Comprehensive Assessment of Collagen/Sodium Alginate-Based Sponges as Hemostatic Dressings
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of C/SA-Based Dressings
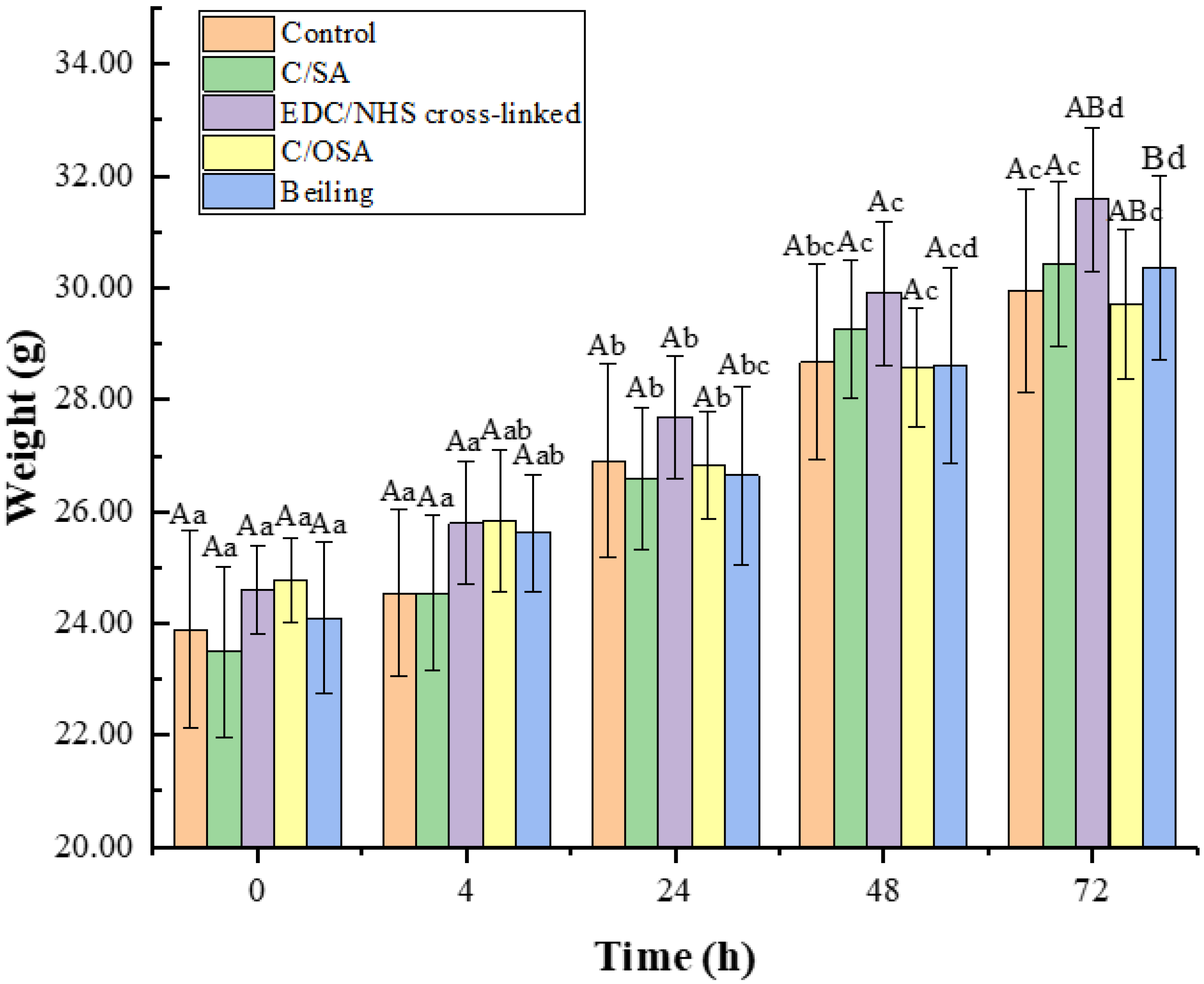
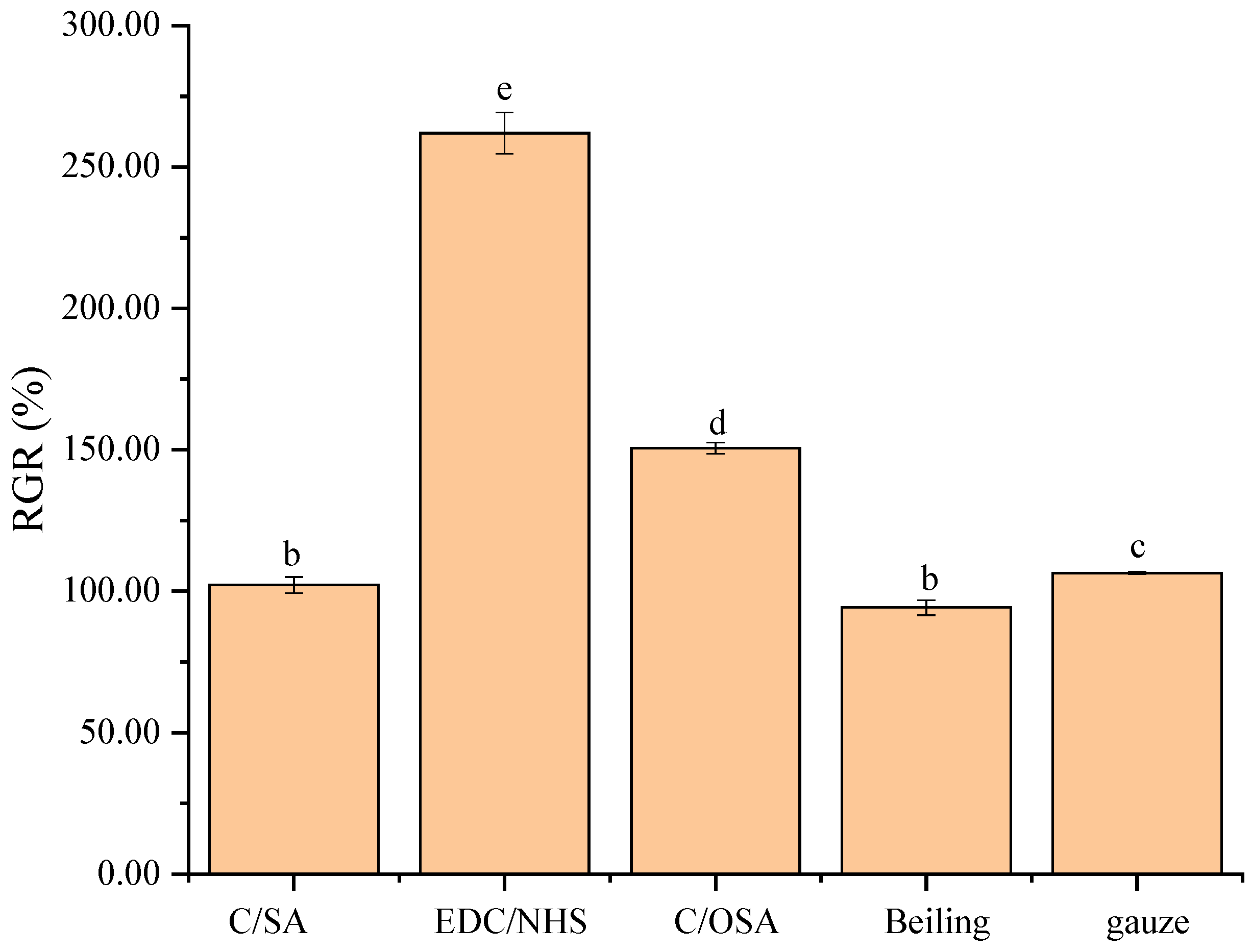
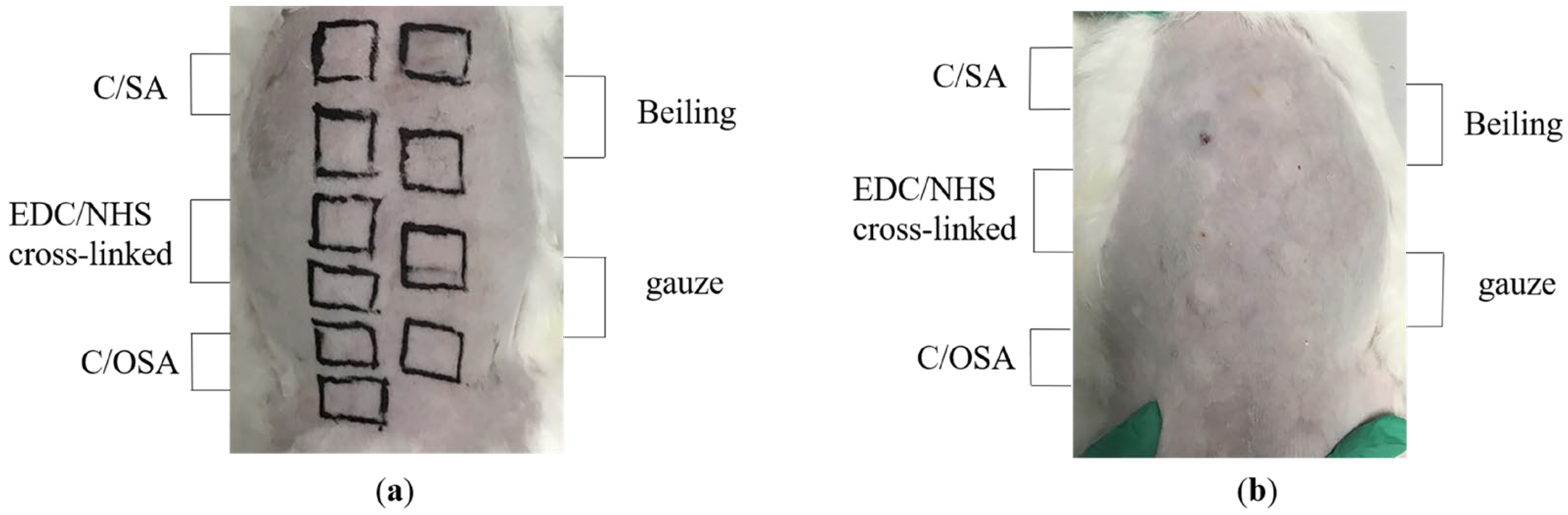
2.2. Biocompatibility of C/SA-Based Dressings
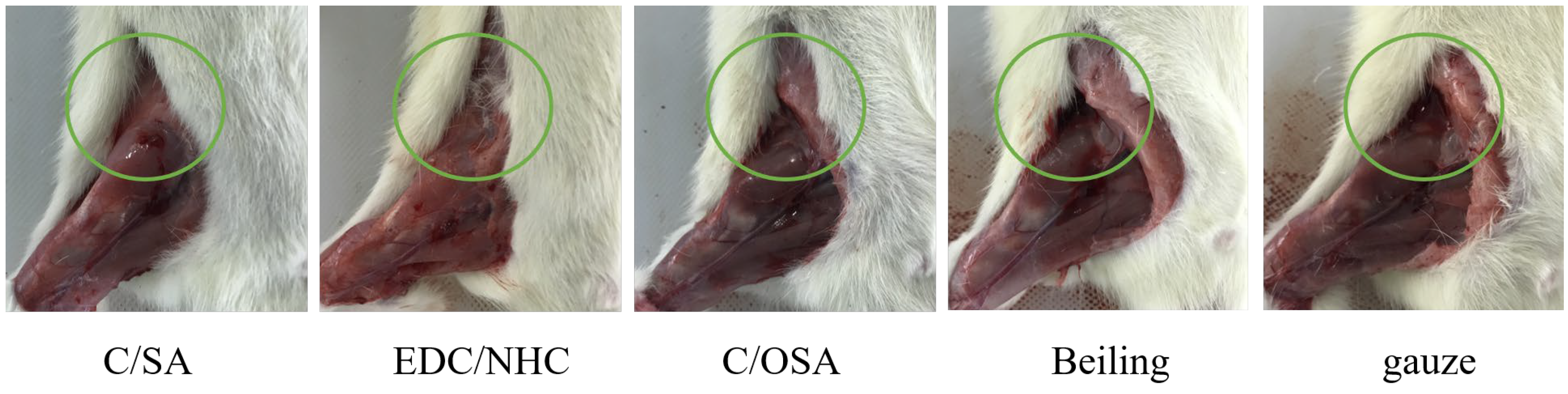
2.3. Hemostatic Evaluation In Vivo
3. Materials and Methods
3.1. Reagents and Experimental Materials
3.2. Fabrication of C/SA-Based Dressings
3.2.1. Fabrication of C/SA Dressings
3.2.2. Fabrication of EDC/NHS Cross-Linked C/SA Dressings
3.2.3. Fabrication of OSA and C/OSA Dressings
3.3. Physicochemical Properties of C/SA-Based Dressings
3.4. Biocompatibility Evaluation
3.4.1. Acute Systemic Toxicity Assay
3.4.2. Irritation Test
Dermal Irritation Test
Intradermal Reaction Test
Sensitization Test
3.4.3. Cytotoxicity In Vitro
3.5. In Vivo Model of Femoral Artery Hemorrhage
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bardes, J.M.; Inaba, K.; Schellenberg, M.; Grabo, D.; Strumwasser, A.; Matsushima, K.; Clark, D.; Brown, N.; Demetriades, D. The contemporary timing of trauma deaths. J. Trauma. Acute Care Surg. 2018, 84, 893–899. [Google Scholar] [CrossRef]
- Evans, J.A.; van Wessem, K.J.; McDougall, D.; Lee, K.A.; Lyons, T.; Balogh, Z.J. Epidemiology of traumatic deaths: Comprehensive population-based assessment. World J. Surg. 2010, 34, 158–163. [Google Scholar] [CrossRef]
- Kalkwarf, K.J.; Drake, S.A.; Yang, Y.; Thetford, C.; Myers, L.; Brock, M.; Wolf, D.A.; Persse, D.; Wade, C.E.; Holcomb, J.B. Bleeding to death in a big city: An analysis of all trauma deaths from hemorrhage in a metropolitan area during 1 year. J. Trauma Acute Care Surg. 2020, 89, 716–722. [Google Scholar] [CrossRef]
- Yang, X.; Liu, W.; Li, N.; Wang, M.; Liang, B.; Ullah, I.; Luis Neve, A.; Feng, Y.; Chen, H.; Shi, C. Design and development of polysaccharide hemostatic materials and their hemostatic mechanism. Biomater. Sci. 2017, 5, 2357–2368. [Google Scholar] [CrossRef]
