Bi/CeO2–Decorated CuS Electrocatalysts for CO2-to-Formate Conversion
Abstract
:1. Introduction
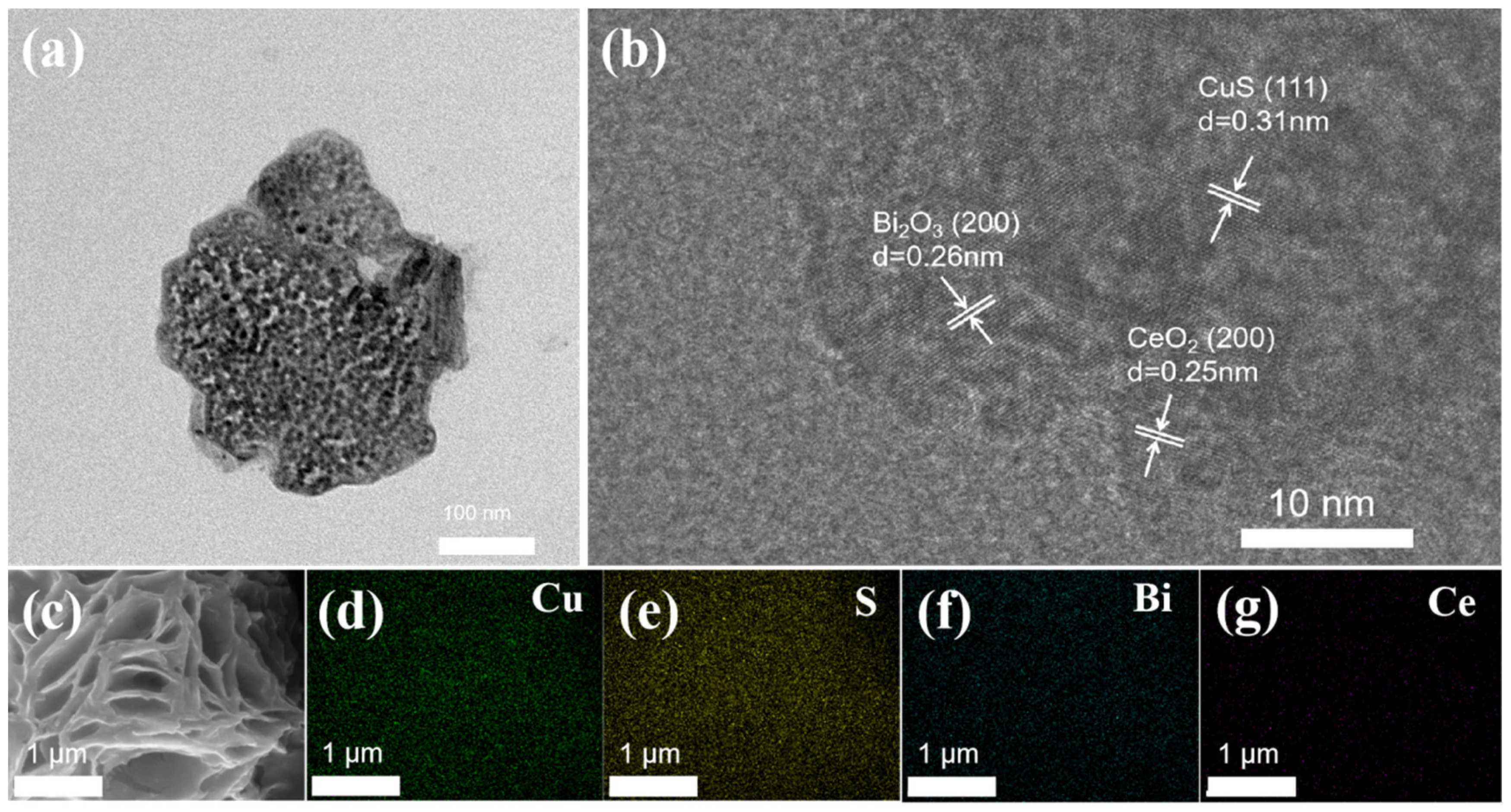
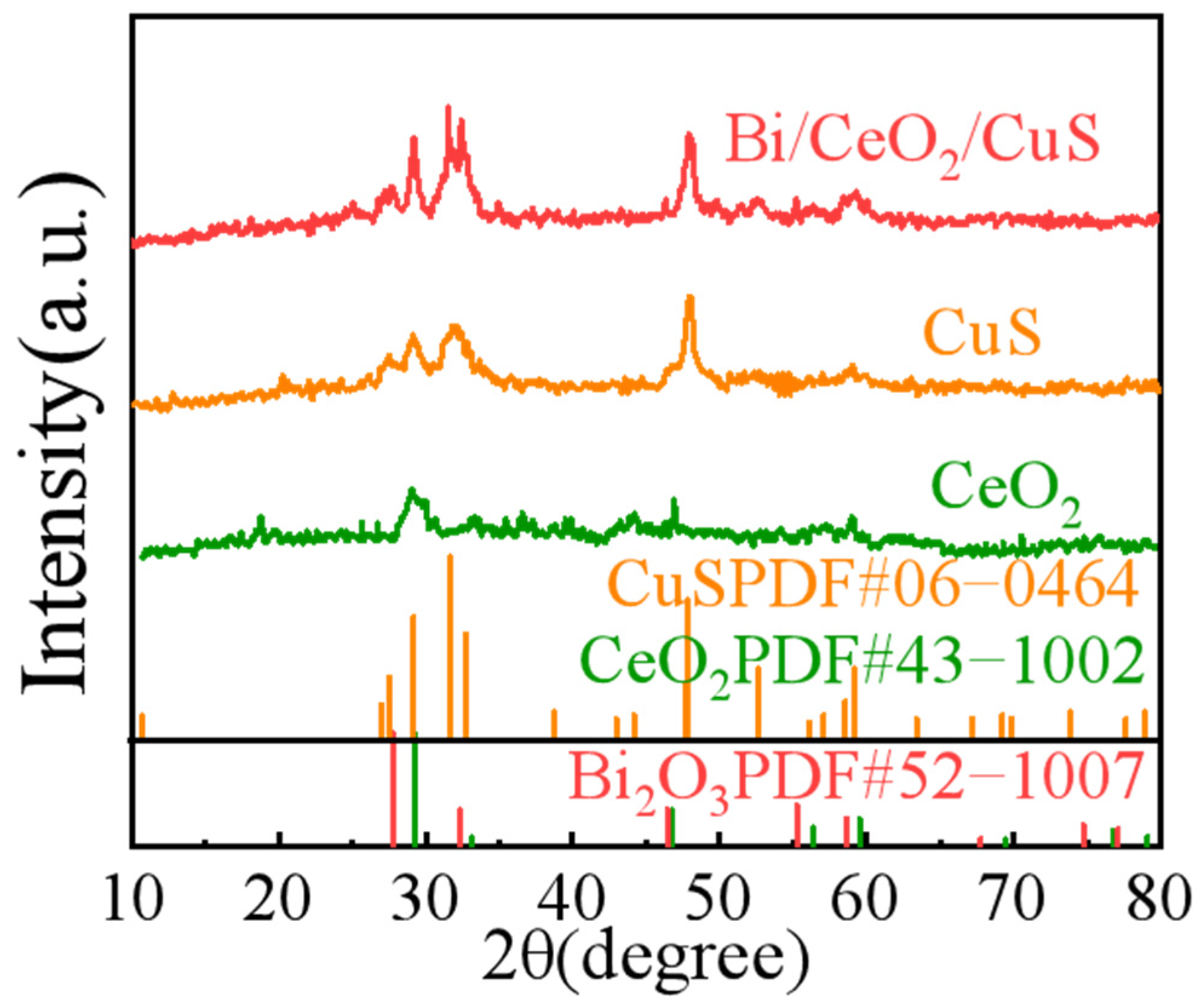
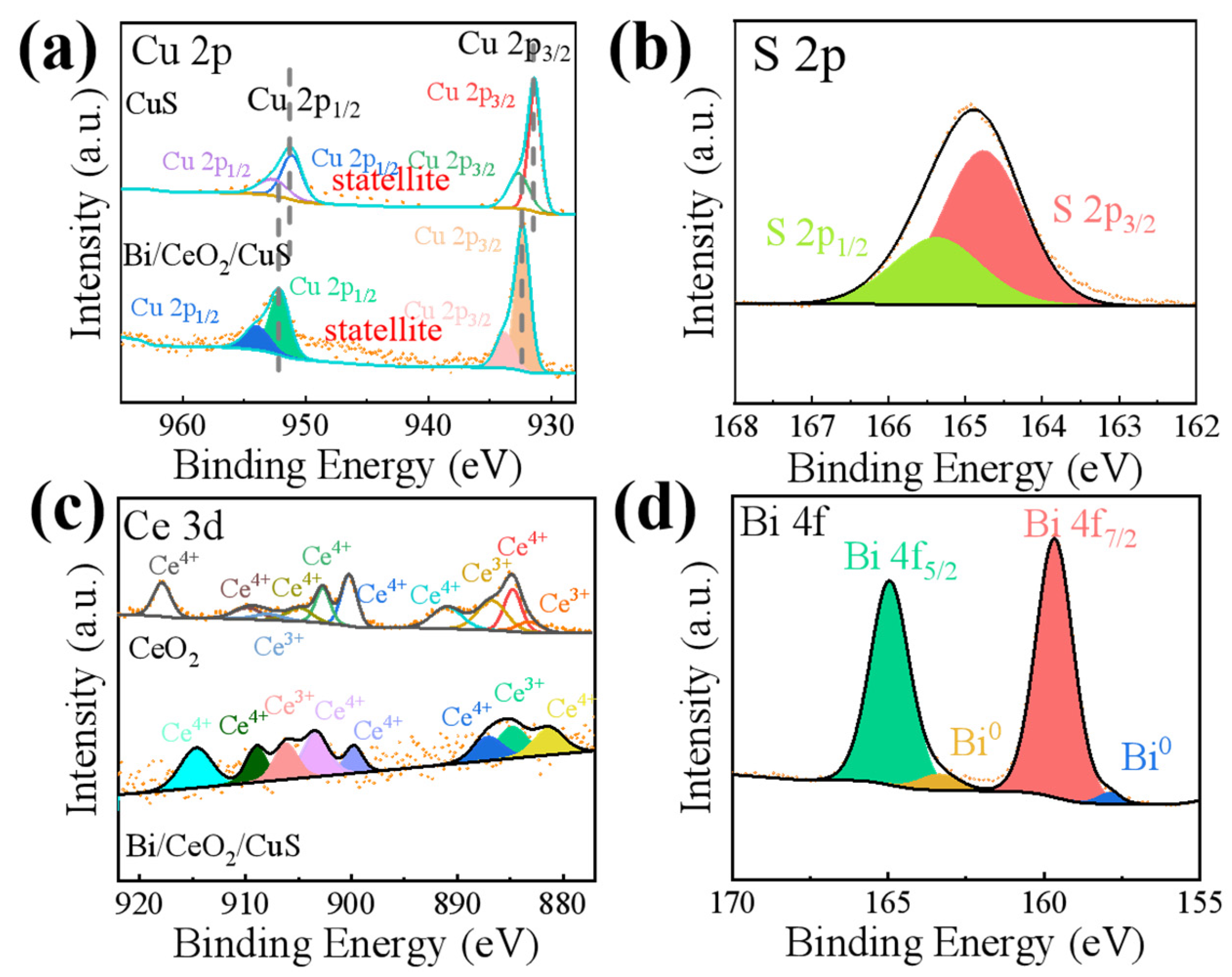
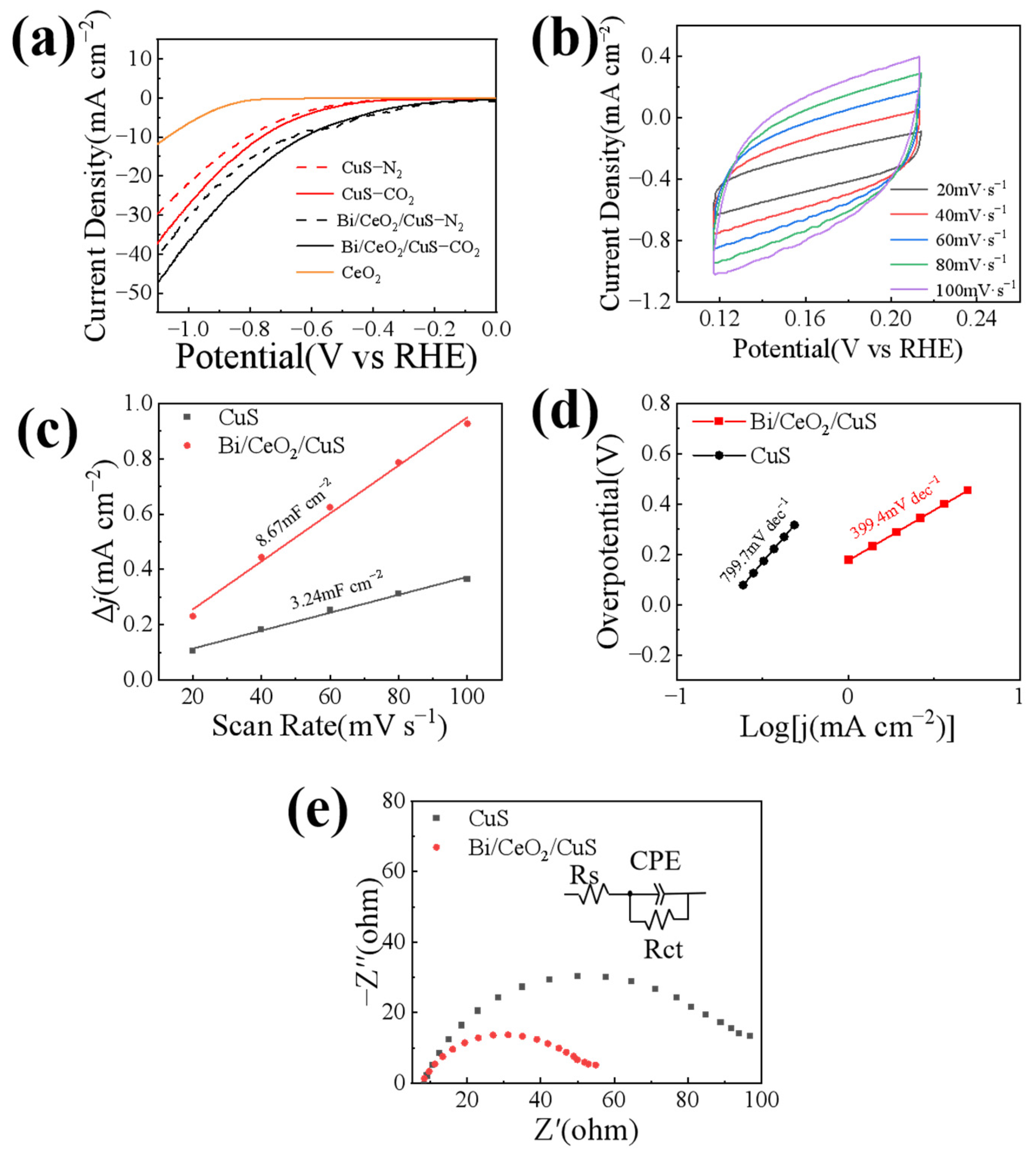
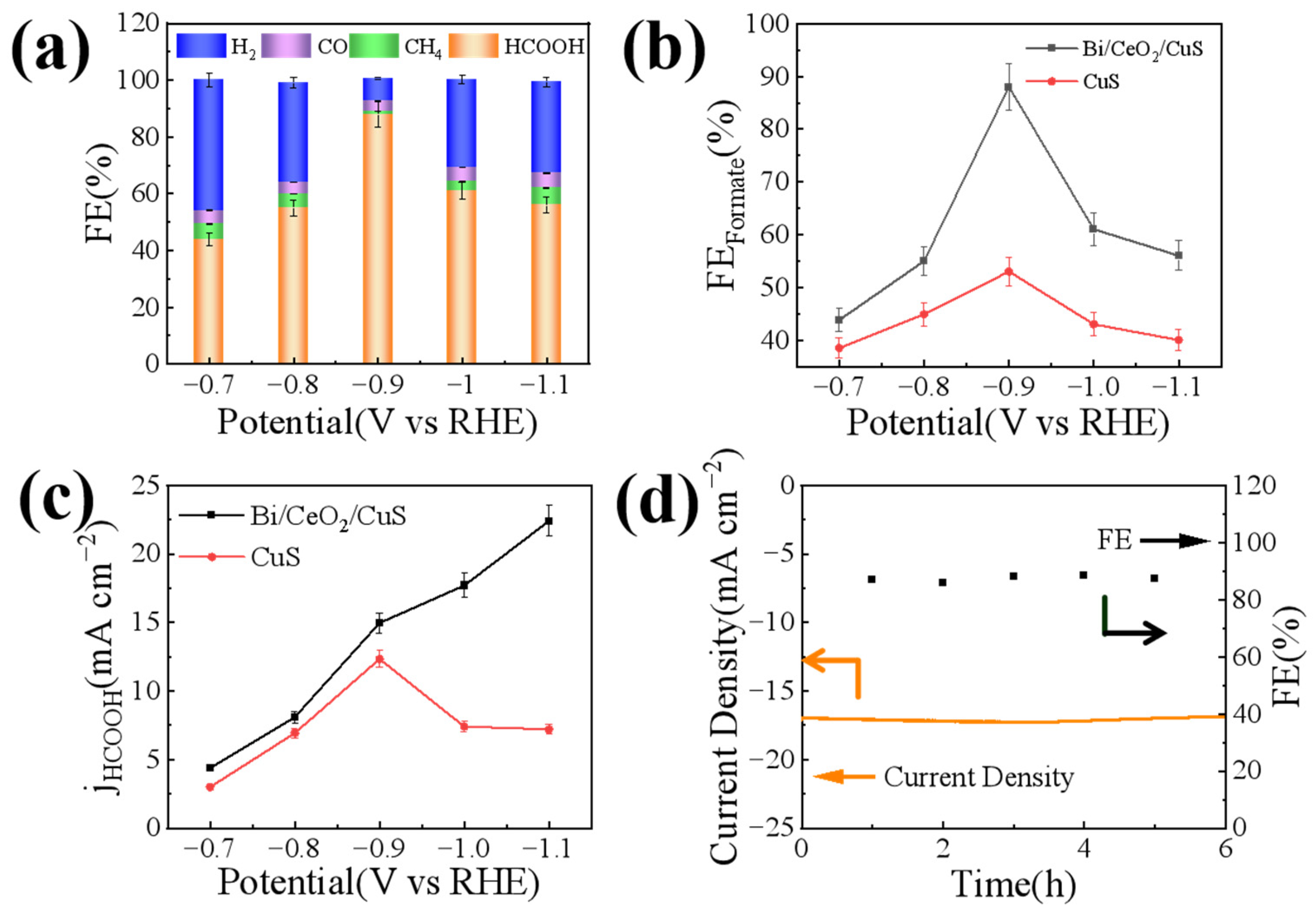
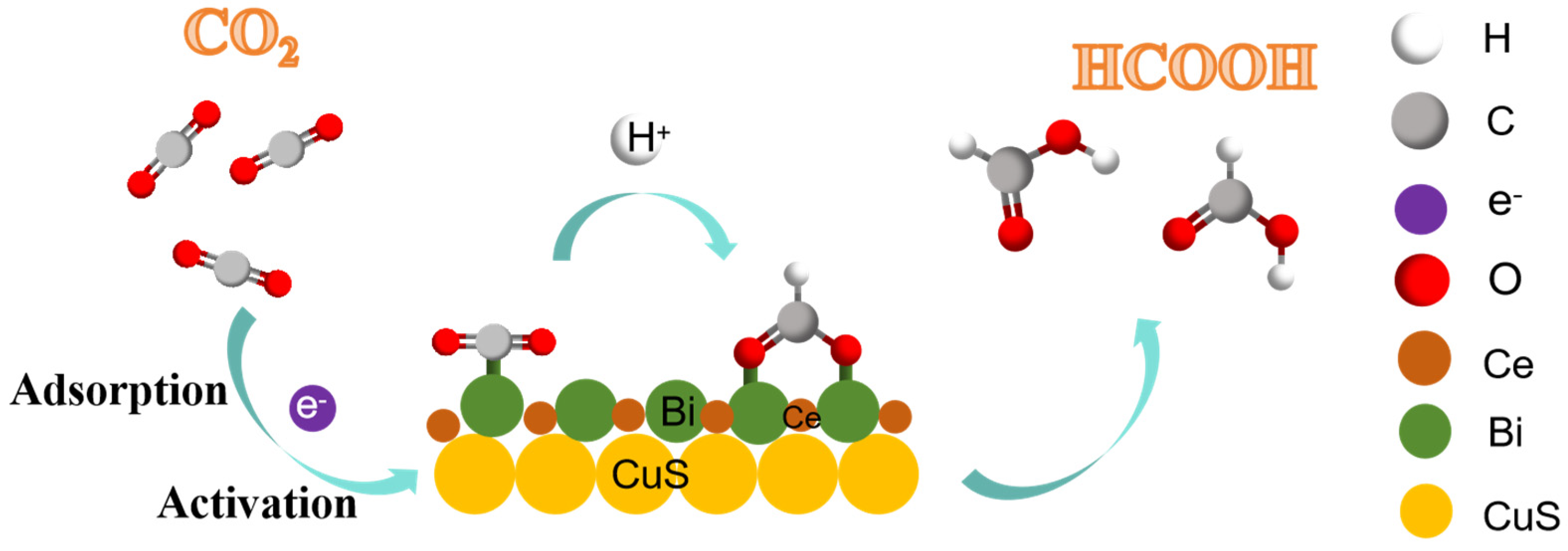
2. Results and Discussion
| Catalysts | Cell Type | Electrolyte | FE(%) | Current Density (mA cm−2) | Ref. |
|---|---|---|---|---|---|
| Cu-xS | H-cell | 0.1 M KHCO3 | 74 | 13.9 | [52] |
| CuSx | H-cell | 0.1 M KHCO3 | 75 | 12 | [24] |
| Cu7S4 NSs | Flow cell | 1 M KOH | 82.7 | 456 | [53] |
| GDY/CuSx | H-cell | 0.1 M KHCO3 | 70 | 65.6 | [54] |
| CuS/N,S-rGO | H-cell | 0.5 M KHCO3 | 82 | 24.2 | [36] |
| Sn–Cu@Sn | GDE | 1 M KHCO3 | 84.2 | 30 | [55] |
| CuBi3 | H-cell | 0.1 M KHCO3 | 98.4 | 21.2 | [23] |
| Bi@NCA | H-cell | 0.5 M KHCO3 | 95 | – | [43] |
| Bi-PVP/CC-600 | H-cell | 0.5 M KHCO3 | 81 | 54 | [56] |
| Bi-NFs | H-cell | 0.1 M KHCO3 | 92.3 | 28.5 | [41] |
| Bi/CeO2/CuS | H-cell | 0.5 M KHCO3 | 88 | 17 | This work |
3. Experimental Section
3.1. Materials
3.2. Catalyst Preparation
3.2.1. Synthesis of CuS Nanosheets
3.2.2. Synthesis of Bi/CeO2/CuS Nanosheets
3.2.3. Preparation of Working Electrodes
3.3. Catalyst Characterization
3.4. CO2RR Performance
3.5. Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zickfeld, K.; MacDougall, A.H.; Matthews, H.D. On the proportionality between global temperature change and cumulative CO2 emissions during periods of net negative CO2 emissions. Environ. Res. Lett. 2016, 11, 055006. [Google Scholar] [CrossRef]
- Chen, H.; Fu, W.; Geng, Z.; Zeng, J.; Yang, B. Inductive effect as a universal concept to design efficient catalysts for CO2 electrochemical reduction: Electronegativity difference makes a difference. J. Mater. Chem. A 2021, 9, 4626–4647. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, H. High-purity and high-concentration liquid fuels through CO2 electroreduction. Nat. Catal. 2021, 4, 943–951. [Google Scholar] [CrossRef]
