Electronic-Structure-Modulated Cu,Co-Coanchored N-Doped Nanocarbon as a Difunctional Electrocatalyst for Hydrogen Evolution and Oxygen Reduction Reactions
Abstract
1. Introduction
2. Results and Discussion
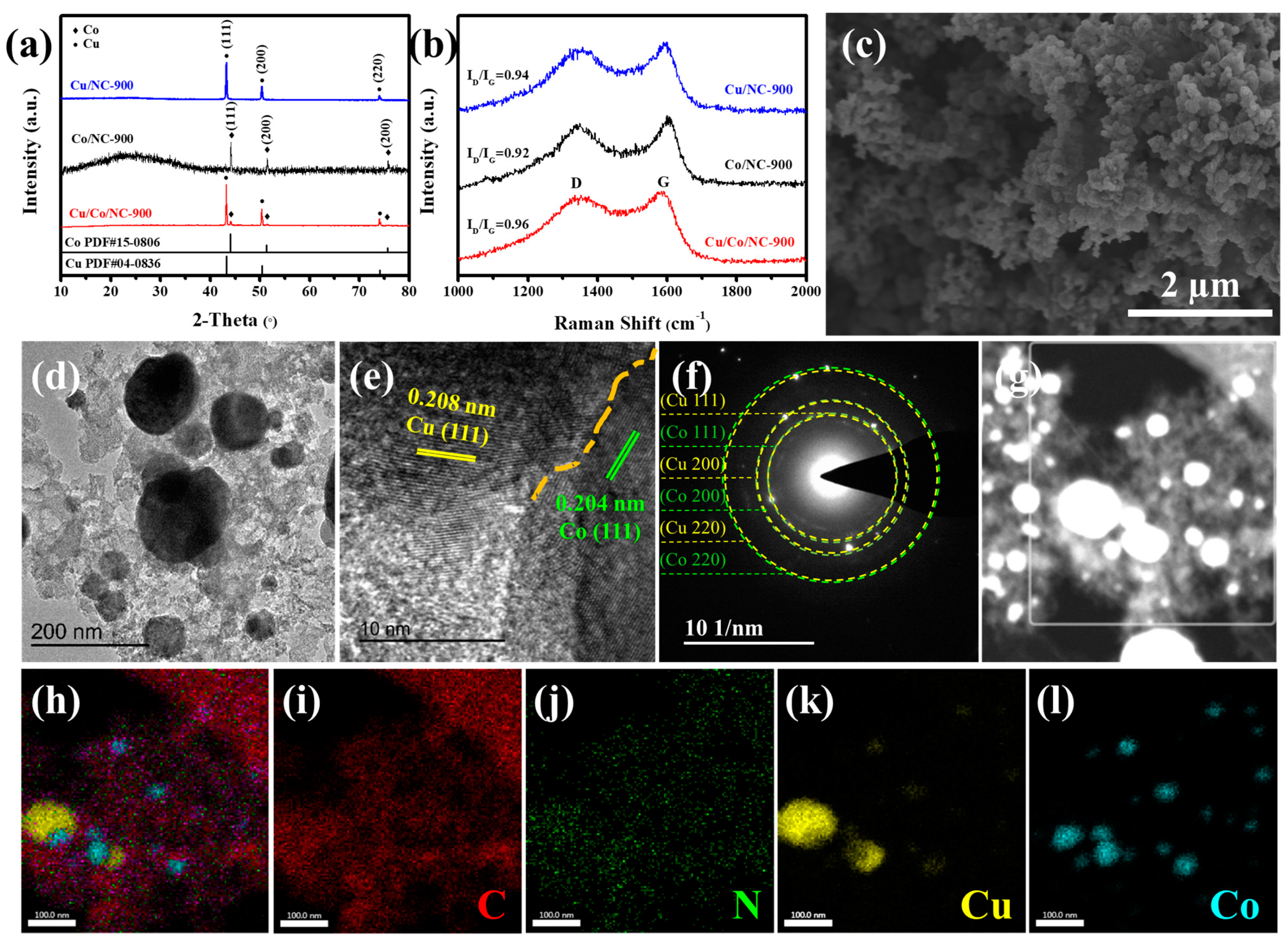
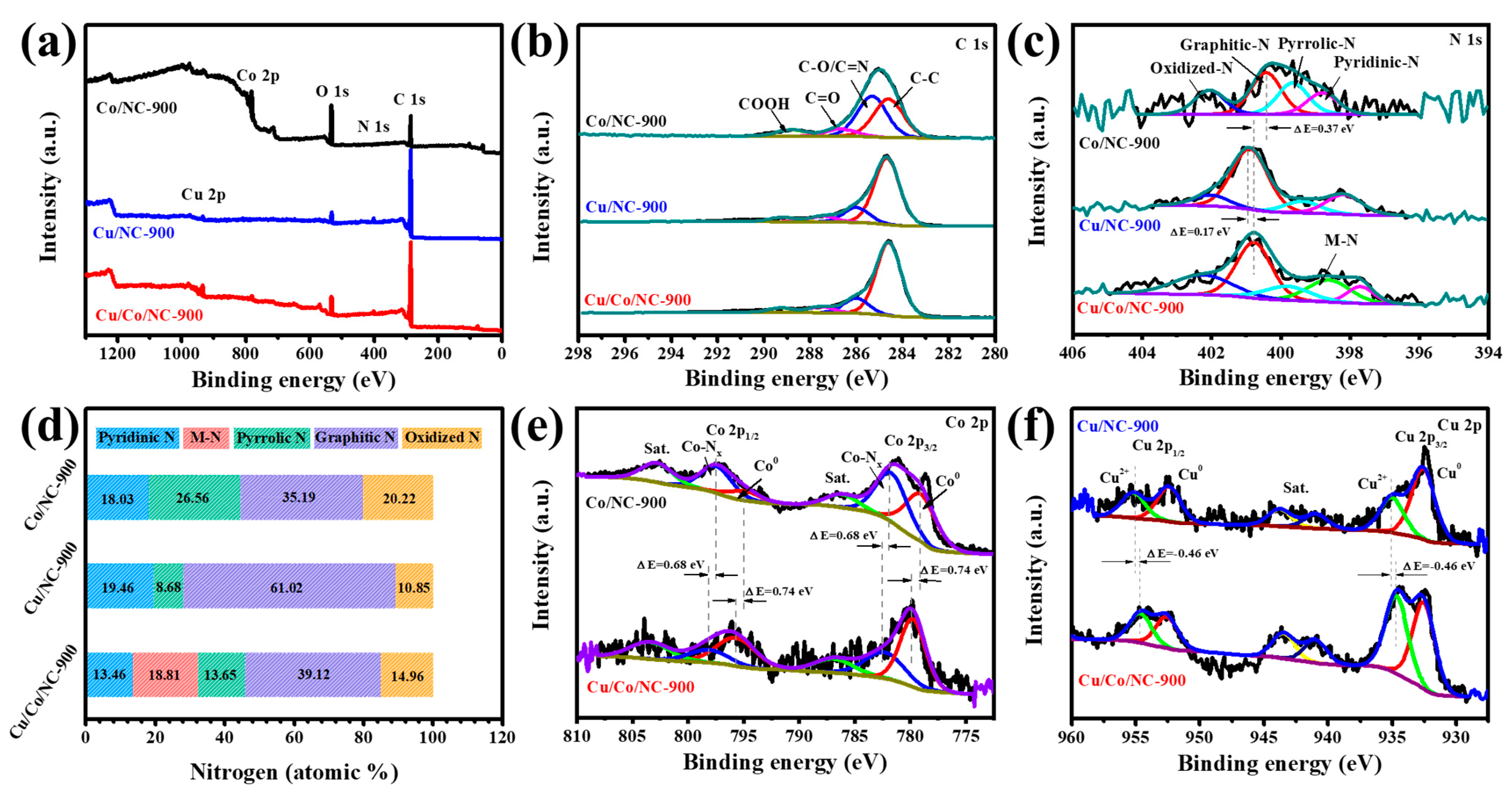
2.1. Structure and Characterisation of Cu/Co/NC Materials
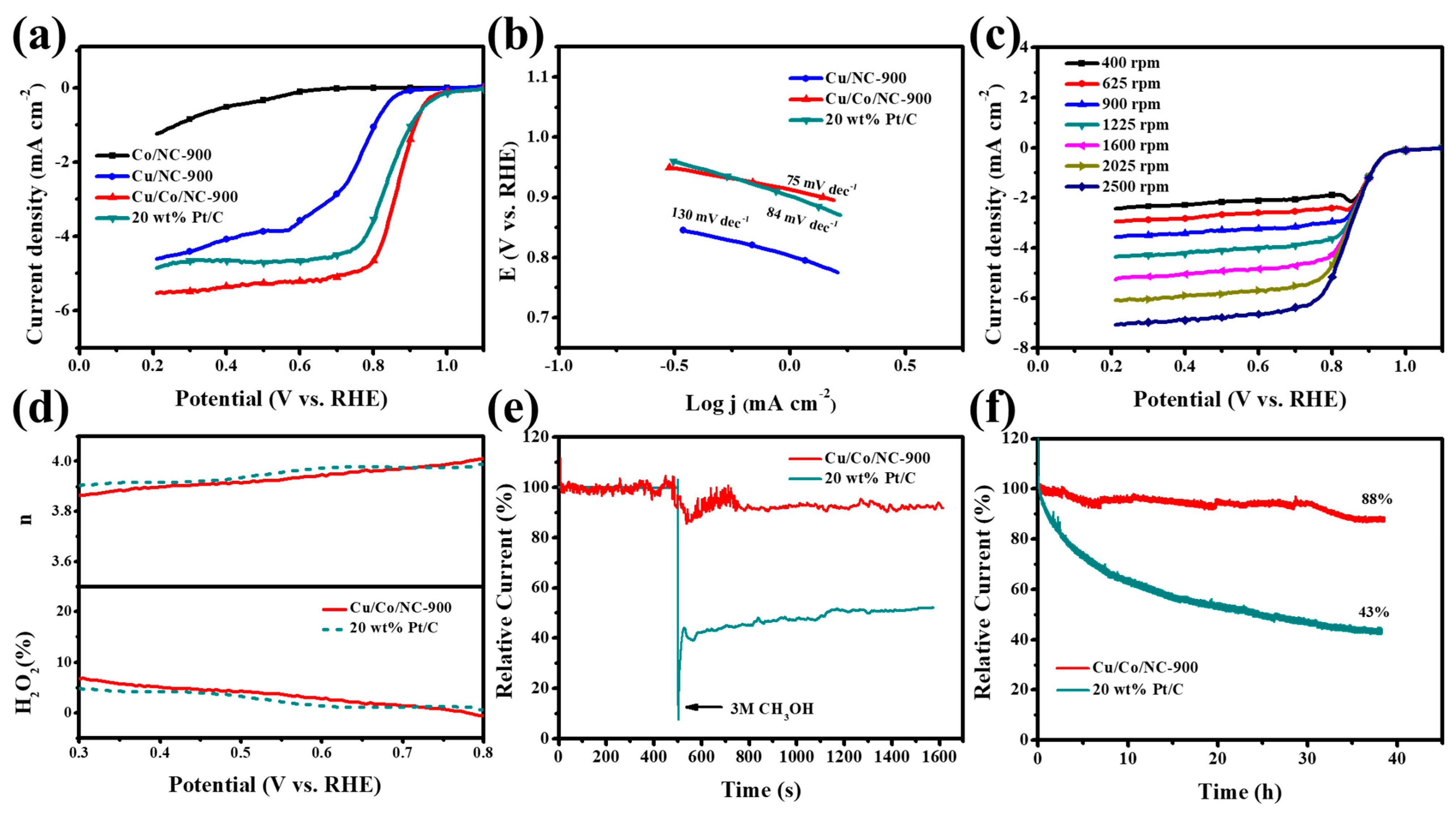
2.2. Characterisation of Electrocatalytic HER Properties of Cu/Co/NC Materials
2.3. Characterisation of Electrocatalytic ORR Properties of Cu/Co/NC Materials
3. Materials and Methods
3.1. Preparation of Materials
3.1.1. Chemicals and Reagents
3.1.2. Synthesis of Co-CuO2/Cu@Ppy
3.1.3. Synthesis of Cu/Co/NC
3.1.4. Synthesis of Cu/NC-900
3.1.5. Synthesis of Co/NC-900
3.2. Material Characterization
3.3. Electrochemical Performance Test Characterization
3.3.1. ORR Electrochemical Characterization
3.3.2. HER Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, H.; Yan, M.; Yang, C.; He, H.; Jiang, Q.; Yang, L.; Lu, Z.; Sun, Z.; Xu, X.; Bando, Y. Graphene Nanoarchitectonics: Recent Advances in Graphene-based Electrocatalysts for Hydrogen Evolution Reaction. Adv. Mater. 2019, 31, 1903415. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, J.; Li, F.; Jung, S.-M.; Okyay, M.S.; Ahmad, I.; Kim, S.J.; Park, N.; Jeong, H.Y.; Baek, J.B. An Efficient and pH-Universal Ruthenium-Based Catalyst for the Hydrogen Evolution Reaction. Nat. Nanotechnol. 2017, 12, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Strmcnik, D.; Uchimura, M.; Wang, C.; Subbaraman, R.; Danilovic, N.; Van Der Vliet, D.; Paulikas, A.P.; Stamenkovic, V.R.; Markovic, N.M. Improving the Hydrogen Oxidation Reaction Rate by Promotion of Hydroxyl Adsorption. Nat. Chem. 2013, 5, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zhang, Q.; Guan, J. Applications of Metal-Organic Framework-Derived Materials in Fuel Cells and Metal-Air Batteries. Coord. Chem. Rev. 2020, 409, 213214. [Google Scholar] [CrossRef]
- Gu, P.; Zheng, M.; Zhao, Q.; Xiao, X.; Xue, H.; Pang, H. Rechargeable Zinc-Air Batteries: A Promising Way to Green Energy. J. Mater. Chem. A 2017, 5, 7651–7666. [Google Scholar] [CrossRef]