- Duarte, A.P.; Coelho, J.F.; Bordado, J.C.; Cidade, M.T.; Gil, M.H. Surgical adhesives: Systematic review of the main types and development forecast. Prog. Polym. Sci. 2012, 37, 1031–1050. [Google Scholar] [CrossRef]
- Khan, M.A.; Mujahid, M. A review on recent advances in chitosan based composite for hemostatic dressings. Int. J. Biol. Macromol. 2019, 124, 138–147. [Google Scholar] [CrossRef]
- Cheng, X.; Shao, Z.; Li, C.; Yu, L.; Raja, M.A.; Liu, C. Isolation, Characterization and Evaluation of Collagen from Jellyfish Rhopilema esculentum Kishinouye for Use in Hemostatic Applications. PLoS ONE 2017, 12, e0169731. [Google Scholar] [CrossRef]
- Hajosch, R.; Suckfuell, M.; Oesser, S.; Ahlers, M.; Flechsenhar, K.; Schlosshauer, B. A novel gelatin sponge for accelerated hemostasis. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 372–379. [Google Scholar] [CrossRef]
- Xie, X.; Li, D.; Chen, Y.; Shen, Y.; Yu, F.; Wang, W.; Yuan, Z.; Morsi, Y.; Wu, J.; Mo, X. Conjugate Electrospun 3D Gelatin Nanofiber Sponge for Rapid Hemostasis. Adv. Healthc. Mater. 2021, 10, e2100918. [Google Scholar] [CrossRef]
- Chen, H.; Shang, X.; Yu, L.; Xiao, L.; Fan, J. Safety evaluation of a low-heat producing zeolite granular hemostatic dressing in a rabbit femoral artery hemorrhage model. J. Biomater. Appl. 2020, 34, 988–997. [Google Scholar] [CrossRef]
- Wang, L.; Hao, F.; Tian, S.; Dong, H.; Nie, J.; Ma, G. Targeting polysaccharides such as chitosan, cellulose, alginate and starch for designing hemostatic dressings. Carbohydr. Polym. 2022, 291, 119574. [Google Scholar] [CrossRef]
- Chowdhry, S.A.; Nieves-Malloure, Y.; Camardo, M.; Robertson, J.M.; Keys, J. Use of oxidised regenerated cellulose/collagen dressings versus standard of care over multiple wound types: A systematic review and meta-analysis. Int. Wound J. 2022, 19, 241–252. [Google Scholar] [CrossRef]
- Loffroy, R.; Mouillot, T.; Bardou, M.; Chevallier, O. Current role of cyanoacrylate glue transcatheter embolization in the treatment of acute nonvariceal gastrointestinal bleeding. Expert. Rev. Gastroenterol. Hepatol. 2020, 14, 975–984. [Google Scholar] [CrossRef]
