Synthesis of Substituted 1,2-Dihydroisoquinolines by Palladium-Catalyzed Cascade Cyclization–Coupling of Trisubstituted Allenamides with Arylboronic Acids
Abstract
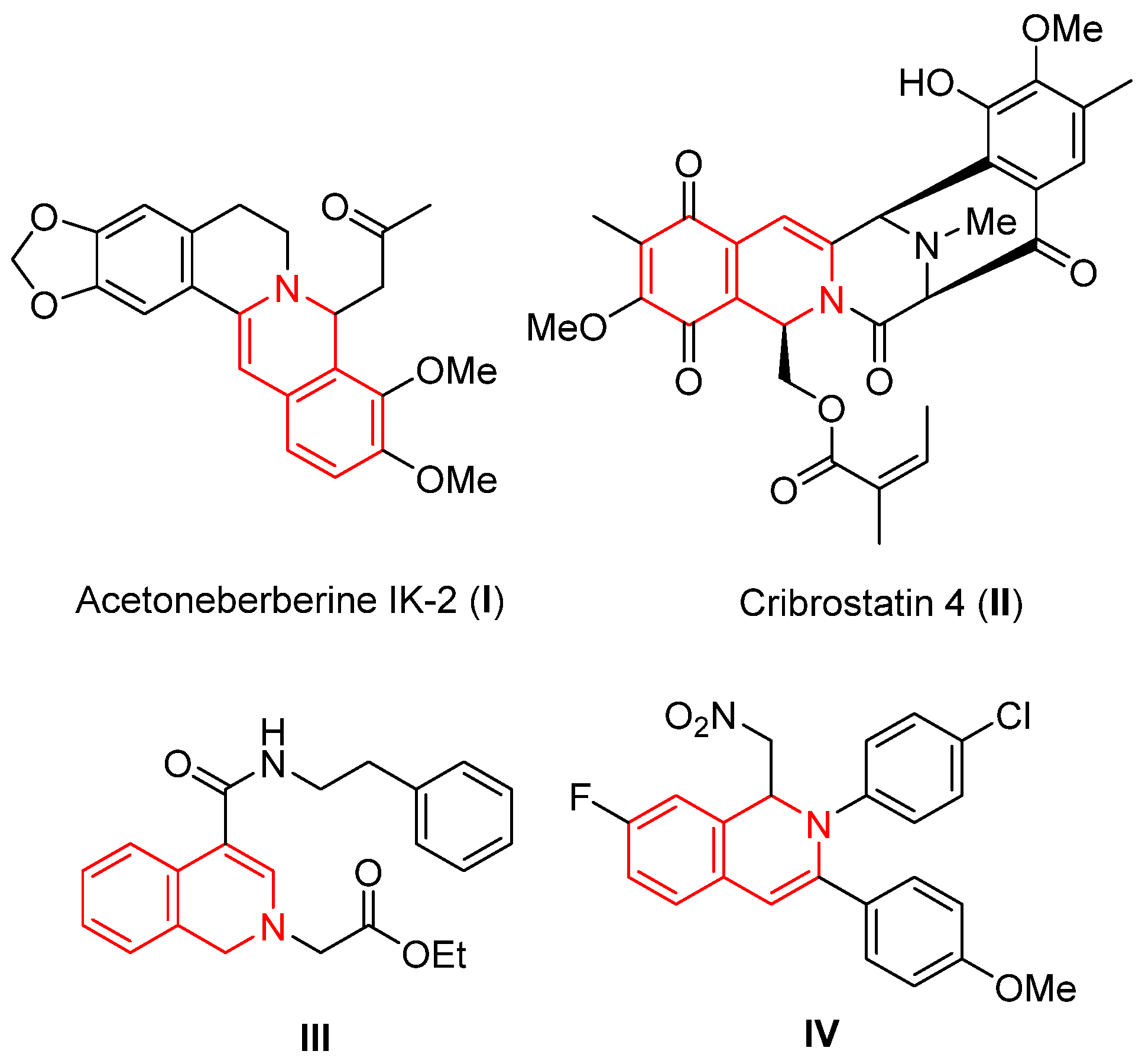
:1. Introduction
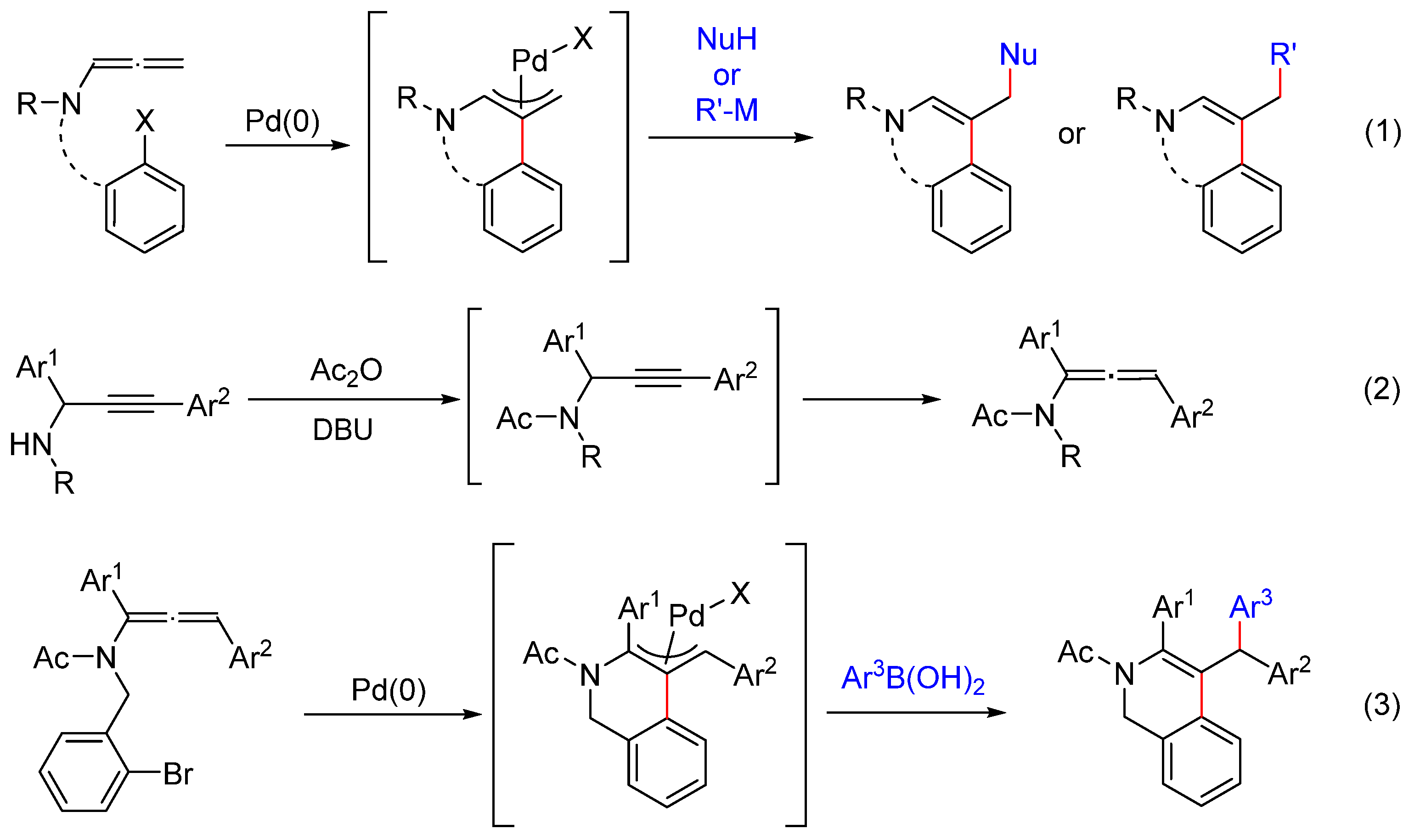
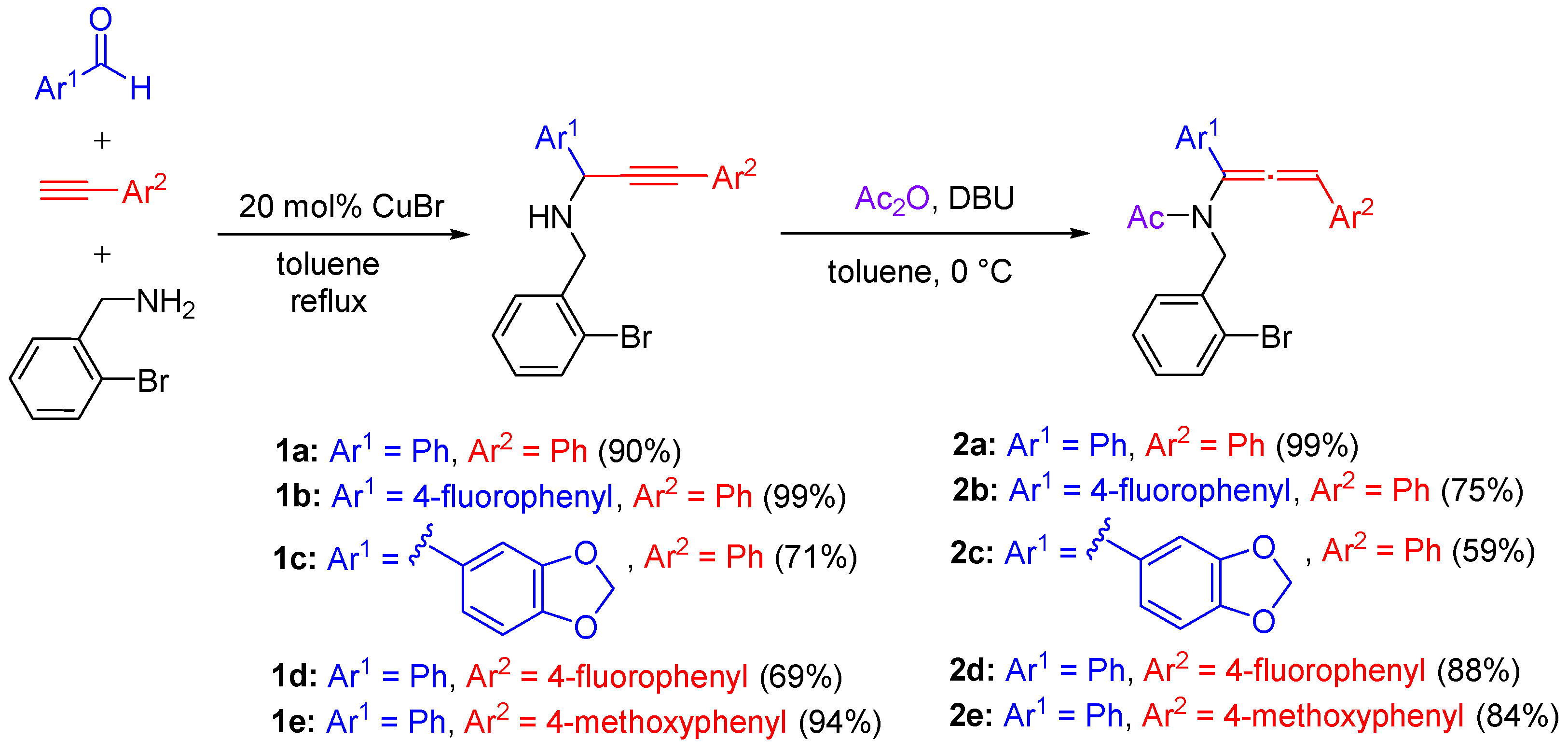
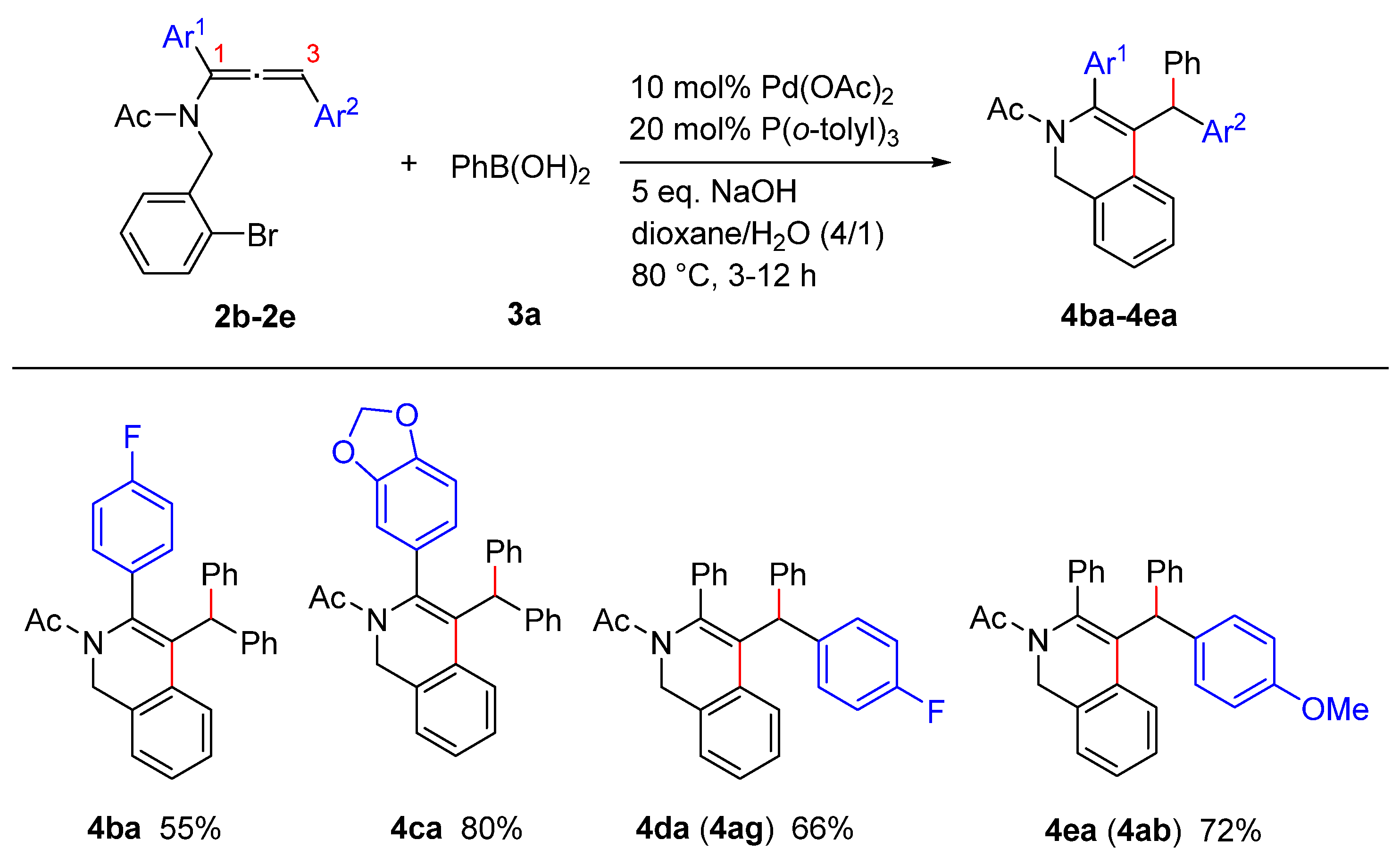
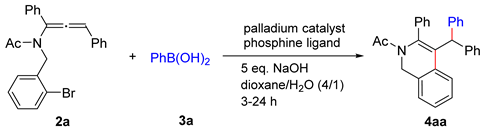
2. Results and Discussion
3. Materials and Methods
3.1. General Procedure for the Three-Component Reaction of Arylaldehyde, Alkyne and Amine in Scheme 2: Synthesis of Propargylamine 1a
3.2. N-(2-Bromobenzyl)-1,3-diphenylprop-2-yn-1-amine (1a)
3.3. N-(2-Bromobenzyl)-1-(4-fluorophenyl)-3-phenylprop-2-yn-1-amine (1b)
3.4. 1-(Benzo[d][1,3]dioxol-5-yl)-N-(2-bromobenzyl)-3-phenylprop-2-yn-1-amine (1c)
3.5. N-(2-Bromobenzyl)-3-(4-fluorophenyl)-1-phenylprop-2-yn-1-amine (1d)
3.6. N-(2-Bromobenzyl)-3-(4-methoxyphenyl)-1-phenylprop-2-yn-1-amine (1e)
3.7. General Procedure for the One-pot Synthesis of Trisubstituted Allenamide in Scheme 2: Synthesis of Allenamide 2a
3.8. N-(2-Bromobenzyl)-N-(1,3-diphenylpropa-1,2-dien-1-yl)acetamide (2a)
3.9. N-(2-Bromobenzyl)-N-(1-(4-fluorophenyl)-3-phenylpropa-1,2-dien-1-yl)acetamide (2b)
3.10. N-(1-(Benzo[d][1,3]dioxol-5-yl)-3-phenylpropa-1,2-dien-1-yl)-N-(2-bromobenzyl)acetamide (2c)
3.11. N-(2-Bromobenzyl)-N-(3-(4-fluorophenyl)-1-phenylpropa-1,2-dien-1-yl)acetamide (2d)
3.12. N-(2-Bromobenzyl)-N-(3-(4-methoxyphenyl)-1-phenylpropa-1,2-dien-1-yl)acetamide (2e)
3.13. General Procedure for the Palladium-Catalyzed Cascade Reaction of Allenamide with Arylboronic Acid: Synthesis of 1,2-dihydroisoquinoline 4aa
3.14. 1-(4-Benzhydryl-3-phenylisoquinolin-2(1H)-yl)ethan-1-one (4aa)