- Torrero, J.; Morawietz, T.; García Sanchez, D.; Galyamin, D.; Retuerto, M.; Martin-Diaconescu, V.; Rojas, S.; Alonso, J.A.; Gago, A.S.; Friedrich, K.A. High Performance and Durable Anode with 10-Fold Reduction of Iridium Loading for Proton Exchange Membrane Water Electrolysis. Adv. Energy Mater. 2023, 13, 2204169. [Google Scholar] [CrossRef]
- Yao, M.; Wang, B.; Sun, B.; Luo, L.; Chen, Y.; Wang, J.; Wang, N.; Komarneni, S.; Niu, X.; Hu, W. Rational Design of Self-Supported Cu@ WC Core-Shell Mesoporous Nanowires for pH-Universal Hydrogen Evolution Reaction. Appl. Catal. B Environ. 2021, 280, 119451. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble Metal-Free Hydrogen Evolution Catalysts for Water Splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Liu, Y.; Luo, Z.; Veder, J.P.; Zhong, Y.; Shao, Z. Defects-Rich Porous Carbon Microspheres as Green Electrocatalysts for Efficient and Stable Oxygen-Reduction Reaction over a Wide Range of pH Values. Chem. Eng. J. 2021, 406, 126883. [Google Scholar] [CrossRef]
- Yan, L.; Li, P.; Zhu, Q.; Kumar, A.; Sun, K.; Tian, S.; Sun, X. Atomically Precise Electrocatalysts for Oxygen Reduction Reaction. Chem 2023, 9, 280–342. [Google Scholar] [CrossRef]
- Han, J.; Bao, H.; Wang, J.Q.; Zheng, L.; Sun, S.; Wang, Z.L.; Sun, C. 3D N-Doped Ordered Mesoporous Carbon Supported Single-Atom Fe-NC Catalysts with Superior Performance for Oxygen Reduction Reaction and Zinc-Air Battery. Appl. Catal. B Environ. 2021, 280, 119411. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Zhang, J.; Pan, K.M.; Xu, H.; Chen, H.; Liu, G.; Wu, N.; Yuan, C.; Liu, X. Tailoring Supports for Enhancing Electrocatalytic Hydrogen Evolution Performance of Platinum Species: A Review. J. Mater. Chem. A 2023, 11, 19812–19844. [Google Scholar] [CrossRef]
- Gao, G.; Zhu, G.; Chen, X.; Sun, Z.; Cabot, A. Optimizing Pt-Based Alloy Electrocatalysts for Improved Hydrogen Evolution Performance in Alkaline Electrolytes: A Comprehensive Review. ACS Nano 2023, 17, 20804–20824. [Google Scholar] [CrossRef]
