- COLIPA -The European Cosmetics Association, In vitro method for the determination of the UVA protection factor and “critical wavelength” values of sunscreen products-Guideline. 2009. Available online: https://downloads.regulations.gov/FDA-1978-N-0018-0698/attachment_70.pdf (accessed on 1 September 2023).
- Güler, E. Polyphenols, organic acids, and their relationships in red grapes of Vitis vinifera and Isabella (Vitis labrusca) under arid conditions. Eur. Food Res. Technol. 2023, 249, 913–921. [Google Scholar] [CrossRef]
- Bonilla, F.; Mayen, M.; Merida, J.; Medina, M. Extraction of phenolic compounds from red grape marc for use as food lipid antioxidants. Food Chem. 1999, 66, 209–215. [Google Scholar] [CrossRef]
- Mansur, J.S.; Breder, M.N.R.; Mansur, M.C.A.; Azulay, R.D. Determinação do fator de proteção solar por espectrofotometria. An. Bras. Dermatol. 1986, 61, 121–124. [Google Scholar]
- Sayre, R.M.; Agin, P.P.; Levee, G.J.; Marlowe, E. Comparison of in vivo and in vitro testing of sunscreening formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef] [PubMed]
- European Commission. European Commission recommendation. On the efficacy of sunscreen products and the claims made relating there to. Off. J. Eur. Union 2006, 265, 39–43. [Google Scholar]