- Bagger, A.; Ju, W.; Varela, A.S.; Strasser, P.; Rossmeisl, J. Electrochemical CO2 reduction: Classifying Cu facets. ACS Catal. 2019, 9, 7894–7899. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Z.; Wei, X.; Liu, S.; Guo, P.; Han, P.; Wang, H.; Zhang, J.; Lu, X.; Wei, B. Promotion of electrochemical CO2 reduction to ethylene on phosphorus-doped copper nanocrystals with stable Cuδ+ sites. Appl. Surf. Sci. 2021, 544, 148965. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.; Niu, D.; Manke, I.; Yang, C.; Zhu, M.; Zhang, X.; Chen, R. Pyridine-grafted nitrogen-doped carbon nanotubes achieving efficient electroreduction of CO2 to CO within a wide electrochemical window. J. Mater. Chem. A 2022, 10, 1852–1860. [Google Scholar] [CrossRef]
- Lin, L.; He, X.; Zhang, X.G.; Ma, W.; Zhang, B.; Wei, D.; Xie, S.; Zhang, Q.; Yi, X.; Wang, Y. A nanocomposite of bismuth clusters and Bi2O2CO3 sheets for highly efficient electrocatalytic reduction of CO2 to formate. Angew. Chem. Int. Ed. 2023, 62, e202214959. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Zhang, M.; Wu, L.; Guo, S.; Liu, X.; Zhao, J.; Xue, W.; Li, J.; Liu, C.; Li, X.; et al. Upcycling CO2 into energy-rich long-chain compounds via electrochemical and metabolic engineering. Nat. Catal. 2022, 5, 388–396. [Google Scholar] [CrossRef]
- Verma, S.; Kim, B.; Jhong, H.R.M.; Ma, S.; Kenis, P.J. A gross-margin model for defining technoeconomic benchmarks in the electroreduction of CO2. ChemSusChem 2016, 9, 1972–1979. [Google Scholar] [CrossRef]
- Seong, H.; Efremov, V.; Park, G.; Kim, H.; Yoo, J.S.; Lee, D. Atomically precise gold nanoclusters as model catalysts for identifying active sites for electroreduction of CO2. Angew. Chem. Int. Ed. 2021, 133, 14684–14691. [Google Scholar] [CrossRef]
- Lim, C.; Lee, W.H.; Won, J.H.; Ko, Y.J.; Kim, S.; Min, B.K.; Lee, K.Y.; Jung, W.S.; Oh, H.S. Enhancement of catalytic activity and selectivity for the gaseous electroreduction of CO2 to CO: Guidelines for the selection of carbon supports. Adv. Sustain. Syst. 2021, 5, 2100216. [Google Scholar] [CrossRef]
