Fluorinated Fullerenes as Electrolyte Additives for High Ionic Conductivity Lithium-Ion Batteries
Abstract
:1. Introduction
2. Results and Discussion
3. Computational Details
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Li, M.; Fang, S.; Wang, Y.; He, H.; Wang, C.; Zhang, Z.; Yuan, B.; Jiang, L.; Baughman, R.H.; et al. Water-induced strong isotropic MXene-bridged graphene sheets for electrochemical energy storage. Science 2024, 383, 771–777. [Google Scholar] [CrossRef]
- Zhang, F.; Wei, M.; Viswanathan, V.V.; Swart, B.; Shao, Y.; Wu, G.; Zhou, C. 3D printing technologies for electrochemical energy storage. Nano Energy 2017, 40, 418–431. [Google Scholar] [CrossRef]
- Wan, H.; Wang, Z.; Zhang, W.; He, X.; Wang, C. Interface design for all-solid-state lithium batteries. Nature 2023, 623, 739–744. [Google Scholar] [CrossRef]
- Quilty, C.D.; Wu, D.; Li, W.; Bock, D.C.; Wang, L.; Housel, L.M.; Abraham, A.; Takeuchi, K.J.; Marschilok, A.C.; Takeuchi, E.S. Electron and ion transport in lithium and lithium-ion battery negative and positive composite electrodes. Chem. Rev. 2023, 123, 1327–1363. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Yu, W.; Xiao, R.; Huang, F.; Tian, H.; Wang, C.; Chen, X.; Shao, J. Scalable fabrication of turbostratic graphene with high density and high ion conductivity for compact capacitive energy storage. Matter 2023, 6, 4032–4049. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, B.; Liu, L.; Lang, J.; Qiu, J. A low-concentration and high ionic conductivity aqueous electrolyte toward ultralow-temperature zinc-ion hybrid capacitors. Small Struct. 2023, 4, 2200345. [Google Scholar] [CrossRef]
- Shi, P.; Ma, J.; Liu, M.; Guo, S.; Huang, Y.; Wang, S.; Zhang, L.; Chen, L.; Yang, K.; Liu, X.; et al. A dielectric electrolyte composite with high lithium-ion conductivity for high-voltage solid-state lithium metal batteries. Nat. Nanotechnol. 2023, 18, 602–610. [Google Scholar] [CrossRef]
