PyBox–La(OTf)3-Catalyzed Enantioselective Diels–Alder Cycloadditions of 2-Alkenoylpyridines with Cyclopentadiene
Abstract
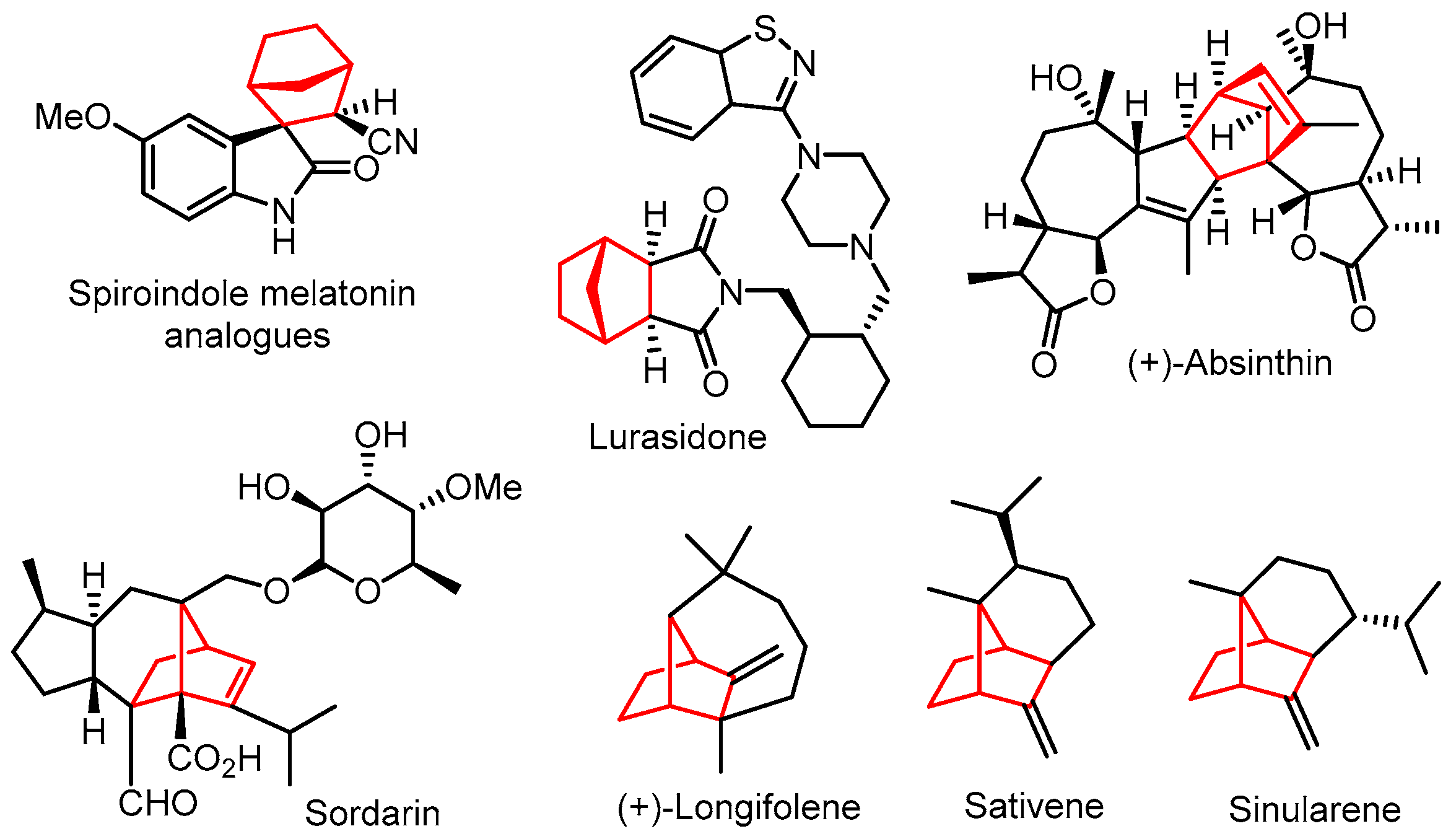
1. Introduction
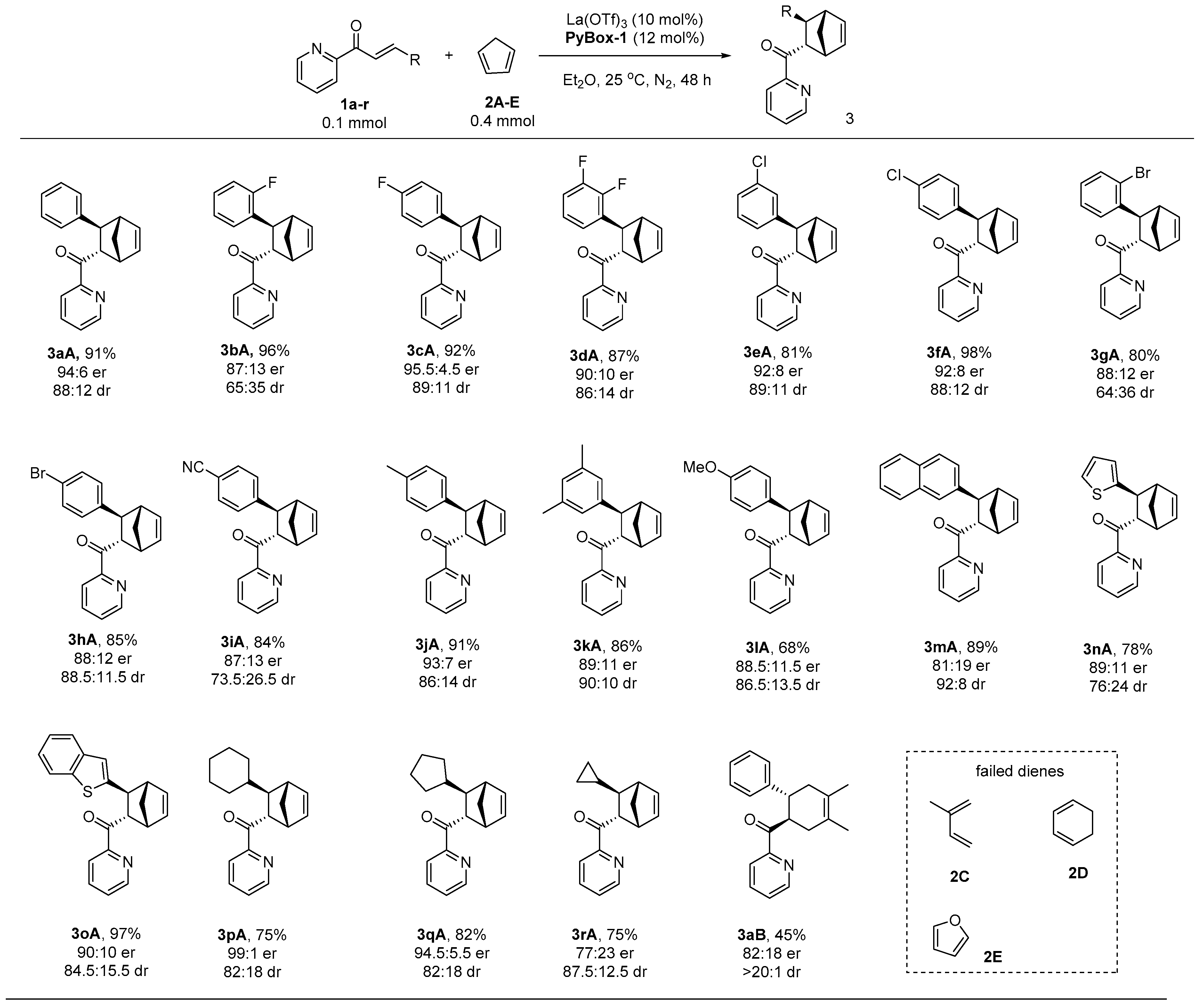
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Instruments
3.2. General Procedure for Reduction of 2-Alkenoylpyridines
3.2.1. (E)-3-(2,3-Difluorophenyl)-1-(pyridin-2-yl)prop-2-en-1-one (1d)
3.2.2. (E)-3-Cyclopentyl-1-(pyridin-2-yl)prop-2-en-1-one (1q)
3.3. General Procedure for Reduction of PyBim-1 and PyBox
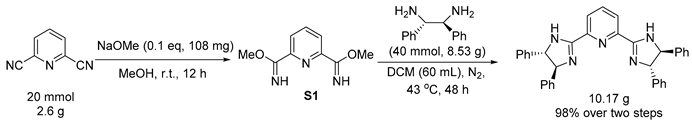
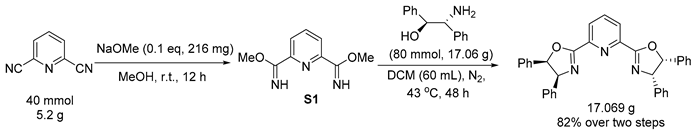
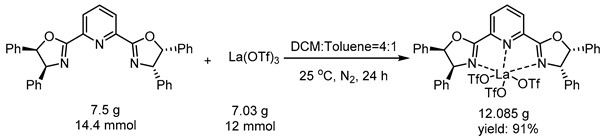
3.4. General Procedure for Reduction of Chiral Product 3
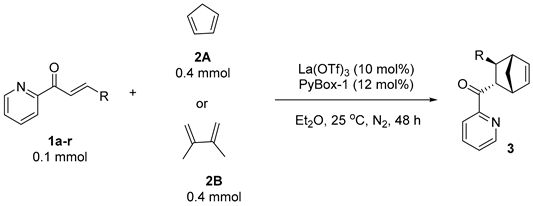
3.4.1. ((1R,2S,3S,4S)-3-Phenylbicyclo[2.2.1]hept-5-en-2-yl)(pyridin-2-yl)methanone (3aA)
3.4.2. ((1R,2S,3S,4S)-3-(2-Fluorophenyl)bicyclo[2.2.1]hept-5-en-2-yl)(pyridin-2-yl)methanone (3bA)
3.4.3. ((1R,2S,3S,4S)-3-(4-Fluorophenyl)bicyclo[2.2.1]hept-5-en-2-yl)(pyridin-2-yl)methanone (3cA)
3.4.4. ((1R,2S,3S,4S)-3-(2,3-Difluorophenyl)bicyclo[2.2.1]hept-5-en-2-yl)(pyridin-2-yl)methanone (3dA)
3.4.5. ((1S,2R,3R,4R)-3-(3-Chlorophenyl)bicyclo[2.2.1]hept-5-en-2-yl)(pyridin-2-yl)methanone (3eA)
3.4.6. ((1R,2S,3S,4S)-3-(4-Chlorophenyl)bicyclo[2.2.1]hept-5-en-2-yl)(pyridin-2-yl)methanone (3fA)
3.4.7. ((1R,2S,3S,4S)-3-(2-Bromophenyl)bicyclo[2.2.1]hept-5-en-2-yl)(pyridin-2-yl)methanone (3gA)
3.4.8. ((1R,2S,3S,4S)-3-(4-Bromophenyl)bicyclo[2.2.1]hept-5-en-2-yl)(pyridin-2-yl)methanone (3hA)
3.4.9. 4-((1S,2S,3S,4R)-3-Picolinoylbicyclo[2.2.1]hept-5-en-2-yl)benzonitrile (3iA)
3.4.10. Pyridin-2-yl((1R,2S,3S,4S)-3-(p-tolyl)bicyclo[2.2.1]hept-5-en-2-yl)methanone (3jA)