3.15. 1-(4-((4-Methoxyphenyl)(phenyl)methyl)-3-phenylisoquinolin-2(1H)-yl)ethan-1-one (4ab/4ea)
3.16. 1-(4-((3,5-Dimethoxyphenyl)(phenyl)methyl)-3-phenylisoquinolin-2(1H)-yl)ethan-1-one (4ac)
3.17. 1-(4-((3,4-Dimethoxyphenyl)(phenyl)methyl)-3-phenylisoquinolin-2(1H)-yl)ethan-1-one (4ad)
3.18. 1-(4-((4-(tert-Butyl)phenyl)(phenyl)methyl)-3-phenylisoquinolin-2(1H)-yl)ethan-1-one (4ae)
3.19. 1-(4-((4-Chlorophenyl)(phenyl)methyl)-3-phenylisoquinolin-2(1H)-yl)ethan-1-one (4af)
3.20. 1-(4-((4-Fluorophenyl)(phenyl)methyl)-3-phenylisoquinolin-2(1H)-yl)ethan-1-one (4ag/4da)
3.21. 1-(4-((2-Acetyl-3-phenyl-1,2-dihydroisoquinolin-4-yl)(phenyl)methyl)phenyl)ethan-1-one (4ah)
3.22. 1-(4-(Naphthalen-1-yl(phenyl)methyl)-3-phenylisoquinolin-2(1H)-yl)ethan-1-one (4ai)
3.23. 1-(4-Benzhydryl-3-(4-fluorophenyl)isoquinolin-2(1H)-yl)ethan-1-one (4ba)
3.24. 1-(4-Benzhydryl-3-(benzo[d][1,3]dioxol-5-yl)isoquinolin-2(1H)-yl)ethan-1-one (4ca)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bentley, K.W. The Isoquinoline Alkaloids; Harwood Academic Publishers: Amsterdam, The Netherlands, 1998; Volume 1. [Google Scholar]
- Scholz, D.; Schmidt, H.; Prieschl, E.E.; Csonga, R.; Scheirer, W.; Weber, V.; Lembachner, A.; Seidl, G.; Werner, G.; Mayer, P.; et al. Inhibition of FcεRI-Mediated Activation of Mast Cells by 2,3,4-Trihydropyrimidino [2,1-a]Isoquinolines. J. Med. Chem. 1998, 41, 1050–1059. [Google Scholar] [CrossRef]
- Ramesh, P.; Reddy, N.S.; Venkateswarlu, Y. A New 1,2-Dihydroisoquinoline from the Sponge Petrosiasimilis. J. Nat. Prod. 1999, 62, 780–781. [Google Scholar] [CrossRef]