- Addor, F.A.S.; Barcaui, C.B.; Gomes, E.E.; Lupi, O.; Marçon, C.R.; Miot, H.A. Sunscreen lotions in the dermatological prescription: Review of concepts and controversies. An. Bras. Dermatol. 2022, 97, 204–222. [Google Scholar] [CrossRef]
- Mataixa, E.; Luque de Castroa, M.D. Simultaneous (or sequential) determination of the total polyphenol index (or I280) and density in wines by flow injection. Analyst 2001, 126, 251–255. [Google Scholar] [CrossRef]
- Manasia, T.; Manolache, A.F.; Ionescu, V.; Mulţescu, M.; Eftimie, M.; Todaşcă, C. Evolution of phenolic compounds during the red grapes fermentation in the presence of trace metals. U.P.B. Sci. Bull. Ser. B 2021, 83, 28–36. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Apak, R.; Ozyurek, M.; Guclu, K.; Bekdeser, B.; Bener, M. Chapter 24—The CUPRAC Methods of Antioxidant Measurement for Beverages. In Processing and Impact on Antioxidants in Beverages; Preedy, V., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 235–244. [Google Scholar]
- Trofin, A.E.; Trincă, L.C.; Ungureanu, E.; Ariton, A.M. CUPRAC Voltammetric Determination of Antioxidant Capacity in Tea Samples by Using Screen-Printed Microelectrodes. J. Anal. Methods Chem. 2019, 2019, 8012758. [Google Scholar] [CrossRef] [PubMed]
- Diffey, B.L.; Robson, J. A new substrate to measure sunscreen protection factors throughout the ultraviolet spectrum. J. Soc. Cosmet. Chem. 1989, 40, 127–133. [Google Scholar]