- Wee, G.; Larsson, O.; Srinivasan, M.; Berggren, M.; Crispin, X.; Mhaisalkar, S. Effect of the ionic conductivity on the performance of polyelectrolyte-based supercapacitors. Adv. Funct. Mater. 2010, 20, 4344–4350. [Google Scholar] [CrossRef]
- Lu, D.-L.; Zhao, R.-R.; Wu, J.-L.; Ma, J.-M.; Huang, M.-L.; Yao, Y.-B.; Tao, T.; Liang, B.; Zhai, J.-W.; Lu, S.-G. Investigations on the properties of Li3xLa2/3−xTiO3 based all-solid-state supercapacitor: Relationships between the capacitance, ionic conductivity, and temperature. J. Eur. Ceram. Soc. 2020, 40, 2396–2403. [Google Scholar] [CrossRef]
- Hu, E.; Xu, K. An electrolyte additive allows stable high-voltage cycling of a nickel-rich layered cathode. Nat. Energy 2022, 7, 482–483. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Pollard, T.P.; Li, Q.; Tan, S.; Hou, S.; Wan, H.; Chen, F.; He, H.; Hu, E.; et al. Electrolyte design for Li-ion batteries under extreme operating conditions. Nature 2023, 614, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Tölle, P.; Köhler, C.; Marschall, R.; Sharifi, M.; Wark, M.; Frauenheim, T. Proton transport in functionalised additives for PEM fuel cells: Contributions from atomistic simulations. Chem. Soc. Rev. 2012, 41, 5143–5159. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xie, X.; Xing, Z.; Chen, X.; Fang, G.; Lu, B.; Zhou, J.; Liang, S.; Fan, H.J. Mechanistic insights of Mg2+-electrolyte additive for high-energy and long-life zinc-ion hybrid capacitors. Adv. Energy Mater. 2021, 11, 2101158. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Liu, A.; Zhang, Y.; Li, Z.; Chen, H.; Shi, Z. Effect of LiOH solution additives on ionic conductivity of Li6.25Al0.25La3Zr2O12 electrolytes prepared by cold sintering. J. Mater. Sci. Mater. Electron. 