Preparation of Pinoresinol and Dehydrodiconiferyl Alcohol from Eucommiae Cortex Extract by Fermentation with Traditional Mucor
Abstract
1. Introduction
2. Results
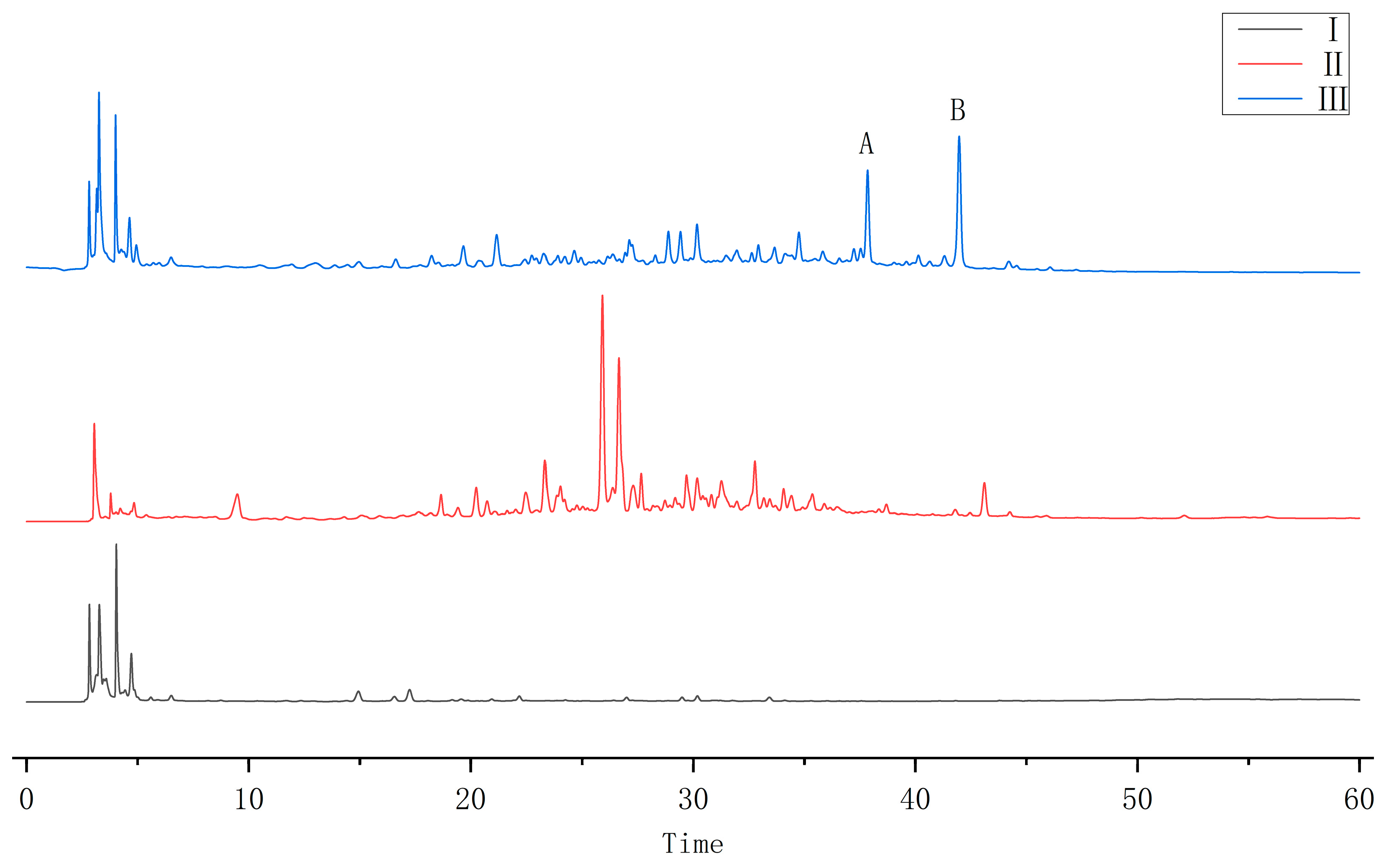
2.1. HPLC Results
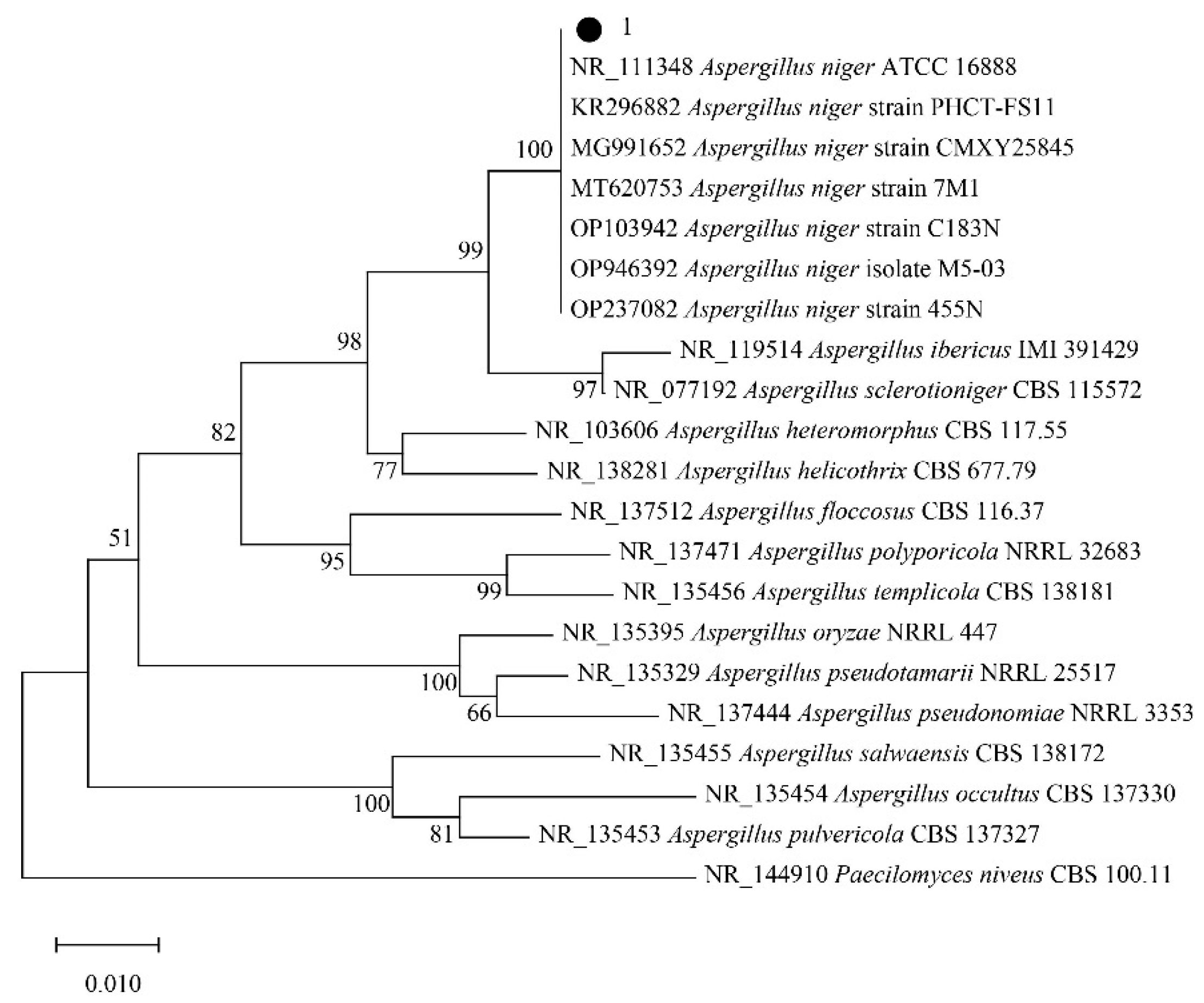
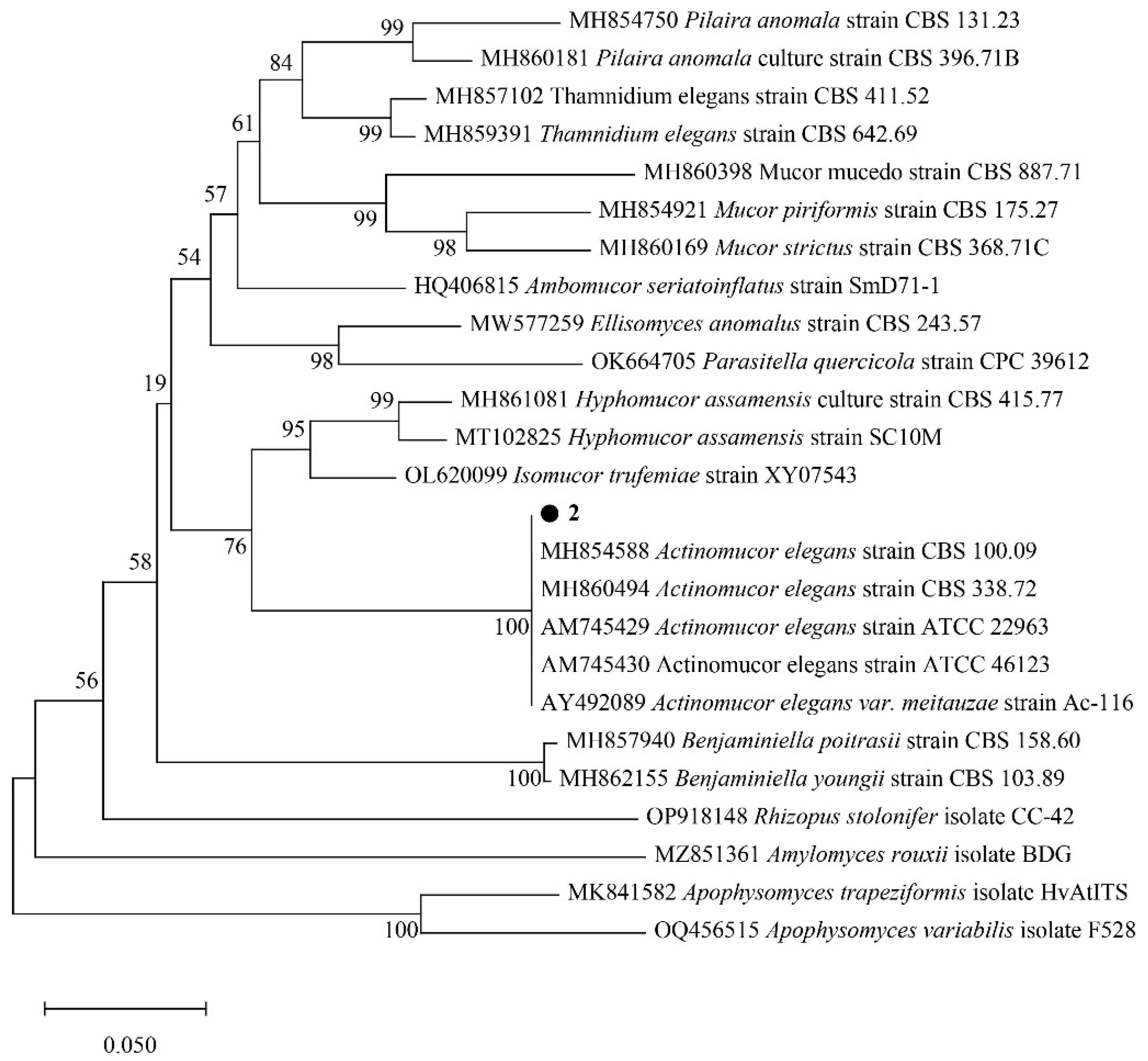
2.2. Strain Identification
2.2.1. ITS Region Sequence
2.2.2. Phylogenetic Analysis of Strains
2.3. Identification of A and B
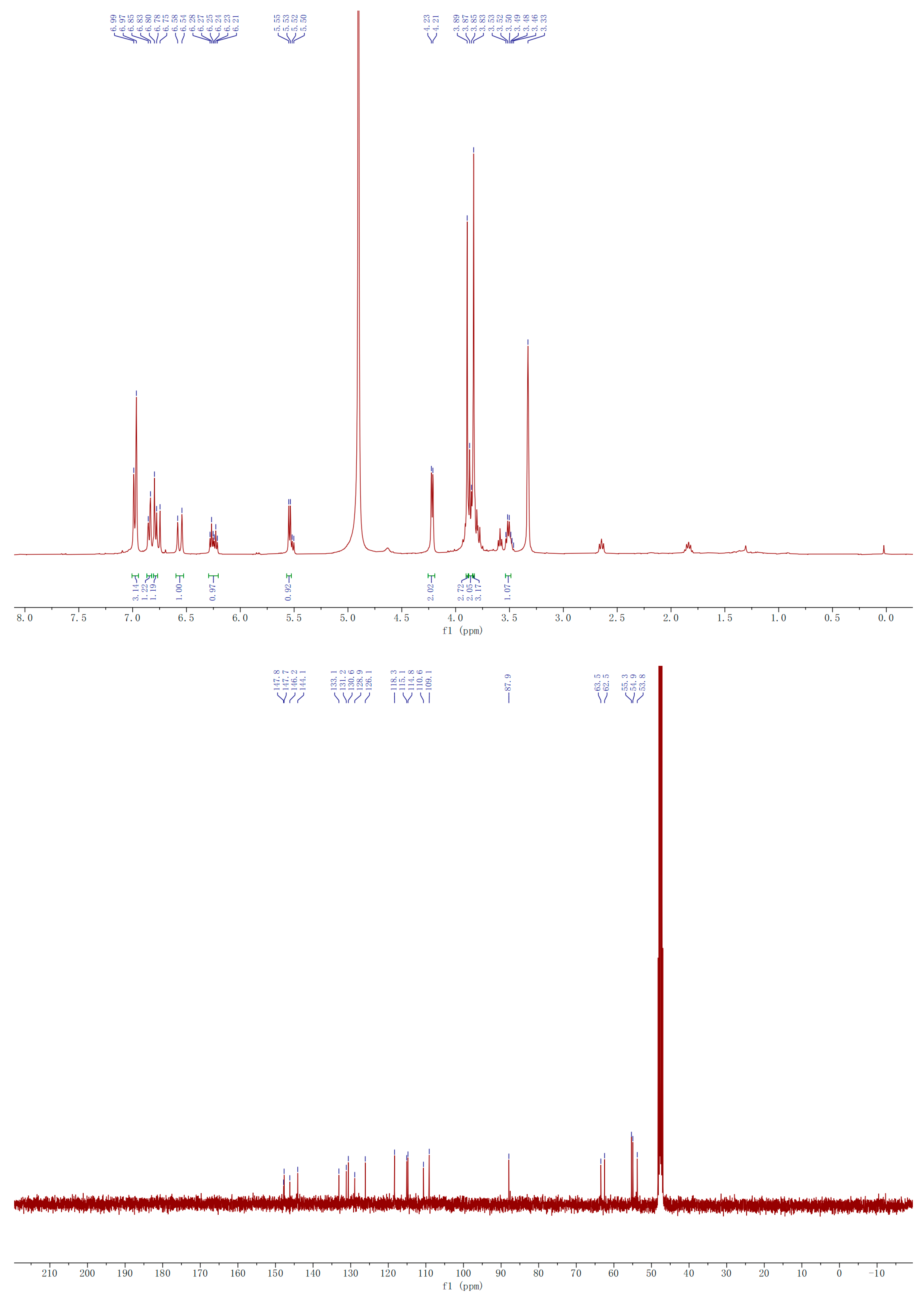
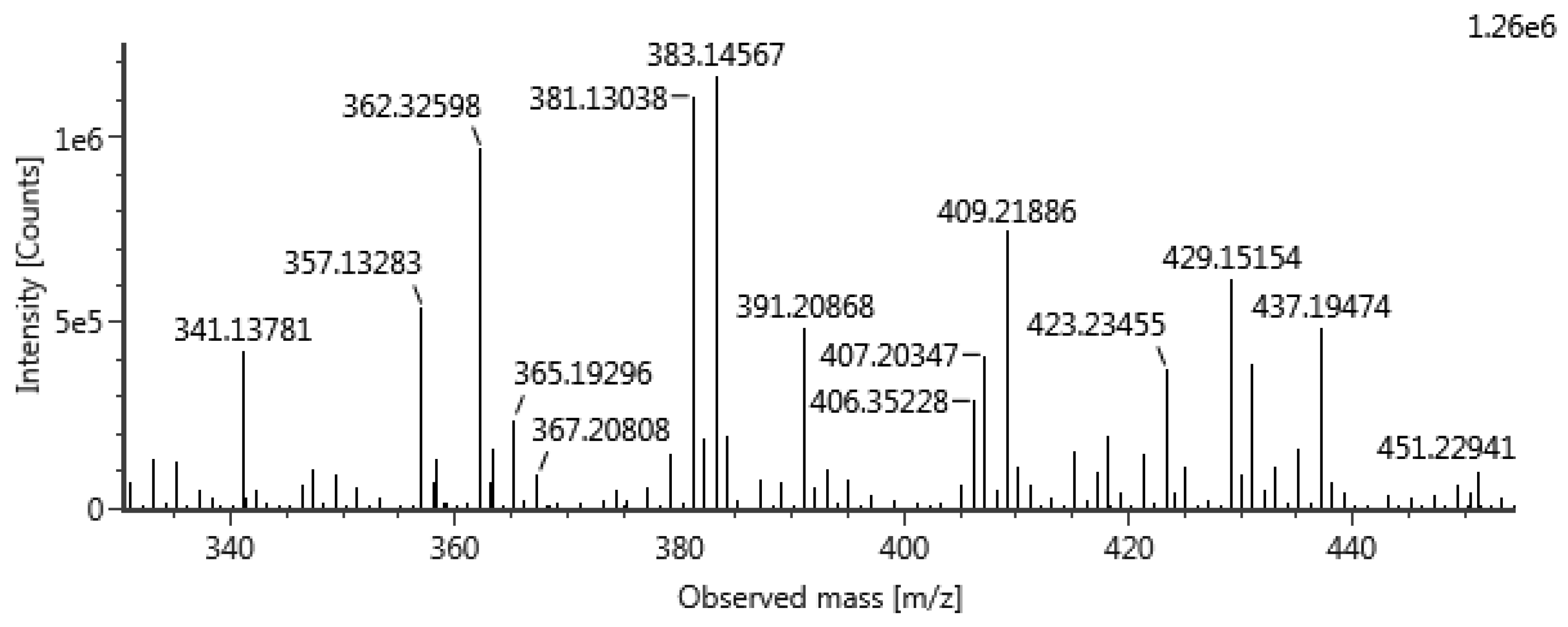
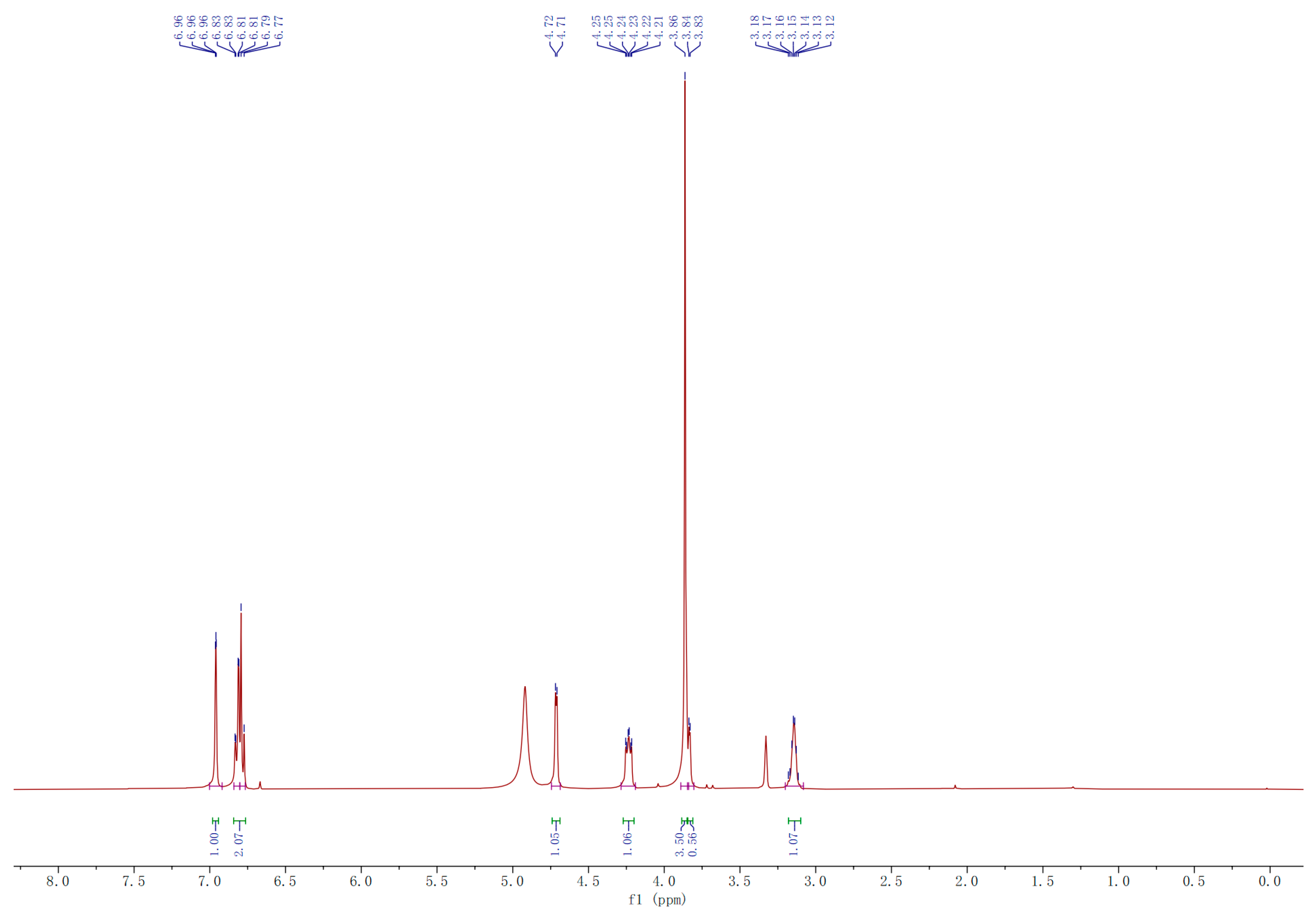
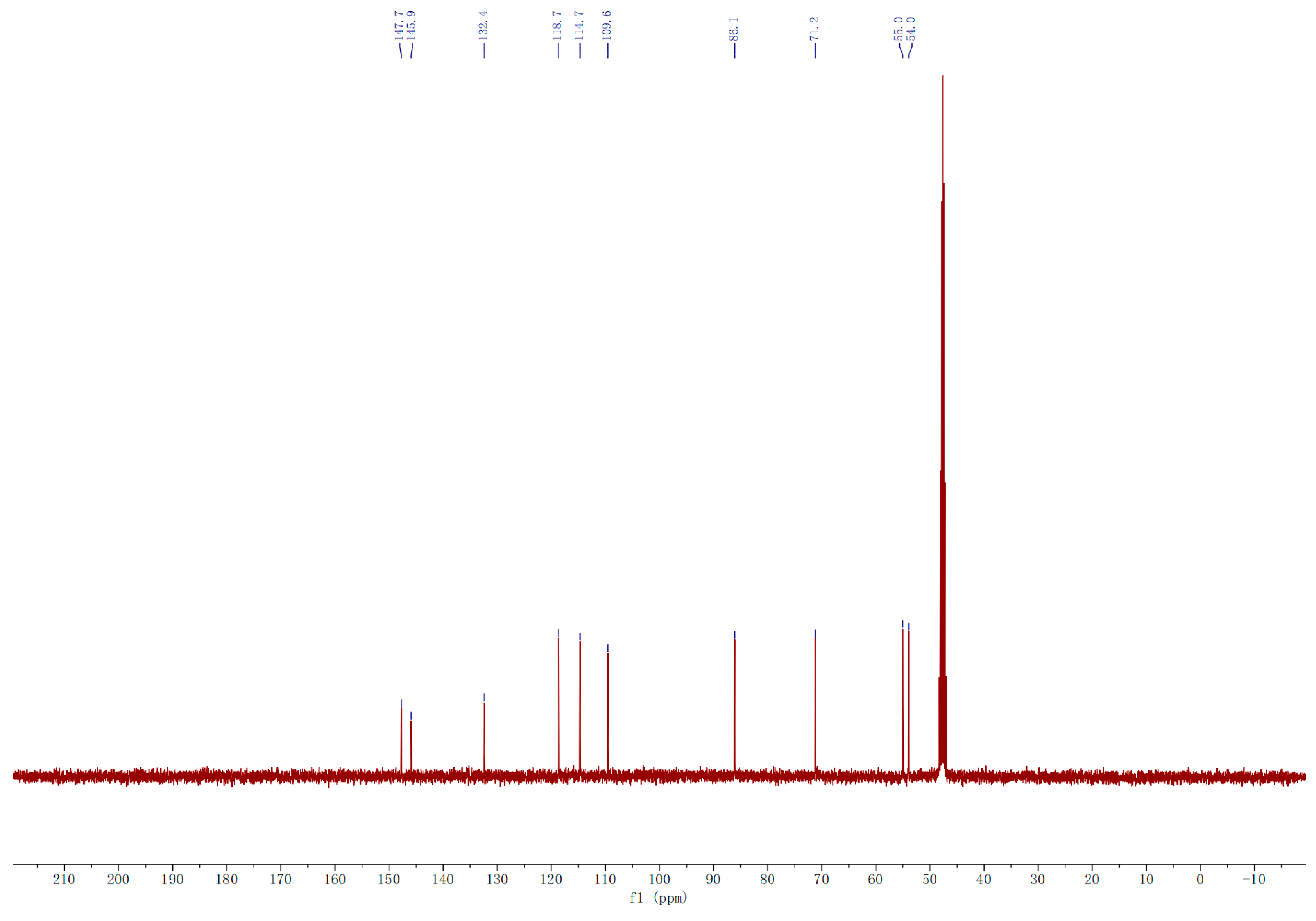
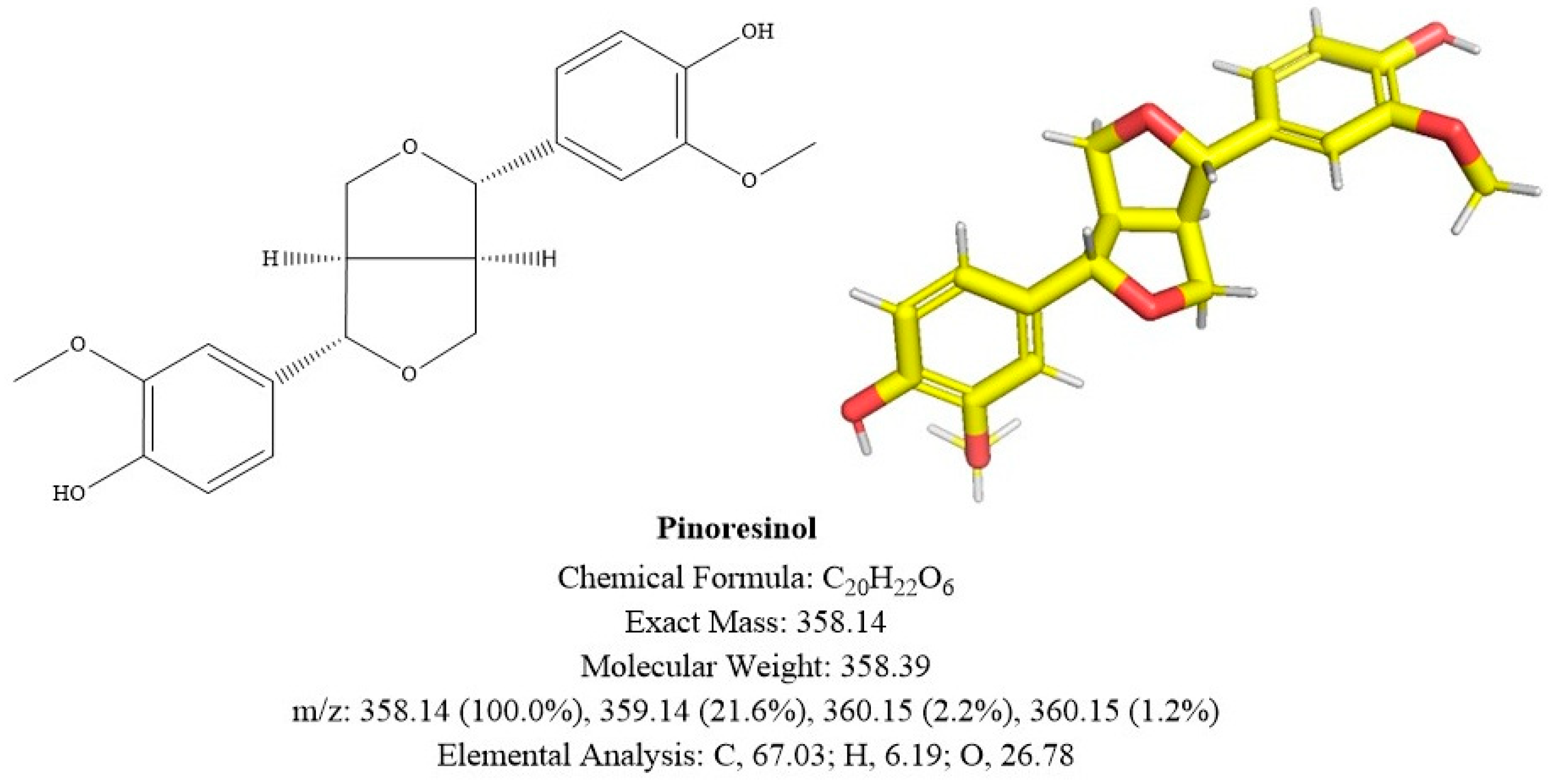
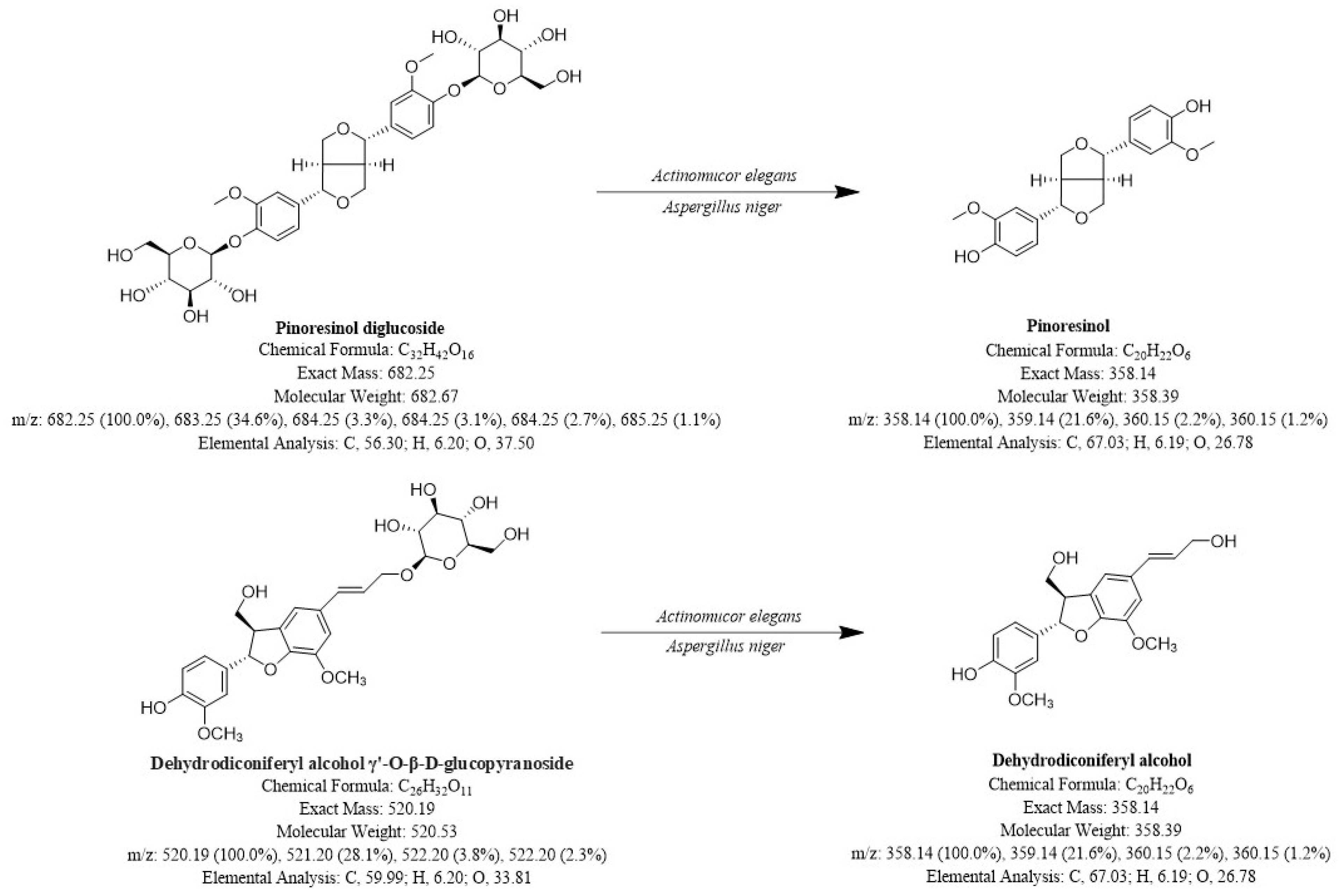
2.3.1. Compound A
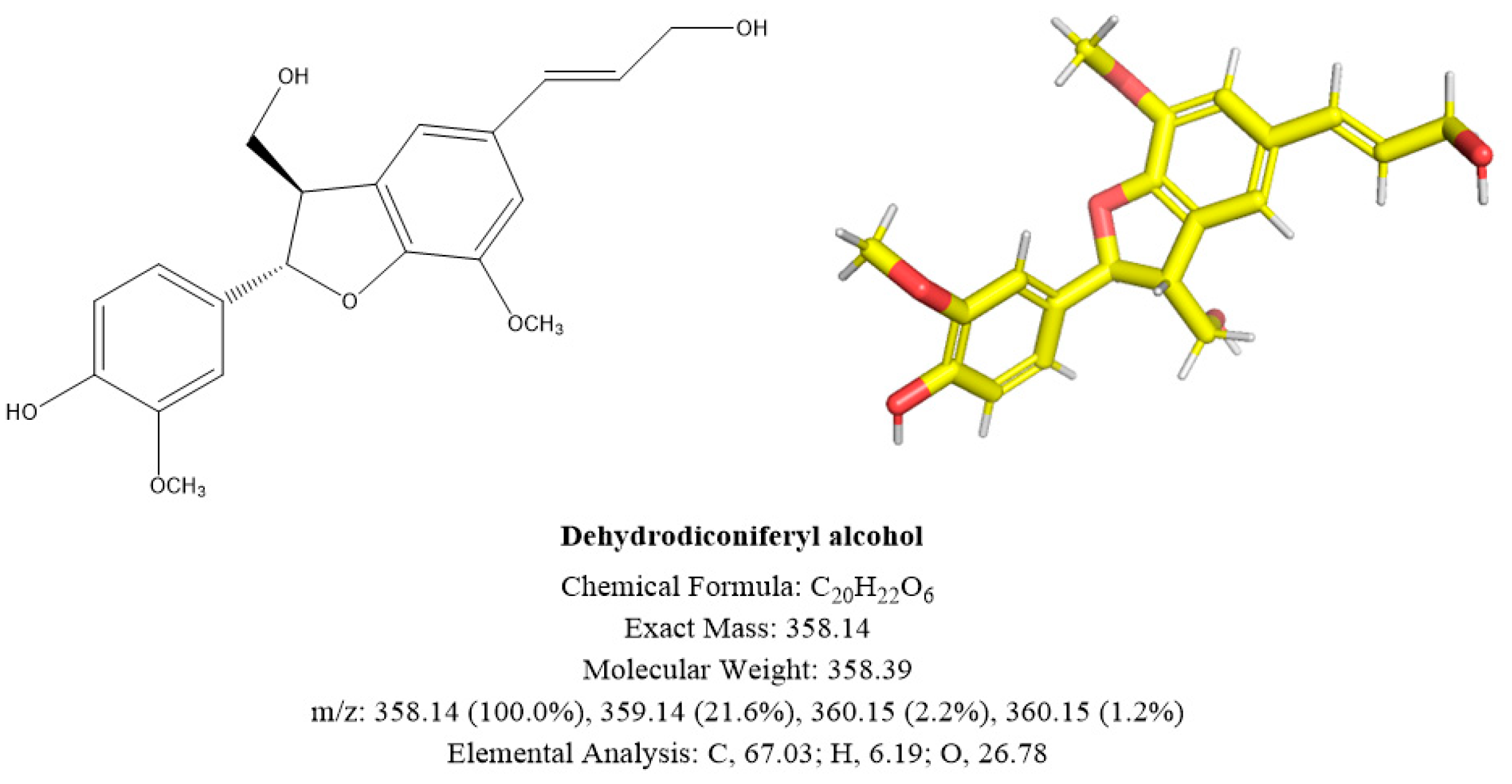
2.3.2. Compound B
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Extraction of EC Components
3.2.2. Fermentation Media
3.2.3. Biotransformation