- Diffey, B.L.; Tanner, P.R.; Matts, P.J.; Nash, J.F. In vitro assessment of the broad spectrum ultraviolet protection of sunscreen products. J. Am. Acad. Dermatol. 2000, 43, 1024–1035. [Google Scholar] [CrossRef] [PubMed]

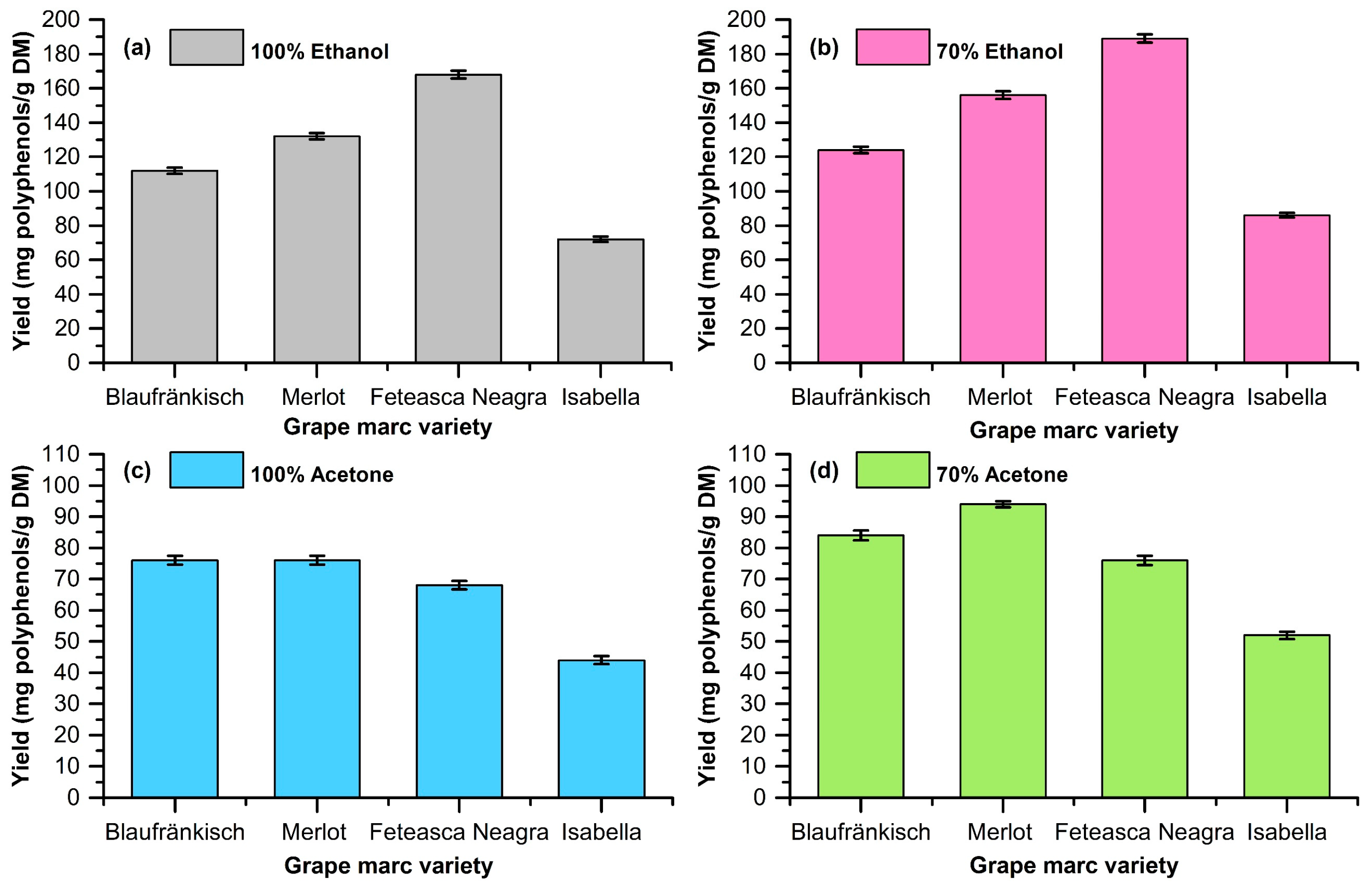
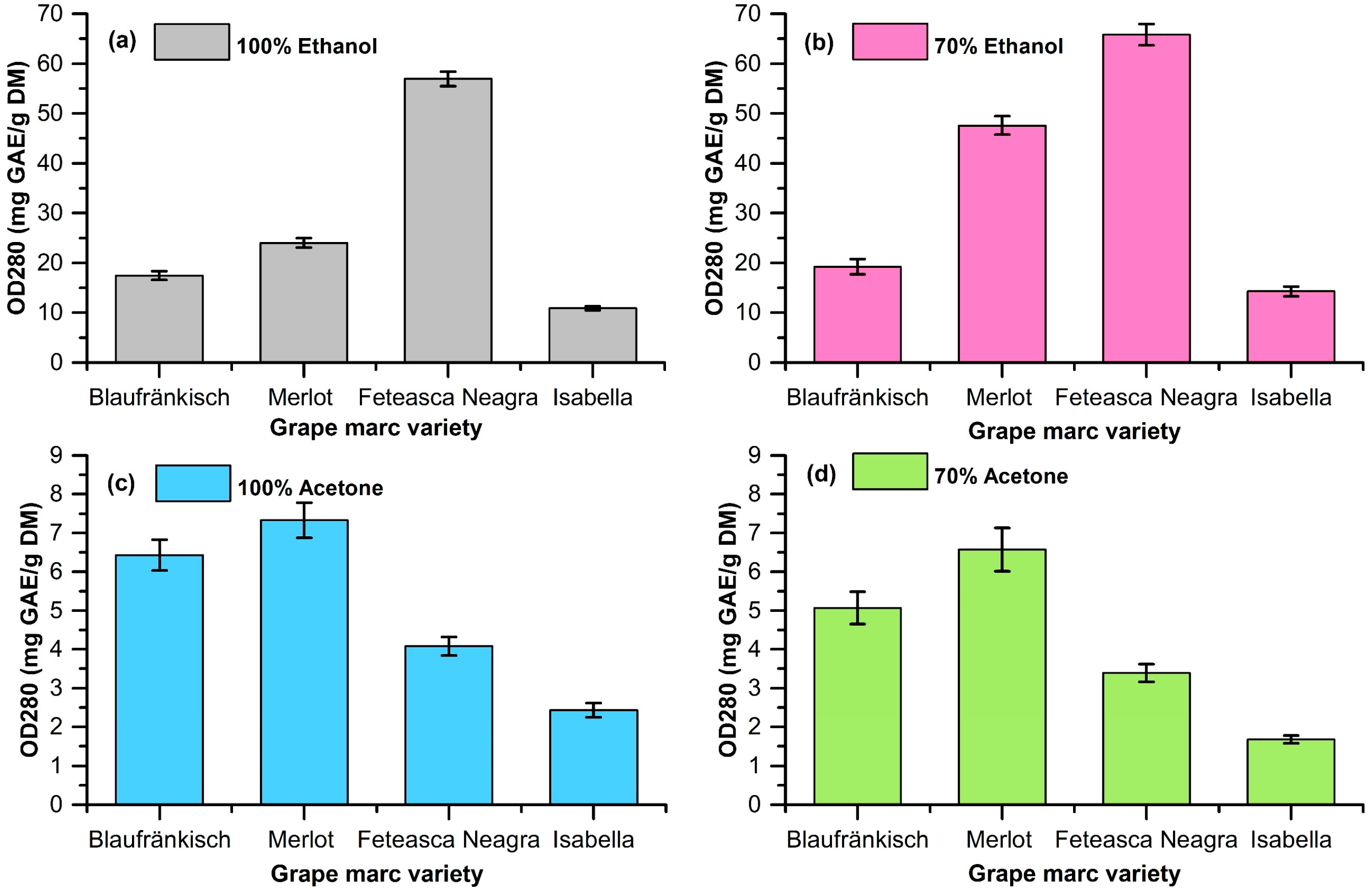

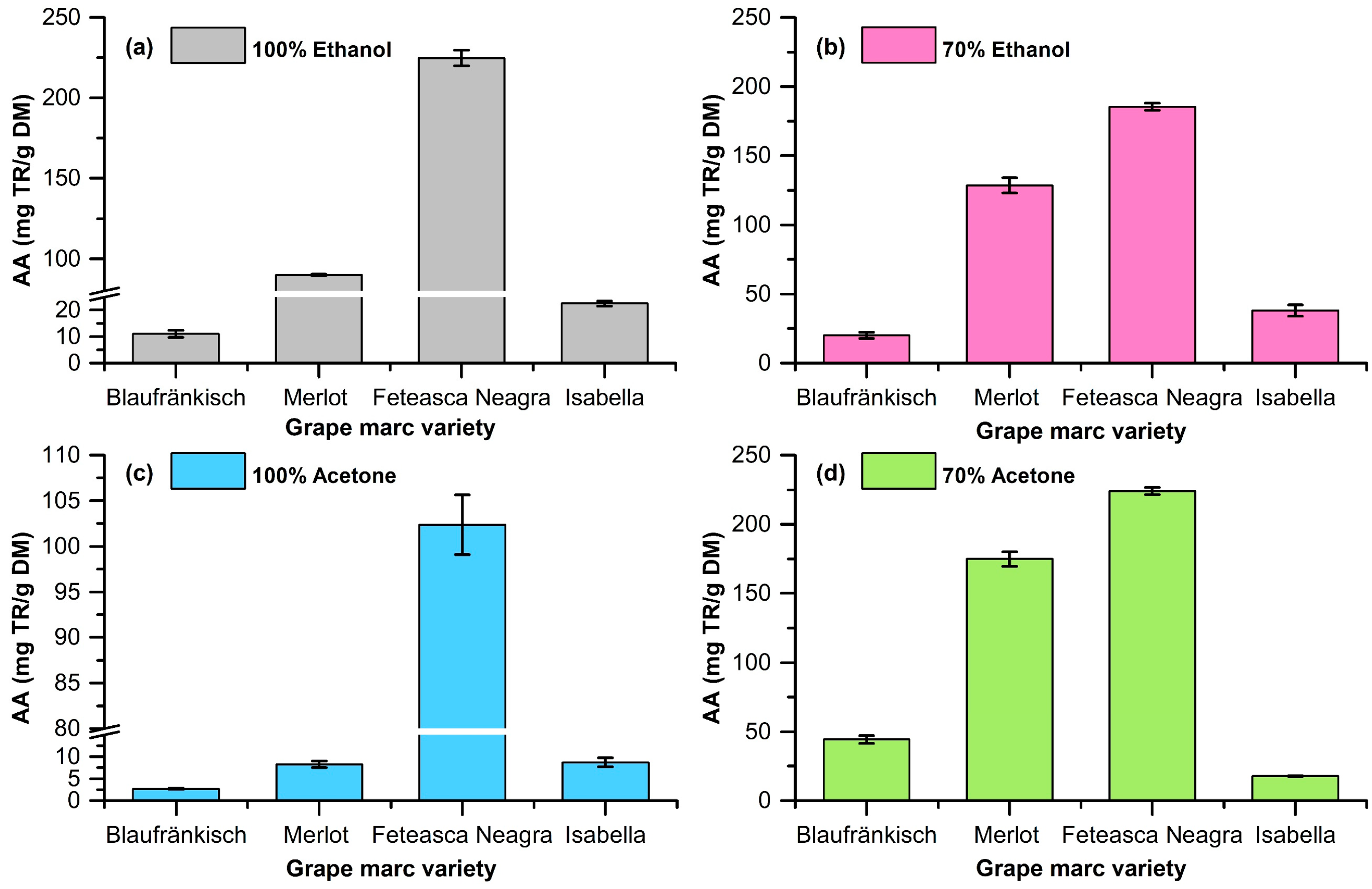

| Grape Marc | Solvent | Yield (mg Polyphenols/g DM) | OD280 (mg GA/g DM) | TPC (mg GA/g DM) | Antioxidant Activity via the CUPRAC Assay (mg TROLOX/g DM) |
|---|---|---|---|---|---|
| Blaufränkisch | 100% ethanol | 112 ± 1.85 | 24.01 ± 0.94 | 63.99 ± 1.68 | 89.92 ± 0.54 |
| 70% ethanol | 124 ± 1.96 | 19.20 ± 1.54 | 58.57 ± 2.32 | 20.11 ± 2.22 | |
| 100% acetone | 76 ± 1.43 | 6.43 ± 0.40 | 0.19 ± 0.02 | 2.69 ± 0.13 | |
| 70% acetone | 84 ± 1.58 | 5.07 ± 0.42 | 62.54 ± 1.20 | 44.39 ± 2.78 | |
| Merlot | 100% ethanol | 132 ± 1.85 | 24.01 ± 0.94 | 63.99 ± 1.68 | 89.92 ± 0.54 |
| 70% ethanol | 156 ± 2.20 | 47.59 ± 1.88 | 113.12 ± 2.39 | 128.50 ± 5.55 | |
| 100% acetone | 76 ± 1.44 | 7.33 ± 0.45 | 5.98 ± 0.19 | 8.26 ± 0.76 | |
| 70% acetone | 94 ± 1.02 | 6.58 ± 0.56 | 243.39 ± 5.88 | 174.85 ± 5.24 | |
| Fetească Neagră | 100% ethanol | 168 ± 2.26 | 56.95 ± 1.45 | 77.60 ± 1.92 | 224.77 ± 4.94 |
| 70% ethanol | 189 ± 2.34 | 65.84 ± 2.13 | 237.35 ± 3.44 | 185.40 ± 2.43 | |
| 100% acetone | 68 ± 1.34 | 4.08 ± 0.24 | 58.20 ± 2.39 | 102.36 ± 3.28 | |