- Zhao, S.; Li, S.; Guo, T.; Zhang, S.; Wang, J.; Wu, Y.; Chen, Y. Advances in Sn-Based Catalysts for Electrochemical CO2 Reduction. Nano-Micro Lett. 2019, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.A.; Abild-Pedersen, F.; Studt, F.; Rossmeisl, J.; Nørskov, J.K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 2010, 3, 1311–1315. [Google Scholar] [CrossRef]
- Yoo, J.S.; Christensen, R.; Vegge, T.; Nørskov, J.K.; Studt, F. Theoretical Insight into the Trends that Guide the Electrochemical Reduction of Carbon Dioxide to Formic Acid. ChemSusChem 2016, 9, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Liu, Y.; Murphy, E.; Ly, A.; Xu, M.; Matanovic, I.; Pan, X.; Atanassov, P. Robust palladium hydride catalyst for electrocatalytic formate formation with high CO tolerance. Appl. Catal. B Environ. 2022, 316, 121659. [Google Scholar] [CrossRef]
- Koh, J.H.; Won, D.H.; Eom, T.; Kim, N.-K.; Jung, K.D.; Kim, H.; Hwang, Y.J.; Min, B.K. Facile CO2 Electro-Reduction to Formate via Oxygen Bidentate Intermediate Stabilized by High-Index Planes of Bi Dendrite Catalyst. ACS Catal. 2017, 7, 5071–5077. [Google Scholar] [CrossRef]
- Feaster, J.T.; Shi, C.; Cave, E.R.; Hatsukade, T.; Abram, D.N.; Kuhl, K.P.; Hahn, C.; Nørskov, J.K.; Jaramillo, T.F. Understanding Selectivity for the Electrochemical Reduction of Carbon Dioxide to Formic Acid and Carbon Monoxide on Metal Electrodes. ACS Catal. 2017, 7, 4822–4827. [Google Scholar] [CrossRef]
- Birdja, Y.Y.; Pérez-Gallent, E.; Figueiredo, M.C.; Göttle, A.J.; Calle-Vallejo, F.; Koper, M.T.M. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat. Energy 2019, 4, 732–745. [Google Scholar] [CrossRef]
- Wu, M.; Xu, B.; Zhang, Y.; Qi, S.; Ni, W.; Hu, J.; Ma, J. Perspectives in emerging bismuth electrochemistry. Chem. Eng. J. 2020, 381, 122558. [Google Scholar] [CrossRef]
- Xue, J.; Fu, X.; Geng, S.; Wang, K.; Li, Z.; Li, M. Boosting electrochemical CO2 reduction via valence state and oxygen vacancy controllable Bi–Sn/CeO2 nanorod. J. Environ. Manag. 2023, 342, 118354. [Google Scholar] [CrossRef]
- Peng, L.; Wang, Y.; Wang, Y.; Xu, N.; Lou, W.; Liu, P.; Cai, D.; Huang, H.; Qiao, J. Separated growth of Bi-Cu bimetallic electrocatalysts on defective copper foam for highly converting CO2 to formate with alkaline anion-exchange membrane beyond KHCO3 electrolyte. Appl. Catal. B Environ. 2021, 288, 120003. [Google Scholar] [CrossRef]