2022, 33, 19187–19194. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, Y.M.; Cho, K.Y.; Yoon, S. Metal iodides (LiI, MgI2, AlI3, TiI4, and SnI4) potentiality as electrolyte additives for Li−S batteries. Electrochim. Acta 2021, 391, 138927. [Google Scholar] [CrossRef]
- Goo, D.E.; Lee, G.R.; Hong, S.H.; Moon, H.C. Effect of novel ionic additives on the performance of lithium batteries. J. Ind. Eng. Chem. 2024, 132, 546–551. [Google Scholar] [CrossRef]
- Palluzzi, M.; Tsurumaki, A.; Mozhzhukhina, N.; Rizell, J.; Matic, A.; D’Angelo, P.; Navarra, M.A. Ionic liquids as cathode additives for high voltage lithium batteries. Batter. Supercaps 2024, e202400068. [Google Scholar] [CrossRef]
- Huang, Q.; Weng, J.; Ouyang, D.; Chen, M.; Wang, X.; Wang, J. Comparative studies on the combustion characteristics of electrolytes and carbonate mixed solvents with flame retardant additives under low pressures. Case Stud. Therm. Eng. 2023, 43, 102810. [Google Scholar] [CrossRef]
- Chen, M.; Mei, J.; Wang, S.; Chen, Q.; Zhao, L.; Kong, Q.; Wu, X. Comparative studies on the combustion characters of the lithium-ion battery electrolytes with composite flame-retardant additives. J. Energy Chem. 2022, 47, 103642. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, Z.; Fu, S.; Zhang, Y.; Wang, R.; Mu, H.; Lian, C.; Wang, W.; Wang, G. A multifunctional electrolyte additive for zinc-ion capacitors with low temperature resistant and long lifespan. J. Energy Chem. 2024, 94, 477–485. [Google Scholar] [CrossRef]
- Haregewoin, A.M.; Wotango, A.S.; Hwang, B.-J. Electrolyte additives for lithium ion battery electrodes: Progress and perspectives. Energy Environ. Sci. 2016, 9, 1955–1988. [Google Scholar] [CrossRef]
