Laponite®—From Dispersion to Gel—Structure, Properties, and Applications
Abstract
:1. Introduction
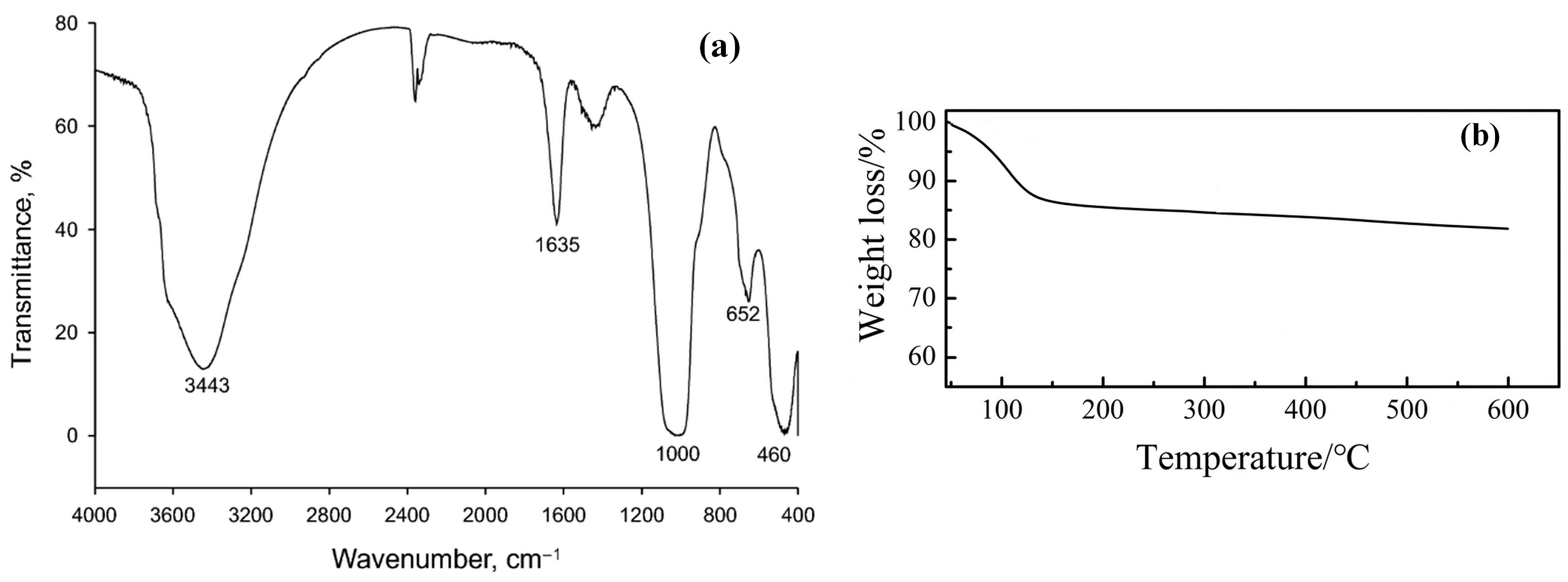
2. Laponite® Structure
3. Laponite® Aqueous Dispersion—Effect of Various Factors on the Clay Platelets Organization
3.1. Effect of Environment Ionic Strength and LAP Concentration
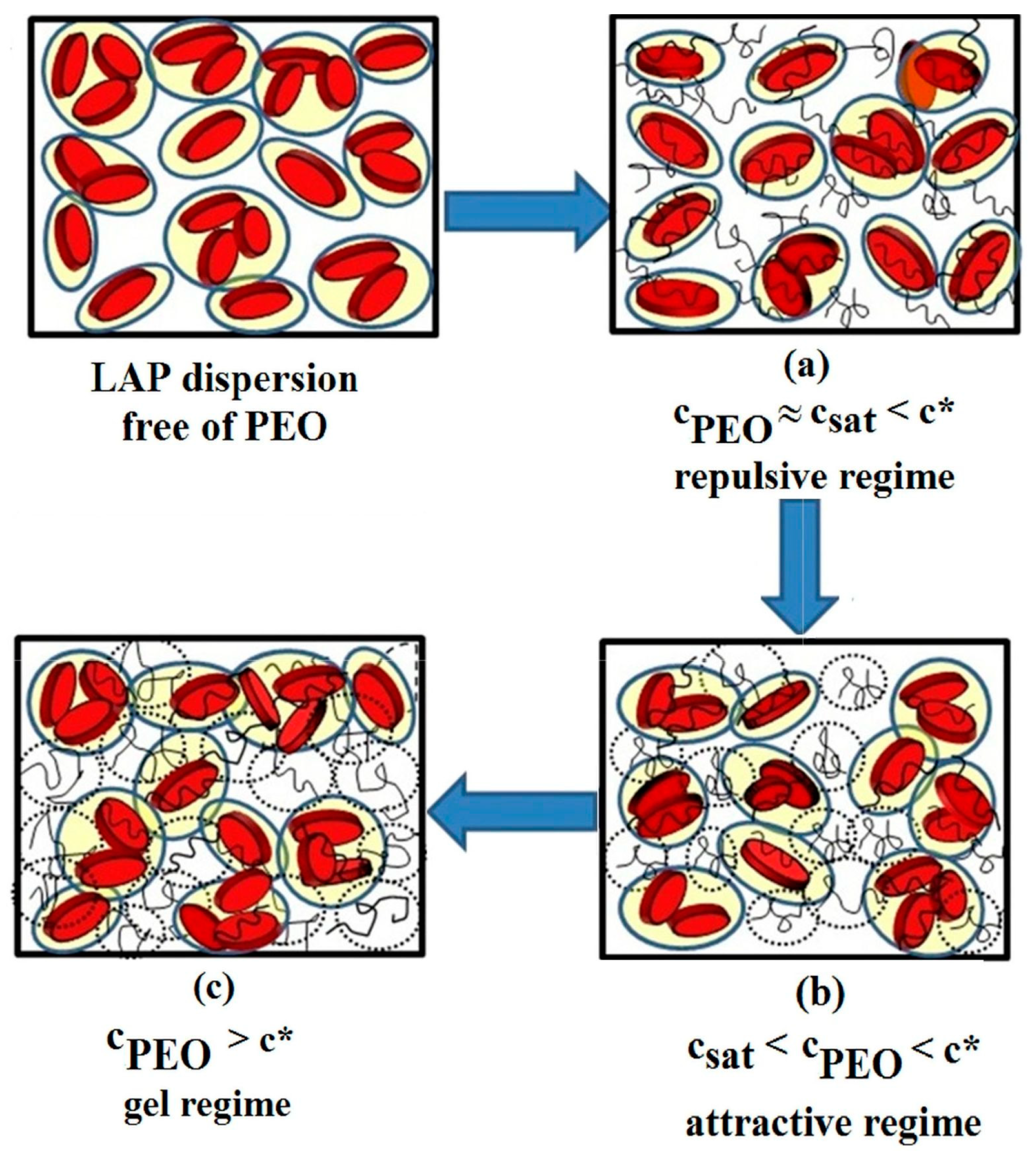
3.2. Effect of Polymer Addition
3.3. Effect of pH
3.4. Effect of Temperature
4. Properties of Laponite® Aqueous Dispersions and Hydrogels
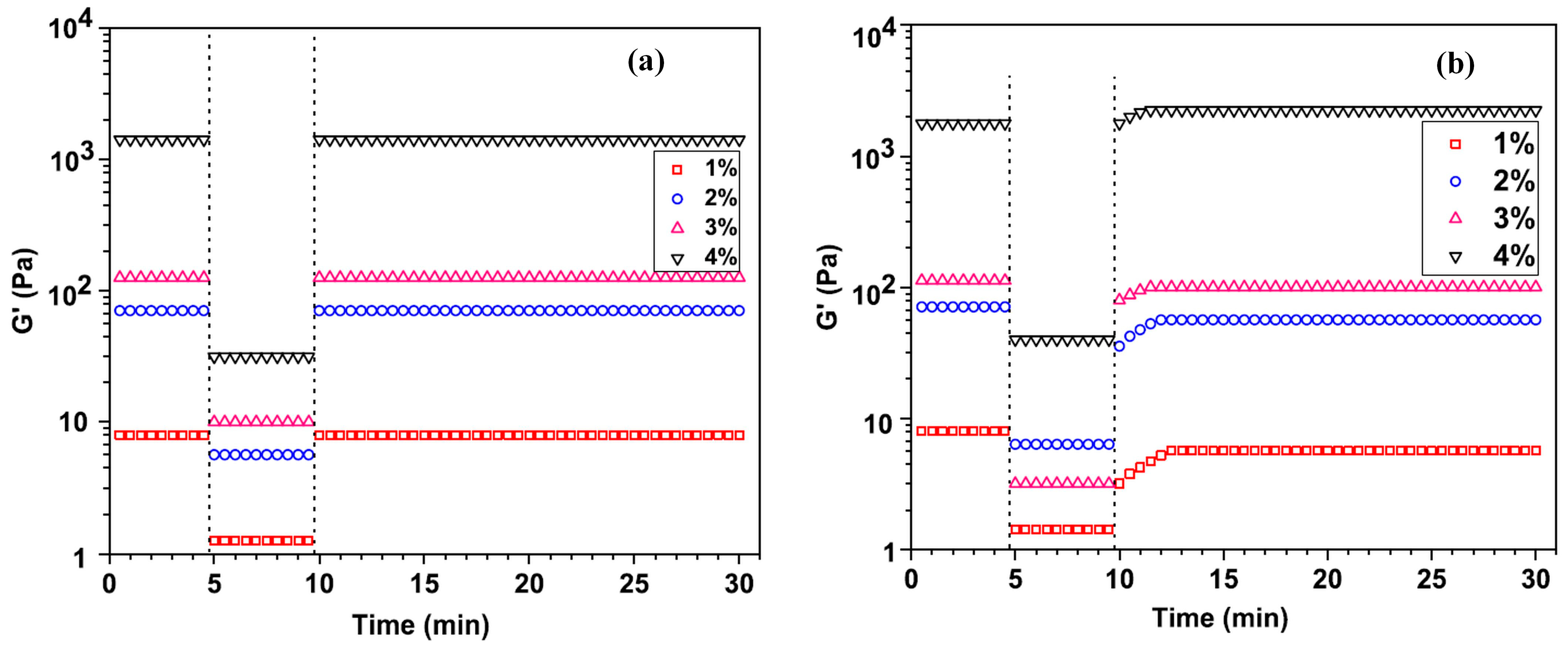
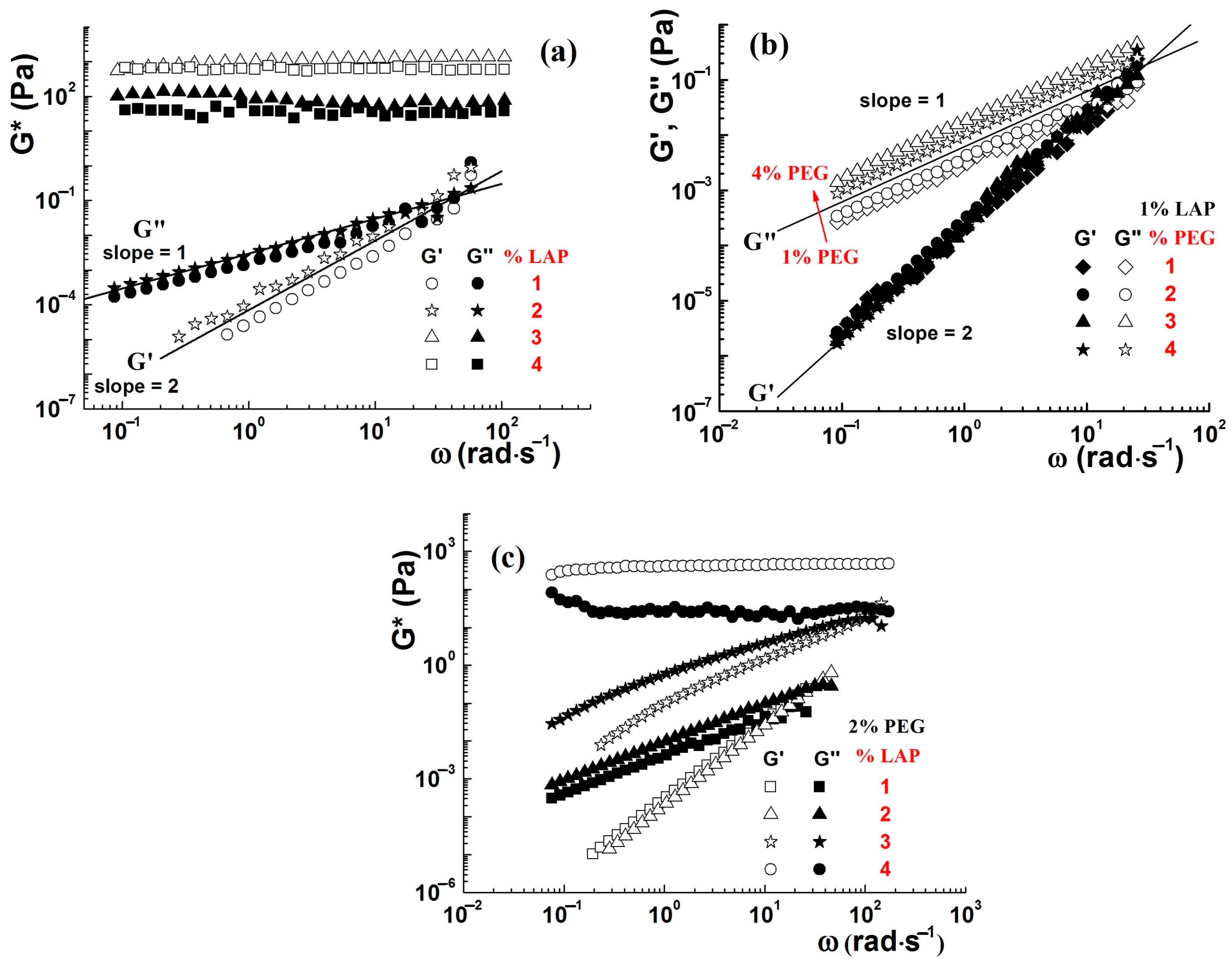
4.1. Rheological Properties
4.2. Mechanical Properties
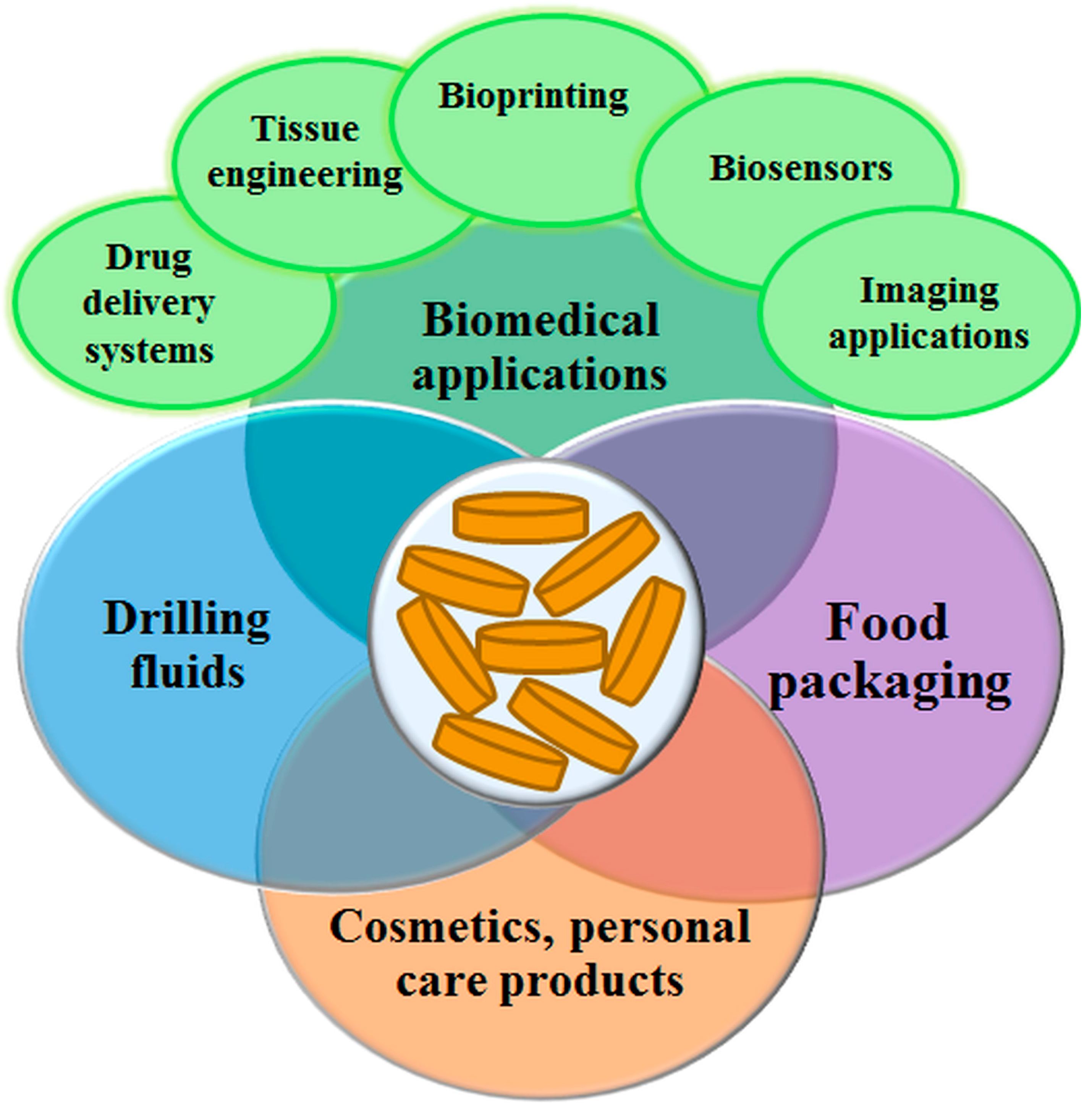
5. Laponite® Applications
5.1. Biomedical Applications
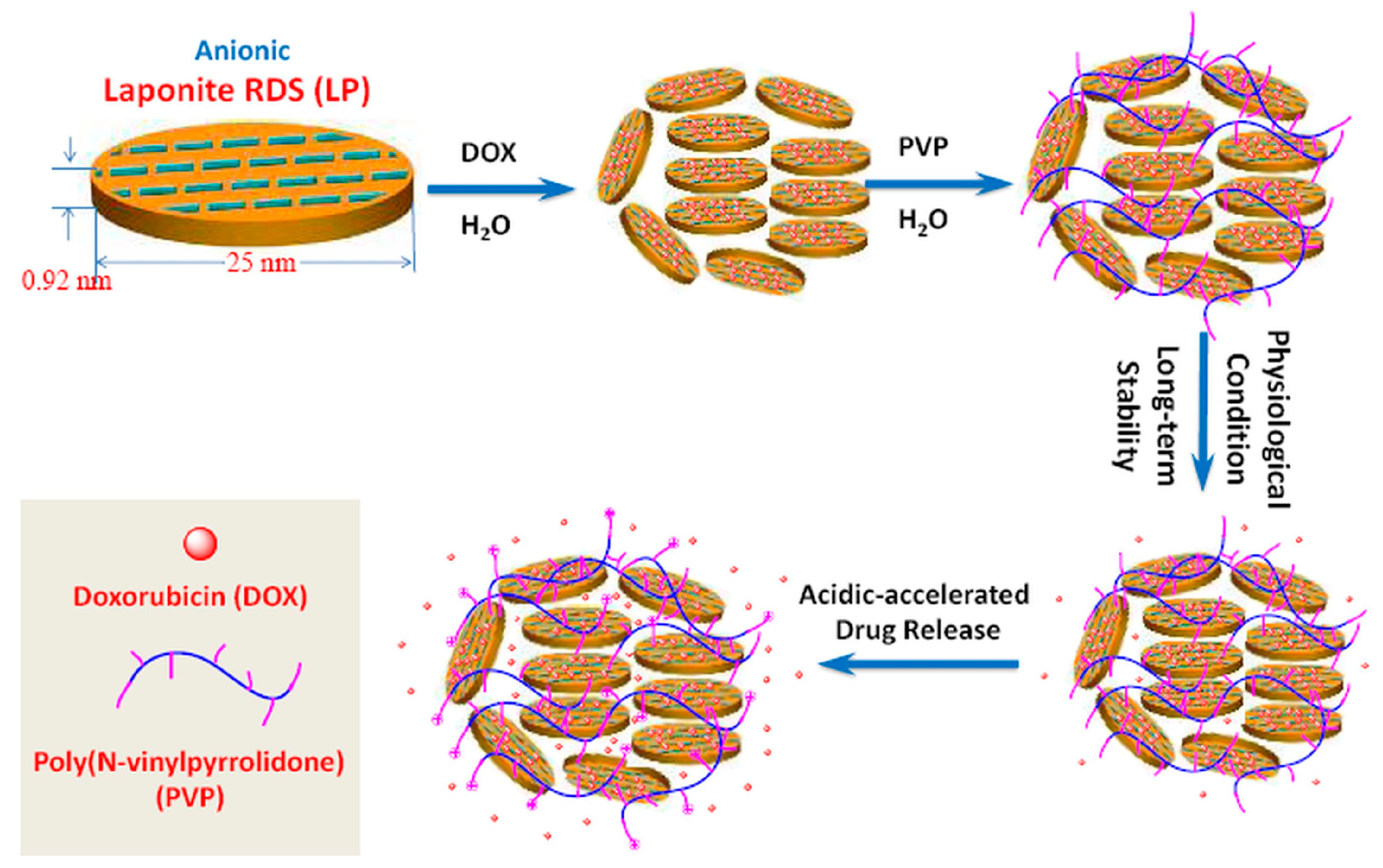
5.1.1. Drug Delivery
5.1.2. Tissue Engineering
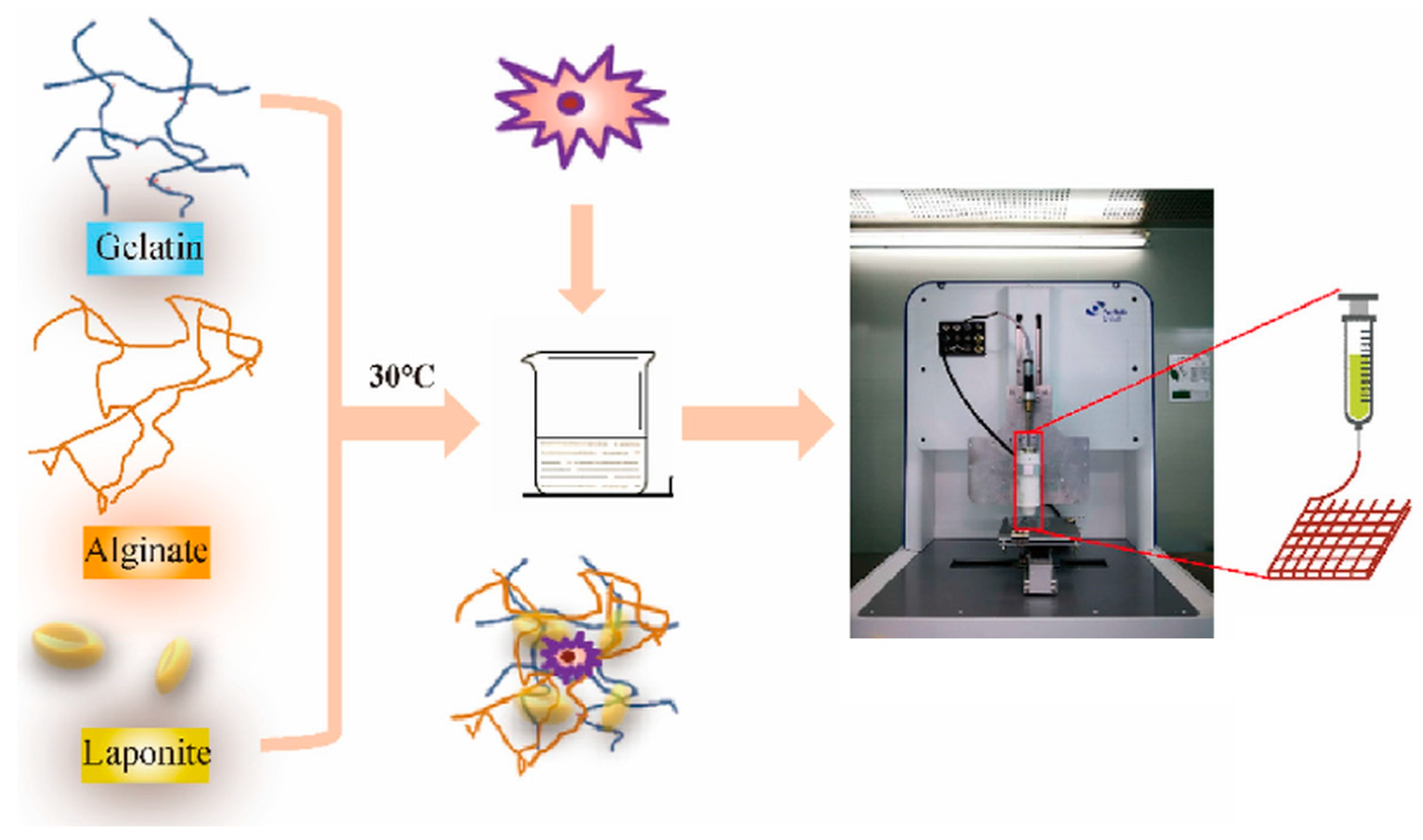
5.1.3. Bioprinting
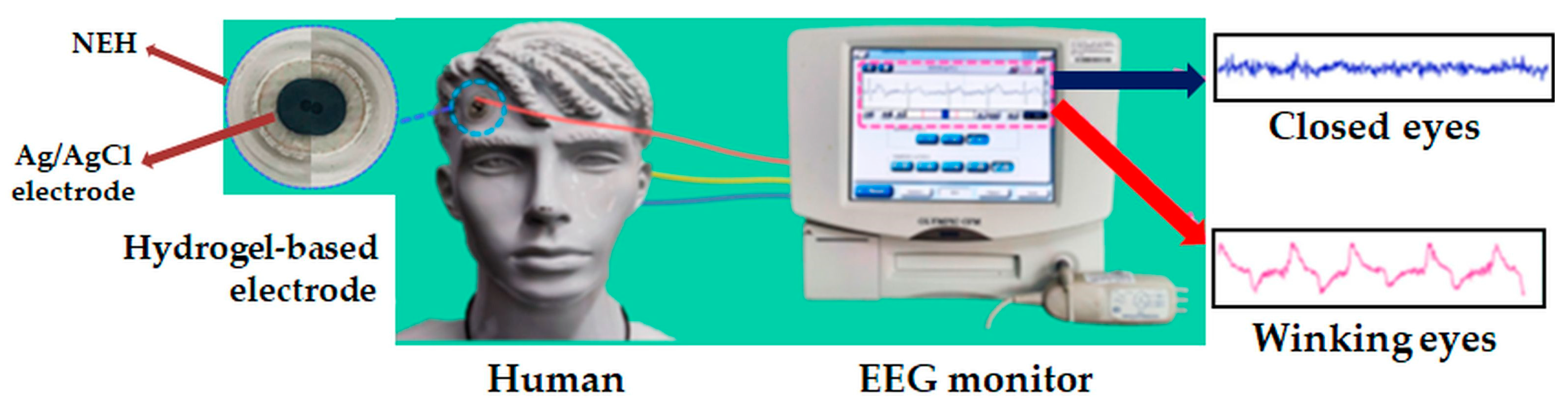
5.1.4. Biosensors
5.1.5. Biomedical Imaging
5.2. Food Packaging
5.3. Drilling Fluids (DF)
5.4. Cosmetics and Personal Care Products
6. Conclusions
7. Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | ascorbic acid |
| AAm | acrylamide |
| AMPS | 2-acrylamido-2-methylpropanesulfonic acid |
| ANDP | nanocomposite filter reducer based on 2-acrylamide-2-methylpropane sulfonic acid, acrylamide, and modified Laponite® |
| c* | overlap concentration of poly(ethylene oxide) |
| cLAP | Laponite® concentration |
| csat | critical polymer concentration corresponding to a complete cover of the clay platelet surface |
| CB[6] | curcubit[6]uril |
| cPEG | cyclic poly(ethylene glycol) |
| CS | chitosan |
| DA | dopamine |
| DF | drilling fluids |
| DNA | deoxyribonucleic acid |
| DOX | doxorubicin |
| FFA | flufenamic acid |
| HP copolymer | heparin–poloxamer 407 |
| LAP | Laponite® |
| MYR | isopropyl myristate |
| Mw | polymer molecular weight |
| OA | oxalic acid |
| PAA | poly(acrylic acid) |
| PAAm | poly(acrylamide) |
| PDT | photodynamic therapy |
| PEG | poly(ethylene glycol) |
| PEGDA | poly(ethylene glycol) diacrylate |
| PEO | poly(ethylene oxide) |
| PLA–PEG–COOH | poly(lactic acid)–poly(ethylene glycol) |
| PPG | poly(propylene glycol) |
| PTT | photothermal therapy |
| PVA | poly(vinyl alcohol) |
| PZC | point of zero charge |
| SQ | squalene |
| tw | aging time |
| UA | uric acid |
| WBDF | water-based drilling fluid |
References
- Jatav, S.; Joshi, Y.M. Chemical Stability of Laponite in Aqueous Media. Appl. Clay Sci. 2014, 97–98, 72–77. [Google Scholar] [CrossRef]
- Tanaka, H.; Meunier, J.; Bonn, D. Nonergodic States of Charged Colloidal Suspensions: Repulsive and Attractive Glasses and Gels. Phys. Rev. E 2004, 69, 031404. [Google Scholar] [CrossRef] [PubMed]
- Le Coeur, C.; Lorthioir, C.; Feoktystov, A.; Wu, B.; Volet, G.; Amiel, C. Laponite/Poly(2-Methyl-2-Oxazoline) Hydrogels: Interplay between Local Structure and Rheological Behaviour. J. Colloid Interface Sci. 2021, 582, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Morariu, S.; Bercea, M.; Gradinaru, L.M.; Rosca, I.; Avadanei, M. Versatile Poly(Vinyl Alcohol)/Clay Physical Hydrogels with Tailorable Structure as Potential Candidates for Wound Healing Applications. Mater. Sci. Eng. C 2020, 109, 110395. [Google Scholar] [CrossRef]
- Morariu, S.; Brunchi, C.-E.; Honciuc, M.; Iftime, M.-M. Development of Hybrid Materials Based on Chitosan, Poly(Ethylene Glycol) and Laponite® RD: Effect of Clay Concentration. Polymers 2023, 15, 841. [Google Scholar] [CrossRef] [PubMed]
- Mahdavinia, G.R.; Hoseinzadeh, H.; Labib, P.; Jabbari, P.; Mohebbi, A.; Barzeger, S.; Jafari, H. (Magnetic Laponite/κ-Carrageenan)@chitosan Core–Shell Carrier for pH-Sensitive Release of Doxorubicin. Polym. Bull. 2023, 80, 12923–12943. [Google Scholar] [CrossRef]
- Mohammed, S.; Liu, M.; Zhang, Q.; Narayanan, S.; Zhang, F.; Gadikota, G. Resolving Salt-Induced Agglomeration of Laponite Suspensions Using X-Ray Photon Correlation Spectroscopy and Molecular Dynamics Simulations. Materials 2023, 16, 101. [Google Scholar] [CrossRef] [PubMed]
- Lysenkov, E.; Klepko, V.; Bulavin, L.; Lebovka, N. Physico-Chemical Properties of Laponite®/Polyethylene-Oxide Based Composites. Chem. Rec. 2024, 24, e202300166. [Google Scholar] [CrossRef]
- Corzo, I.J.M.; da Fonsêca, J.H.L.; d’Ávila, M.A. Influence of Carboxymethyl Cellulose Solutions on Rheological Properties of Laponite Dispersions. Rheol. Acta 2023, 62, 393–404. [Google Scholar] [CrossRef]
- Morariu, S.; Teodorescu, M.; Bercea, M. Rheological Investigation of Polymer/Clay Dispersions as Potential Drilling Fluids. J. Pet. Sci. Eng. 2022, 210, 110015. [Google Scholar] [CrossRef]
- Jafari, H.; Namazi, H. pH-Sensitive Biosystem Based on Laponite RD/Chitosan/Polyvinyl Alcohol Hydrogels for Controlled Delivery of Curcumin to Breast Cancer Cells. Colloids Surf. B Biointerface 2023, 231, 113585. [Google Scholar] [CrossRef]
- Zhou, X.; Xie, X.; Ma, X.; Mu, Q.; Gong, X.; Huang, Z.; Zhao, Y.; Chu, X.; Ma, H.; Xu, W. Highly Stretchable and Adhesive Poly(N, N-Dimethylacrylamide)/Laponite Nanocomposite Hydrogels for Wearable Sensor Devices. ChemistrySelect 2022, 7, e202201724. [Google Scholar] [CrossRef]
- Bailey, L.; Lekkerkerker, H.N.W.; Maitland, G.C. Smectite Clay—Inorganic Nanoparticle Mixed Suspensions: Phase Behaviour and Rheology. Soft Matter 2015, 11, 222–236. [Google Scholar] [CrossRef]
- Neumann, B.S. Improvements in or Relating to Synthetic Clay-Like Minerals. UK Patent GB1054111, 26 June 1962. [Google Scholar]
- Kroon, M.; Vos, W.L.; Wegdam, G.H. Structure and Formation of a Gel of Colloidal Disks. Int. J. Thermophys. 1998, 19, 887–894. [Google Scholar] [CrossRef]
- Avery, R.G.; Ramsay, J.D.F. Colloidal Properties of Synthetic Hectorite Clay Dispersions: II. Light and Small Angle Neutron Scattering. J. Colloid Interface Sci. 1986, 109, 448–454. [Google Scholar] [CrossRef]
- Li, L.; Harnau, L.; Rosenfeldt, S.; Ballauff, M. Effective Interaction of Charged Platelets in Aqueous Solution: Investigations of Colloid Laponite Suspensions by Static Light Scattering and Small-Angle X-Ray Scattering. Phys. Rev. E 2005, 72, 051504. [Google Scholar] [CrossRef]