- Lilloja, J.; Kibena-Poldsepp, E.; Sarapuu, A.; Kikas, A.; Kisand, V.; Käärik, M.; Merisalu, M.; Treshchalov, A.; Leis, J.; Sammelselg, V. Nitrogen-Doped Carbide-Derived Carbon/Carbon Nanotube Composites as Cathode Catalysts for Anion Exchange Membrane Fuel Cell Application. Appl. Catal. B Environ. 2020, 272, 119012. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, Y.; You, B. Dynamic Electrodeposition on Bubbles: An Effective Strategy toward Porous Electrocatalysts for Green Hydrogen Cycling. Acc. Chem. Res. 2023, 56, 1421–1432. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, Y.; Wang, J.; Geng, Y.; Zhang, B.; He, J.; Xue, J.; Frauenheim, T.; Li, M. Ni/Mo Bimetallic-oxide-derived Heterointerface-rich Sulfide Nanosheets with Co-doping for Efficient Alkaline Hydrogen Evolution by Boosting Volmer Reaction. Small 2021, 17, 2006730. [Google Scholar] [CrossRef]
- Lu, Z.; Cao, Y.; Xie, J.; Hu, J.; Wang, K.; Jia, D. Construction of Co2P/CoP@Co@NCNT Rich-Interface to Synergistically Promote Overall Water Splitting. Chem. Eng. J. 2022, 430, 132877. [Google Scholar] [CrossRef]
- Feng, L.L.; Li, G.D.; Liu, Y.; Wu, Y.; Chen, H.; Wang, Y.; Zou, Y.C.; Wang, D.; Zou, X. Carbon-Armored Co9S8 Nanoparticles as All-pH Efficient and Durable H2-Evolving Electrocatalysts. ACS Appl. Mater. Interfaces 2015, 7, 980–988. [Google Scholar] [CrossRef]
- Chen, W.F.; Muckerman, J.T.; Fujita, E. Recent Developments in Transition Metal Carbides and Nitrides as Hydrogen Evolution Electrocatalysts. Chem. Commun. 2013, 49, 8896–8909. [Google Scholar] [CrossRef]
- Huang, H.; Huang, W.; Xu, Y.; Ye, X.; Wu, M.; Shao, Q.; Ou, G.; Peng, Z.; Shi, J.; Chen, J. Catalytic Oxidation of Gaseous Benzene with Ozone over Zeolite-Supported Metal Oxide Nanoparticles at Room Temperature. Catal. Today 2015, 258, 627–633. [Google Scholar] [CrossRef]
- Hemmati, S.; Naderi, A.; Ghadermazi, M.; Veisi, H. SiO2-Functionalized Melamine-Pyridine Group-Supported Cu(OAc)2 as an Efficient Heterogeneous and Recyclable Nanocatalyst for the N-Arylation of Amines through Ullmann Coupling Reactions. Comptes Rendus. Chimie 2018, 21, 659–668. [Google Scholar] [CrossRef]