- Dou, T.; Song, D.; Wang, Y.; Zhao, X.; Zhang, F.; Lei, X. Hierarchical Bi/S-modified Cu/brass mesh used as structured highly performance catalyst for CO2 electroreduction to formate. Nano Res. 2023, 17, 3644–3652. [Google Scholar] [CrossRef]
- Fu, Y.; Leng, K.; Zhuo, H.; Liu, W.; Liu, L.; Zhou, G.; Tang, J. Nanoconfinement effects on CuBi3 alloy catalyst for efficient CO2 electroreduction to formic acid. J. CO2 Util. 2023, 70, 102456. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, Y.; Ren, D.; Handoko, A.D.; Seh, Z.W.; Hirunsit, P.; Yeo, B.S. On the Role of Sulfur for the Selective Electrochemical Reduction of CO2 to Formate on CuSx Catalysts. ACS Appl. Mater. Interfaces 2018, 10, 28572–28581. [Google Scholar] [CrossRef]
- Dou, T.; Qin, Y.; Zhang, F.; Lei, X. CuS Nanosheet Arrays for Electrochemical CO2 Reduction with Surface Reconstruction and the Effect on Selective Formation of Formate. ACS Appl. Energy Mater. 2021, 4, 4376–4384. [Google Scholar] [CrossRef]
- Chen, J.; Tu, Y.; Zou, Y.; Li, X.; Jiang, J. Morphology and composition-controllable synthesis of copper sulfide nanocrystals for electrochemical reduction of CO2 to HCOOH. Mater. Lett. 2021, 284, 128919. [Google Scholar] [CrossRef]
- Wang, J.; Tan, H.Y.; Zhu, Y.; Chu, H.; Chen, H.M. Linking the dynamic chemical state of catalysts with the product profile of electrocatalytic CO2 reduction. Angew. Chem. Int. Ed. 2021, 133, 17394–17407. [Google Scholar] [CrossRef]
- Chu, S.; Yan, X.; Choi, C.; Hong, S.; Robertson, A.W.; Masa, J.; Han, B.; Jung, Y.; Sun, Z. Stabilization of Cu+ by tuning a CuO–CeO2 interface for selective electrochemical CO2 reduction to ethylene. Green Chem. 2020, 22, 6540–6546. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, C.; Meng, S.; Lu, Z.; Zhao, R.; Wang, H.; Liu, Z.; Wang, J. Ag Single Atoms Anchored on CeO2 with Interfacial Oxygen Vacancies for Efficient CO2 Electroreduction. ACS Appl. Mater. Interfaces 2023, 15, 30262–30271. [Google Scholar] [CrossRef]