- Lebovka, N.; Lisetski, L.; Bulavin, L.A. Organization of Nano-Disks of Laponite® in Soft Colloidal Systems. In Proceedings of the Modern Problems of the Physics of Liquid Systems, Kyiv, Ukraine, 18–22 May 2018; Bulavin, L.A., Xu, L., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 137–164. [Google Scholar]
- Tawari, S.L.; Koch, D.L.; Cohen, C. Electrical Double-Layer Effects on the Brownian Diffusivity and Aggregation Rate of Laponite Clay Particles. J. Colloid Interface Sci. 2001, 240, 54–66. [Google Scholar] [CrossRef]
- Thompson, D.W.; Butterworth, J.T. The Nature of Laponite and Its Aqueous Dispersions. J. Colloid Interface Sci. 1992, 151, 236–243. [Google Scholar] [CrossRef]
- Madejová, J. FTIR Techniques in Clay Mineral Studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Xiong, Z.-Q.; Li, X.-D.; Fu, F.; Li, Y.-N. Performance Evaluation of Laponite as a Mud-Making Material for Drilling Fluids. Pet. Sci. 2019, 16, 890–900. [Google Scholar] [CrossRef]
- Xiong, Z.; Fu, F.; Li, X. Experimental Investigation on Laponite as Ultra-High-Temperature Viscosifier of Water-Based Drilling Fluids. SN Appl. Sci. 2019, 1, 1374. [Google Scholar] [CrossRef]
- Ruzicka, B.; Zaccarelli, E. A Fresh Look at the Laponite Phase Diagram. Soft Matter 2011, 7, 1268–1286. [Google Scholar] [CrossRef]
- Jatav, S.; Joshi, Y.M. Phase Behavior of Aqueous Suspension of Laponite: New Insights with Microscopic Evidence. Langmuir 2017, 33, 2370–2377. [Google Scholar] [CrossRef] [PubMed]
- Suman, K.; Joshi, Y.M. Microstructure and Soft Glassy Dynamics of an Aqueous Laponite Dispersion. Langmuir 2018, 34, 13079–13103. [Google Scholar] [CrossRef] [PubMed]
- Mourchid, A.; Delville, A.; Lambard, J.; LeColier, E.; Levitz, P. Phase Diagram of Colloidal Dispersions of Anisotropic Charged Particles: Equilibrium Properties, Structure, and Rheology of Laponite Suspensions. Langmuir 1995, 11, 1942–1950. [Google Scholar] [CrossRef]
- Gabriel, J.-C.P.; Sanchez, C.; Davidson, P. Observation of Nematic Liquid-Crystal Textures in Aqueous Gels of Smectite Clays. J. Phys. Chem. 1996, 100, 11139–11143. [Google Scholar] [CrossRef]
- Mongondry, P.; Tassin, J.F.; Nicolai, T. Revised State Diagram of Laponite Dispersions. J. Colloid Interface Sci. 2005, 283, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Jabbari-Farouji, S.; Tanaka, H.; Wegdam, G.H.; Bonn, D. Multiple Nonergodic Disordered States in Laponite Suspensions: A Phase Diagram. Phys. Rev. E 2008, 78, 061405. [Google Scholar] [CrossRef]
- Bonn, D.; Kellay, H.; Tanaka, H.; Wegdam, G.; Meunier, J. Laponite: What Is the Difference between a Gel and a Glass? Langmuir 1999, 15, 7534–7536. [Google Scholar] [CrossRef]
- Tanaka, H.; Jabbari-Farouji, S.; Meunier, J.; Bonn, D. Kinetics of Ergodic-to-Nonergodic Transitions in Charged Colloidal Suspensions: Aging and Gelation. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2005, 71, 021402. [Google Scholar] [CrossRef]
- Davidson, P.; Gabriel, J.-C.P. Self-Assemblies of Anisotropic Nanoparticles: Mineral Liquid Crystals. In Nanocrystals Forming Mesoscopic Structures; Wiley: Hoboken, NJ, USA, 2005; pp. 173–212. ISBN 978-3-527-60758-7. [Google Scholar]
- Ruzicka, B.; Zaccarelli, E.; Zulian, L.; Angelini, R.; Sztucki, M.; Moussaïd, A.; Narayanan, T.; Sciortino, F. Observation of Empty Liquids and Equilibrium Gels in a Colloidal Clay. Nat. Mater. 2011, 10, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Andersson, O.; Johari, G.P. Effects of Nanometer-Size Laponite Disks on Thermal Conductivity and Specific Heat of Water and Ice, and the Gelation Time. Colloid Polym. Sci. 2015, 293, 901–911. [Google Scholar] [CrossRef]
- Shahin, A.; Joshi, Y.M. Irreversible Aging Dynamics and Generic Phase Behavior of Aqueous Suspensions of Laponite. Langmuir 2010, 26, 4219–4225. [Google Scholar] [CrossRef] [PubMed]
- Shahin, A.; Joshi, Y.M. Physicochemical Effects in Aging Aqueous Laponite Suspensions. Langmuir 2012, 28, 15674–15686. [Google Scholar] [CrossRef] [PubMed]
- Shahin, A.; Joshi, Y.M. Hyper-Aging Dynamics of Nanoclay Suspension. Langmuir 2012, 28, 5826–5833. [Google Scholar] [CrossRef] [PubMed]
- Bonn, D.; Tanaka, H.; Wegdam, G.; Kellay, H.; Meunier, J. Aging of a Colloidal “Wigner” Glass. Europhys. Lett. 1999, 45, 52. [Google Scholar] [CrossRef]
- Pilavtepe, M.; Recktenwald, S.M.; Schuhmann, R.; Emmerich, K.; Willenbacher, N. Macro- and Microscale Structure Formation and Aging in Different Arrested States of Laponite Dispersions. J. Rheol. 2018, 62, 593–605. [Google Scholar] [CrossRef]
- Morariu, S.; Bercea, M. Effect of Temperature and Aging Time on the Rheological Behavior of Aqueous Poly(Ethylene Glycol)/Laponite RD Dispersions. J. Phys. Chem. B 2012, 116, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Bellour, M.; Knaebel, A.; Harden, J.L.; Lequeux, F.; Munch, J.-P. Aging Processes and Scale Dependence in Soft Glassy Colloidal Suspensions. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2003, 67, 031405. [Google Scholar] [CrossRef]
- Gili, T.; Capuani, S.; Maraviglia, B. Nonergodic Arrested State in Diluted Clay Suspensions Monitored by Triple-Quantum 23Na Nuclear Magnetic Resonance. J. Phys. Chem. B 2007, 111, 7092–7097. [Google Scholar] [CrossRef]
- Porion, P.; Al Mukhtar, M.; Meyer, S.; Faugère, A.M.; van der Maarel, J.R.C.; Delville, A. Nematic Ordering of Suspensions of Charged Anisotropic Colloids Detected by 23Na Nuclear Magnetic Resonance. J. Phys. Chem. B 2001, 105, 10505–10514. [Google Scholar] [CrossRef]
- Mathur, S.; Moudgil, B.M. Adsorption Mechanism(s) of Poly(Ethylene Oxide) on Oxide Surfaces. J Colloid. Interface Sci. 1997, 196, 92–98. [Google Scholar] [CrossRef]
- Su, C.-C.; Shen, Y.-H. Effects of Poly(Ethylene Oxide) Adsorption on the Dispersion of Smectites. Colloids Surf. A Physicochem. Eng. Asp. 2008, 312, 1–6. [Google Scholar] [CrossRef]
- Nelson, A.; Cosgrove, T. A Small-Angle Neutron Scattering Study of Adsorbed Poly(Ethylene Oxide) on Laponite. Langmuir 2004, 20, 2298–2304. [Google Scholar] [CrossRef]