- Guo, B.; Dong, R.; Liang, Y.; Li, M. Haemostatic materials for wound healing applications. Nat. Rev. Chem. 2021, 5, 773–791. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Nourbakhsh, N.; Akbari Kenari, M.; Zare, M.; Ramakrishna, S. Collagen-based biomaterials for biomedical applications. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1986–1999. [Google Scholar] [CrossRef]
- Lebbink, R.J.; de Ruiter, T.; Adelmeijer, J.; Brenkman, A.B.; van Helvoort, J.M.; Koch, M.; Farndale, R.W.; Lisman, T.; Sonnenberg, A.; Lenting, P.J.; et al. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J. Exp. Med. 2006, 203, 1419–1425. [Google Scholar] [CrossRef]
- Courtenay, J.C.; Sharma, R.I.; Scott, J.L. Recent Advances in Modified Cellulose for Tissue Culture Applications. Molecules 2018, 23, 654. [Google Scholar] [CrossRef]
- Ruszczak, Z. Effect of collagen matrices on dermal wound healing. Adv. Drug Deliv. Rev. 2003, 55, 1595–1611. [Google Scholar] [CrossRef]
- Aszodi, A.; Legate, K.R.; Nakchbandi, I.; Fassler, R. What mouse mutants teach us about extracellular matrix function. Annu. Rev. Cell Dev. Biol. 2006, 22, 591–621. [Google Scholar] [CrossRef]
- D’Amico, E.; Pierfelice, T.V.; Lepore, S.; Iezzi, G.; D’Arcangelo, C.; Piattelli, A.; Covani, U.; Petrini, M. Hemostatic Collagen Sponge with High Porosity Promotes the Proliferation and Adhesion of Fibroblasts and Osteoblasts. Int. J. Mol. Sci. 2023, 24, 7749. [Google Scholar] [CrossRef]
- He, Y.; Wang, J.; Si, Y.; Wang, X.; Deng, H.; Sheng, Z.; Li, Y.; Liu, J.; Zhao, J. A novel gene recombinant collagen hemostatic sponge with excellent biocompatibility and hemostatic effect. Int. J. Biol. Macromol. 2021, 178, 296–305. [Google Scholar] [CrossRef]
- Shekhter, A.B.; Fayzullin, A.L.; Vukolova, M.N.; Rudenko, T.G.; Osipycheva, V.D.; Litvitsky, P.F. Medical applications of collagen and collagen-based materials. Curr. Med. Chem. 2019, 26, 506–516. [Google Scholar] [CrossRef]
- Khajouei, R.A.; Keramat, J.; Hamdami, N.; Ursu, A.V.; Delattre, C.; Laroche, C.; Gardarin, C.; Lecerf, D.; Desbrieres, J.; Djelveh, G.; et al. Extraction and characterization of an alginate from the Iranian brown seaweed Nizimuddinia zanardini. Int. J. Biol. Macromol. 2018, 118, 1073–1081. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Li, Y.; Han, Y.; Wang, X.; Peng, J.; Xu, Y.; Chang, J. Multifunctional Hydrogels Prepared by Dual Ion Cross-Linking for Chronic Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 16054–16062. [Google Scholar] [CrossRef]