- Evano, G.; Blanchard, N.; Toumi, M. Copper-Mediated Coupling Reactions and Their Applications in Natural Products and Designed Biomolecules Synthesis. Chem. Rev. 2008, 108, 3054–3131. [Google Scholar] [CrossRef] [PubMed]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jonsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Kannimuthu, K.; Sangeetha, K.; Sankar, S.S.; Karmakar, A.; Madhu, R.; Kundu, S. Investigation on Nanostructured Cu-Based Electrocatalysts for Improvising Water Splitting: A Review. Inorg. Chem. Front. 2021, 8, 234–272. [Google Scholar] [CrossRef]
- Feng, H.; Tang, L.; Zeng, G.; Tang, J.; Deng, Y.; Yan, M.; Liu, Y.; Zhou, Y.; Ren, X.; Chen, S. Carbon-Based Core-Shell Nanostructured Materials for Electrochemical Energy Storage. J. Mater. Chem. A 2018, 6, 7310–7337. [Google Scholar] [CrossRef]
- Nunes, M.; Fernandes, D.M.; Morales, M.V.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A.; Freire, C. Cu and Pd Nanoparticles Supported on a Graphitic Carbon Material as Bifunctional HER/ORR Electrocatalysts. Catal. Today 2020, 357, 279–290. [Google Scholar] [CrossRef]
- Sanad, M.F.; Puente Santiago, A.R.; Tolba, S.A.; Ahsan, M.A.; Fernandez-Delgado, O.; Shawky Adly, M.; Hashem, E.M.; Mahrous Abodouh, M.; El-Shall, M.S.; Sreenivasan, S.T. Co-Cu Bimetallic Metal Organic Framework Catalyst Outperforms the Pt/C Benchmark for Oxygen Reduction. J. Am. Chem. Soc. 2021, 143, 4064–4073. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Fujibayashi, N.; Abe, D.; Matsubara, N.; Yasuda, S.; Yagi, I. Impact of Heterometallic Cooperativity of Iron and Copper Active Sites on Electrocatalytic Oxygen Reduction Kinetics. ACS Catal. 2021, 11, 2356–2365. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y.; Chen, Y.Y.; Zhao, L.; Huang, L.-B.; Luo, H.; Jiang, W.J.; Zhang, X.; Niu, S.; Gao, D. Encased Copper Boosts the Electrocatalytic Activity of N-Doped Carbon Nanotubes for Hydrogen Evolution. ACS Appl. Mater. Interfaces 2017, 9, 36857–36864. [Google Scholar] [CrossRef]
- Mondol, P.; Barile, C.J. Four-Electron Electrocatalytic O2 Reduction by a Ferrocene-Modified Glutathione Complex of Cu. ACS Appl. Energy Mater. 2021, 4, 9611–9617. [Google Scholar] [CrossRef]
