The Acid Roles of PtSn@Al2O3 in the Synthesis and Performance of Propane Dehydrogenation
Abstract
:1. Introduction
2. Results
2.1. Dispersed PtSn Catalyst
2.2. Citric Acid Regulates Immersion Depth
2.3. Influence of Acid Impregnation on Catalyst Performance
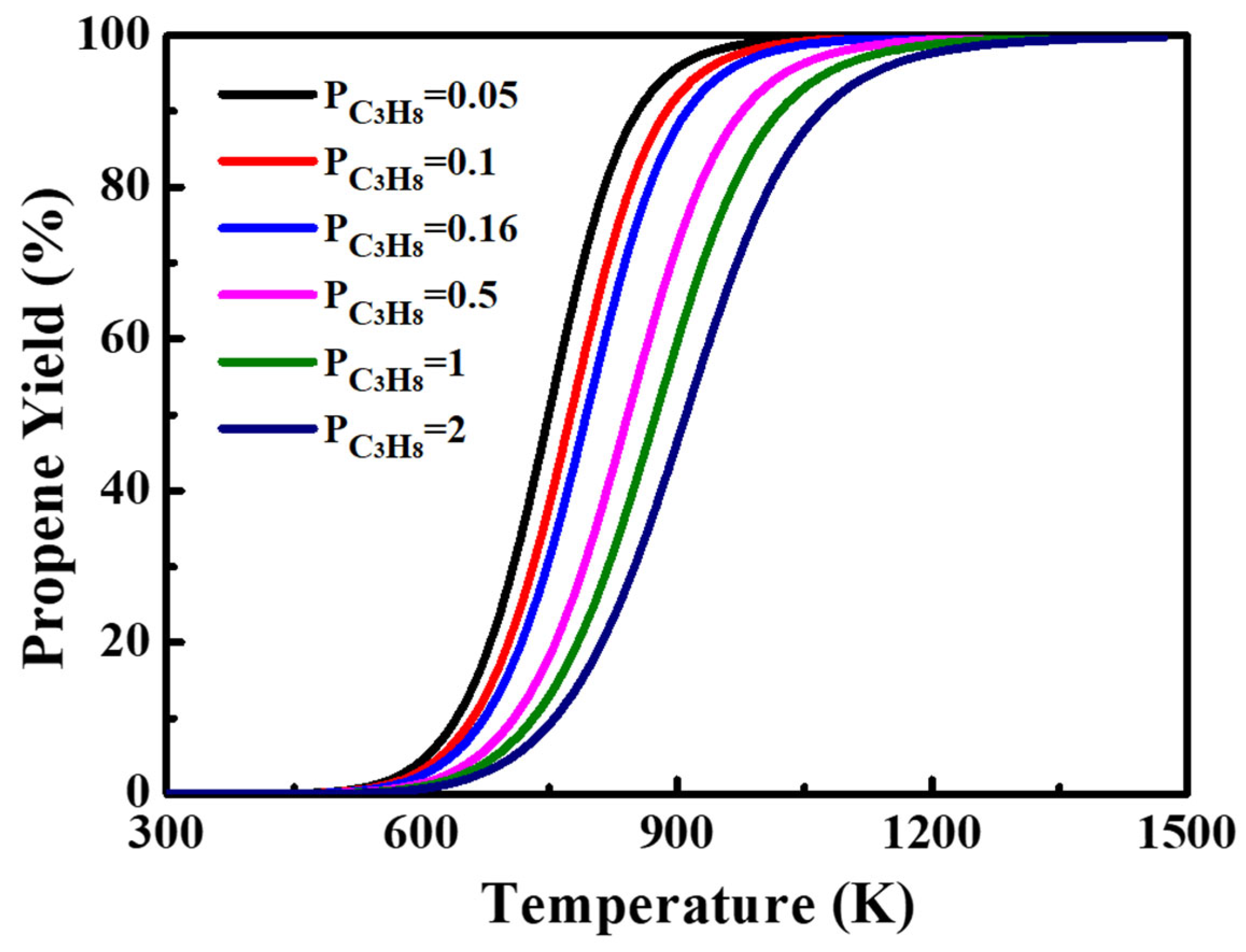
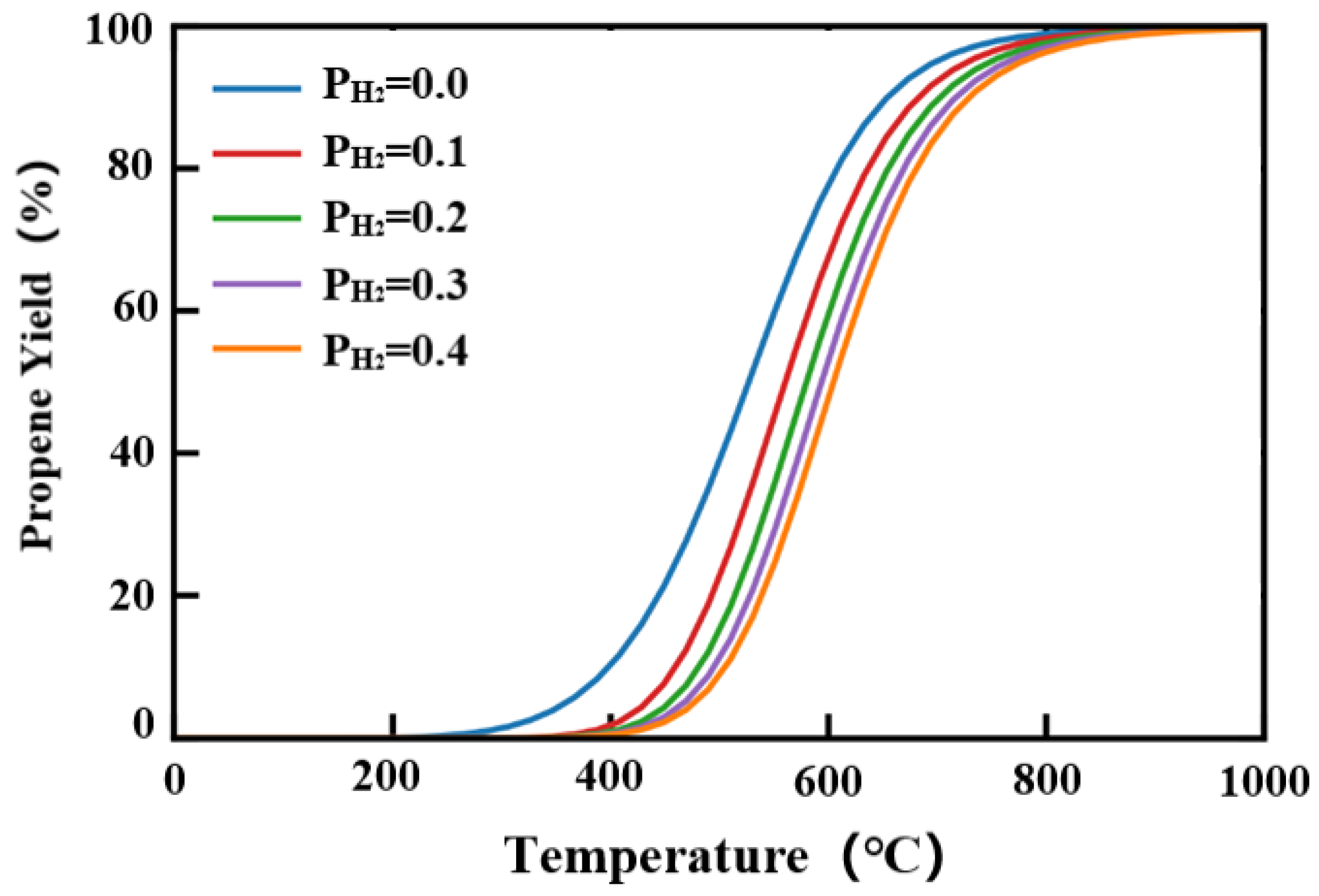