- Singh Chandel, A.K.; Ohta, S.; Taniguchi, M.; Yoshida, H.; Tanaka, D.; Omichi, K.; Shimizu, A.; Isaji, M.; Hasegawa, K.; Ito, T. Balance of antiperitoneal adhesion, hemostasis, and operability of compressed bilayer ultrapure alginate sponges. Biomater. Adv. 2022, 137, 212825. [Google Scholar] [CrossRef]
- Xie, M.; Zeng, Y.; Wu, H.; Wang, S.; Zhao, J. Multifunctional carboxymethyl chitosan/oxidized dextran/sodium alginate hydrogels as dressing for hemostasis and closure of infected wounds. Int. J. Biol. Macromol. 2022, 219, 1337–1350. [Google Scholar] [CrossRef]
- Chen, Y.W.; Lu, C.H.; Shen, M.H.; Lin, S.Y.; Chen, C.H.; Chuang, C.K.; Ho, C.C. In vitro evaluation of the hyaluronic acid/alginate composite powder for topical haemostasis and wound healing. Int. Wound J. 2020, 17, 394–404. [Google Scholar] [CrossRef]
- Lv, C.; Li, L.; Jiao, Z.; Yan, H.; Wang, Z.; Wu, Z.; Guo, M.; Wang, Y.; Zhang, P. Improved hemostatic effects by Fe(3+) modified biomimetic PLLA cotton-like mat via sodium alginate grafted with dopamine. Bioact. Mater. 2021, 6, 2346–2359. [Google Scholar] [CrossRef]
- Lee, S.M.; Park, I.K.; Kim, Y.S.; Kim, H.J.; Moon, H.; Mueller, S.; Jeong, Y.I. Physical, morphological, and wound healing properties of a polyurethane foam-film dressing. Biomater. Res. 2016, 20, 15. [Google Scholar] [CrossRef]
- Cheng, F.; Liu, C.; Wei, X.; Yan, T.; Li, H.; He, J.; Huang, Y. Preparation and Characterization of 2,2,6,6-Tetramethylpiperidine-1-oxyl (TEMPO)-Oxidized Cellulose Nanocrystal/Alginate Biodegradable Composite Dressing for Hemostasis Applications. ACS Sustain. Chem. Eng. 2017, 5, 3819–3828. [Google Scholar] [CrossRef]
- Chandel, A.K.S.; Shimizu, A.; Hasegawa, K.; Ito, T. Advancement of Biomaterial-Based Postoperative Adhesion Barriers. Macromol. Biosci. 2021, 21, e2000395. [Google Scholar] [CrossRef]