- Yuan, L.J.; Sui, X.L.; Liu, C.; Zhuo, Y.L.; Li, Q.; Pan, H.; Wang, Z.B. Electrocatalysis Mechanism and Structure-Activity Relationship of Atomically Dispersed Metal-Nitrogen-Carbon Catalysts for Electrocatalytic Reactions. Small Methods 2023, 7, 2201524. [Google Scholar] [CrossRef]
- Hu, H.; Liang, J.H.; Zu, Z.Y.; Mi, J.L.; Xiao, B.B.; Zhang, P. Ni/Cu Regulating Nitrogen-Doped Porous Carbon as Electrocatalyst for Oxygen Reduction Reaction. ChemistrySelect 2021, 6, 6949–6956. [Google Scholar] [CrossRef]
- Bu, M.; Liu, Y.; Liao, S.; Liu, W.; Yang, Z.; Jiang, J.; Gao, X.; Yang, Y.; Liu, H. In-Site Grown Carbon Nanotubes Connecting Fe/Cu-NC Polyhedrons as Robust Electrocatalysts for Zn-Air Batteries. Carbon 2023, 214, 118365. [Google Scholar] [CrossRef]
- Wang, B.; Xu, L.; Liu, G.; Zhang, P.; Zhu, W.; Xia, J.; Li, H. Biomass Willow Catkin-Derived Co3O4/N-Doped Hollow Hierarchical Porous Carbon Microtubes as an Effective Tri-Functional Electrocatalyst. J. Mater. Chem. A 2017, 5, 20170–20179. [Google Scholar] [CrossRef]
- Zhou, N.; Zhang, R.; Wang, R.; Li, Y. Preparation of PtNiCo Alloy/Nitrogen-Doped Porous Carbon Composites and Their Electrocatalytic Properties. Chem. Eng. J. 2023, 474, 146010. [Google Scholar] [CrossRef]
- Tao, L.; Wang, Q.; Dou, S.; Ma, Z.; Huo, J.; Wang, S.; Dai, L. Edge-Rich and Dopant-Free Graphene as a Highly Efficient Metal-Free Electrocatalyst for the Oxygen Reduction Reaction. Chem. Commun. 2016, 52, 2764–2767. [Google Scholar] [CrossRef]
- Xue, Y.; Sun, S.; Wang, Q.; Dong, Z.; Liu, Z. Transition Metal Oxide-Based Oxygen Reduction Reaction Electrocatalysts for Energy Conversion Systems with Aqueous Electrolytes. J. Mater. Chem. A 2018, 6, 10595–10626. [Google Scholar] [CrossRef]
- Jose, V.; Nsanzimana, J.M.V.; Hu, H.; Choi, J.; Wang, X.; Lee, J.M. Highly Efficient Oxygen Reduction Reaction Activity of N-doped Carbon-Cobalt Boride Heterointerfaces. Adv. Energy Mater. 2021, 11, 2100157. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Balamurugan, J.; Lau, K.T.; Kim, N.H.; Lee, J.H. Novel Cobalt-Doped Molybdenum Oxynitride Quantum Dot@N-Doped Carbon Nanosheets with Abundant Oxygen Vacancies for Long-Life Rechargeable Zinc-Air Batteries. J. Mater. Chem. A 2021, 9, 9092–9104. [Google Scholar] [CrossRef]