- Deore, R.R.; Chen, G.S.; Chen, C.-S.; Chang, P.-T.; Chuang, M.-H.; Chern, T.-R.; Wang, H.-C.; Chern, J.-W. 2-Hydroxy-1-oxo-1,2-dihydroisoquinoline-3-carboxylic acid with inbuilt β-N-hydroxy-γ-keto-acid pharmacophore as HCV NS5B polymerase inhibitors. Curr. Med. Chem. 2012, 19, 613–624. [Google Scholar] [CrossRef]
- Inoue, K.; Kulsum, U.; Chowdhury, S.A.; Fujisawa, S.; Ishihara, M.; Yokoe, I.; Sakagami, H. Tumor-specific Cytotoxicity and Apoptosis-inducing Activity of Berberines. Anticancer. Res. 2005, 25, 4053–4059. [Google Scholar]
- Gonzalez, J.F.; de la Cuesta, E.; Avendano, C. Synthesis and cytotoxic activity of pyrazino [1,2-b]-isoquinolines, 1-(3-isoquinolyl)isoquinolines, and 6,15-iminoisoquino-[3,2-b]-3-benzazocines. Bioorg. Med. Chem. 2007, 15, 112–118. [Google Scholar] [CrossRef]
- Mahmoud, S.; Sheha, M.; Aboul-Fadl, T.; Farag, H. 1,2-Dihydroisoquinoline-N-acetic Acid Derivatives as New Carriers for Brain-specific Delivery II: Delivery of Phenethylamine as Model Drug. Arch. Pharm. Pharm. Med. Chem. 2003, 336, 258–263. [Google Scholar] [CrossRef]
- Tandon, V.; Urvashi; Yadav, P.; Sur, S.; Abbat, S.; Tiwari, V.; Hewer, R.; Papathanasopoulos, M.A.; Raja, R.; Banerjea, A.C.; et al. Design, Synthesis, and Biological Evaluation of 1,2-Dihydroisoquinolines as HIV-1 Integrase Inhibitors. ACS Med. Chem. Lett. 2015, 6, 1065–1070. [Google Scholar] [CrossRef]
- Hajinasiri, R. A review of the synthesis of 1,2-dihydroisoquinoline, [2,1-a] isoquinoline and [5,1-a] isoquinoline since 2006. Tetrahedron 2022, 104, 132576. [Google Scholar] [CrossRef]
- Ohtaka, M.; Nakamura, H.; Yamamoto, Y. Synthesis of 1,2-dihydroisoquinolines via the reaction of ortho-alkynylarylimines with bis-π-allylpalladium. Tetrahedron Lett. 2004, 4, 7339–7341. [Google Scholar] [CrossRef]