- Baghdadi, H.A.; Sardinha, H.; Bhatia, S.R. Rheology and Gelation Kinetics in Laponite Dispersions Containing Poly(Ethylene Oxide). J. Polym. Sci. Part B Polym. Phys. 2005, 43, 233–240. [Google Scholar] [CrossRef]
- Morariu, S.; Bercea, M. Viscoelastic Properties of Laponite RD Dispersions Containing PEO with Different Molecular Weights. Rev. Roum. Chim. 2015, 60, 777–785. [Google Scholar]
- Baghdadi, H.A.; Jensen, E.C.; Easwar, N.; Bhatia, S.R. Evidence for Re-Entrant Behavior in Laponite–PEO Systems. Rheol. Acta 2008, 47, 121–127. [Google Scholar] [CrossRef]
- Atmuri, A.K.; Peklaris, G.A.; Kishore, S.; Bhatia, S.R. A Re-Entrant Glass Transition in Colloidal Disks with Adsorbing Polymer. Soft Matter 2012, 8, 8965–8971. [Google Scholar] [CrossRef]
- Can, V.; Okay, O. Shake Gels Based on Laponite–PEO Mixtures: Effect of Polymer Molecular Weight. Des. Monomers Polym. 2005, 8, 453–462. [Google Scholar] [CrossRef]
- Srivastava, S.; Kishore, S.; Narayanan, S.; Sandy, A.R.; Bhatia, S.R. Multiple Dynamic Regimes in Colloid-Polymer Dispersions: New Insight Using X-Ray Photon Correlation Spectroscopy. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 752–760. [Google Scholar] [CrossRef]
- Kishore, S.; Chen, Y.; Ravindra, P.; Bhatia, S.R. The Effect of Particle-Scale Dynamics on the Macroscopic Properties of Disk-Shaped Colloid–Polymer Systems. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 585–595. [Google Scholar] [CrossRef]
- Zulian, L.; Ruzicka, B.; Ruocco, G. Influence of an Adsorbing Polymer on the Aging Dynamics of Laponite Clay Suspensions. Philos. Mag. 2008, 88, 4213–4221. [Google Scholar] [CrossRef]
- Zulian, L.; Augusto de Melo Marques, F.; Emilitri, E.; Ruocco, G.; Ruzicka, B. Dual Aging Behaviour in a Clay–Polymer Dispersion. Soft Matter 2014, 10, 4513–4521. [Google Scholar] [CrossRef] [PubMed]
- Baghdadi, H.A.; Parrella, J.; Bhatia, S.R. Long-Term Aging Effects on the Rheology of Neat Laponite and Laponite–PEO Dispersions. Rheol. Acta 2008, 47, 349–357. [Google Scholar] [CrossRef]
- Au, P.-I.; Hassan, S.; Liu, J.; Leong, Y.-K. Behaviour of LAPONITE® Gels: Rheology, Ageing, pH Effect and Phase State in the Presence of Dispersant. Chem. Eng. Res. Des. 2015, 101, 65–73. [Google Scholar] [CrossRef]
- Awasthi, V.; Joshi, Y.M. Effect of Temperature on Aging and Time–Temperature Superposition in Nonergodic Laponite Suspensions. Soft Matter 2009, 5, 4991–4996. [Google Scholar] [CrossRef]
- Thuresson, A.; Segad, M.; Turesson, M.; Skepö, M. Flocculated Laponite–PEG/PEO Dispersions with Monovalent Salt, a SAXS and Simulation Study. J. Colloid Interface Sci. 2016, 466, 330–342. [Google Scholar] [CrossRef]
- Thuresson, A.; Segad, M.; Plivelic, T.S.; Skepö, M. Flocculated Laponite-PEG/PEO Dispersions with Multivalent Salt: A SAXS, Cryo-TEM, and Computer Simulation Study. J. Phys. Chem. C 2017, 121, 7387–7396. [Google Scholar] [CrossRef]
- Chen, L.; Li, G.; Chen, Y.; Zeng, H.; Mao, Z.; Liu, L.; Wang, X.; Xu, S. Thixotropy Research of Laponite-Hydrogel Composites for Water Shutoff in Horizontal Wells. J. Pet. Sci. Eng. 2022, 208, 109600. [Google Scholar] [CrossRef]
- Li, N.; Du, J.; Tan, Y.; Ling, J.; Yang, X.; Ma, J.; Wu, R.; Xu, S.; Zhang, Y. Dispersion and Rheological Behaviors of Laponite in 2-Acrylamido-2-Methylpropanesulfonic Acid Solution. Appl. Clay Sci. 2017, 137, 94–100. [Google Scholar] [CrossRef]
- Šebenik, U.; Lapasin, R.; Krajnc, M. Rheology of Aqueous Dispersions of Laponite and TEMPO-Oxidized Nanofibrillated Cellulose. Carbohydr. Polym. 2020, 240, 116330. [Google Scholar] [CrossRef] [PubMed]
- Lapasin, R.; Abrami, M.; Grassi, M.; Šebenik, U. Rheology of Laponite-Scleroglucan Hydrogels. Carbohydr. Polym. 2017, 168, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Morariu, S.; Bercea, M. Effect of Addition of Polymer on the Rheology and Electrokinetic Features of Laponite RD Aqueous Dispersions. J. Chem. Eng. Data 2009, 54, 54–59. [Google Scholar] [CrossRef]
- Morariu, S.; Bercea, M.; Sacarescu, L. Tailoring of Clay/Poly(Ethylene Oxide) Hydrogel Properties by Chitosan Incorporation. Ind. Eng. Chem. Res. 2014, 53, 13690–13698. [Google Scholar] [CrossRef]
- Liu, S.F.; Lafuma, F.; Audebert, R. Rheological Behavior of Moderately Concentrated Silica Suspensions in the Presence of Adsorbed Poly(Ethylene Oxide). Colloid Polym. Sci. 1994, 272, 196–203. [Google Scholar] [CrossRef]
- Cabane, B.; Wong, K.; Lindner, P.; Lafuma, F. Shear Induced Gelation of Colloidal Dispersions. J. Rheol. 1997, 41, 531–547. [Google Scholar] [CrossRef]
- Mar Ramos-Tejada, M.; Luckham, P.F. Shaken but Not Stirred: The Formation of Reversible Particle—Polymer Gels under Shear. Colloids Surf. A Physicochem. Eng. Asp. 2015, 471, 164–169. [Google Scholar] [CrossRef]
- Pozzo, D.C.; Walker, L.M. Reversible Shear Gelation of Polymer–Clay Dispersions. Colloids Surf. A Physicochem. Eng. Asp. 2004, 240, 187–198. [Google Scholar] [CrossRef]
- Zebrowski, J.; Prasad, V.; Zhang, W.; Walker, L.M.; Weitz, D.A. Shake-Gels: Shear-Induced Gelation of Laponite–PEO Mixtures. Colloids Surf. A Physicochem. Eng. Asp. 2003, 213, 189–197. [Google Scholar] [CrossRef]
- Huang, Y.; Kobayashi, M. Direct Observation of Relaxation of Aqueous Shake-Gel Consisting of Silica Nanoparticles and Polyethylene Oxide. Polymers 2020, 12, 1141. [Google Scholar] [CrossRef]
- Fall, A.; Bonn, D. Shear Thickening of Laponite Suspensions with Poly(Ethylene Oxide). Soft Matter 2012, 8, 4645–4651. [Google Scholar] [CrossRef]
- Schmidt, G.; Nakatani, A.I.; Butler, P.D.; Han, C.C. Small-Angle Neutron Scattering from Viscoelastic Polymer–clay Solutions. Macromolecules 2002, 35, 4725–4732. [Google Scholar] [CrossRef]
- Kawasaki, S.; Kobayashi, M. Affirmation of the Effect of pH on Shake-Gel and Shear Thickening of a Mixed Suspension of Polyethylene Oxide and Silica Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 236–242. [Google Scholar] [CrossRef]