- Liu, L.; Meng, H.; Chai, Y.; Chen, X.; Xu, J.; Liu, X.; Liu, W.; Guldi, D.M.; Zhu, Y. Enhancing built-in electric fields for efficient photocatalytic hydrogen evolution by encapsulating C60 fullerene into zirconium-based metal-organic frameworks. Angew. Chem. Int. Ed. 2023, 62, e202217897. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, Y.; Li, Y.; Wang, Y.; Peng, B.; Davey, K.; Sun, L.; Li, G.; Zhang, S.; Guo, Z. C60 and derivatives boost electrocatalysis and photocatalysis: Electron buffers to heterojunctions. Adv. Energy Mater. 2023, 13, 2302438. [Google Scholar] [CrossRef]
- Shen, W.; Azmy, A.; Li, G.; Mishra, A.; Syrgiannis, Z.; Zheng, W.; Volonakis, G.; Kepenekian, M.; Even, J.; Wojtas, L.; et al. A crystalline 2D fullerene-based metal halide semiconductor for efficient and stable ideal-bandgap perovskite solar cells. Adv. Energy Mater. 2024, 14, 2400582. [Google Scholar] [CrossRef]
- Szala-Bilnik, J.; Costa Gomes, M.F.; Pádua, A.A.H. Solvation of C60 fullerene and C60F48 fluorinated fullerene in molecular and ionic liquids. J. Phys. Chem. C 2016, 120, 19396–19408. [Google Scholar] [CrossRef]
- Okino, F.; Yajima, S.; Suganuma, S.; Mitsumoto, R.; Seki, K.; Touhara, H. Fluorination of fullerene C60 and electrochemical properties of C60Fx. Synth. Met. 1995, 70, 1447–1448. [Google Scholar] [CrossRef]
- Oreshkin, A.I.; Muzychenko, D.A.; Oreshkin, S.I.; Panov, V.I.; Bakhtizin, R.Z.; Petukhov, M.N. Fluorinated fullerene molecule on Cu(001) surface as a controllable source of fluorine atoms. J. Phys. Chem. C 2018, 122, 24454–24458. [Google Scholar] [CrossRef]
- Kalika, E.B.; Katin, K.P.; Kochaev, A.I.; Kaya, S.; Elik, M.; Maslov, M.M. Fluorinated carbon and boron nitride fullerenes for drug delivery: Computational study of structure and adsorption. J. Mol. Liq. 2022, 353, 118773. [Google Scholar] [CrossRef]