- Asao, N.; Yudha, S.S.; Nogami, T.; Yamamoto, Y. Direct Mannich and Nitro-Mannich Reactions with Non-Activated Imines: AgOTf-Catalyzed Addition of Pronucleophiles to ortho-Alkynylaryl Aldimines Leading to 1,2-Dihydroisoquinolines. Angew. Chem. Int. Ed. 2005, 44, 5526–5528. [Google Scholar] [CrossRef]
- Obika, S.; Kono, H.; Yasui, Y.; Yanada, R.; Takemoto, Y. Concise Synthesis of 1,2-Dihydroisoquinolines and 1H-Isochromenes by Carbophilic Lewis Acid-Catalyzed Tandem Nucleophilic Addition and Cyclization of 2-(1-Alkynyl)arylaldimines and 2-(1-Alkynyl)arylaldehydes. J. Org. Chem. 2007, 72, 4462–4468. [Google Scholar] [CrossRef]
- Su, S.; Porco, J.A. 1,2-Dihydroisoquinolines as Templates for Cascade Reactions To Access Isoquinoline Alkaloid Frameworks. Org. Lett. 2007, 9, 4983–4986. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, X.; Wu, J. AgOTf-catalyzed tandem reaction of N′-(2-alkynylbenzylidene)hydrazide with alkyne. Chem. Commun. 2009, 3469–3471. [Google Scholar] [CrossRef]
- Guo, Z.; Cai, M.; Jiang, J.; Yang, L.; Hu, W. Rh2(OAc)4-AgOTf Cooperative Catalysis in Cyclization/Three-Component Reactions for Concise Synthesis of 1,2-Dihydroisoquinolines. Org. Lett. 2010, 12, 652–655. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, H.-J.; Ruan, W.; Wen, T.-B. Construction of Isoquinolin-1(2H)-ones by Copper-Catalyzed Tandem Reactions of 2-(1-Alkynyl)benzaldimines with Water. Eur. J. Org. Chem. 2015, 2015, 5914–5918. [Google Scholar] [CrossRef]
- Chaudhari, T.Y.; Ginotra, S.K.; Yadav, P.; Kumar, G.; Tandon, V. Regioselective synthesis of functionalized dihydroisoquinolines from: O -alkynylarylaldimines via the Reformatsky reaction. Org. Biomol. Chem. 2016, 14, 9896–9906. [Google Scholar] [CrossRef]