- Jin, S.; Sun, F.; Zou, Q.; Huang, J.; Zuo, Y.; Li, Y.; Wang, S.; Cheng, L.; Man, Y.; Yang, F.; et al. Fish Collagen and Hydroxyapatite Reinforced Poly(lactide- co-glycolide) Fibrous Membrane for Guided Bone Regeneration. Biomacromolecules 2019, 20, 2058–2067. [Google Scholar] [CrossRef]
- Liu, S.; Lau, C.-S.; Liang, K.; Wen, F.; Teoh, S.H. Marine collagen scaffolds in tissue engineering. Curr. Opin. Biotechnol. 2022, 74, 92–103. [Google Scholar] [CrossRef]
- Lim, Y.S.; Ok, Y.J.; Hwang, S.Y.; Kwak, J.Y.; Yoon, S. Marine Collagen as A Promising Biomaterial for Biomedical Applications. Mar. Drugs 2019, 17, 467. [Google Scholar] [CrossRef]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish Collagen: Extraction, Characterization, and Applications for Biomaterials Engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef]
- Xu, N.; Peng, X.-L.; Li, H.-R.; Liu, J.-X.; Cheng, J.-S.-Y.; Qi, X.-Y.; Ye, S.-J.; Gong, H.-L.; Zhao, X.-H.; Yu, J. Marine-derived collagen as biomaterials for human health. Front. Nutr. 2021, 8, 702108. [Google Scholar] [CrossRef]
- Nepal, A.; Tran, H.D.N.; Nguyen, N.T.; Ta, H.T. Advances in haemostatic sponges: Characteristics and the underlying mechanisms for rapid haemostasis. Bioact. Mater. 2023, 27, 231–256. [Google Scholar] [CrossRef]
- Li, H.; Tan, C.; Li, L. Review of 3D printable hydrogels and constructs. Mater. Des. 2018, 159, 20–38. [Google Scholar] [CrossRef]
- Wei, Q.; Zhou, J.; An, Y.; Li, M.; Zhang, J.; Yang, S. Modification, 3D printing process and application of sodium alginate based hydrogels in soft tissue engineering: A review. Int. J. Biol. Macromol. 2023, 232, 123450. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Davidenko, N.; Bax, D.V.; Schuster, C.F.; Farndale, R.W.; Hamaia, S.W.; Best, S.M.; Cameron, R.E. Optimisation of UV irradiation as a binding site conserving method for crosslinking collagen-based scaffolds. J. Mater. Sci. Mater. Med. 2016, 27, 14. [Google Scholar] [CrossRef]
- Nair, M.; Best, S.M.; Cameron, R.E. Crosslinking Collagen Constructs: Achieving Cellular Selectivity Through Modifications of Physical and Chemical Properties. Appl. Sci. 2020, 10, 6911. [Google Scholar] [CrossRef]