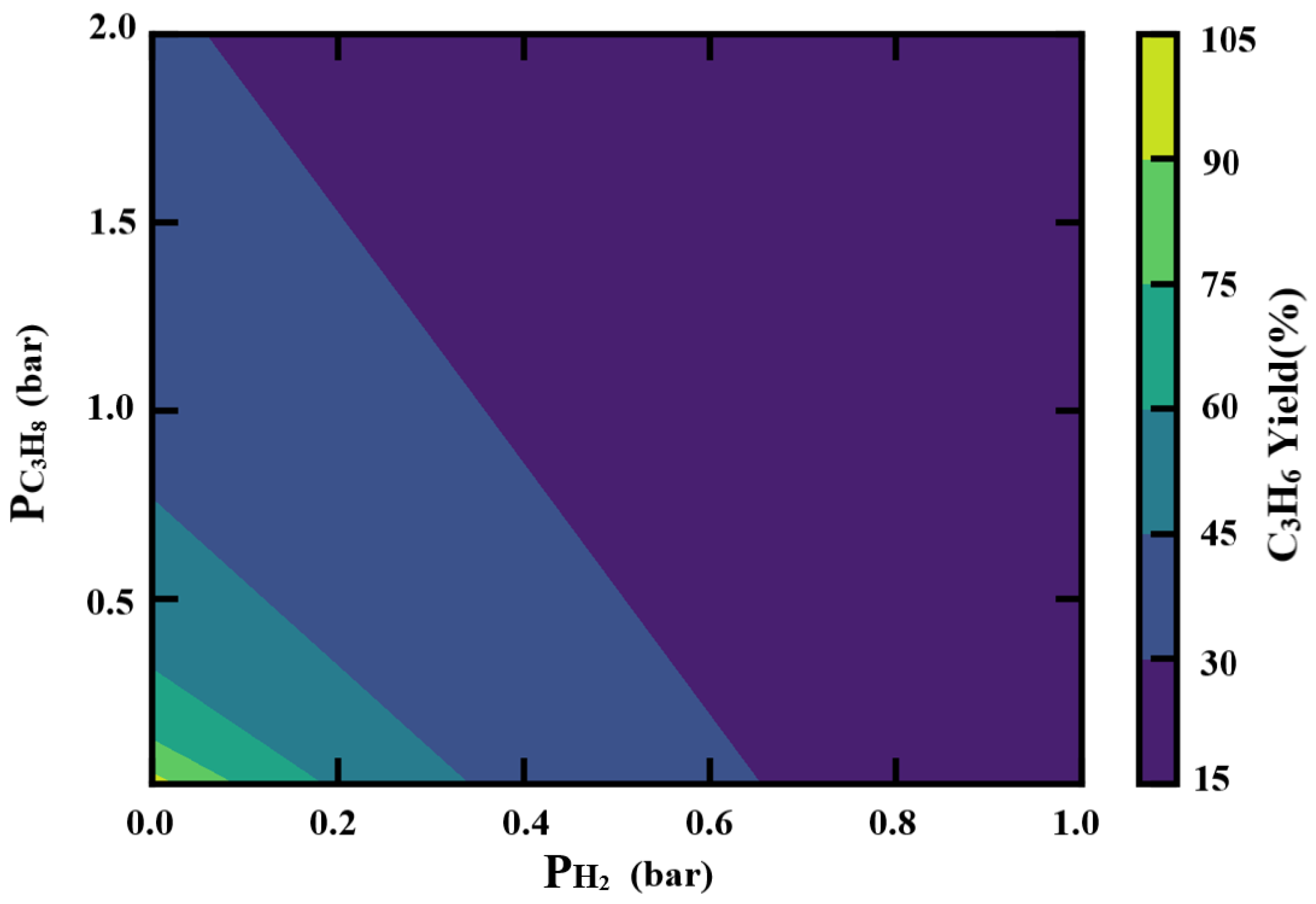
2.4. Reaction Thermodynamic Analysis of Catalyst
3. Discussion
4. Materials and Methods
4.1. Synthesis of Catalysts
4.2. Characterization of Catalysts
4.3. Catalytic Performance Test
4.4. Reaction Equilibrium Calculation
5. Conclusions
- (1)
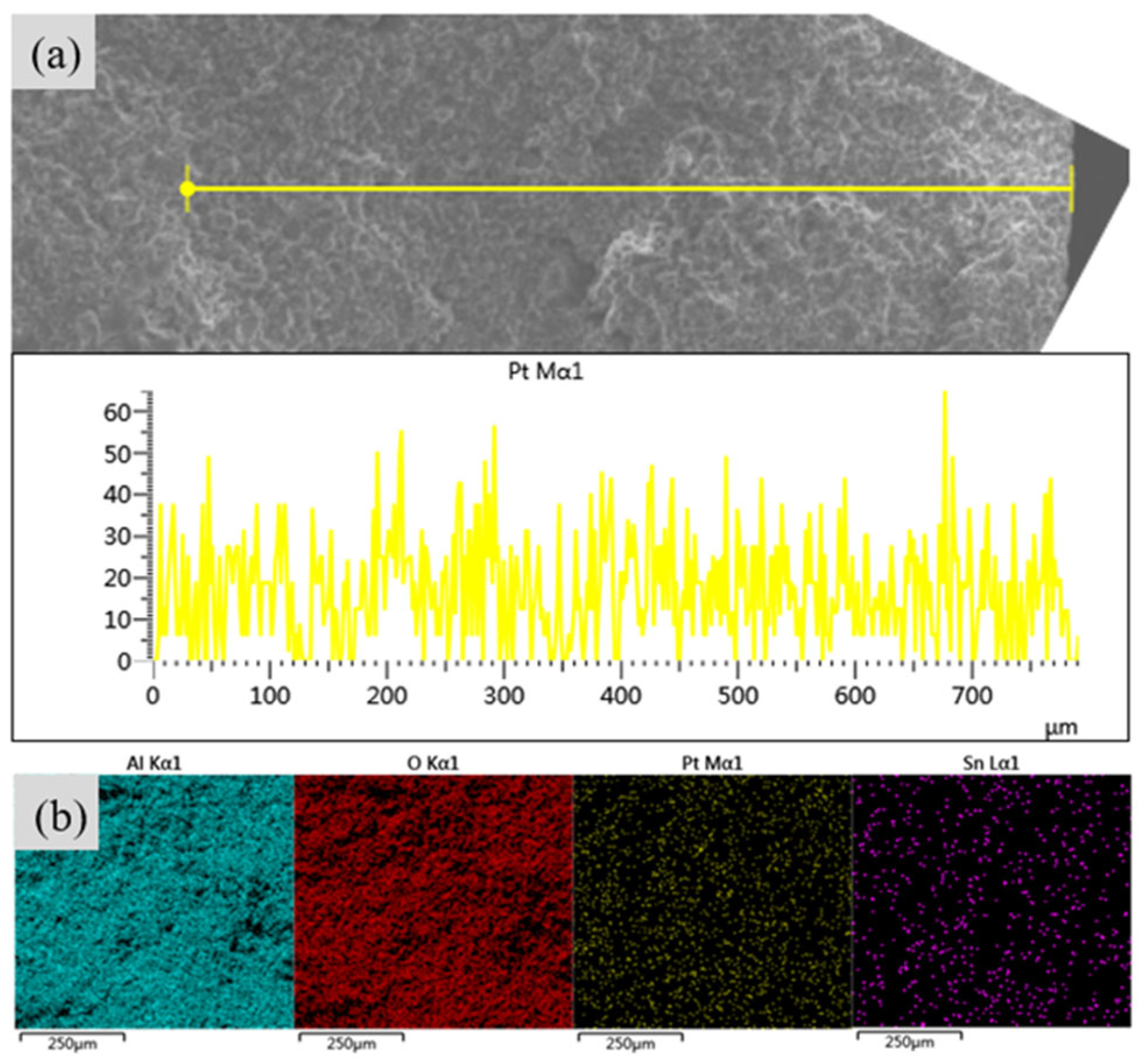
- Through characterization analyses, such as EDS, XRD, and N2 adsorption–desorption, Pt and Sn active metals are well dispersed. SEM scanning showed that the addition of an appropriate amount of citric acid increased the apparent concentration and immersion depth of the active metal Pt. Citric acid, as a competitive adsorbent, increased the number of Pt1 sites during the impregnation process.