- de Bruyn, J.R.; Pignon, F.; Tsabet, E.; Magnin, A. Micron-Scale Origin of the Shear-Induced Structure in Laponite–Poly(Ethylene Oxide) Dispersions. Rheol. Acta 2008, 47, 63–73. [Google Scholar] [CrossRef]
- Trens, P.; Denoyel, R. Conformation of Poly(Ethylene Glycol) Polymers at the Silica/Water Interface: A Microcalorimetric Study. Langmuir 1993, 9, 519–522. [Google Scholar] [CrossRef]
- Liu, X.; Niu, X.; Fu, Z.; Liu, L.; Bai, S.; Wang, J.; Li, L.; Wang, Y.; Guo, X. A Facile Approach to Obtain Highly Tough and Stretchable LAPONITE®-Based Nanocomposite Hydrogels. Soft Matter 2020, 16, 8394–8399. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Yu, H.; Wen, J.; Zeng, H.; Liang, T.; Zuo, F.; Cheng, C. Super-Adsorbent Poly(Acrylic Acid)/Laponite Hydrogel with Ultrahigh Mechanical Property for Adsorption of Methylene Blue. J. Environ. Chem. Eng. 2021, 9, 106346. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Peng, W.; Zare, Y.; Rhee, K.Y. Effects of Size and Aggregation/Agglomeration of Nanoparticles on the Interfacial/Interphase Properties and Tensile Strength of Polymer Nanocomposites. Nanoscale Res. Lett. 2018, 13, 214. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Su, X. Poly(Vinyl Alcohol)/Laponite/Layered Double Hydroxide/Hydroxyapatite Nanocomposite Hydrogels for Stimulus-Responsive Devices. ACS Appl. Nano Mater. 2024, 7, 2270–2279. [Google Scholar] [CrossRef]
- Kiaee, G.; Dimitrakakis, N.; Sharifzadeh, S.; Kim, H.-J.; Avery, R.K.; Moghaddam, K.M.; Haghniaz, R.; Yalcintas, E.P.; de Barros, N.R.; Karamikamkar, S.; et al. Laponite-Based Nanomaterials for Drug Delivery. Adv. Healthc. Mater. 2022, 11, 2102054. [Google Scholar] [CrossRef]
- Stealey, S.T.; Gaharwar, A.K.; Zustiak, S.P. Laponite-Based Nanocomposite Hydrogels for Drug Delivery Applications. Pharmaceuticals 2023, 16, 821. [Google Scholar] [CrossRef] [PubMed]
- Morariu, S.; Teodorescu, M. Laponite®—A Versatile Component in Hybrid Materials for Biomedical Applications. Mem. Sci. Sect. Rom. Acad. 2020, XLIII, 141–155. [Google Scholar]
- Jung, H.; Kim, H.-M.; Choy, Y.B.; Hwang, S.-J.; Choy, J.-H. Itraconazole–Laponite: Kinetics and Mechanism of Drug Release. Appl. Clay Sci. 2008, 40, 99–107. [Google Scholar] [CrossRef]
- Park, J.K.; Choy, Y.B.; Oh, J.-M.; Kim, J.Y.; Hwang, S.-J.; Choy, J.-H. Controlled Release of Donepezil Intercalated in Smectite Clays. Int. J. Pharm. 2008, 359, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.R.; Hutcheon, G.A.; Roberts, M.; Gaskell, E.E. Formulation and Antibacterial Profiles of Clay–Ciprofloxacin Composites. Appl. Clay Sci. 2014, 87, 129–135. [Google Scholar] [CrossRef]
- Fraile, J.M.; Garcia-Martin, E.; Gil, C.; Mayoral, J.A.; Pablo, L.E.; Polo, V.; Prieto, E.; Vispe, E. Laponite as Carrier for Controlled in Vitro Delivery of Dexamethasone in Vitreous Humor Models. Eur. J. Pharm. Biopharm. 2016, 108, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Malekkhaiat Häffner, S.; Nyström, L.; Browning, K.L.; Mörck Nielsen, H.; Strömstedt, A.A.; van der Plas, M.J.A.; Schmidtchen, A.; Malmsten, M. Interaction of Laponite with Membrane Components—Consequences for Bacterial Aggregation and Infection Confinement. ACS Appl. Mater. Interfaces 2019, 11, 15389–15400. [Google Scholar] [CrossRef]
- Basu, S.; Pacelli, S.; Paul, A. Self-Healing DNA-Based Injectable Hydrogels with Reversible Covalent Linkages for Controlled Drug Delivery. Acta Biomater. 2020, 105, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Suterio, N.; Bazzo, G.C.; Rauber, G.S.; Silva, A.H.; Caon, T.; Parize, A.L.; Creczynski-Pasa, T.B.; Stulzer, H.K. Laponite® Gel Formulation Containing Simvastatin for Melanoma Treatment. Appl. Clay Sci. 2022, 228, 106651. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Sun, Y.; Wang, Y.; Yang, Y.; Yuan, Y.; Li, Y.; Liu, C. In Situ Formation of pH-/Thermo-Sensitive Nanohybrids via Friendly-Assembly of Poly(N-Vinylpyrrolidone) onto LAPONITE®. RSC Adv. 2016, 6, 31816–31823. [Google Scholar] [CrossRef]
- Tang, S.; Chen, J.; Cannon, J.; Chekuri, M.; Farazuddin, M.; Baker, J.R.; Wang, S.H. Delicate Hybrid Laponite–Cyclic Poly(Ethylene Glycol) Nanoparticles as a Potential Drug Delivery System. Pharmaceutics 2023, 15, 1998. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Cinà, G.; Borrego-Sánchez, A.; Sainz-Díaz, C.I.; Viseras-Iborra, C.; Sánchez-Espejo, R.; de Melo Barbosa, R.; Leone, F.; Pibiri, I.; Noto, R.; et al. Thixotropic Hydrogels Based on Laponite® and Cucurbituril for Delivery of Lipophilic Drug Molecules. ChemPlusChem 2024, 89, e202300592. [Google Scholar] [CrossRef] [PubMed]
- Frelichowska, J.; Bolzinger, M.-A.; Pelletier, J.; Valour, J.-P.; Chevalier, Y. Topical Delivery of Lipophilic Drugs from o/w Pickering Emulsions. Int. J. Pharm. 2009, 371, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, T.; Mieszkowska, A.; Kempińska-Kupczyk, D.; Kot-Wasik, A.; Namieśnik, J.; Mazerska, Z. The Impact of Lipophilicity on Environmental Processes, Drug Delivery and Bioavailability of Food Components. Microchem. J. 2019, 146, 393–406. [Google Scholar] [CrossRef]
- Capanema, N.S.V.; Carvalho, I.C.; Mansur, A.A.P.; Carvalho, S.M.; Lage, A.P.; Mansur, H.S. Hybrid Hydrogel Composed of Carboxymethylcellulose–Silver Nanoparticles–Doxorubicin for Anticancer and Antibacterial Therapies against Melanoma Skin Cancer Cells. ACS Appl. Nano Mater. 2019, 2, 7393–7408. [Google Scholar] [CrossRef]
- Akbarzadeh, I.; Keramati, M.; Azadi, A.; Afzali, E.; Shahbazi, R.; Chiani, M.; Norouzian, D.; Bakhshandeh, H. Optimization, Physicochemical Characterization, and Antimicrobial Activity of a Novel Simvastatin Nano-Niosomal Gel against E. Coli and S. Aureus. Chem. Phys. Lipids 2021, 234, 105019. [Google Scholar] [CrossRef]
- Becher, T.B.; Mendonça, M.C.P.; de Farias, M.A.; Portugal, R.V.; de Jesus, M.B.; Ornelas, C. Soft Nanohydrogels Based on Laponite Nanodiscs: A Versatile Drug Delivery Platform for Theranostics and Drug Cocktails. ACS Appl. Mater. Interfaces 2018, 10, 21891–21900. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pan, H.; Qiao, S.; Li, Y.; Wang, J.; Liu, W.; Pan, W. The Utilization of Low Molecular Weight Heparin-Poloxamer Associated Laponite Nanoplatform for Safe and Efficient Tumor Therapy. Int. J. Biol. Macromol. 2019, 134, 63–72. [Google Scholar] [CrossRef]