3.4.11. ((1R,2S,3S,4S)-3-(3,5-Dimethylphenyl)bicyclo[2.2.1]hept-5-en-2-yl)(pyridin-2-yl)methanone (3kA)
3.4.12. ((1R,2S,3S,4S)-3-(4-Methoxyphenyl)bicyclo[2.2.1]hept-5-en-2-yl)(pyridin-2-yl)methanone (3lA)
3.4.13. ((1R,2S,3S,4S)-3-(Naphthalen-2-yl)bicyclo[2.2.1]hept-5-en-2-yl)(pyridin-2-yl)methanone (3mA)
3.4.14. Pyridin-2-yl((1R,2S,3S,4S)-3-(thiophen-2-yl)bicyclo[2.2.1]hept-5-en-2-yl)methanone (3nA)
3.4.15. ((1R,2S,3S,4S)-3-(Benzo[b]thiophen-2-yl)bicyclo[2.2.1]hept-5-en-2-yl)(pyridin-2-yl)methanone (3oA)
3.4.16. ((1R,2S,3S,4S)-3-Cyclohexylbicyclo[2.2.1]hept-5-en-2-yl)(pyridin-2-yl)methanone (3pA)
3.4.17. ((1R,2S,3S,4S)-3-Cyclopentylbicyclo[2.2.1]hept-5-en-2-yl)(pyridin-2-yl)methanone (3qA)
3.4.18. ((1R,2S,3S,4S)-3-Cyclopropylbicyclo[2.2.1]hept-5-en-2-yl)(pyridin-2-yl)methanone (3rA)
3.4.19. ((1S,2S)-4,5-Dimethyl-1,2,3,6-tetrahydro-[1,1’-biphenyl]-2-yl)(pyridin-2-yl)methanone (3aB)
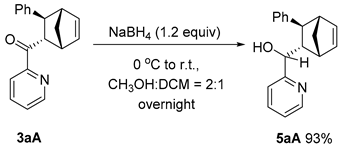
3.5. Synthesis and Characterization of Product 5

((1R,2R,4S,5S,6R,7S)-7-Phenyl-3-oxatricyclo[3.2.1.02,4]octan-6-yl)(pyridin-2-yl)methanone (5)
3.6. Synthesis and Characterization of Product 6
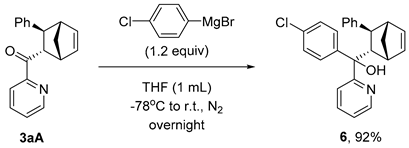
(4-Chlorophenyl)((1R,2S,3S,4S)-3-phenylbicyclo[2.2.1]hept-5-en-2-yl)(pyridin-2-yl)methanol (6)
3.7. Synthesis and Characterization of Product 7
(S)-((1R,2S,3S,4S)-3-Phenylbicyclo[2.2.1]hept-5-en-2-yl)(pyridin-2-yl)methanol (7)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Luo, S.; Fang, F.; Chen, Q.; Hu, H.; Jia, X.; Zhai, H. Total Synthesis of Absinthin. J. Am. Chem. Soc. 2005, 127, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Schule, A.; Liang, H.; Vors, J.P.; Ciufolini, M.A. Synthetic