| 70% acetone | 76 ± 1.45 | 3.39 ± 0.23 | 238.62 ± 4.83 | 223.98 ± 2.52 | |
| Isabella | 100% ethanol | 72 ± 1.65 | 10.89 ± 0.40 | 10.37 ± 0.05 | 22.52 ± 0.96 |
| 70% ethanol | 86 ± 1.46 | 14.28 ± 0.98 | 46.48 ± 0.34 | 38.01 ± 4.13 | |
| 100% acetone | 44 ± 1.34 | 2.43 ± 0.18 | 0.99 ± 0.02 | 8.69 ± 1.03 | |
| 70% acetone | 52 ± 1.16 | 1.67 ± 0.10 | 125.08 ± 2.39 | 17.83 ± 0.30 |
| Variety | SPFspectrophotometric | |||
|---|---|---|---|---|
| 100% Ethanol | 100% Acetone | 70% Ethanol | 70% Acetone | |
| Blaufränkisch | 2.76 ± 0.24 | 0.97 ± 0.12 | 4.33 ± 0.46 | 2.05 ± 0.16 |
| Merlot | 3.89 ± 0.42 | 1.38 ± 0.23 | 7.83 ± 0.76 | 2.89 ± 0.22 |
| Fetească Neagră | 5.19 ± 0.52 | 1.12 ± 0.32 | 6.15 ± 0.58 | 1.30 ± 0.23 |
| Isabella | 1.60 ± 0.60 | 0.29 ± 0.08 | 2.24 ± 0.32 | 1.03 ± 0.15 |
| Sample | Stability—24 Ore | Stability—48 Ore | SPF In Vitro | UVAPF0 |
|---|---|---|---|---|
| P1A | Very good | Very good | 5.65 ± 0.87 | 1.63 ± 0.35 |
| P2A | Very good | Very good | 6.84 ± 0.92 | 1.72 ± 0.24 |
| P3A | Very good | Very good | 10.46 ± 1.10 | 2.15 ± 0.44 |
| P4A | Good | Good | 14.07 ± 1.50 | 2.15 ± 0.38 |
| P1E | Very good | Very good | 3.27 ± 0.87 | 1.42 ± 0.32 |
| P2E | Good | Good | 4.77 ± 0.94 | 1.59 ± 0.34 |
| P3E | Good | Good | 8.94 ± 1.10 | 2.21 ± 0.43 |
| P4E | Good | Poor | 11.46 ± 1.32 | 2.42 ± 0.46 |
| Wavelength (nm) | E × I |
|---|---|
| 290 | 0.0150 |
| 295 | 0.0817 |