- Dong, H.; Zuo, Y.; Song, N.; Hong, S.; Xiao, M.; Zhu, D.; Sun, J.; Chen, G.; Li, C. Bimetallic Synergetic Regulating Effect on Electronic Structure in Cobalt/Vanadium Co-Doped Carbon Nitride for Boosting Photocatalytic Performance. Appl. Catal. B Environ. 2021, 287, 119954. [Google Scholar] [CrossRef]
- Low, J.; Dai, B.; Tong, T.; Jiang, C.; Yu, J. In Situ Irradiated X-ray Photoelectron Spectroscopy Investigation on a Direct Z-scheme TiO2/CdS Composite Film Photocatalyst. Adv. Mater. 2019, 31, 1802981. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Sun, W.; Shang, H.; Chen, W.; Sun, T.; Li, H.; Dong, J.; Zhou, J.; Li, Z.; Wang, Y. Atomic Interface Effect of a Single Atom Copper Catalyst for Enhanced Oxygen Reduction Reactions. Energy Environ. Sci. 2019, 12, 3508–3514. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, X.; Shi, Y.; Ye, Y.; Lin, S. Heterostructural Co||Cu Coated with Nitrogen-Doped Carbon as a Highly Efficient Electrocatalyst for Oxygen Reduction Reaction and Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2022, 10, 5986–5997. [Google Scholar] [CrossRef]
- Geng, W.; Li, W.; Liu, L.; Liu, J.; Liu, L.; Kong, X. Facile Assembly of Cu-Cu2O/N-Reduced Graphene Oxide Nanocomposites for Efficient Synthesis of 2-Methylfuran. Fuel 2020, 259, 116267. [Google Scholar] [CrossRef]
- Huang, Z.; Zhou, Q.; Wang, J.; Yu, Y. Fermi-Level-Tuned MOF-Derived N-ZnO@NC for Photocatalysis: A Key Role of Pyridine-N-Zn Bond. J. Mater. Sci. Technol. 2022, 112, 68–76. [Google Scholar] [CrossRef]
- Swathi, S.; Yuvakkumar, R.; Ravi, G.; Thambidurai, M.; Arunmetha, S.; Velauthapillai, D. Nanoplatelets assemble CuCo2S4/N doped rGO nanocomposites for hydrogen evolution reaction. Int. J. Hydrog. 2024, 65, 704–716. [Google Scholar] [CrossRef]