studies toward sordarin: Building blocks for the terpenoid core and for analogues thereof. J. Org. Chem. 2009, 74, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Antczak, K.; Kingston, J.F.; Fallis, A.G.; Hanson, A.W. A general intramolecular Diels–Alder approach to tricyclic sesquiterpenes: Stereoselective total syntheses of (±)-sinularene and (±)-5-epi-sinularene. Can. J. Chem. 1987, 65, 114–123. [Google Scholar] [CrossRef]
- Bo, L.; Fallis, A.G. Direct total synthesis of (+)-longifolene via an intramolecular Diels-Alder strategy. J. Am. Chem. Soc. 2002, 112, 4609–4610. [Google Scholar] [CrossRef]
- Lozinskaya, N.A.; Volkova, M.S.; Seliverstov, M.Y.; Temnov, V.V.; Sosonyuk, S.E.; Proskurnina, M.V.; Zefirov, N.S. Syntheses of spiroindole melatonin analogues via 2-(indolin-3-ylidene)acetonitrile cycloadditions. Mendeleev Commun. 2014, 24, 260–261. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Xu, Y.; Hu, W.; Zheng, G.; Zheng, L.; Ren, T. Synthesis and Tribological Behavior of Bridged Bicyclic Polymers as Lubricants. Ind. Eng. Chem. Res. 2020, 59, 20730–20739. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, R. Recent Developments in Catalytic Asymmetric Inverse-Electron-Demand Diels–Alder Reaction. Chem. Rev. 2013, 113, 5515–5546. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Boccaletti, G.; Engberts, J.B.F.N. A Chiral Lewis-Acid-Catalyzed Diels−Alder Reaction. Water-Enhanced Enantioselectivity. J. Am. Chem. Soc. 1998, 120, 4238–4239. [Google Scholar] [CrossRef]