- Gao, Y.-N.; Shi, F.-C.; Xu, Q.; Shi, M. Enantioselective Synthesis of Isoquinolines: Merging Chiral-Phosphine and Gold Catalysis. Chem. Eur. J. 2016, 22, 6803–6807. [Google Scholar] [CrossRef]
- De Abreu, M.; Tangm, Y.; Brachet, E.; Selkti, M.; Michelet, V.; Belmony, P. Silver-catalyzed tandem cycloisomerization/hydroarylation reactions and mechanistic investigations for an efficient access to 1,2-dihydroisoquinolines. Org. Biomol. Chem. 2021, 19, 1037–1046. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Chen, J.-H.; Teng, M.-Y.; Li, X.; Jiang, T.-Y.; Huang, F.-R.; Yao, Q.-J.; Shi, B.-F. Cobalt-Catalyzed Enantioselective C-H Annulation of Benzylamines with Alkynes: Application to the Modular and Asymmetric Syntheses of Bioactive Molecules. J. Am. Chem. Soc. 2023, 145, 24499–24505. [Google Scholar] [CrossRef]
- Seoane-Carabel, F.; Alonso-Marañón, L.; Sarandeses, L.A.; Sestelo, J.P. Synthesis of 1H-Isochromenes and 1,2-Dihydroisoquinolines by Indium(III)-Catalyzed Cycloisomerization of ortho-(Alkynyl)benzyl Derivatives. Synthesis 2023, 55, 1714–1723. [Google Scholar]
- Umeda, R.; Ishida, T.; Mori, S.; Yashima, H.; Yajima, T.; Osaka, I.; Takata, R.; Nishiyama, Y. Rhenium-catalyzed synthetic method of 1,2-dihydroisoquinolines and isoquinolines by the intramolecular cyclization of 2-alkynylaldimines or 2-alkynylbenzylamines. Tetrahedron 2024, 154, 133854. [Google Scholar] [CrossRef]
- Wei, L.L.; Xiong, H.; Hsung, R.P. The Emergence of Allenamides in Organic Synthesis. Acc. Chem. Res. 2003, 36, 773–782. [Google Scholar] [CrossRef]