- (2)
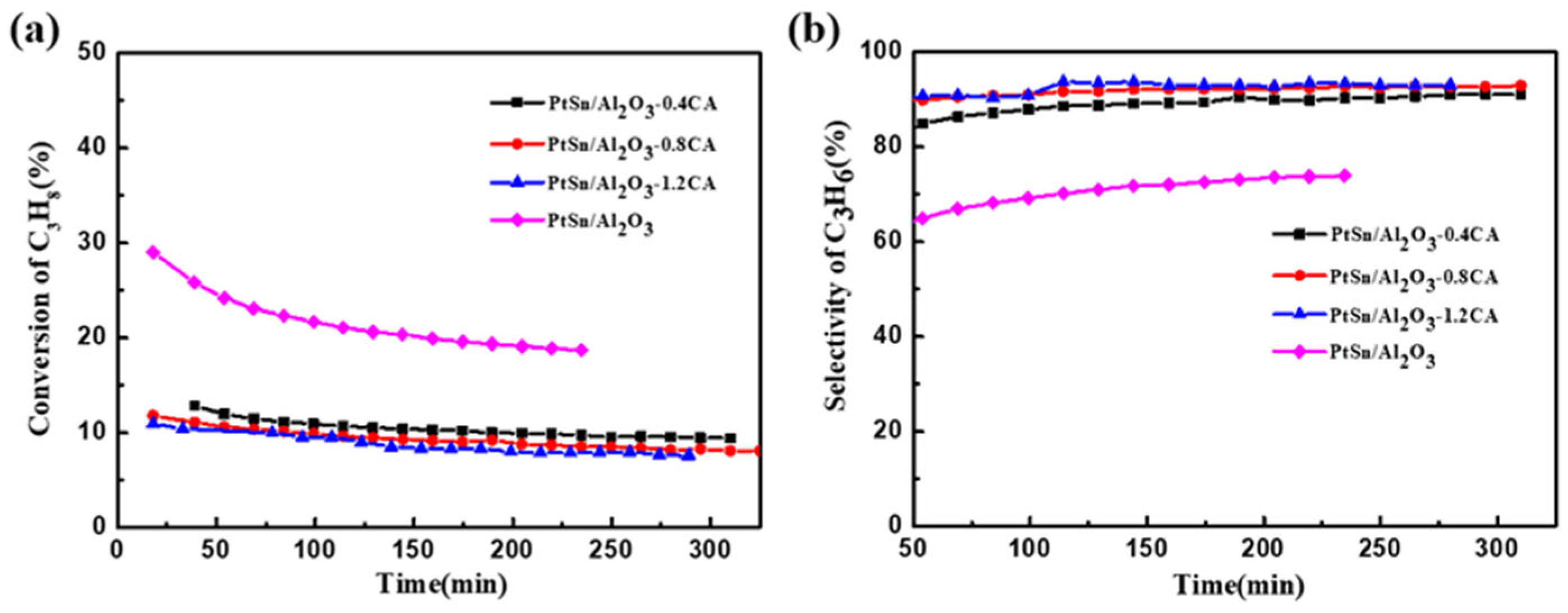
- With the effects of acid sites, compared to a fresh PtSn/Al2O3 catalyst, the addition of citric acid led to a slight decrease in PDH conversion. However, the selectivity of the catalyst increased significantly from 74% to 93%. After neutralization with alkali and washing treatment, the selectivity of the catalyst further improved to 96%.
- (3)
- The fresh PtSn/Al2O3 catalyst possesses the strongest acid sites. During the PDH reaction, the strong acid sites promote the cleavage of C–C bonds, leading to the generation of more by-products, such as methane and ethylene. This, in turn, reduces the selectivity of the catalyst.
- (4)
- From the PDH reaction thermodynamic analysis of catalyst, a relatively sub-atmospheric pressure environment with a lower propane pressure could be the reasonable choice. When recovering reaction gases, hydrogen should be removed as much as possible to ensure higher propane utilization efficiency.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, Z.-P.; Yang, D.; Wang, Z.; Yuan, Z.-Y. State-of-the-art catalysts for direct dehydrogenation of propane to propylene. Chin. J. Catal. 2019, 40, 1233–1254. [Google Scholar] [CrossRef]
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic Dehydrogenation of Light Alkanes on Metals and Metal Oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, D.; Miracca, I. Dehydrogenation of paraffins: Synergies between catalyst design and reactor engineering. Catal. Today 2006, 111, 133–139. [Google Scholar] [CrossRef]
- Atanga, M.A.; Rezaei, F.; Jawad, A.; Fitch, M.; Rownaghi, A.A. Oxidative dehydrogenation of propane to propylene with carbon dioxide. Appl. Catal. B Environ. 2018, 220, 429–445. [Google Scholar] [CrossRef]
- Cavani, F.; Ballarini, N.; Cericola, A. Oxidative dehydrogenation of ethane and propane: How far from commercial implementation? Catal. Today 2007, 127, 113–131. [Google Scholar] [CrossRef]
- Yarulina, I.; De Wispelaere, K.; Bailleul, S.; Goetze, J.; Radersma, M.; Abou-Hamad, E.; Vollmer, I.; Goesten, M.; Mezari, B.; Hensen, E.J.M.; et al. Structure–performance descriptors and the role of Lewis acidity in the methanol-to-propylene process. Nat. Chem. 2018, 10, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Torres Galvis, H.M.; de Jong, K.P. Catalysts for Production of Lower Olefins from Synthesis Gas: A Review. ACS Catal. 2013, 3, 2130–2149. [Google Scholar] [CrossRef]