- Li, G.; Duan, X.; Liu, X.; Zhan, R.; Wang, X.; Du, J.; Chen, Z.; Li, Y.; Cai, Z.; Shen, Y.; et al. Locking active Li metal through localized redistribution of fluoride enabling stable Li-metal batteries. Adv. Mater. 2023, 35, 2207310. [Google Scholar] [CrossRef]
- Li, L.F.; Lee, H.S.; Li, H.; Yang, X.Q.; Huang, X.J. A pentafluorophenylboron oxalate additive in non-aqueous electrolytes for lithium batteries. Electrochem. Commun. 2009, 11, 2296–2299. [Google Scholar] [CrossRef]
- Wang, R.; Parent, L.R.; Zhong, Y. Sulfur poisoning mechanism of LSCF cathode material in the presence of SO2: A computational and experimental study. J. Mater. Inf. 2023, 3, 3. [Google Scholar] [CrossRef]
- Ma, B.; Yu, F.; Zhou, P.; Wu, X.; Zhao, C.; Lin, C.; Gao, M.; Lin, T.; Sa, B. Machine learning accelerated discovery of high transmittance in (K0.5Na0.5)NbO3-based ceramics. J. Mater. Inf. 2023, 3, 13. [Google Scholar] [CrossRef]
- Bauer, B.; Bravyi, S.; Motta, M.; Chan, G.K.-L. Quantum algorithms for quantum chemistry and quantum materials science. Chem. Rev. 2020, 120, 12685–12717. [Google Scholar] [CrossRef]
- Goryunkov, A.A.; Kareev, I.E.; Ioffe, I.N.; Popov, A.A.; Kuvychko, I.V.; Markov, V.Y.; Goldt, I.V.; Pimenova, A.S.; Serov, M.G.; Avdoshenko, S.M.; et al. Reaction of C60 with KMnF4: Isolation and characterization of a new isomer of C60F8 and re-evaluation of the structures of C60F7(CF3) and the known isomer of C60F8. J. Fluor. Chem. 2006, 127, 1423–1435. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Z.; Si, Y.; Sa, B.; Li, H.; Yu, T.; Wen, C.; Wu, B. Structural, electronic, and nonlinear optical properties of C66H4 and C70Cl6 encapsulating Li and F atoms. ACS Omega 2021, 6, 16234–16240. [Google Scholar] [CrossRef]