- Lu, T.; Lu, Z.; Ma, Z.-X.; Zhang, Y.; Hsung, R.P. Allenamides: A Powerful and Versatile Building Block in Organic Synthesis. Chem. Rev. 2013, 113, 4862–4904. [Google Scholar] [CrossRef]
- Braun, M.-G.; Katcher, M.H.; Doyle, A.G. Carbofluorination via a palladium-catalyzed cascade reaction. Chem. Sci. 2013, 4, 1216–1220. [Google Scholar] [CrossRef]
- Xiong, W.; Cheng, R.; Wu, B.; Wu, W.; Qi, C.; Jiang, H.F. Palladium-catalyzed regioselective cascade reaction of carbon dioxide, amines and allenes for the synthesis of functionalized carbamates. Sci. China Chem. 2020, 63, 331–335. [Google Scholar] [CrossRef]
- Higuchi, Y.; Mita, T.; Sato, Y. Palladium-Catalyzed Intramolecular Arylative Carboxylation of Allenes with CO2 for the Construction of 3-Substituted Indole-2-carboxylic Acids. Org. Lett. 2017, 19, 2710–2713. [Google Scholar] [CrossRef]
- Zhu, X.; Li, R.; Yao, H.; Lin, A. Palladium-Catalyzed Allenamide Carbopalladation/Allylation with Active Methine Compounds. Org. Lett. 2021, 23, 4630–4634. [Google Scholar] [CrossRef]
- Wang, D.-C.; Yang, T.-T.; Qu, G.-R.; Guo, H.-M. Substrate-Dependent Regioselectivity: Pd/PTC Cooperatively Catalyzed Domino Heck/Allylation of Allenamides with α-Carbon of Carbonyl Compounds. J. Org. Chem. 2022, 87, 14284–14298. [Google Scholar] [CrossRef]
- Hédouin, J.; Carpentier, V.; Renard, R.M.Q.; Schneider, C.; Gillaizeau, I.; Hoarau, C. Regioselective Pd-Catalyzed Carbopalladation/Decarboxylative Allylic Alkynylation of ortho-Iodoallenamides with Alkynyl Carboxylic Acids. J. Org. Chem. 2019, 84, 10535–10545. [Google Scholar] [CrossRef]
- Shen, Q.-W.; Wen, W.; Guo, Q.-X. Chiral Aldehyde–Palladium Catalysis Enables Asymmetric Synthesis of α-Alkyl Tryptophans via Cascade Heck-Alkylation Reaction. Org. Lett. 2023, 25, 3163–3167. [Google Scholar] [CrossRef]