- Dimatteo, R.; Darling, N.J.; Segura, T. In Situ Forming Injectable Hydrogels for Drug Delivery and Wound Repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef]
- Almawash, S.; Osman, S.K.; Mustafa, G.; El Hamd, M.A. Current and Future Prospective of Injectable Hydrogels-Design Challenges and Limitations. Pharmaceuticals 2022, 15, 371. [Google Scholar] [CrossRef]
- Madhavikutty, A.S.; Ohta, S.; Chandel, A.K.S.; Qi, P.; Ito, T. Analysis of Endoscopic Injectability and Post-Ejection Dripping of Yield Stress Fluids: Laponite, Carbopol and Xanthan Gum. J. Chem. Eng. Jpn. 2021, 54, 500–511. [Google Scholar] [CrossRef]
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Foroughi, J.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G.; et al. Self-Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering? Adv. Sci. 2019, 6, 1801664. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Liu, J.; Moussa, Z.L.; Collins, J.E.; McDonnell, S.; Hayward, A.M.; Jajoo, K.; Langer, R.; Traverso, G. Endoscopically Injectable Shear-Thinning Hydrogels Facilitating Polyp Removal. Adv. Sci. 2019, 6, 1901041. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mynar, J.L.; Yoshida, M.; Lee, E.; Lee, M.; Okuro, K.; Kinbara, K.; Aida, T. High-Water-Content Mouldable Hydrogels by Mixing Clay and a Dendritic Molecular Binder. Nature 2010, 463, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Tamesue, S.; Ohtani, M.; Yamada, K.; Ishida, Y.; Spruell, J.M.; Lynd, N.A.; Hawker, C.J.; Aida, T. Linear versus Dendritic Molecular Binders for Hydrogel Network Formation with Clay Nanosheets: Studies with ABA Triblock Copolyethers Carrying Guanidinium Ion Pendants. J. Am. Chem. Soc. 2013, 135, 15650–15655. [Google Scholar] [CrossRef] [PubMed]
- Kostina, N.Y.; Sharifi, S.; de los Santos Pereira, A.; Michálek, J.; Grijpma, D.W.; Rodriguez-Emmenegger, C. Novel Antifouling Self-Healing Poly(Carboxybetaine Methacrylamide-Co-HEMA) Nanocomposite Hydrogels with Superior Mechanical Properties. J. Mater. Chem. B 2013, 1, 5644–5650. [Google Scholar] [CrossRef] [PubMed]
- Hirose, R.; Nakaya, T.; Naito, Y.; Daidoji, T.; Dohi, O.; Yoshida, N.; Yasuda, H.; Konishi, H.; Itoh, Y. Identification of the Critical Viscoelastic Factor in the Performance of Submucosal Injection Materials. Mater. Sci. Eng. C 2019, 94, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Hirose, R.; Naito, Y.; Inoue, K.; Dohi, O.; Yoshida, N.; Kamada, K.; Uchiyama, K.; Ishikawa, T.; Takagi, T.; et al. Viscosity: An Important Factor in Predicting the Performance of Submucosal Injection Materials. Mater. Des. 2020, 195, 109008. [Google Scholar] [CrossRef]
- Ranjbardamghani, F.; Eslahi, N.; Jahanmardi, R. An Injectable Chitosan/Laponite Hydrogel Synthesized via Hybrid Cross-Linking System: A Smart Platform for Cartilage Regeneration. Polym. Adv. Technol. 2023, 34, 2298–2311. [Google Scholar] [CrossRef]
- Magalhães, L.S.S.M.; Andrade, D.B.; Bezerra, R.D.S.; Morais, A.I.S.; Oliveira, F.C.; Rizzo, M.S.; Silva-Filho, E.C.; Lobo, A.O. Nanocomposite Hydrogel Produced from PEGDA and Laponite for Bone Regeneration. J. Funct. Biomater. 2022, 13, 53. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Cardiel, M.J.; Fraile, J.M.; Mayoral, J.A.; Pablo, L.E.; Garcia-Martin, E. Laponite for Biomedical Applications: An Ophthalmological Perspective. Mater. Today Bio 2024, 24, 100935. [Google Scholar] [CrossRef] [PubMed]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.-C.; Reid, R.R. 3D Bioprinting Technologies in Tissue Engineering and Regenerative Medicine: Current and Future Trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Afewerki, S.; Magalhães, L.S.S.M.; Silva, A.D.R.; Stocco, T.D.; Silva Filho, E.C.; Marciano, F.R.; Lobo, A.O. Bioprinting a Synthetic Smectic Clay for Orthopedic Applications. Adv. Healthc. Mater. 2019, 8, 1900158. [Google Scholar] [CrossRef] [PubMed]
- Jungst, T.; Smolan, W.; Schacht, K.; Scheibel, T.; Groll, J. Strategies and Molecular Design Criteria for 3D Printable Hydrogels. Chem. Rev. 2016, 116, 1496–1539. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma. 2019, 33, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Mihaila, S.M.; Swami, A.; Patel, A.; Sant, S.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Bioactive Silicate Nanoplatelets for Osteogenic Differentiation of Human Mesenchymal Stem Cells. Adv. Mater. 2013, 25, 3329–3336. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Zhou, J.; Liu, B.; Lei, X.; Wang, T.; Hao, X.; Cheng, P.; Wu, H.; Song, Y.; Pei, G.; et al. A 3D Bioprinted Nano-Laponite Hydrogel Construct Promotes Osteogenesis by Activating PI3K/AKT Signaling Pathway. Mater. Today Bio 2022, 16, 100342. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Mahurubin, S.; Sooriyaarachchi, D.; Tan, G.Z. The Effect of Nanoclays on Nanofiber Density Gradient in 3D Scaffolds Fabricated by Divergence Electrospinning. Procedia Manuf. 2019, 34, 110–117. [Google Scholar] [CrossRef]
- Cidonio, G.; Cooke, M.; Glinka, M.; Dawson, J.I.; Grover, L.; Oreffo, R.O.C. Printing Bone in a Gel: Using Nanocomposite Bioink to Print Functionalised Bone Scaffolds. Mater. Today Bio 2019, 4, 100028. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, R.; Zhang, L.; Cao, X. 4D Printing of Robust Hydrogels Consisted of Agarose Nanofibers and Polyacrylamide. ACS Macro Lett. 2018, 7, 442–446. [Google Scholar] [CrossRef]
- Zhu, W.; Webster, T.J.; Zhang, L.G. 4D Printing Smart Biosystems for Nanomedicine. Nanomedicine 2019, 14, 1643–1645. [Google Scholar] [CrossRef]
- Salehabadi, A.; Enhessari, M.; Ahmad, M.I.; Ismail, N.; Gupta, B.D. Chapter 1—Introduction. In Metal Chalcogenide Biosensors; Salehabadi, A., Enhessari, M., Ahmad, M.I., Ismail, N., Gupta, B.D., Eds.; Woodhead Publishing: Sawston, UK, 2023; pp. 1–7. ISBN 978-0-323-85381-1. [Google Scholar]