- Yang, X.; Du, C.; Zhu, Y.; Peng, H.; Liu, B.; Cao, Y.; Zhang, Y.; Ma, X.; Cao, C. Constructing defect-rich unconventional phase Cu7.2S4 nanotubes via microwave-induced selective etching for ultra-stable rechargeable magnesium batteries. Chem. Eng. J. 2022, 430, 133108. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, X.; Wang, J.; Lv, X.; Wu, H.B. CeO2-modified Cu electrode for efficient CO2 electroreduction to multi-carbon products. J. CO2 Util. 2021, 54, 101741. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y.; Gao, W.; Wang, Y.; Jiao, L.; Yuan, H.; Liu, S. Synthesis of novel CuS with hierarchical structures and its application in lithium-ion batteries. Powder Technol. 2011, 212, 64–68. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, J.; Shen, H.-J.; Zhang, L.; Yang, G.-X.; Zhou, X.-C.; Feng, J.-J.; Wang, A.-J. Reconstituting Cu0/Cu+ synergy with heterostructured CeO2 enabling energy-efficient bipolar hydrogen generation. Chem. Eng. J. 2023, 475, 146506. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Huang, Y.-T.; Yang, H.-Y. Crystallization mechanism and photocatalytic performance of vanadium-modified bismuth oxide through precipitation processes at room temperature. CrystEngComm 2016, 18, 6881–6888. [Google Scholar] [CrossRef]
- Zhuang, T.-T.; Liang, Z.-Q.; Seifitokaldani, A.; Li, Y.; De Luna, P.; Burdyny, T.; Che, F.; Meng, F.; Min, Y.; Quintero-Bermudez, R.; et al. Steering post-C-C coupling selectivity enables high efficiency electroreduction of carbon dioxide to multi-carbon alcohols. Nat. Catal. 2018, 1, 421–428. [Google Scholar] [CrossRef]
- Wu, Z.; Yu, J.; Wu, K.; Song, J.; Gao, H.; Shen, H.; Xia, X.; Lei, W.; Hao, Q. Ultrafine CuS anchored on nitrogen and sulfur Co-doped graphene for selective CO2 electroreduction to formate. Appl. Surf. Sci. 2022, 575, 151796. [Google Scholar] [CrossRef]
- Zhao, S.; Jiang, J.; Zhang, C.; Chen, F.; Song, Y.; Tang, Y. Construction of a novel double S-scheme heterojunction CeO2/g-C3N4/Bi2O4 for significantly boosted degradation of tetracycline: Insight into the dual charge transfer mode. Chem. Eng. J. 2023, 479, 147333. [Google Scholar] [CrossRef]