- Raghavachari, K. Perspective on “Density functional thermochemistry. III. The role of exact exchange”. Theor. Chem. Acc. 2000, 103, 361–363. [Google Scholar] [CrossRef]
- Frisch, M.J.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; Li, X.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2011, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and testing of the opls all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Wang, C.L.W.; Liao, K.; Wang, Z.; Wang, Y.; Gong, K. AuToFF Program; Version 1.0; Hzwtech: Shanghai, China, 2023. [Google Scholar]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Lai, P.; Huang, B.; Deng, X.; Li, J.; Hua, H.; Zhang, P.; Zhao, J. A localized high concentration carboxylic ester-based electrolyte for high-voltage and low temperature lithium batteries. Chem. Eng. J. 2023, 461, 141904. [Google Scholar] [CrossRef]



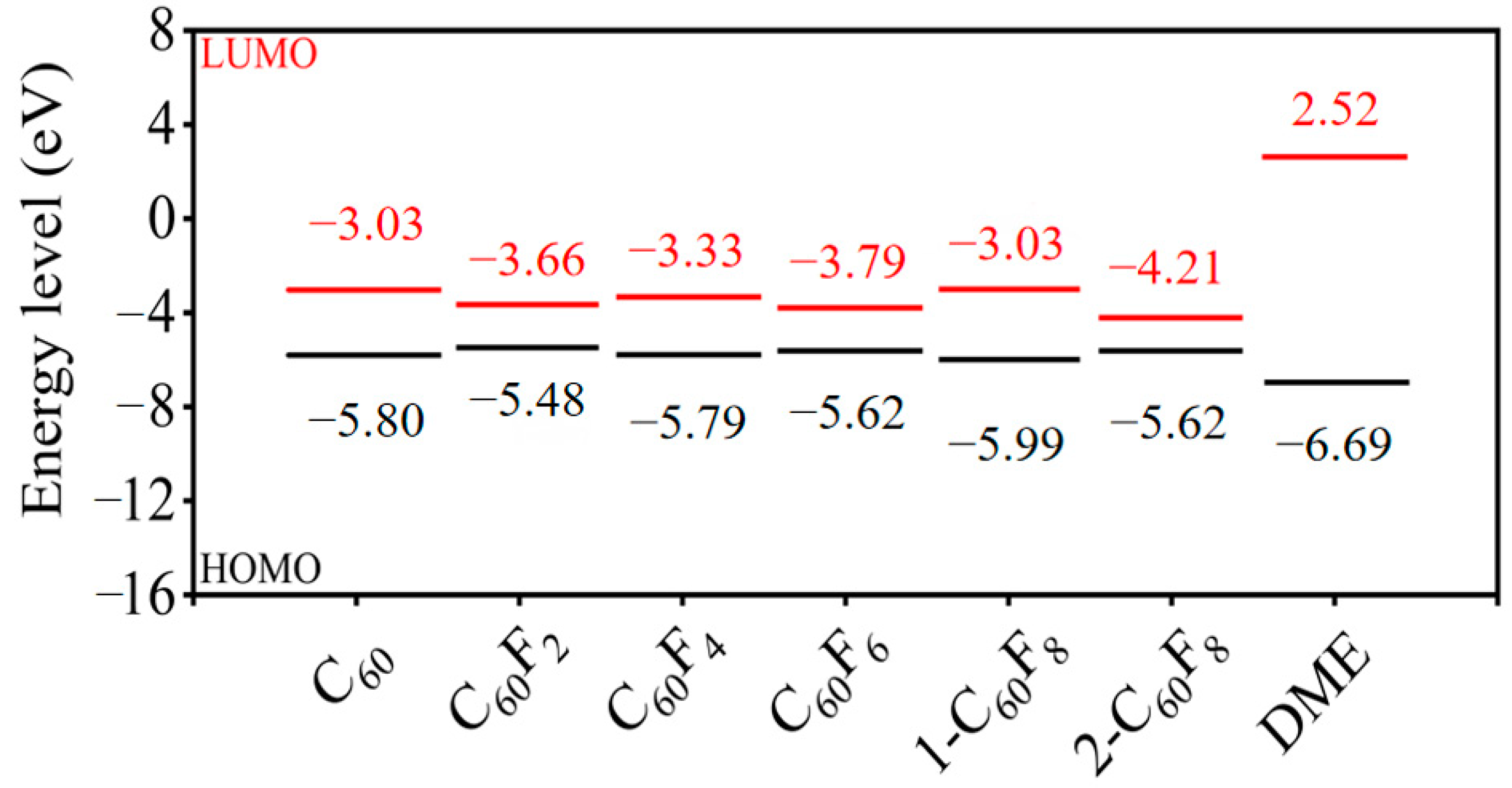
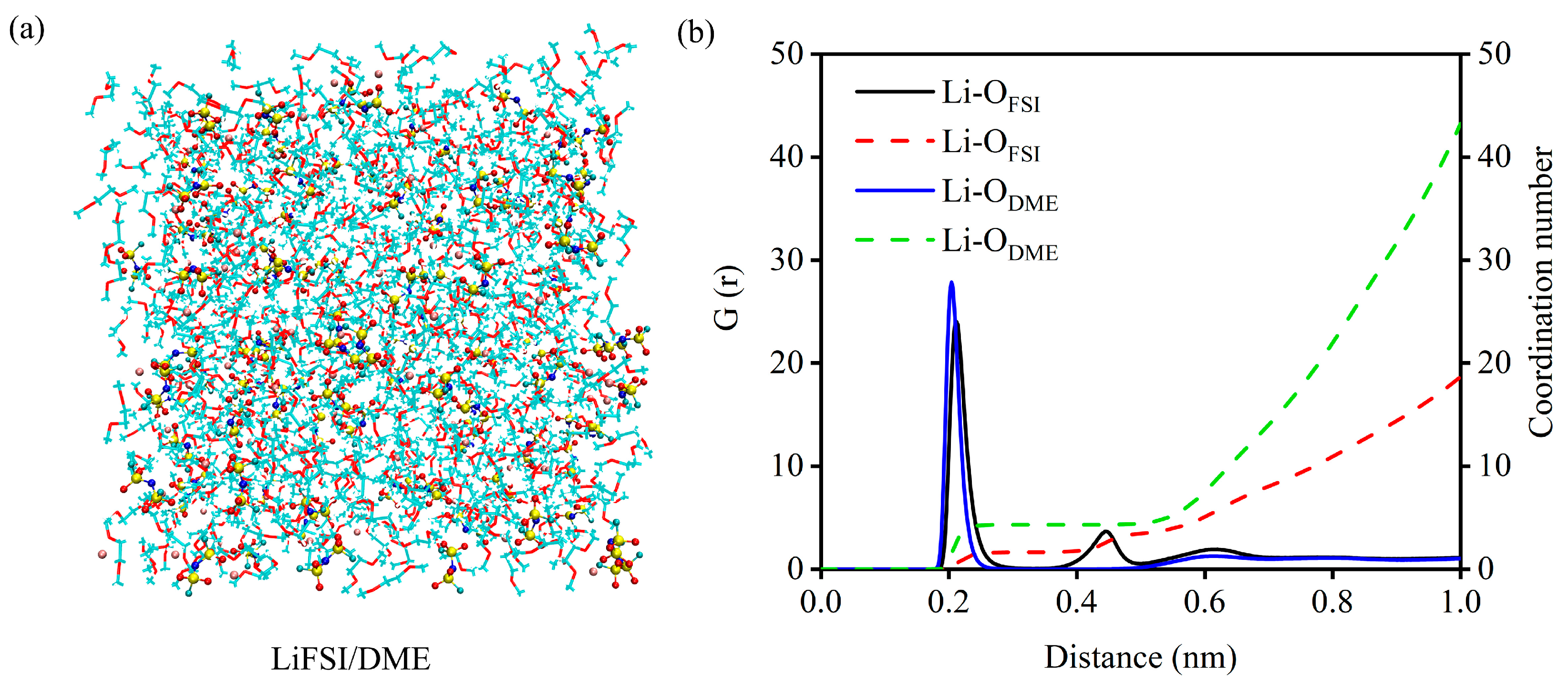

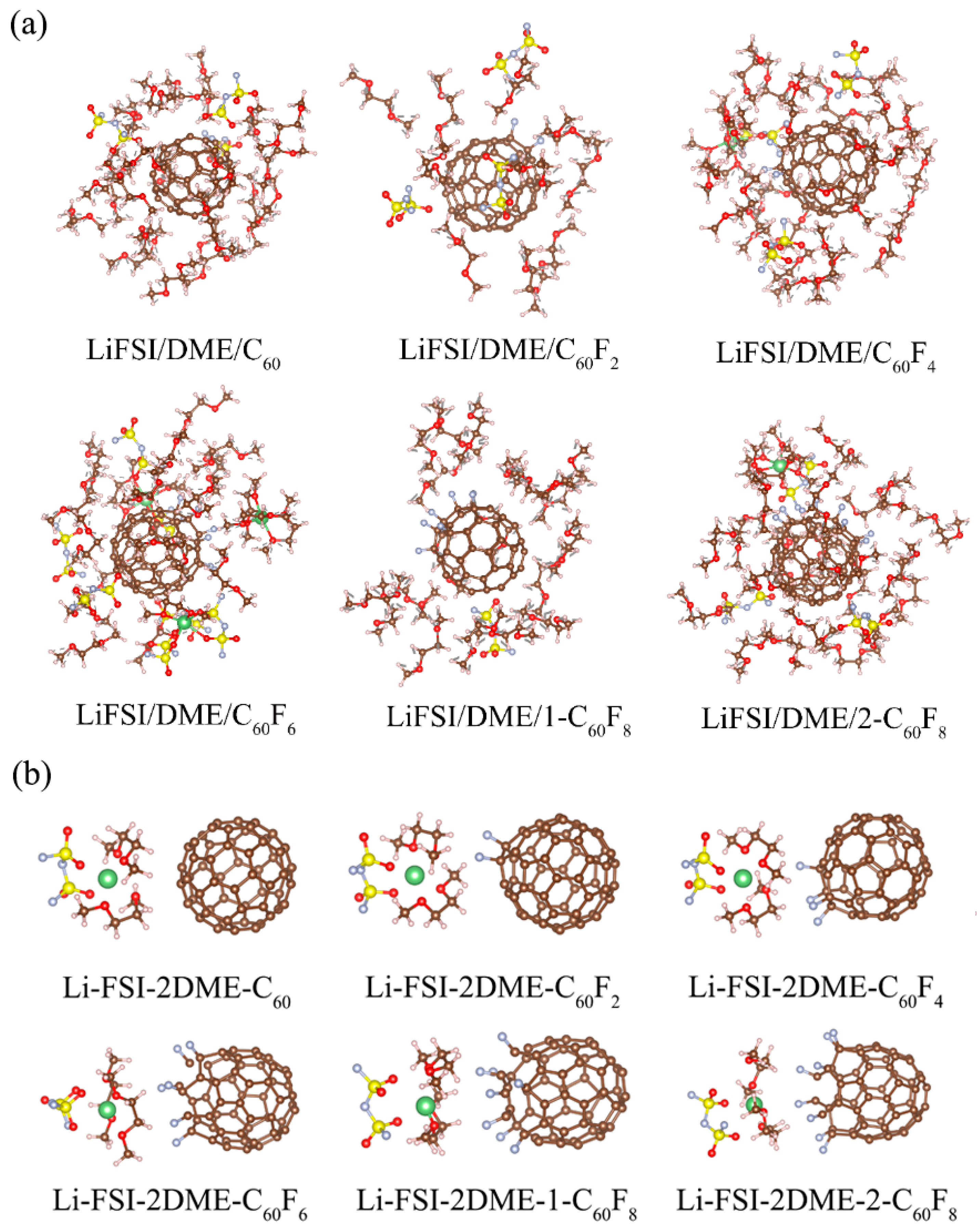

| Features | C60 | C60F2 | C60F4 | C60F6 | 1-C60F8 | 2-C60F8 | |
|---|---|---|---|---|---|---|---|
| C-C bonding | L (Å) | 1.45 | 1.62 | 1.60 | 1.62 | 1.58 | 1.58 |
| ρBCP | 0.28 | 0.22 | 0.26 | 0.22 | 0.23 | 0.21 | |
| HBCP | −0.25 | −0.16 | −0.21 | −0.16 | −0.18 | −0.15 | |
| |VBCP|/GBCP | 4.11 | 4.67 | 4.60 | 4.67 | 4.87 | 4.71 | |
| MBO | 1.16 | 0.87 | 0.89 | 0.89 | 0.89 | 0.88 | |
| C-F bonding | L (Å) | / | 1.39 | 1.38 | 1.39 | 1.36 | 1.38 |
| ρBCP | / | 0.24 | 0.24 | 0.24 | 0.25 | 0.24 | |
| HBCP | / | −0.32 | −0.34 | −0.33 | −0.35 | −0.33 | |
| |VBCP|/GBCP | / | 2.12 | 2.05 | 2.07 | 2.04 | 2.07 | |
| MBO | / | 0.83 | 0.81 | 0.82 | 0.84 | 0.83 | |
| F atom | NPA charge | / | −0.37 | −0.36 | −0.35 | −0.35 | −0.35 |
| Electrolytes | CN-Li+-OFSI− | CN-Li+-ODME |
|---|---|---|
| LiFSI/DME | 1.90 | 4.07 |