- Poyard, S.; Jaffrezic-Renault, N.; Martelet, C.; Cosnier, S.; Labbe, P.; Besombes, J.L. A New Method for the Controlled Immobilization of Enzyme in Inorganic Gels (Laponite) for Amperometric Glucose Biosensing. Sens. Actuators B Chem. 1996, 33, 44–49. [Google Scholar] [CrossRef]
- Pecheu, C.N.; Tchieda, V.K.; Tajeu, K.Y.; Jiokeng, S.L.; Lesch, A.; Tonle, I.K.; Ngameni, E.; Janiak, C. Electrochemical Determination of Epinephrine in Pharmaceutical Preparation Using Laponite Clay-Modified Graphene Inkjet-Printed Electrode. Molecules 2023, 28, 5487. [Google Scholar] [CrossRef]
- Ding, S.-N.; Zheng, C.-L.; Wan, N.; Cosnier, S. Graphene/Clay Composite Electrode Formed by Exfoliating Graphite with Laponite for Simultaneous Determination of Ascorbic Acid, Dopamine, and Uric Acid. Monatsh. Chem. 2014, 145, 1389–1394. [Google Scholar] [CrossRef]
- Shimohigoshi, M.; Karube, I. Development of Uric Acid and Oxalic Acid Sensors Using a Bio-Thermochip. Sens. Actuators B Chem. 1996, 30, 17–21. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, L.; Wang, M.; Li, Z.; Liu, H.; Li, J. A Novel Room Temperature Ionic Liquid Sol–Gel Matrix for Amperometric Biosensor Application. Green Chem. 2005, 7, 655–658. [Google Scholar] [CrossRef]
- Maiyalagan, T.; Kannan, P.; Jönsson-Niedziolka, M.; Niedziolka-Jönsson, J. Tungsten Carbide Nanotubes Supported Platinum Nanoparticles as a Potential Sensing Platform for Oxalic Acid. Anal. Chem. 2014, 86, 7849–7857. [Google Scholar] [CrossRef]
- Hong, F.; Nilvebrant, N.O.; Jönsson, L.J. Rapid and Convenient Determination of Oxalic Acid Employing a Novel Oxalate Biosensor Based on Oxalate Oxidase and SIRE Technology. Biosens. Bioelectron. 2003, 18, 1173–1181. [Google Scholar] [CrossRef]
- Joshi, N.; Rawat, K.; Solanki, P.R.; Bohidar, H.B. Biocompatible Laponite Ionogels Based Non-Enzymatic Oxalic Acid Sensor. Sens. Bio-Sens. Res. 2015, 5, 105–111. [Google Scholar] [CrossRef]
- Wang, F.; Yang, L.; Sun, Y.; Cai, Y.; Xu, X.; Liu, Z.; Liu, Q.; Zhao, H.; Ma, C.; Liu, J. A Nanoclay-Enhanced Hydrogel for Self-Adhesive Wearable Electrophysiology Electrodes with High Sensitivity and Stability. Gels 2023, 9, 323. [Google Scholar] [CrossRef]
- Fan, Q.; Shan, D.; Xue, H.; He, Y.; Cosnier, S. Amperometric Phenol Biosensor Based on Laponite Clay-Chitosan Nanocomposite Matrix. Biosens. Bioelectron. 2007, 22, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Tomás, H.; Alves, C.S.; Rodrigues, J. Laponite®: A Key Nanoplatform for Biomedical Applications? Nanomedicine 2018, 14, 2407–2420. [Google Scholar] [CrossRef]
- Mustafa, R.; Zhou, B.; Yang, J.; Zheng, L.; Zhang, G.; Shi, X. Dendrimer-Functionalized Laponite® Nanodisks Loaded with Gadolinium for T1-Weighted MR Imaging Applications. RSC Adv. 2016, 6, 95112–95119. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhao, L.; Zheng, L.; Hu, Y.; Ding, L.; Li, X.; Liu, C.; Zhao, J.; Shi, X.; Guo, R. Laponite-Polyethylenimine Based Theranostic Nanoplatform for Tumor-Targeting CT Imaging and Chemotherapy. ACS Biomater. Sci. Eng. 2017, 3, 431–442. [Google Scholar] [CrossRef]
- Ding, L.; Wang, R.; Hu, Y.; Xu, F.; Zhang, N.; Cao, X.; Wang, X.; Shi, X.; Guo, R. Folic Acid-Modified Laponite®-Stabilized Fe3O4 Nanoparticles for Targeted T2-Weighted MR Imaging of Tumor. Appl. Clay Sci. 2020, 186, 105447. [Google Scholar] [CrossRef]
- Liu, R.; Xu, F.; Wang, L.; Liu, M.; Cao, X.; Shi, X.; Guo, R. Polydopamine-Coated Laponite Nanoplatforms for Photoacoustic Imaging-Guided Chemo-Phototherapy of Breast Cancer. Nanomaterials 2021, 11, 394. [Google Scholar] [CrossRef]
- Wu, H.; Wang, W.; Zhang, Z.; Li, J.; Zhao, J.; Liu, Y.; Wu, C.; Huang, M.; Li, Y.; Wang, S. Synthesis of a Clay-Based Nanoagent for Photonanomedicine. ACS Appl. Mater. Interfaces 2020, 12, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Nath, D.; Santhosh, R.; Pal, K.; Sarkar, P. Nanoclay-Based Active Food Packaging Systems: A Review. Food Packag. Shelf Life 2022, 31, 100803. [Google Scholar] [CrossRef]
- Ahari, H.; Soufiani, S.P. Smart and Active Food Packaging: Insights in Novel Food Packaging. Front. Microbiol. 2021, 12, 657233. [Google Scholar] [CrossRef]
- Perera, K.Y.; Hopkins, M.; Jaiswal, A.K.; Jaiswal, S. Nanoclays-Containing Bio-Based Packaging Materials: Properties, Applications, Safety, and Regulatory Issues. J. Nanostruct. Chem. 2024, 14, 71–93. [Google Scholar] [CrossRef]
- González-López, M.E.; de Calva-Estrada, S.J.; Gradilla-Hernández, M.S.; Barajas-Álvarez, P. Current Trends in Biopolymers for Food Packaging: A Review. Front. Sustain. Food Syst. 2023, 7, 1225371. [Google Scholar] [CrossRef]
- Valencia, G.A.; Lourenço, R.V.; Bittante, A.M.Q.B.; do Amaral Sobral, P.J. Physical and Morphological Properties of Nanocomposite Films Based on Gelatin and Laponite. Appl. Clay Sci. 2016, 124–125, 260–266. [Google Scholar] [CrossRef]
- Sharma, C.; Manepalli, P.H.; Thatte, A.; Thomas, S.; Kalarikkal, N.; Alavi, S. Biodegradable Starch/PVOH/Laponite RD-Based Bionanocomposite Films Coated with Graphene Oxide: Preparation and Performance Characterization for Food Packaging Applications. Colloid Polym. Sci. 2017, 295, 1695–1708. [Google Scholar] [CrossRef]
- Olivera, N.; Rouf, T.B.; Bonilla, J.C.; Carriazo, J.G.; Dianda, N.; Kokini, J.L. Effect of Laponite® Addition on the Mechanical, Barrier and Surface Properties of Novel Biodegradable Kafirin Nanocomposite Films. J. Food Eng. 2019, 245, 24–32. [Google Scholar] [CrossRef]
- Soares, K.S.; Souza, M.P.; Silva-Filho, E.C.; Barud, H.S.; Ribeiro, C.A.; Santos, D.D.; Rocha, K.N.S.; de Moura, J.F.P.; Oliveira, R.L.; Bezerra, L.R. Effect of Edible Onion (Allium cepa L.) Film on Quality, Sensory Properties and Shelf Life of Beef Burger Patties. Molecules 2021, 26, 7202. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.L.; de Oliveira, L.M.; Paiva, R.; Dametto, A.C.; dos Dias, D.S.; Ribeiro, C.A.; Wrona, M.; Nerín, C.; Barud, H.d.S.; Cruz, S.A. Evaluation the Potential of Onion/Laponite Composites Films for Sustainable Food Packaging with Enhanced UV Protection and Antioxidant Capacity. Molecules 2023, 28, 6829. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, A.; Ye, R.; Wang, Y.; Wang, W. Fabrication of Gelatin–Laponite Composite Films: Effect of the Concentration of Laponite on Physical Properties and the Freshness of Meat during Storage. Food Hydrocoll. 2015, 44, 390–398. [Google Scholar] [CrossRef]