- Barroso, S.; Blay, G.; Pedro, J.R. 2-alkenoyl pyridine N-oxides, highly efficient dienophiles for the enantioselective Cu(II)-bis(oxazoline) catalyzed Diels-Alder reaction. Org. Lett. 2007, 9, 1983–1986. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, Y.; Lin, L.; Zheng, H.; Fu, K.; Liu, X.; Feng, X. N,N′-Dioxide/nickel(ii)-catalyzed asymmetric Diels–Alder reaction of cyclopentadiene with 2,3-dioxopyrrolidines and 2-alkenoyl pyridines. Chem. Commun. 2016, 52, 8255–8258. [Google Scholar] [CrossRef]
- Meng, D.; Li, D.; Ollevier, T. Recyclable iron(ii) caffeine-derived ionic salt catalyst in the Diels-Alder reaction of cyclopentadiene and alpha,beta-unsaturated N-acyl-oxazolidinones in dimethyl carbonate. RSC Adv. 2019, 9, 21956–21963. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.; Fusetti, F.; Driessen, A.J.; Roelfes, G. Enantioselective artificial metalloenzymes by creation of a novel active site at the protein dimer interface. Angew. Chem. Int. Ed. 2012, 51, 7472–74725. [Google Scholar] [CrossRef] [PubMed]
- Filice, M.; Romero, O.; Gutierrez-Fernandez, J.; de Las Rivas, B.; Hermoso, J.A.; Palomo, J.M. Synthesis of a heterogeneous artificial metallolipase with chimeric catalytic activity. Chem. Commun. 2015, 51, 9324–9327. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Meng, Y.; Zhang, L.; Liu, M. Self-Assembled Single-Walled Metal-Helical Nanotube (M-HN): Creation of Efficient Supramolecular Catalysts for Asymmetric Reaction. J. Am. Chem. Soc. 2016, 138, 15629–15635. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.; Hao, J.; Cheng, M.; Jia, G.; Li, C. Higher-order human telomeric G-quadruplex DNA metalloenzyme catalyzed Diels-Alder reaction: An unexpected inversion of enantioselectivity modulated by K+ and NH4+ ions. Chem. Commun. 2015, 51, 13174–13177. [Google Scholar] [CrossRef] [PubMed]