| LiFSI/DME/C60 | 1.74 | 4.25 |
| LiFSI/DME/C60F2 | 1.73 | 4.25 |
| LiFSI/DME/C60F4 | 1.68 | 4.29 |
| LiFSI/DME/C60F6 | 1.61 | 4.37 |
| LiFSI/DME/1-C60F8 | 1.33 | 4.65 |
| LiFSI/DME/2-C60F8 | 1.73 | 4.24 |
| Electrolytes | Box Size (nm) | Molar Ratio | Viscosity (103 μPa·s) | Diffusion Coefficient (1 × 107 cm2/s) |
|---|---|---|---|---|
| LiFSI/DME | 4.81 × 4.81 × 4.81 | 100:602 | 3.64 | 5.30 |
| LiFSI/DME/C60 | 4.82 × 4.82 × 4.82 | 100:602:1 | 3.59 | 5.16 |
| LiFSI/DME/C60F2 | 4.81 × 4.81 × 4.81 | 100:602:1 | 4.46 | 4.78 |
| LiFSI/DME/C60F4 | 4.81 × 4.81 × 4.81 | 100:602:1 | 4.41 | 6.43 |
| LiFSI/DME/C60F6 | 4.81 × 4.81 × 4.81 | 100:602:1 | 3.57 | 6.79 |
| LiFSI/DME/1-C60F8 | 4.81 × 4.81 × 4.81 | 100:602:1 | 4.92 | 5.26 |
| LiFSI/DME/2-C60F8 | 4.82 × 4.82 × 4.82 | 100:602:1 | 3.99 | 8.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, H.; Yang, Z.; Chen, J.; Li, H.; Wen, C.; Sa, B. Fluorinated Fullerenes as Electrolyte Additives for High Ionic Conductivity Lithium-Ion Batteries. Molecules 2024, 29, 2955. https://doi.org/10.3390/molecules29132955
Pan H, Yang Z, Chen J, Li H, Wen C, Sa B. Fluorinated Fullerenes as Electrolyte Additives for High Ionic Conductivity Lithium-Ion Batteries. Molecules. 2024; 29(13):2955. https://doi.org/10.3390/molecules29132955
Chicago/Turabian StylePan, Haoyu, Zhanlin Yang, Jianhui Chen, Hengyi Li, Cuilian Wen, and Baisheng Sa. 2024. "Fluorinated Fullerenes as Electrolyte Additives for High Ionic Conductivity Lithium-Ion Batteries" Molecules 29, no. 13: 2955. https://doi.org/10.3390/molecules29132955
APA StylePan, H., Yang, Z., Chen, J., Li, H., Wen, C., & Sa, B. (2024). Fluorinated Fullerenes as Electrolyte Additives for High Ionic Conductivity Lithium-Ion Batteries. Molecules, 29(13), 2955. https://doi.org/10.3390/molecules29132955