- Liu, X.; Yan, W.; Song, J.; Song, H.; Chen, W.; Zhang, Y.; Chen, Y. Kirkendall effect induced the formation of hollow Co2P in Co-N-C for ORR, OER, HER and flexible Zn–Air battery. Chem. Eng. J. 2024, 492, 152301. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, J.; Zhou, H.; Xing, L.; Qiao, S.; Yuan, J.; Mei, B.; Wei, Z.; Zhao, S.; Tang, Y.; et al. Coordination Shell Dependent Activity of CuCo Diatomic Catalysts for Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution Reaction. Adv. Funct. Mater. 2024, 34, 2311664. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Yan, B.; Zhang, F.; Shi, Y.; Guo, X. Single Cu atoms confined in N-doped porous carbon networks by flash nanocomplexation as efficient trifunctional electrocatalysts for Zn-air batteries and water splitting. Compo. Part B-Eng. 2023, 253, 110575. [Google Scholar] [CrossRef]
- Liang, K.; Liang, Y.; Zhang, H.; Tang, F.; Shang, S.-L.; Liu, Z.-K.; Lin, J. Boosting oxygen reduction electrocatalytic performance of Cu-Nx by modulating electron structure via construction of Cu-Nx and Cu nanocluster coupling catalyst. Int. J. Hydrogen Energ. 2024, 69, 21–30. [Google Scholar] [CrossRef]
- Wang, C.; Zou, P.; Xu, W.; Zhang, Y.; Huo, J.; Wang, J.-Q.; Liu, Y.; Qin, C. One-step synthesis of self-supporting porous Cu81(Ni,Co)19 intermetallic catalysts for hydrogen evolution reaction. J. Alloys Compounds 2024, 995, 174790. [Google Scholar] [CrossRef]
- Yu, J.; Jiang, Z.; Huang, T.; Tang, C. BN/Cu/CNT nanoparticles as an efficient tri-functional electrocatalyst for ORR and OER. Int. J. Hydrogen Energ. 2023, 48, 20368–20377. [Google Scholar] [CrossRef]
- Wang, S.; Chen, S.; Wen, C.; Dong, L.; Tan, C.; Li, B.; Fan, M.; He, H.; Chen, Z. Nitrogen-doped MoO2/Cu heterojunction electrocatalyst for highly efficient alkaline hydrogen evolution reaction. Int. J. Hydrogen Energ. 2024, 66, 103–109. [Google Scholar] [CrossRef]
- Li, M.; Selvarajan, P.; Wang, S.; Wan, T.; Xi, S.; Wang, X.; Xue, J.; Indirathankam, S.C.; Geng, X.; Qiao, L.; et al. Thermostable 1T-MoS2 Nanosheets Achieved by Spontaneous Intercalation of Cu Single Atoms at Room Temperature and Their Enhanced HER Performance. Small Struct. 2023, 4, 2300010. [Google Scholar] [CrossRef]