- Marek, J.J.; Hennecke, U. Why DNA Is a More Effective Scaffold than RNA in Nucleic Acid-Based Asymmetric Catalysis-Supramolecular Control of Cooperative Effects. Chemistry 2017, 23, 6009–6013. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Okamura, I.; Sakashita, S.; Yum, J.H.; Acharya, C.; Gao, L.; Sugiyama, H. Development of DNA Metalloenzymes Using a Rational Design Approach and Application in the Asymmetric Diels–Alder Reaction. ACS Catal. 2015, 5, 4708–4712. [Google Scholar] [CrossRef]
- Fukuzawa, S.-I.; Metoki, K.; Esumi, S.-I. Asymmetric Diels–Alder reactions in supercritical carbon dioxide catalyzed by rare earth complexes. Tetrahedron 2003, 59, 10445–10452. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; Yang, Z. Iridium-Catalyzed Stereoselective Transfer Hydrogenation of 1,5-Benzodiazepines. J. Org. Chem. 2022, 87, 12001–12018. [Google Scholar] [CrossRef]
- Yang, S.; Tang, W.; Yang, Z.; Xu, J. Iridium-Catalyzed Highly Efficient and Site-Selective Deoxygenation of Alcohols. ACS Catal. 2018, 8, 9320–9326. [Google Scholar] [CrossRef]
- Wang, J.; Wang, T.; Du, H.; Chen, N.; Xu, J.; Yang, Z. Accessing para-Alkylphenols via Iridium-Catalyzed Site-Specific Deoxygenation of Alcohols. J. Org. Chem. 2023, 88, 12572–12584. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Miao, R.; Luo, R.; Xu, J.; Yang, Z. Furan-2-yl Anions as γ-Oxo/Hydroxyl Acyl Anion Equivalents Enabled by Iridium-Catalyzed Chemoselective Reduction. Org. Lett. 2023, 25, 4705–4710. [Google Scholar] [CrossRef] [PubMed]
- Bhor, S.; Anilkumar, G.; Tse, M.K.; Klawonn, M.; Döbler, C.; Bitterlich, B.; Grotevendt, A.; Beller, M. Synthesis of a New Chiral N,N,N-Tridentate Pyridinebisimidazoline Ligand Library and Its Application in Ru-Catalyzed Asymmetric Epoxidation. Org. Lett. 2005, 7, 3393–3396. [Google Scholar] [CrossRef] [PubMed]