- Grigg, R.; Sansano, J.M.; Santhakumar, V.; Sridharan, V.; Thangavelanthum, R.; Thornton-Pett, M.; Wilson, D. Palladium catalysed tandem cyclisation-anion capture processes. Part 3. Organoboron anion transfer agents. Tetrahedron 1997, 53, 11803–11826. [Google Scholar] [CrossRef]
- Fuwa, H.; Sasaki, M. A strategy for the synthesis of 2,3-disubstituted indoles starting from N-(o-halophenyl)allenamides. Org. Biomol. Chem. 2007, 5, 2214–2218. [Google Scholar] [CrossRef]
- Parthasarathy, K.; Jeganmohan, M.; Cheng, C.-H. Palladium-Catalyzed Multistep Reactions Involving Ring Closure of 2-Iodophenoxyallenes and Ring Opening of Bicyclic Alkenes. Org. Lett. 2006, 8, 621–623. [Google Scholar] [CrossRef]
- Chen, X.; Qiu, G.; Liu, R.; Chen, D.; Chen, Z. Divergent oriented synthesis (DOS) of aza-heterocyclic amides through palladium-catalyzed ketenimination of 2-iodo-N-(propa-1,2-dien-1-yl)anilines. Org. Chem. Front. 2020, 7, 890–895. [Google Scholar] [CrossRef]
- Hédouin, J.; Schneider, C.; Gillaizeau, I.; Hoarau, C. Palladium-Catalyzed Domino Allenamide Carbopalladation/Direct C–H Allylation of Heteroarenes: Synthesis of Primprinine and Papaverine Analogues. Org. Lett. 2018, 20, 6027–6032. [Google Scholar] [CrossRef]
- Xue, Q.; Pu, Y.; Zhao, H.; Xie, X.; Zhang, H.; Wang, J.; Yan, L.; Shang, Y. Palladium-catalysed aryl/monofluoroalkylation of allenamides: Access to fluoroalkyl indoles and isoquinolones. Chem. Commun. 2024, 60, 3794–3797. [Google Scholar] [CrossRef]
- Hirokane, T.; Watanabe, S.; Matsumoto, K.; Yoshida, M. A facile synthesis of trisubstituted allenamides by DBU-promoted isomerization of propargylamides. Tetrahedron Lett. 2020, 61, 152146. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]