- Azenha, C.; Mateos-Pedrero, C.; Alvarez-Guerra, M.; Irabien, A.; Mendes, A. Binary copper-bismuth catalysts for the electrochemical reduction of CO2: Study on surface properties and catalytic activity. Chem. Eng. J. 2022, 445, 136575. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, X.-G.; Jiang, K.; Wu, D.-Y.; Cai, W.-B. Boosting Formate Production in Electrocatalytic CO2 Reduction over Wide Potential Window on Pd Surfaces. J. Am. Chem. Soc. 2018, 140, 2880–2889. [Google Scholar] [CrossRef]
- Li, S.; Sha, X.; Gao, X.; Peng, J. Al-Doped Octahedral Cu2O Nanocrystal for Electrocatalytic CO2 Reduction to Produce Ethylene. Int. J. Mol. Sci. 2023, 24, 12680. [Google Scholar] [CrossRef]
- Anson, F.C. Application of Potentiostatic Current Integration to the Study of the Adsorption of Cobalt(III)−(Ethylenedinitrilo(tetraacetate)) on Mercury Electrodes. Anal. Chem. 1964, 36, 932–934. [Google Scholar] [CrossRef]
- Yang, S.; Jiang, M.; Zhang, W.; Hu, Y.; Liang, J.; Wang, Y.; Tie, Z.; Jin, Z. In Situ Structure Refactoring of Bismuth Nanoflowers for Highly Selective Electrochemical Reduction of CO2 to Formate. Adv. Funct. Mater. 2023, 33, 2301984. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, J.; Yu, L.; Wang, H.; Huang, X. Thermal derived bismuth nanoparticles on nitrogen-doped carbon aerogel enable selective electrochemical production of formate from CO2. J. CO2 Util. 2022, 61, 102031. [Google Scholar] [CrossRef]
- He, C.; Chen, S.; Long, R.; Song, L.; Xiong, Y. Design of CuInS2 hollow nanostructures toward CO2 electroreduction. Sci. China Chem. 2020, 63, 1721–1726. [Google Scholar] [CrossRef]
- Zheng, X.; De Luna, P.; García de Arquer, F.P.; Zhang, B.; Becknell, N.; Ross, M.B.; Li, Y.; Banis, M.N.; Li, Y.; Liu, M.; et al. Sulfur-Modulated Tin Sites Enable Highly Selective Electrochemical Reduction of CO2 to Formate. Joule 2017, 1, 794–805. [Google Scholar] [CrossRef]
- Zhou, X.; Shan, J.; Chen, L.; Xia, B.Y.; Ling, T.; Duan, J.; Jiao, Y.; Zheng, Y.; Qiao, S.-Z. Stabilizing Cu2+ Ions by Solid Solutions to Promote CO2 Electroreduction to Methane. J. Am. Chem. Soc. 2022, 144, 2079–2084. [Google Scholar] [CrossRef]