- Debnath, A.; Diyali, S.; Das, M.; Panda, S.J.; Mondal, D.; Dhak, D.; Purohit, C.S.; Ray, P.P.; Biswas, B. Harnessing the hydrogen evolution reaction (HER) through the electrical mobility of an embossed Ag(i)-molecular cage and a Cu(ii)-coordination polymer. Dalton Trans. 2023, 52, 8850–8856. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, Y.; Xia, S.; Wei, C.; Chen, Y.; Chen, S.; Yan, J. Stabilizing High Density Cu Active Sites with ZrO2 Quantum Dots as Chemical Ligand in N-doped Porous Carbon Nanofibers for Efficient ORR. Small 2023, 19, 2206823. [Google Scholar] [CrossRef]
- Zhang, Q.; Kumar, P.; Zhu, X.; Daiyan, R.; Bedford, N.M.; Wu, K.-H.; Han, Z.; Zhang, T.; Amal, R.; Lu, X. Electronically Modified Atomic Sites Within a Multicomponent Co/Cu Composite for Efficient Oxygen Electroreduction. Adv. Energy Mater. 2021, 11, 2100303. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, L.; Liu, R.; Huang, Y.; Chu, D.; Li, M.; Xu, G.; Li, X.; Huang, J.; Zhao, Y.; Feng, L. Electronic-Structure-Modulated Cu,Co-Coanchored N-Doped Nanocarbon as a Difunctional Electrocatalyst for Hydrogen Evolution and Oxygen Reduction Reactions. Molecules 2024, 29, 2973. https://doi.org/10.3390/molecules29132973
Cao L, Liu R, Huang Y, Chu D, Li M, Xu G, Li X, Huang J, Zhao Y, Feng L. Electronic-Structure-Modulated Cu,Co-Coanchored N-Doped Nanocarbon as a Difunctional Electrocatalyst for Hydrogen Evolution and Oxygen Reduction Reactions. Molecules. 2024; 29(13):2973. https://doi.org/10.3390/molecules29132973
Chicago/Turabian StyleCao, Liyun, Rui Liu, Yixuan Huang, Dewei Chu, Mengyao Li, Guoting Xu, Xiaoyi Li, Jianfeng Huang, Yong Zhao, and Liangliang Feng. 2024. "Electronic-Structure-Modulated Cu,Co-Coanchored N-Doped Nanocarbon as a Difunctional Electrocatalyst for Hydrogen Evolution and Oxygen Reduction Reactions" Molecules 29, no. 13: 2973. https://doi.org/10.3390/molecules29132973
APA StyleCao, L., Liu, R., Huang, Y., Chu, D., Li, M., Xu, G., Li, X., Huang, J., Zhao, Y., & Feng, L. (2024). Electronic-Structure-Modulated Cu,Co-Coanchored N-Doped Nanocarbon as a Difunctional Electrocatalyst for Hydrogen Evolution and Oxygen Reduction Reactions. Molecules, 29(13), 2973. https://doi.org/10.3390/molecules29132973