| 300 | 0.2874 |
| 305 | 0.3278 |
| 310 | 0.1862 |
| 315 | 0.0839 |
| 320 | 0.0180 |
| Total | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Draghici-Popa, A.-M.; Buliga, D.-I.; Popa, I.; Tomas, S.T.; Stan, R.; Boscornea, A.C. Cosmetic Products with Potential Photoprotective Effects Based on Natural Compounds Extracted from Waste of the Winemaking Industry. Molecules 2024, 29, 2775. https://doi.org/10.3390/molecules29122775
Draghici-Popa A-M, Buliga D-I, Popa I, Tomas ST, Stan R, Boscornea AC. Cosmetic Products with Potential Photoprotective Effects Based on Natural Compounds Extracted from Waste of the Winemaking Industry. Molecules. 2024; 29(12):2775. https://doi.org/10.3390/molecules29122775
Chicago/Turabian StyleDraghici-Popa, Ana-Maria, Diana-Ioana Buliga, Ioana Popa, Stefan Theodor Tomas, Raluca Stan, and Aurelian Cristian Boscornea. 2024. "Cosmetic Products with Potential Photoprotective Effects Based on Natural Compounds Extracted from Waste of the Winemaking Industry" Molecules 29, no. 12: 2775. https://doi.org/10.3390/molecules29122775
APA StyleDraghici-Popa, A.-M., Buliga, D.-I., Popa, I., Tomas, S. T., Stan, R., & Boscornea, A. C. (2024). Cosmetic Products with Potential Photoprotective Effects Based on Natural Compounds Extracted from Waste of the Winemaking Industry. Molecules, 29(12), 2775. https://doi.org/10.3390/molecules29122775