- Ren, D.; Fong, J.; Yeo, B.S. The effects of currents and potentials on the selectivities of copper toward carbon dioxide electroreduction. Nat. Commun. 2018, 9, 925. [Google Scholar] [CrossRef]
- Wen, G.; Lee, D.U.; Ren, B.; Hassan, F.M.; Jiang, G.; Cano, Z.P.; Gostick, J.; Croiset, E.; Bai, Z.; Yang, L. Orbital interactions in Bi-Sn bimetallic electrocatalysts for highly selective electrochemical CO2 reduction toward formate production. Adv. Energy Mater. 2018, 8, 1802427. [Google Scholar] [CrossRef]
- Zheng, Y.; Vasileff, A.; Zhou, X.; Jiao, Y.; Jaroniec, M.; Qiao, S.-Z. Understanding the roadmap for electrochemical reduction of CO2 to multi-carbon oxygenates and hydrocarbons on copper-based catalysts. J. Am. Chem. Soc. 2019, 141, 7646–7659. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, Y.; Zou, Y.; Liu, W.; Luo, Y.; Liu, B.; Zhao, C. Hydrothermal Synthesis of CuS Catalysts for Electrochemical CO2 Reduction: Unraveling the Effect of the Sulfur Precursor. ACS Appl. Energy Mater. 2023, 6, 1340–1354. [Google Scholar] [CrossRef]
- Tan, S.M.; Sofer, Z.; Pumera, M. Sulfur poisoning of emergent and current electrocatalysts: Vulnerability of MoS2, and direct correlation to Pt hydrogen evolution reaction kinetics. Nanoscale 2015, 7, 8879–8883. [Google Scholar] [CrossRef]
- Huang, Y.; Deng, Y.; Handoko, A.D.; Goh, G.K.; Yeo, B.S. Rational design of sulfur-doped copper catalysts for the selective electroreduction of carbon dioxide to formate. ChemSusChem 2018, 11, 320–326. [Google Scholar] [CrossRef]
- Wen, Y.; Fang, N.; Liu, W.; Yang, T.; Xu, Y.; Huang, X. Cu7S4 nanosheets enriched with Cu–S bond for highly active and selective CO2 electroreduction to formate. J. Mater. Chem. A 2023, 11, 10823–10827. [Google Scholar] [CrossRef]
- Cao, S.; Xue, Y.; Chen, X.; Zhang, C.; Gao, Y.; Li, Y. Graphdiyne/copper sulfide heterostructure for active conversion of CO2 to formic acid. Mater. Chem. Front. 2023, 7, 2620–2627. [Google Scholar] [CrossRef]