- Anilkumar, G.; Bhor, S.; Tse, M.K.; Klawonn, M.; Bitterlich, B.; Beller, M. Synthesis of a novel class of chiral N,N,N-tridentate pyridinebisimidazoline ligands and their application in Ru-catalyzed asymmetric epoxidations. Tetrahedron Asymmetry 2005, 16, 3536–3561. [Google Scholar] [CrossRef]
- Mendoza, S.D.; Rombola, M.; Tao, Y.; Zuend, S.J.; Götz, R.; McLaughlin, M.J.; Reisman, S.E. Expanding the Chiral Monoterpene Pool: Enantioselective Diels–Alder Reactions of α-Acyloxy Enones. Org. Lett. 2022, 24, 3802–3806. [Google Scholar] [CrossRef] [PubMed]
- Desimoni, G.; Faita, G.; Guala, M.; Pratelli, C. An efficient catalyst for highly enantioselective exo-Diels–Alder reaction between alkenoyl-1,3-oxazolidin-2-ones and cyclopentadiene. Tetrahedron 2002, 58, 2929–2935. [Google Scholar] [CrossRef]
- Brown, H.C.; Kawakami, J.H.; Ikegami, S. Additions to bicyclic olefins. III. Stereochemistry of the epoxidation of norbornene, 7,7-dimethylnorbornene, and related bicyclic olefins. Steric effects in the 7,7-dimethylnorbornyl system. J. Am. Chem. Soc. 1970, 92, 6914–6917. [Google Scholar] [CrossRef]
- Ciupa, A.; Mahon, M.F.; De Bank, P.A.; Caggiano, L. Simple pyrazoline and pyrazole “turn on” fluorescent sensors selective for Cd2+ and Zn2+ in MeCN. Org. Biomol. Chem. 2012, 10, 8753–8757. [Google Scholar] [CrossRef]
- Nguyen, H.; Gagné, M.R. Enantioselective Cascade Cyclization/Protodemetalation of Polyenes with N3Pt2+ Catalysts. ACS Catal. 2014, 4, 855–859. [Google Scholar] [CrossRef]
- Meng, J.-C.; Fokin, V.V.; Finn, M.G. Kinetic resolution by copper-catalyzed azide–alkyne cycloaddition. Tetrahedron Lett. 2005, 46, 4543–4546. [Google Scholar] [CrossRef]