3.2.4. Collection of Transformation Products
3.2.5. HPLC Assay
- Chromatographic conditions
- Preparation of test solutions
3.2.6. Isolation, Purification and Identification of Strains
3.2.7. Isolation, Purification and Identification of A and B
3.3. Experimental Flow
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- China Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China: Volume I; China Medical Science Press: Beijing, China, 2020; p. 172. [Google Scholar]
- He, X.; Wang, J.; Li, M.; Hao, D.; Yang, Y.; Zhang, C.; He, R.; Tao, R. Eucommia ulmoides Oliv.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2014, 151, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Z.; Zhang, L.; Liu, Y.P.; Zhu, L.P.; Wei, Z.G.; Zhou, Y.Z. Study on the bidirectional fermentation of Eucommia ulmoides and poplar flowers by Cordyceps militaris. Mod. Anim. Husb. 2022, 6, 10–14. [Google Scholar]
- Yao, Y.N. Bioconversion and Comprehensive Utilization of Lignocellulosic Residue from Gutta-Percha Extraction Process of Eucommia ulmoides Oliver. Shandong University: Jinan, China, 2023. [Google Scholar] [CrossRef]
- Chen, J.Q.; Wang, Y.W.; Feng, C.L.; Cai, J.; Ji, M. Isolation and identification of endophytic fungi from Eucommia ulmoides and their biotransformation activity of isosteviol. J. Southeast Univ. (Med. Sci. Ed.) 2011, 30, 861–865. [Google Scholar]
- Trees, R.P. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Huang, D.L. One lignan-like constituent in Codonopsis tangshen. Jiangxi J. Tradit. Chin. Med. 2013, 44, 54. [Google Scholar]
- Nan, C.Y.; Zhu, J.X.; Jiang, W.; Zhong, G.Y.; Li, M. Chemical Constituents from Edgeworthia gardneri Flos. J. Chin. Med. Mater. 2017, 40, 1618–1621. [Google Scholar]