- Lim, J.; Garcia-Esparza, A.T.; Lee, J.W.; Kang, G.; Shin, S.; Jeon, S.S.; Lee, H. Electrodeposited Sn–Cu@Sn dendrites for selective electrochemical CO2 reduction to formic acid. Nanoscale 2022, 14, 9297–9303. [Google Scholar] [CrossRef]
- Wu, D.; Wang, X.; Fu, X.-Z.; Luo, J.-L. Ultrasmall Bi nanoparticles confined in carbon nanosheets as highly active and durable catalysts for CO2 electroreduction. Appl. Catal. B Environ. 2021, 284, 119723. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, X.; Chu, Z.; Wang, Q.; Cao, Y.; Li, J.; Lei, W.; Cao, J.; Si, W. Construction of Co-decorated 3D nitrogen doped-carbon nanotube/Ti3C2Tx-MXene as efficient hydrogen evolution electrocatalyst. Int. J. Hydrogen Energy 2023, 48, 15053–15064. [Google Scholar] [CrossRef]
- Guo, D.; Li, X.; Jiao, Y.; Yan, H.; Wu, A.; Yang, G.; Wang, Y.; Tian, C.; Fu, H. A dual-active Co-CoO heterojunction coupled with Ti3C2-MXene for highly-performance overall water splitting. Nano Res. 2022, 15, 238–247. [Google Scholar] [CrossRef]
- Gao, S.; Lin, Y.; Jiao, X.; Sun, Y.; Luo, Q.; Zhang, W.; Li, D.; Yang, J.; Xie, Y. Partially oxidized atomic cobalt layers for carbon dioxide electroreduction to liquid fuel. Nature 2016, 529, 68–71. [Google Scholar] [CrossRef]
- Gong, Q.; Ding, P.; Xu, M.; Zhu, X.; Wang, M.; Deng, J.; Ma, Q.; Han, N.; Zhu, Y.; Lu, J.; et al. Structural defects on converted bismuth oxide nanotubes enable highly active electrocatalysis of carbon dioxide reduction. Nat. Commun. 2019, 10, 2807. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Bao, T.; Zhao, X.; Cao, Y.; Cao, J.; Li, Q.; Si, W. Bi/CeO2–Decorated CuS Electrocatalysts for CO2-to-Formate Conversion. Molecules 2024, 29, 2948. https://doi.org/10.3390/molecules29132948
Wang Q, Bao T, Zhao X, Cao Y, Cao J, Li Q, Si W. Bi/CeO2–Decorated CuS Electrocatalysts for CO2-to-Formate Conversion. Molecules. 2024; 29(13):2948. https://doi.org/10.3390/molecules29132948
Chicago/Turabian StyleWang, Qi, Tianshuang Bao, Xiangchuan Zhao, Yue Cao, Jun Cao, Qiaoling Li, and Weimeng Si. 2024. "Bi/CeO2–Decorated CuS Electrocatalysts for CO2-to-Formate Conversion" Molecules 29, no. 13: 2948. https://doi.org/10.3390/molecules29132948