 | ||||
|---|---|---|---|---|
| Entry | Palladium Catalyst | Phosphine Ligand | Temperature (°C) | Yield (%) |
| 1 | Pd(OAc)2 (5 mol%) | P(o-tolyl)3 (10 mol%) | 80 | 78 |
| 2 | Pd(OAc)2 (5 mol%) | P(o-tolyl)3 (20 mol%) | 80 | 85 |
| 3 | Pd(OAc)2 (10 mol%) | P(o-tolyl)3 (20 mol%) | 80 | 88 |
| 4 | Pd(OAc)2 (10 mol%) | P(o-tolyl)3 (20 mol%) | 50 | 86 |
| 5 | Pd(OAc)2 (10 mol%) | P(o-tolyl)3 (20 mol%) | 25 | 30 |
| 6 | Pd(OAc)2 (10 mol%) | P(o-tolyl)3 (20 mol%) | 100 | 75 |
| 7 | Pd(OAc)2 (10 mol%) | PPh3 (20 mol%) | 80 | 70 |
| 8 | Pd(OAc)2 (10 mol%) | PCy3 (20 mol%) | 80 | 19 |
| 9 | Pd(OAc)2 (10 mol%) | DPPE (10 mol%) | 80 | 47 |
| 10 | Pd(OAc)2 (10 mol%) | DPPF (10 mol%) | 80 | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, M.; Imaji, R.; Shiomi, S. Synthesis of Substituted 1,2-Dihydroisoquinolines by Palladium-Catalyzed Cascade Cyclization–Coupling of Trisubstituted Allenamides with Arylboronic Acids. Molecules 2024, 29, 2917. https://doi.org/10.3390/molecules29122917
Yoshida M, Imaji R, Shiomi S. Synthesis of Substituted 1,2-Dihydroisoquinolines by Palladium-Catalyzed Cascade Cyclization–Coupling of Trisubstituted Allenamides with Arylboronic Acids. Molecules. 2024; 29(12):2917. https://doi.org/10.3390/molecules29122917
Chicago/Turabian StyleYoshida, Masahiro, Ryunosuke Imaji, and Shinya Shiomi. 2024. "Synthesis of Substituted 1,2-Dihydroisoquinolines by Palladium-Catalyzed Cascade Cyclization–Coupling of Trisubstituted Allenamides with Arylboronic Acids" Molecules 29, no. 12: 2917. https://doi.org/10.3390/molecules29122917