- Casariego, A.; Souza, B.W.S.; Cerqueira, M.A.; Teixeira, J.A.; Cruz, L.; Díaz, R.; Vicente, A.A. Chitosan/Clay Films’ Properties as Affected by Biopolymer and Clay Micro/Nanoparticles’ Concentrations. Food Hydrocoll. 2009, 23, 1895–1902. [Google Scholar] [CrossRef]
- Vishnuvarthanan, M.; Rajeswari, N. Food Packaging: Pectin–Laponite–Ag Nanoparticle Bionanocomposite Coated on Polypropylene Shows Low O2 Transmission, Low Ag Migration and High Antimicrobial Activity. Environ. Chem. Lett. 2019, 17, 439–445. [Google Scholar] [CrossRef]
- Rodrigues, R.K.; de Martins, S.F.C.; Naccache, M.F.; de Souza Mendes, P.R. Rheological Modifiers in Drilling Fluids. J. Non-Newton. Fluid Mech. 2020, 286, 104397. [Google Scholar] [CrossRef]
- Huang, X.-B.; Sun, J.-S.; Huang, Y.; Yan, B.-C.; Dong, X.-D.; Liu, F.; Wang, R. Laponite: A Promising Nanomaterial to Formulate High-Performance Water-Based Drilling Fluids. Pet. Sci. 2021, 18, 579–590. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Wang, R.; Rui, Z.; Cheng, R.; Wang, Q.; Wang, J.; Lv, K. Impact of Laponite on the Formation of NGHs and Its Adaptability for Use in NGH Drilling Fluids. J. Nat. Gas Sci. Eng. 2022, 107, 104799. [Google Scholar] [CrossRef]
- Zhang, J.R.; Xu, M.D.; Christidis, G.E.; Zhou, C.H. Clay Minerals in Drilling Fluids: Functions and Challenges. Clay Miner. 2020, 55, 1–11. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, L.; Luo, P.; Li, X.; Ren, W.; Yi, T. Polymer-Laponite Composites as Filtrate Reducer for High Temperature and Salt Resistant Drilling Fluid: Characterization and Performance Evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2024, 688, 133679. [Google Scholar] [CrossRef]
- Ahmed, A.; Mahmoud, A.A.; Elkatatny, S. Curing Time Impacts on the Mechanical and Petrophysical Properties of a Laponite-Based Oil Well Cement. ACS Omega 2022, 7, 31246–31259. [Google Scholar] [CrossRef] [PubMed]
- Baba Hamed, S.; Belhadri, M. Rheological Properties of Biopolymers Drilling Fluids. J. Pet. Sci. Eng. 2009, 67, 84–90. [Google Scholar] [CrossRef]
- Saleh, T.A.; Ibrahim, M.A. Advances in Functionalized Nanoparticles Based Drilling Inhibitors for Oil Production. Energy Reports 2019, 5, 1293–1304. [Google Scholar] [CrossRef]
- Xiumin, M.; Yue, C.; Luheng, Q. Research and Application of Gas-Lift Reverse Circulation Drilling Technology to Geothermal Well Construction in Dalian Jiaoliu Island. Procedia Eng. 2014, 73, 252–257. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Adachi, Y. Effects of Electrolyte Concentration and pH on the Sedimentation Rate of Coagulated Suspension of Sodium Montmorillonite. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 686–693. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, X.; Ji, C.; Zhan, Q.; Li, Z.; Guan, J.; Huang, J. Modification Method of High-Efficiency Organic Bentonite for Drilling Fluids: A Review. Molecules 2023, 28, 7866. [Google Scholar] [CrossRef]
- Wang, W.; Wang, A. Recent Progress in Dispersion of Palygorskite Crystal Bundles for Nanocomposites. Appl. Clay Sci. 2016, 119, 18–30. [Google Scholar] [CrossRef]
- Weng, J.; Gong, Z.; Liao, L.; Lv, G.; Tan, J. Comparison of Organo-Sepiolite Modified by Different Surfactants and Their Rheological Behavior in Oil-Based Drilling Fluids. Appl. Clay Sci. 2018, 159, 94–101. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Wang, A. Superior Dispersion Properties of Palygorskite in Dimethyl Sulfoxide via High-Pressure Homogenization Process. Appl. Clay Sci. 2013, 86, 174–178. [Google Scholar] [CrossRef]
- Karmous, M.S.; Ben Rhaiem, H.; Robert, J.L.; Lanson, B.; Ben Haj Amara, A. Charge Location Effect on the Hydration Properties of Synthetic Saponite and Hectorite Saturated by Na+, Ca2+ Cations: XRD Investigation. Appl. Clay Sci. 2009, 46, 43–50. [Google Scholar] [CrossRef]
- Liu, F.; Jiang, G.-C.; Wang, K.; Wang, J. Laponite Nanoparticle as a Multi-Functional Additive in Water-Based Drilling Fluids. J. Mater. Sci. 2017, 52, 12266–12278. [Google Scholar] [CrossRef]
- Huang, X.; Shen, H.; Sun, J.; Lv, K.; Liu, J.; Dong, X.; Luo, S. Nanoscale Laponite as a Potential Shale Inhibitor in Water-Based Drilling Fluid for Stabilization of Wellbore Stability and Mechanism Study. ACS Appl. Mater. Interfaces 2018, 10, 33252–33259. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Jiang, G.C.; Wang, K.; Wang, J.X. Laponite Nanoparticle as a High Performance Rheological Modifier in Water-Based Drilling Fluids. Mater. Sci. Forum 2018, 917, 134–139. [Google Scholar] [CrossRef]
- Shen, H.; Lv, K.; Huang, X.; Liu, J.; Bai, Y.; Wang, J.; Sun, J. Hydrophobic-Associated Polymer-Based Laponite Nanolayered Silicate Composite as Filtrate Reducer for Water-Based Drilling Fluid at High Temperature. J. Appl. Polym. Sci. 2020, 137, 48608. [Google Scholar] [CrossRef]
- Yang, J.; Wang, R.; Sun, J.; Wang, J.; Liu, L.; Qu, Y.; Wang, P.; Ren, H.; Gao, S.; Yang, Z. Nanolaponite/Comb Polymer Composite as a Rheological Modifier for Water-Based Drilling Fluids. ACS Appl. Nano Mater. 2023, 6, 13453–13465. [Google Scholar] [CrossRef]
- Bai, Y.; Zhu, Y.; Sun, J.; Shang, X.; Wang, J. High Stability Polymer Gel for Lost Circulation Control When Drilling in Fractured Oil and Gas Formations. Geoenergy Sci. Eng. 2023, 225, 211722. [Google Scholar] [CrossRef]
- Ni, X.; Shi, H.; Zhang, J.; Liu, R.; Wang, J.; Cheng, R. Modified Laponite Synthesized with Special Wettability as a Multifunctional Additive in Oil-Based Drilling Fluids. J. Pet. Sci. Eng. 2023, 220, 111211. [Google Scholar] [CrossRef]
- Li, X.-L.; Jiang, G.-C.; Xu, Y.; Deng, Z.-Q.; Wang, K. A New Environmentally Friendly Water-Based Drilling Fluids with Laponite Nanoparticles and Polysaccharide/Polypeptide Derivatives. Pet. Sci. 2022, 19, 2959–2968. [Google Scholar] [CrossRef]