- Zhang, Y.; Zhao, H.; Di, Y.; Li, Q.; Shao, D.; Shi, J.; Huang, Q. Antitumor activity of Pinoresinol in vitro: Inducing apoptosis and inhibiting HepG2 invasion. J. Funct. Foods 2018, 45, 206–214. [Google Scholar] [CrossRef]
- Yu, J.; Kwon, H.; Cho, E.; Kang, R.H.; Youn, K.; Jun, M.; Lee, Y.C.; Ryu, J.H.; Kim, D.H. The effects of pinoresinol on cholinergic dysfunction-induced memory impairments and synaptic plasticity in mice. Food Chem. Toxicol. 2019, 125, 376–382. [Google Scholar] [CrossRef]
- Yu, J.; Cho, E.; Kwon, H.; Jeon, J.; Sin, J.S.; Park, J.K.; Kim, J.S.; Choi, J.W.; Park, S.J.; Jun, M.; et al. Akt and calcium-permeable AMPA receptor are involved in the effect of pinoresinol on amyloid β-induced synaptic plasticity and memory deficits. Biochem. Pharmacol. 2021, 184, 114366. [Google Scholar] [CrossRef]
- Jung, H.W.; Mahesh, R.; Lee, J.G.; Lee, S.H.; Kim, Y.S.; Park, Y.K. Pinoresinol from the fruits of Forsythia koreana inhibits inflammatory responses in LPS-activated microglia. Neurosci. Lett. 2010, 480, 215–220. [Google Scholar] [CrossRef] [PubMed]
- During, A.; Debouche, C.; Raas, T.; Larondelle, Y. Among Plant Lignans, Pinoresinol Has the Strongest Antiinflammatory Properties in Human Intestinal Caco-2 Cells, 3. J. Nutr. 2012, 142, 1798–1805. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, J.K.; Choi, J.H.; Jung, J.Y.; Oh, W.Y.; Kim, D.C.; Lee, H.S.; Kim, Y.S.; Kang, S.S.; Lee, S.H.; et al. Hepatoprotective effect of pinoresinol on carbon tetrachloride–induced hepatic damage in mice. J. Pharmacol. Sci. 2010, 112, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Ma, Y.; Dong, Z.; Wang, G.; Lan, X.; Liao, Z.; Chen, M. Dehydrodiconiferyl alcohol, a lignan from Herpetospermum pedunculosum, alleviates cholestasis by activating pathways associated with the farnesoid X receptor. Phytomedicine 2021, 80, 153378. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Ko, K.R.; Kim, H.K.; Lim, S.; Kim, S. Dehydrodiconiferyl alcohol promotes BMP-2-induced osteoblastogenesis through its agonistic effects on estrogen receptor. Biochem. Biophys. Res. Commun. 2018, 495, 2242–2248. [Google Scholar] [CrossRef] [PubMed]
- Tsuneyoshi, T.; Kanamori, Y.; Matsutomo, T.; Morihara, N. Dehydrodiconiferyl alcohol suppresses monocyte adhesion to endothelial cells by attenuation of JNK signaling pathway. Biochem. Biophys. Res. Commun. 2015, 465, 408–413. [Google Scholar] [CrossRef]
- Lee, J.; Choi, J.; Lee, W.; Ko, K.; Kim, S. Dehydrodiconiferyl alcohol (DHCA) modulates the differentiation of Th17 and Th1 cells and suppresses experimental autoimmune encephalomyelitis. Mol. Immunol. 2015, 68, 434–444. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S. Upregulation of heme oxygenase-1 expression by dehydrodiconiferyl alcohol (DHCA) through the AMPK–Nrf2 dependent pathway. Toxicol. Appl. Pharmacol. 2014, 281, 87–100. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.; Choi, J.; Choi, H.; Ryu, J.H.; Jeong, J.; Park, E.J.; Kim, S.H.; Kim, S. Dehydrodiconiferyl alcohol isolated from Cucurbita moschata shows anti-adipogenic and anti-lipogenic effects in 3T3-L1 cells and primary mouse embryonic fibroblasts. J. Biol. Chem. 2012, 287, 8839–8851. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; He, Z.; Yao, R.; Chen, Z.; Zeng, X.; Zheng, M.; Wang, J.; Li, J.; Jiang, Y. Preparation of Pinoresinol and Dehydrodiconiferyl Alcohol from Eucommiae Cortex Extract by Fermentation with Traditional Mucor. Molecules 2024, 29, 2979. https://doi.org/10.3390/molecules29132979
Jiang W, He Z, Yao R, Chen Z, Zeng X, Zheng M, Wang J, Li J, Jiang Y. Preparation of Pinoresinol and Dehydrodiconiferyl Alcohol from Eucommiae Cortex Extract by Fermentation with Traditional Mucor. Molecules. 2024; 29(13):2979. https://doi.org/10.3390/molecules29132979
Chicago/Turabian StyleJiang, Wenyi, Zhengyou He, Ruijiao Yao, Zhiyang Chen, Xia Zeng, Miao Zheng, Jing Wang, Jia Li, and Yong Jiang. 2024. "Preparation of Pinoresinol and Dehydrodiconiferyl Alcohol from Eucommiae Cortex Extract by Fermentation with Traditional Mucor" Molecules 29, no. 13: 2979. https://doi.org/10.3390/molecules29132979
APA StyleJiang, W., He, Z., Yao, R., Chen, Z., Zeng, X., Zheng, M., Wang, J., Li, J., & Jiang, Y. (2024). Preparation of Pinoresinol and Dehydrodiconiferyl Alcohol from Eucommiae Cortex Extract by Fermentation with Traditional Mucor. Molecules, 29(13), 2979. https://doi.org/10.3390/molecules29132979