- Motagamwala, A.H.; Almallahi, R.; Wortman, J.; Igenegbai, V.O.; Linic, S. Stable and selective catalysts for propane dehydrogenation operating at thermodynamic limit. Science 2021, 373, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhou, Y.; Zhang, Y.; Zhou, S.; Zhang, Z.; Kong, J.; Guo, M. Synthesis of magnesium-modified mesoporous Al2O3 with enhanced catalytic performance for propane dehydrogenation. J. Mater. Sci. 2014, 49, 5772–5781. [Google Scholar] [CrossRef]
- Chen, M.; Xu, J.; Su, F.-Z.; Liu, Y.-M.; Cao, Y.; He, H.-Y.; Fan, K.-N. Dehydrogenation of propane over spinel-type gallia–alumina solid solution catalysts. J. Catal. 2008, 256, 293–300. [Google Scholar] [CrossRef]
- Hu, B.; Bean Getsoian, A.; Schweitzer, N.M.; Das, U.; Kim, H.; Niklas, J.; Poluektov, O.; Curtiss, L.A.; Stair, P.C.; Miller, J.T.; et al. Selective propane dehydrogenation with single-site CoII on SiO2 by a non-redox mechanism. J. Catal. 2015, 322, 24–37. [Google Scholar] [CrossRef]
- Schweitzer, N.M.; Hu, B.; Das, U.; Kim, H.; Greeley, J.; Curtiss, L.A.; Stair, P.C.; Miller, J.T.; Hock, A.S. Propylene Hydrogenation and Propane Dehydrogenation by a Single-Site Zn2+ on Silica Catalyst. ACS Catal. 2014, 4, 1091–1098. [Google Scholar] [CrossRef]
- Ponomaryov, A.B.; Smirnov, A.V.; Pisarenko, E.V.; Shostakovsky, M.V. Enhanced Pt dispersion and catalytic properties of NaCl-promoted Pt/MFI zeolite catalysts for propane dehydrogenation. Microporous Mesoporous Mater. 2022, 339, 112010. [Google Scholar] [CrossRef]
- Jang, E.J.; Lee, J.; Jeong, H.Y.; Kwak, J.H. Controlling the acid-base properties of alumina for stable PtSn-based propane dehydrogenation catalysts. Appl. Catal. A Gen. 2019, 572, 1–8. [Google Scholar] [CrossRef]
- Kley, I.; Traa, Y. Influence of acid sites on the propene selectivity during propane dehydrogenation on zeolite Pt/Zn,Na-MCM-22. Microporous Mesoporous Mater. 2012, 164, 145–147. [Google Scholar] [CrossRef]
- Choi, S.-W.; Kim, W.-G.; So, J.-S.; Moore, J.S.; Liu, Y.; Dixit, R.S.; Pendergast, J.G.; Sievers, C.; Sholl, D.S.; Nair, S.; et al. Propane dehydrogenation catalyzed by gallosilicate MFI zeolites with perturbed acidity. J. Catal. 2017, 345, 113–123. [Google Scholar] [CrossRef]
- Ji, Z.; Miao, D.; Gao, L.; Pan, X.; Bao, X. Effect of pH on the catalytic performance of PtSn/B-ZrO2 in propane dehydrogenation. Chin. J. Catal. 2020, 41, 719–729. [Google Scholar] [CrossRef]