- Dong, X.; Sun, J.; Huang, X.; Lv, K.; Zhou, Z.; Gao, C. Nano-Laponite/Polymer Composite as Filtration Reducer on Water-Based Drilling Fluid and Mechanism Study. R. Soc. Open Sci. 2022, 9, 220385. [Google Scholar] [CrossRef] [PubMed]
- López-Galindo, A.; Viseras, C.; Cerezo, P. Compositional, Technical and Safety Specifications of Clays to Be Used as Pharmaceutical and Cosmetic Products. Appl. Clay Sci. 2007, 36, 51–63. [Google Scholar] [CrossRef]
- Lull, M.A.; Howell, A.L.; Novack, C.D. Laponite Clay in Cosmetic and Personal Care Products 2015.
- Sarruf, F.D.; Contreras, V.J.; Martinez, R.M.; Velasco, M.V.; Baby, A.R. The Scenario of Clays and Clay Minerals Use in Cosmetics/Dermocosmetics. Cosmetics 2024, 11, 7. [Google Scholar] [CrossRef]
- Mayes, B.J. Synthetic Hectorite—A New Toothpaste Binder. Int. J. Cosmet. Sci. 1979, 1, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Viseras, C.; Sánchez-Espejo, R.; Palumbo, R.; Liccardi, N.; García-Villén, F.; Borrego-Sánchez, A.; Massaro, M.; Riela, S.; López-Galindo, A. Clays in Cosmetics and Personal-Care Products. Clays Clay Miner. 2021, 69, 561–575. [Google Scholar] [CrossRef]
- Disalvo, A.L.; Mordas, C.J. Composite Materials Comprising Metal-Loaded Nanoparticles. AU2004285579A1, 10 February 2011. [Google Scholar]
- Wu, C.-J.; Gaharwar, A.K.; Chan, B.K.; Schmidt, G. Mechanically Tough Pluronic F127/Laponite Nanocomposite Hydrogels from Covalently and Physically Cross-Linked Networks. Macromolecules 2011, 44, 8215–8224. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Schexnailder, P.J.; Kline, B.P.; Schmidt, G. Assessment of Using Laponite Cross-Linked Poly(Ethylene Oxide) for Controlled Cell Adhesion and Mineralization. Acta Biomater. 2011, 7, 568–577. [Google Scholar] [CrossRef]
- Carretero, M.I.; Pozo, M. Clay and Non-Clay Minerals in the Pharmaceutical and Cosmetic Industries Part II. Active Ingredients. Appl. Clay Sci. 2010, 47, 171–181. [Google Scholar] [CrossRef]
- Nezadi, M.; Keshvari, H.; Shokrolahi, F.; Shokrollahi, P. Injectable, Self-Healing Hydrogels Based on Gelatin, Quaternized Chitosan, and Laponite as Localized Celecoxib Delivery System for Nucleus Pulpous Repair. Int. J. Biol. Macromol. 2024, 266, 131337. [Google Scholar] [CrossRef] [PubMed]
- Poeta, M.; Cioffi, V.; Buccigrossi, V.; Nanayakkara, M.; Baggieri, M.; Peltrini, R.; Amoresano, A.; Magurano, F.; Guarino, A. Diosmectite Inhibits the Interaction between SARS-CoV-2 and Human Enterocytes by Trapping Viral Particles, Thereby Preventing NF-kappaB Activation and CXCL10 Secretion. Sci. Rep. 2021, 11, 21725. [Google Scholar] [CrossRef] [PubMed]







| LAP Type | Composition | Physical Properties | Applications | Differences |
|---|---|---|---|---|
| RD |
SiO2: 59.5% MgO: 27.5% Na2O: 2.8% Li2O: 0.8% Loss on ignition: 8.2% |
bulk density: ~1.0 g/cm3 specific surface area: ~370 m2/g pH (2% dispersion): 9.8 |
|
|
| RDS |
SiO2: 54.5% MgO: 26% Na2O: 5.6% P2O5: 4.4% Li2O: 0.8% Loss on ignition: 8% |
bulk density: ~1.0 g/cm3 specific surface area: ~330 m2/g pH (2% dispersion): 9.7 |
| similar to RD, with slight differences in surface area and viscosity for specific applications. |
| XLG |
SiO2: 59.5% MgO: 27.5% Na2O: 2.8% Li2O: 0.8% Loss on ignition: 7% |
bulk density: ~1.0 g/cm3 specific surface area: ~370 m2/g pH (2% dispersion): 9.8 |
|
|
| XLS |
SiO2: 54.5% MgO: 26% Na2O: 5.6% P2O5: 4.1% Li2O: 0.8% Loss on ignition: 8.2% |
bulk density: ~1.0 g/cm3 specific surface area: ~330 m2/g pH (2% dispersion): 9.7 |
| optimized for transparent formulations with lower turbidity and enhanced clarity. |
| JS |
SiO2: 50.2% MgO: 22.2% Na2O: 7.5% P2O5: 5.4% Li2O: 0.8% Loss on ignition: 8.2% |
bulk density: ~0.950 g/cm3 specific surface area: ~300 m2/g pH (2% dispersion): 10 |
| designed for industrial applications such as drilling fluids, where higher performance in suspension is needed. |
| LAP | Natural Clay | |
|---|---|---|
| Advantages |
|
|
| Disadvantages |
|
|
| Field | Reviews | Application | Composition | Research Papers/Patents |
|---|---|---|---|---|
| Biomedical Applications | [83,84,85] [111,114] [116,118] | Drug delivery | LAP/CS/PVA LAP/DNA/Oxidized Alginate LAP/cPEG/DOX LAP/Poly(acrylate)/Sodium phosphate LAP/Heparin/Poloxamer 407 LAP/quaternized CS/gelatin LAP/Antiviral Agents | [11] [91] [94] [100] [101] [187] [188] |
| Tissue engineering | LAP/Sodium Alginate LAP/CS LAP/PEGDA | [106] [112] [113] | ||
| Bioprinting | LAP/Stromal cells LAP/Caprolactona LAP/Gelan Gum LAP/PAAm/Agarose | [120] [121] [122] [123] | ||
| Biosensors | LAP/Graphene Electrode LAP/PAAm | [127] [134] | ||
| Biomedical imaging | LAP/Polyethylenimine LAP/Fe3O4 NP LAP/Polydopamine LAP/Polypyrrole | [138] [139] [140] [141] | ||
| Food Packaging | [142,143] [144,145] | Food properties (i.e., sensory, quality, shell life, etc.) Packaging properties | LAP/Gelatin LAP/(Lactic Acid/Glycerine/PEG) mixture (1:1:1) LAP/Onion LAP/Gelatin LAP/Pectin/Ag NP | [146] [148] [150] [151] [153] |
| Drilling fluids | [157,161] | Drilling operations Lubrication of drilling equipment Wellbore integrity Cleaning hole | LAP/Polymer Nanocomposites LAP/Polyionic Cellulose LAP/PEG; LAP/PPG LAP/Isopentenol Polyoxyethylene Ether LAP/Perfluorohexylethyltrimethoxysilane LAP/Polysaccharide/Polypeptide | [156,157,158,159,172,177] [171] [10] [173] [175] [176] |
| Cosmetics and Personal Care Products | [180] | Emulsifying, thickening, suspending, anticaking and moisturizing agent | LAP/Sunscreen Formulation | [179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunchi, C.-E.; Morariu, S. Laponite®—From Dispersion to Gel—Structure, Properties, and Applications. Molecules 2024, 29, 2823. https://doi.org/10.3390/molecules29122823
Brunchi C-E, Morariu S. Laponite®—From Dispersion to Gel—Structure, Properties, and Applications. Molecules. 2024; 29(12):2823. https://doi.org/10.3390/molecules29122823
Chicago/Turabian StyleBrunchi, Cristina-Eliza, and Simona Morariu. 2024. "Laponite®—From Dispersion to Gel—Structure, Properties, and Applications" Molecules 29, no. 12: 2823. https://doi.org/10.3390/molecules29122823