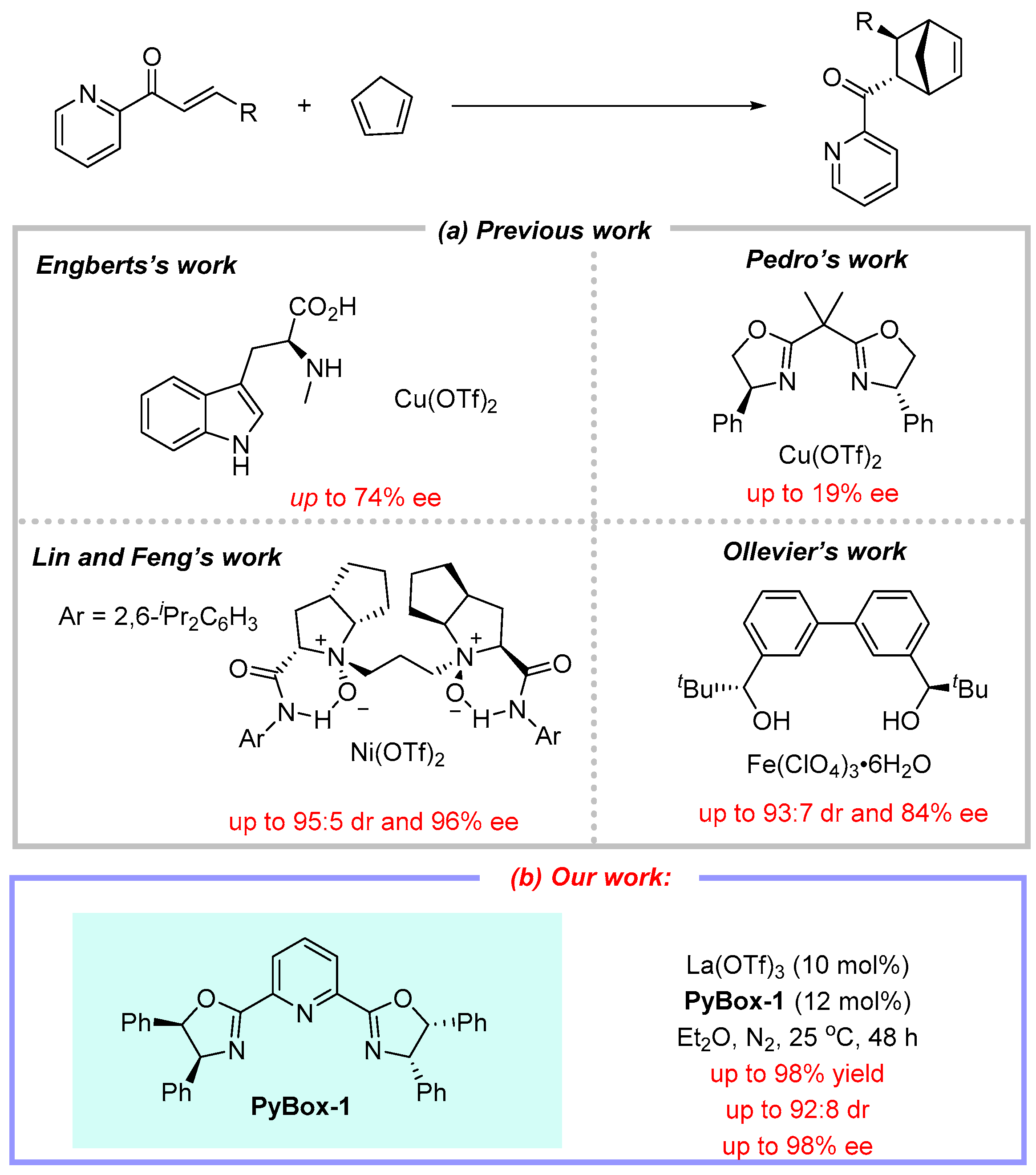


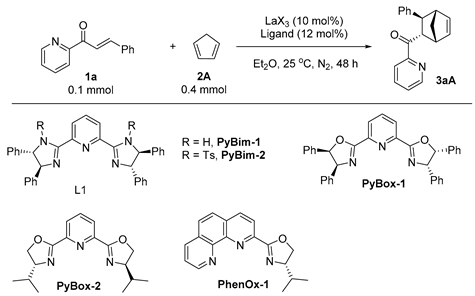 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Ligand | Yield a (%) | Er b | Dr b |
| 1 | La(OTf)3 | PyBim-1 | 54 | 84:16 | 51:49 |
| 2 | La(OTf)3 | PyBim-2 | 67 | 85.5:14.5 | 79.5:20.5 |
| 3 | La(OTf)3 | PyBox-1 | 91 | 94:6 | 88:12 |
| 4 | La(OTf)3 | PyBox-2 | 95 | 68:32 | 89:11 |
| 5 | La(OTf)3 | PhenOx-1 | 87 | 58:42 | 86.5:13.5 |
| 6 | LaCl3 | PyBox-1 | 48 | 51.5:48.5 | 84:16 |
| 7 | La(BF4)3 | PyBox-1 | 71 | 51:49 | 89:11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, H.; Zhang, Y.; Jin, S.; Yu, Y.; Chen, N.; Xu, J.; Yang, Z. PyBox–La(OTf)3-Catalyzed Enantioselective Diels–Alder Cycloadditions of 2-Alkenoylpyridines with Cyclopentadiene. Molecules 2024, 29, 2978. https://doi.org/10.3390/molecules29132978
Wei H, Zhang Y, Jin S, Yu Y, Chen N, Xu J, Yang Z. PyBox–La(OTf)3-Catalyzed Enantioselective Diels–Alder Cycloadditions of 2-Alkenoylpyridines with Cyclopentadiene. Molecules. 2024; 29(13):2978. https://doi.org/10.3390/molecules29132978
Chicago/Turabian StyleWei, Hao, Yujie Zhang, Sanlin Jin, Ying Yu, Ning Chen, Jiaxi Xu, and Zhanhui Yang. 2024. "PyBox–La(OTf)3-Catalyzed Enantioselective Diels–Alder Cycloadditions of 2-Alkenoylpyridines with Cyclopentadiene" Molecules 29, no. 13: 2978. https://doi.org/10.3390/molecules29132978
APA StyleWei, H., Zhang, Y., Jin, S., Yu, Y., Chen, N., Xu, J., & Yang, Z. (2024). PyBox–La(OTf)3-Catalyzed Enantioselective Diels–Alder Cycloadditions of 2-Alkenoylpyridines with Cyclopentadiene. Molecules, 29(13), 2978. https://doi.org/10.3390/molecules29132978