- Jiang, F.; Zeng, L.; Li, S.; Liu, G.; Wang, S.; Gong, J. Propane Dehydrogenation over Pt/TiO2–Al2O3 Catalysts. ACS Catal. 2015, 5, 438–447. [Google Scholar] [CrossRef]
- Ardiles, D.R.; Miguel, S.; Castro, A.; Scelza, O. Pt-Re catalysts: Study of the impregnation step. Appl. Catal. 1986, 24, 12. [Google Scholar] [CrossRef]
- Prakash, N.; Lee, M.-H.; Yoon, S.; Jung, K.-D. Role of acid solvent to prepare highly active PtSn/θ-Al2O3 catalysts in dehydrogenation of propane to propylene. Catal. Today 2017, 293, 33–41. [Google Scholar] [CrossRef]
- Kim, C.H.; Lee, M.-W.; Jang, J.S.; Lee, S.H.; Lee, K.-Y. Enhanced activity of a WOx-incorporated Pt/Al2O3 catalyst for the dehydrogenation of homocyclic LOHCs: Effects of impregnation sequence on Pt–WOx interactions. Fuel 2022, 313, 122654. [Google Scholar] [CrossRef]
- Araújo JC, S.; Oton, L.F.; Oliveira, A.C.; Lang, R.; Otubo, L.; Bueno JM, C. On the role of size controlled Pt particles in nanostructured Pt-containing Al2O3 catalysts for partial oxidation of methane. Int. J. Hydrog. Energy 2019, 44, 27329–27342. [Google Scholar] [CrossRef]
- Kianpoor, Z.; Falamaki, C.; Parvizi, M.R. Exceptional catalytic performance of Au–Pt/γ-Al2O3 in naphtha reforming at very low Au dosing levels. React. Kinet. Mech. Catal. 2019, 128, 427–441. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Huber, G.W.; Sánchez-Castillo, M.A.; Dumesic, J.A.; Rodríguez-Reinoso, F.; Sepúlveda-Escribano, A. Effect of Sn addition to Pt/CeO2–Al2O3 and Pt/Al2O3 catalysts: An XPS, 119Sn Mössbauer and microcalorimetry study. J. Catal. 2006, 241, 378–388. [Google Scholar] [CrossRef]
- Xu, Z.; Yue, Y.; Bao, X.; Xie, Z.; Zhu, H. Propane Dehydrogenation over Pt Clusters Localized at the Sn Single-Site in Zeolite Framework. ACS Catal. 2020, 10, 818–828. [Google Scholar] [CrossRef]
- Srisakwattana, T.; Suriye, K.; Praserthdam, P.; Panpranot, J. Preparation of aluminum magnesium oxide by different methods for use as PtSn catalyst supports in propane dehydrogenation. Catal. Today 2020, 358, 90–99. [Google Scholar] [CrossRef]
- Gao, X.; Xu, W.; Li, X.; Cen, J.; Xu, Y.; Lin, L.; Yao, S. Non-oxidative dehydrogenation of propane to propene over Pt-Sn/Al2O3 catalysts: Identification of the nature of active site. Chem. Eng. J. 2022, 443, 136393. [Google Scholar] [CrossRef]
- Sun, G.; Zhao, Z.-J.; Mu, R.; Zha, S.; Li, L.; Chen, S.; Zang, K.; Luo, J.; Li, Z.; Purdy, S.C.; et al. Breaking the scaling relationship via thermally stable Pt/Cu single atom alloys for catalytic dehydrogenation. Nat. Commun. 2018, 9, 4454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, H.; Jiang, J.; Sui, Z.; Zhu, Y.; Chen, D.; Zhou, X. Size Dependence of Pt Catalysts for Propane Dehydrogenation: From Atomically Dispersed to Nanoparticles. ACS Catal. 2020, 10, 12932–12942. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Asakura, H.; Zhang, B.; Zhang, J.; Zhou, M.; Han, Y.; Tanaka, T.; Wang, A.; Zhang, T.; et al. Thermally stable single atom Pt/m-Al2O3 for selective hydrogenation and CO oxidation. Nat. Commun. 2017, 8, 16100. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Gulec, A.; Johnson, A.M.; Schweitzer, N.M.; Stucky, G.D.; Marks, L.D.; Stair, P.C. Identification of active sites in CO oxidation and water-gas shift over supported Pt catalysts. Science 2015, 350, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Hadjiivanov, K.I.; Vayssilov, G.N. Characterization of oxide surfaces and zeolites by carbon monoxide as an IR probe molecule. Adv. Catal. 2002, 47, 307–511. [Google Scholar]
- Nakaya, Y.; Hirayama, J.; Yamazoe, S.; Shimizu, K.-I.; Furukawa, S. Single-atom Pt in intermetallics as an ultrastable and selective catalyst for propane dehydrogenation. Nat. Commun. 2020, 11, 2838. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zong, X.; Patra, A.; Caratzoulas, S.; Vlachos, D.G. Propane Dehydrogenation on PtxSny (x, y ≤ 4) Clusters on Al2O3(110). ACS Catal. 2023, 13, 2802–2812. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Shi, J. Effect of zinc addition on catalytic properties of PtSnK/γ-Al2O3 catalyst for isobutane dehydrogenation. Fuel Process. Technol. 2012, 96, 220–227. [Google Scholar] [CrossRef]
- Rodrigues, V.D.O.; Eon, J.-G.; Faro, A.C., Jr. Correlations between Dispersion, Acidity, Reducibility, and Propane Aromatization Activity of Gallium Species Supported on HZSM5 Zeolites. J. Phys. Chem. C 2010, 114, 4557–4567. [Google Scholar] [CrossRef]
- Kim, W.-G.; So, J.; Choi, S.-W.; Liu, Y.; Dixit, R.S.; Sievers, C.; Sholl, D.S.; Nair, S.; Jones, C.W. Hierarchical Ga-MFI Catalysts for Propane Dehydrogenation. Chem. Mater. 2017, 29, 7213–7222. [Google Scholar] [CrossRef]
- Zhang, M.; Zou, S.; Mo, S.; Zhong, J.; Chen, D.; Ren, Q.; Fu, M.; Chen, P.; Ye, D. Enhancement of catalytic toluene combustion over Pt–Co3O4 catalyst through in-situ metal-organic template conversion. Chemosphere 2021, 262, 127738. [Google Scholar] [CrossRef] [PubMed]

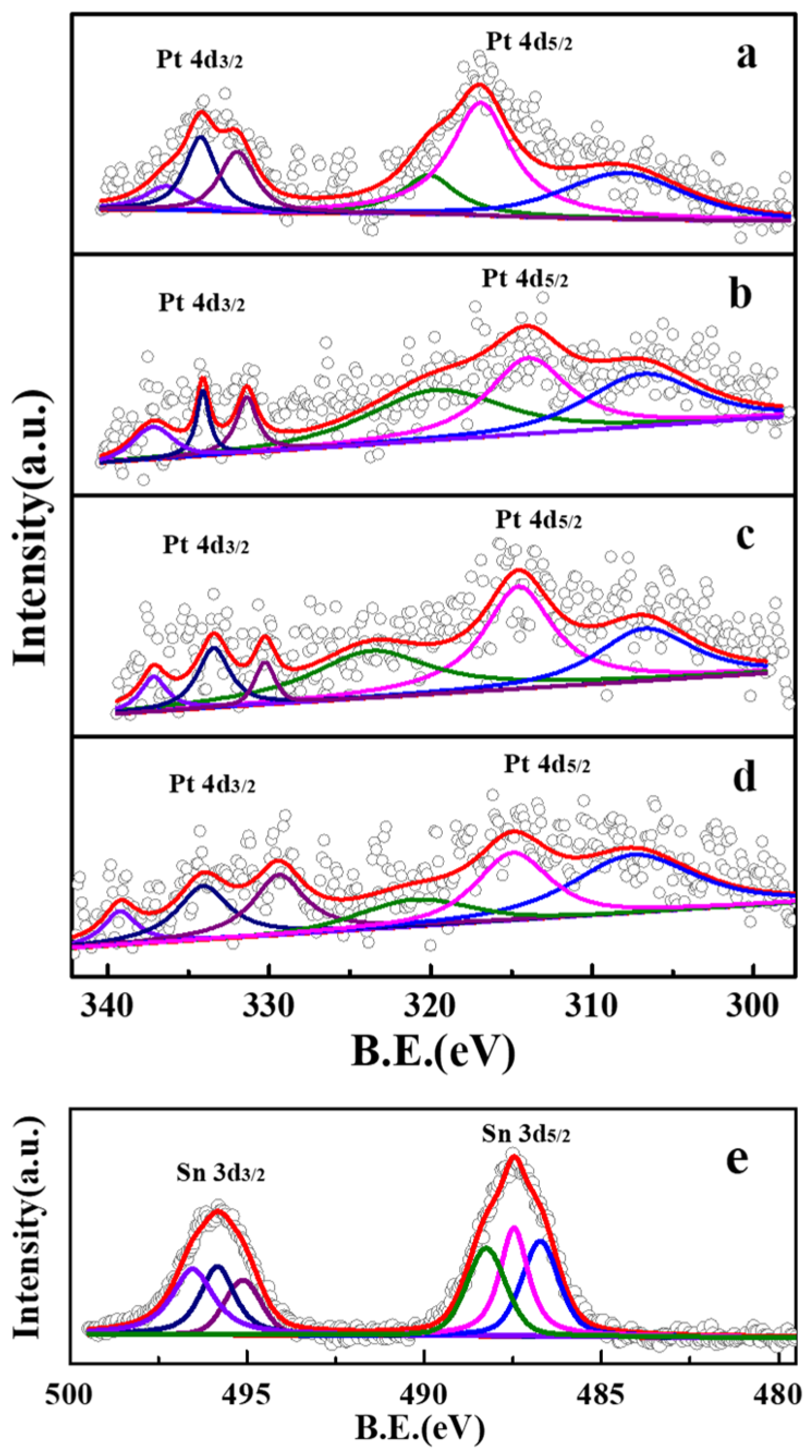
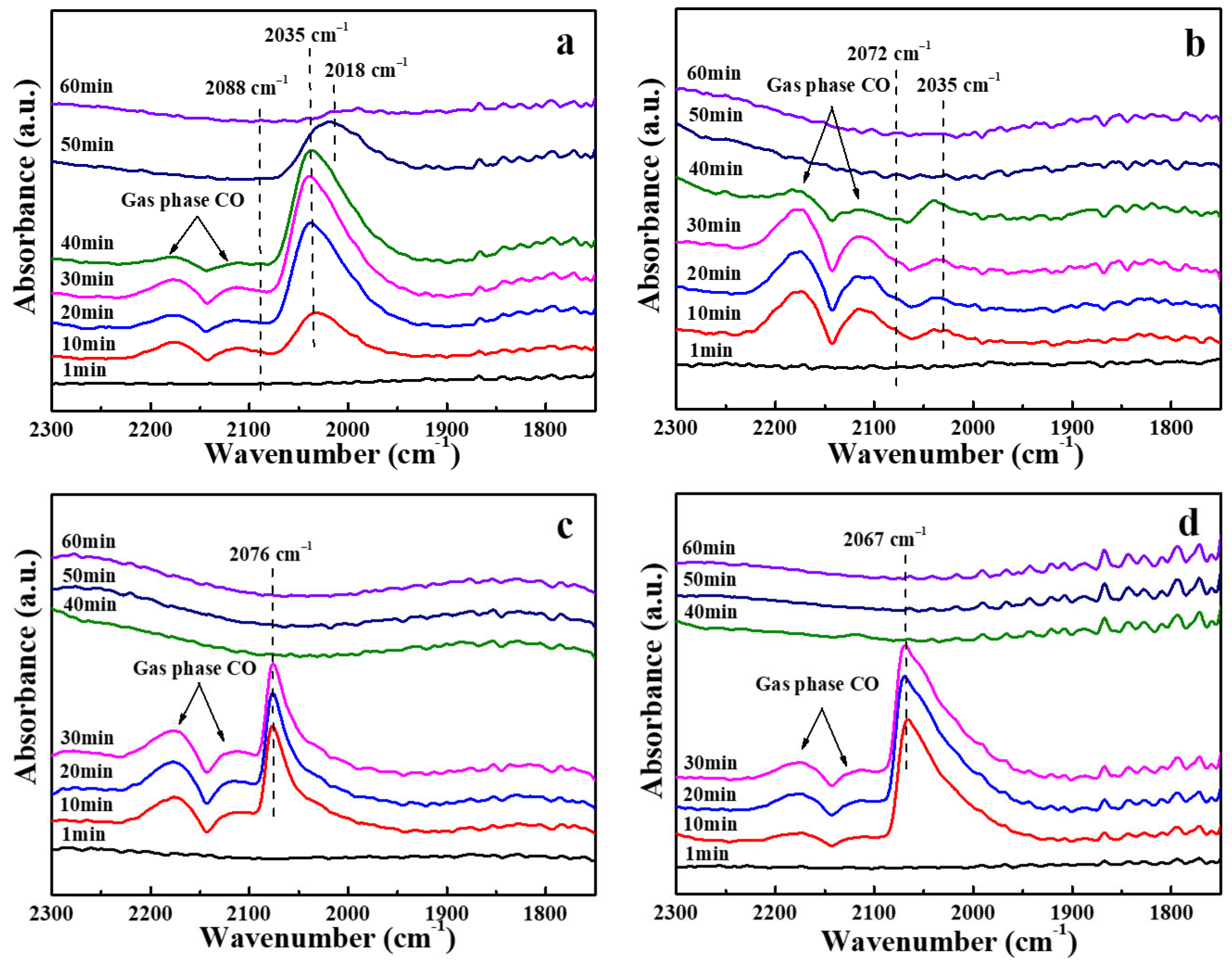


| Sample | Binding Energy (eV) | |||||
|---|---|---|---|---|---|---|
| Pt 4d5/2 | Pt 4d3/2 | |||||
| Pt0 | Pt2+ | Pt4+ | Pt0 | Pt2+ | Pt4+ | |
| PtSn/Al2O3 | 309.5 | 316.9 | 320.1 | 332.0 | 334.2 | 336.4 |
| PtSn/Al2O3-0.4CA | 306.9 | 314.0 | 319.7 | 331.4 | 334.1 | 337.2 |
| PtSn/Al2O3-0.8CA | 306.7 | 314.6 | 323.7 | 330.3 | 333.4 | 337.1 |
| PtSn/Al2O3-1.2CA | 307.4 | 314.9 | 321.0 | 329.4 | 334.1 | 339.2 |
| Sample | Sn 4d5/2 | Sn 4d3/2 | ||||
| Sn0 | Sn2+ | Sn4+ | Sn0 | Sn2+ | Sn4+ | |
| Sn/Al2O3 | 495.1 | 495.8 | 496.5 | 486.7 | 487.5 | 488.2 |
| Sample | Conversion (%) | Selectivity (%) | K d a (h−1) | ||
|---|---|---|---|---|---|
| Initial | Final | Initial | Final | ||
| PtSn/Al2O3 | 29.0 | 18.7 | 56.2 | 74.0 | 0.0024 |
| PtSn/Al2O3-0.4 CA | 12.9 | 9.4 | 81.8 | 91.0 | 0.0011 |
| PtSn/Al2O3-0.8 CA | 11.8 | 8.1 | 87.4 | 93.9 | 0.0013 |
| PtSn/Al2O3-1.2 CA | 11.0 | 7.6 | 88.7 | 93.1 | 0.0014 |
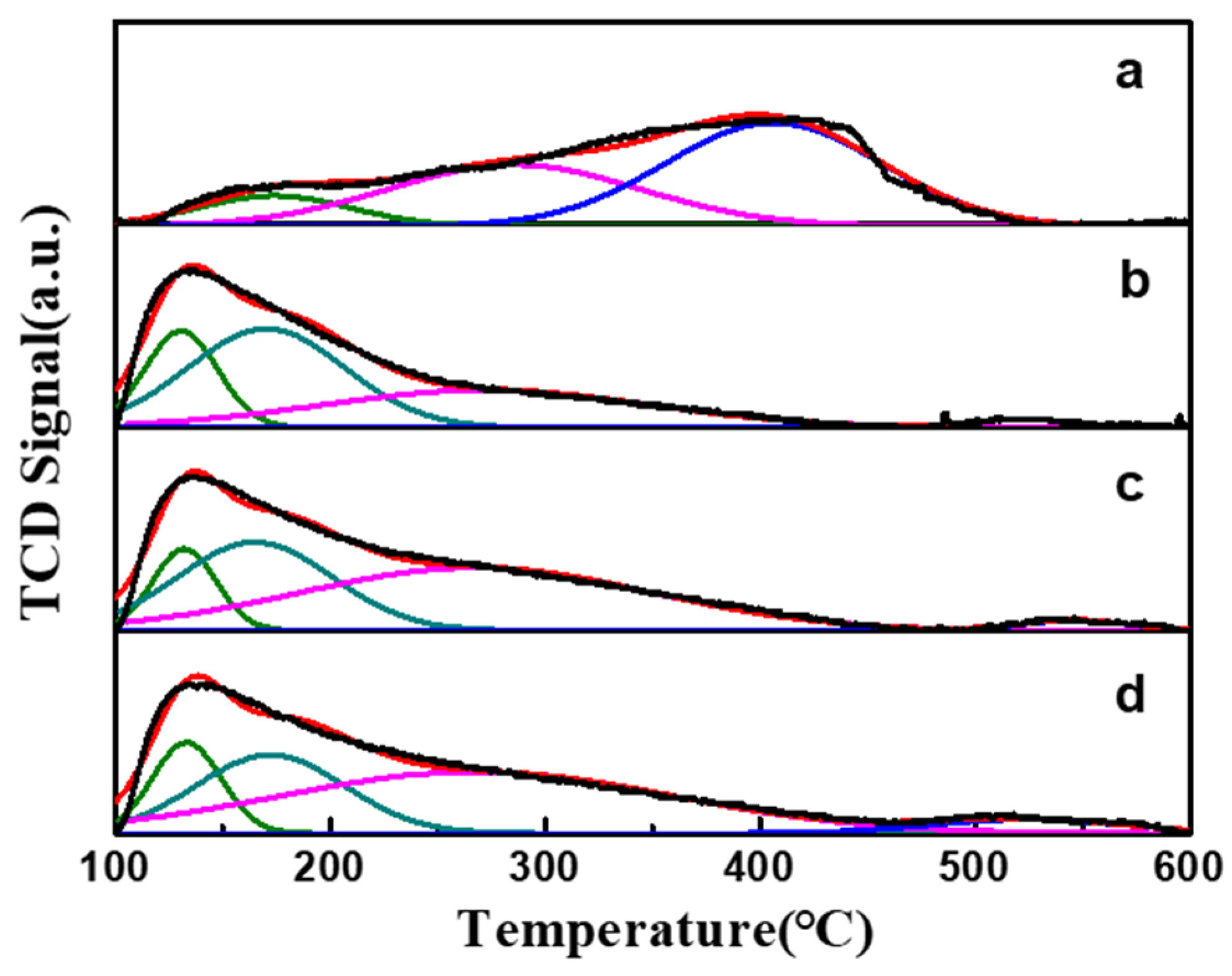
| Sample | TM (°C) | Peak Area (a.u.) | Total Area (a.u.) | |||||
|---|---|---|---|---|---|---|---|---|
| Weak | Medium | Strong | Weak | Medium | Strong | |||
| PtSn/Al2O3 | 173 | 286 | 406 | 52.7 | 176.6 | 256.9 | 486.2 | |
| PtSn/Al2O3-0.4CA | 131 | 170 | 273 | 523 | 258.2 | 140.1 | 9.6 | 407.9 |
| PtSn/Al2O3-0.8CA | 132 | 165 | 268 | 547 | 215.8 | 252.3 | 12.3 | 480.4 |
| PtSn/Al2O3-1.2CA | 133 | 172 | 268 | 524 | 206.3 | 278.9 | 30.7 | 515.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, H.; Ma, J.; Gan, L.; Li, K. The Acid Roles of PtSn@Al2O3 in the Synthesis and Performance of Propane Dehydrogenation. Molecules 2024, 29, 2959. https://doi.org/10.3390/molecules29132959
Niu H, Ma J, Gan L, Li K. The Acid Roles of PtSn@Al2O3 in the Synthesis and Performance of Propane Dehydrogenation. Molecules. 2024; 29(13):2959. https://doi.org/10.3390/molecules29132959
Chicago/Turabian StyleNiu, Hejingying, Jinhua Ma, Lina Gan, and Kezhi Li. 2024. "The Acid Roles of PtSn@Al2O3 in the Synthesis and Performance of Propane Dehydrogenation" Molecules 29, no. 13: 2959. https://doi.org/10.3390/molecules29132959