- Bax, D.V.; Davidenko, N.; Hamaia, S.W.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Impact of UV- and carbodiimide-based crosslinking on the integrin-binding properties of collagen-based materials. Acta Biomater. 2019, 100, 280–291. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Liu, A.; Wang, W. Improved thermal-stability and mechanical properties of type I collagen by crosslinking with casein, keratin and soy protein isolate using transglutaminase. Int. J. Biol. Macromol. 2017, 98, 292–301. [Google Scholar] [CrossRef]
- Jin, J.; Ji, Z.; Xu, M.; Liu, C.; Ye, X.; Zhang, W.; Li, S.; Wang, D.; Zhang, W.; Chen, J.; et al. Microspheres of Carboxymethyl Chitosan, Sodium Alginate, and Collagen as a Hemostatic Agent in Vivo. ACS Biomater. Sci. Eng. 2018, 4, 2541–2551. [Google Scholar] [CrossRef]
- Huang, H.; Chen, H.; Wang, X.; Qiu, F.; Liu, H.; Lu, J.; Tong, L.; Yang, Y.; Wang, X.; Wu, H. Degradable and Bioadhesive Alginate-Based Composites: An Effective Hemostatic Agent. ACS Biomater. Sci. Eng. 2019, 5, 5498–5505. [Google Scholar] [CrossRef]
- Gu, L.; Shan, T.; Ma, Y.-x.; Tay, F.R.; Niu, L. Novel Biomedical Applications of Crosslinked Collagen. Trends Biotechnol. 2019, 37, 464–491. [Google Scholar] [CrossRef]
- Li, S.; Wu, P.; Ji, Z.; Zhang, Y.; Zhang, P.; He, Y.; Shen, Y. In vitro biocompatibility study of EDC/NHS cross-linked silk fibroin scaffold with olfactory ensheathing cells. J. Biomater. Sci. Polym. Ed. 2023, 34, 482–496. [Google Scholar] [CrossRef]
- Sun, L.; Shen, Y.; Li, M.; Wang, Q.; Li, R.; Gong, S. Preparation and Modification of Collagen/Sodium Alginate-Based Biomedical Materials and Their Characteristics. Polymers 2024, 16, 171. [Google Scholar] [CrossRef]
- Rong, J.J.; Liang, M.; Xuan, F.Q.; Sun, J.Y.; Zhao, L.J.; Zheng, H.Z.; Tian, X.X.; Liu, D.; Zhang, Q.Y.; Peng, C.F.; et al. Thrombin-loaded alginate-calcium microspheres: A novel hemostatic embolic material for transcatheter arterial embolization. Int. J. Biol. Macromol. 2017, 104, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Gantenbein-Ritter, B.; Sakai, D. Biomaterials for Intervertebral Disc Regeneration. In Comprehensive Biomaterials; Elsevier: Amsterdam, The Netherlands, 2011; pp. 161–169. [Google Scholar] [CrossRef]
- Tsuyukubo, A.; Kubota, R.; Sato, Y.; Fujimoto, I. The Toughness-Enhanced Atelocollagen Double-Network Gel for Biomaterials. Polymers 2024, 16, 283. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.J.; Liang, M.; Xuan, F.Q.; Sun, J.Y.; Zhao, L.J.; Zhen, H.Z.; Tian, X.X.; Liu, D.; Zhang, Q.Y.; Peng, C.F.; et al. Alginate-calcium microsphere loaded with thrombin: A new composite biomaterial for hemostatic embolization. Int. J. Biol. Macromol. 2015, 75, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Rostamzadeh, P.; Nooryshokry, N.; Le, H.C.; Golzarian, J. In vitro and in vivo evaluation of biodegradable embolic microspheres with tunable anticancer drug release. Acta Biomater. 2013, 9, 6823–6833. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices-Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Bax, D.V.; Davidenko, N.; Gullberg, D.; Hamaia, S.W.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Fundamental insight into the effect of carbodiimide crosslinking on cellular recognition of collagen-based scaffolds. Acta Biomater. 2017, 49, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Seon, G.M.; Lee, M.H.; Kwon, B.J.; Kim, M.S.; Koo, M.A.; Kim, D.; Seomun, Y.; Kim, J.T.; Park, J.C. Functional improvement of hemostatic dressing by addition of recombinant batroxobin. Acta Biomater. 2017, 48, 175–185. [Google Scholar] [CrossRef]
- Sun, L.; Li, B.; Song, W.; Zhang, K.; Fan, Y.; Hou, H. Comprehensive assessment of Nile tilapia skin collagen sponges as hemostatic dressings. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110532. [Google Scholar] [CrossRef] [PubMed]
- Stuart, S.M.; Zarow, G.; Walchak, A.; McLean, J.; Roszko, P. Pilot Study of a Novel Swine Model for Controlling Junctional Hemorrhage Using the iTClamp in Conjunction With Hemostatic Agents. Mil. Med. 2019, 184, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Yang, Y.; Zhang, S.; Liu, K.; Chen, J. Multifunctional dopamine modification of green antibacterial hemostatic sponge. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 127, 112227. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, R.; Wang, H.; Li, Y.; Fu, C. Application of chitosan-based materials in surgical or postoperative hemostasis. Front. Mater. 2022, 9, 994265. [Google Scholar] [CrossRef]
- Teng, K.; An, Q.A.-O.; Chen, Y.; Zhang, Y.A.-O.; Zhao, Y. Recent Development of Alginate-Based Materials and Their Versatile Functions in Biomedicine, Flexible Electronics, and Environmental Uses. ACS Biomater. Sci. Eng. 2021, 7, 1302–1337. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.L.; Hou, H.; Li, B.F.; Zhang, Y. Characterization of acid- and pepsin-soluble collagen extracted from the skin of Nile tilapia (Oreochromis niloticus). Int. J. Biol. Macromol. 2017, 99, 8–14. [Google Scholar] [CrossRef] [PubMed]
- The State Pharmacopoeia Commission of P.R.China. China Pharmacopeia 2015, fourth section, Pharmaceutical Excipients. 2017. [Google Scholar]
- ISO 10993-11:2017; Biological Evaluation of Medical Devices-Part 11: Tests for Systemic Toxicity. ISO: Geneva, Switzerland, 2017.
- ISO 10993-10:2021; Biological Evaluation of Medical Devices-Part 10: Tests for Irritation and Skin Sensitization. ISO: Geneva, Switzerland, 2021.
- Wu, B.; Du, F.; A, W.; Liu, F.; Liu, Y.; Zheng, W.; Li, G.; Wang, X. Graphene-ophicalcite heterogeneous composite sponge for rapid hemostasis. Colloids Surf. B Biointerfaces 2022, 216, 112596. [Google Scholar] [CrossRef] [PubMed]


| C/SA | EDC/NHS Cross-Linked C/SA | C/OSA | |
|---|---|---|---|
| Loss on drying (%) | 16.30 ± 0.09 | 13.52 ± 0.42 | 16.77 ± 0.01 |
| Total ash content (%) | 9.17 ± 0.22 | 0.29 ± 0.02 | 6.99 ± 0.12 |
| Carbodiimide residue (mg) | - | 0 | - |
| C/SA | EDC/NHS Cross-Linked C/SA | C/OSA | Beiling | Gauze | |
|---|---|---|---|---|---|
| Dermal irritation test | |||||
| 24 h | 0 | 0 | 0 | 0 | 0 |
| 48 h | 0 | 0 | 0 | 0 | 0 |
| 72 h | 0 | 0 | 0 | 0 | 0 |
| PII | 0 | 0 | 0 | 0 | 0 |
| Intradermal reaction test | |||||
| 24 h | 0 | 0 | 0 | 0 | 0 |
| 48 h | 0 | 0 | 0 | 0 | 0 |
| 72 h | 0 | 0 | 0 | 0 | 0 |
| PII | 0 | 0 | 0 | 0 | 0 |
| Sensitization test | |||||
| 24 h | 0 | 0 | 0 | 0 | 0 |
| 48 h | 0 | 0 | 0 | 0 | 0 |
| 72 h | 0 | 0 | 0 | 0 | 0 |
| Blood Clotting Time (s) | Blood Loss (g) | Success Rate of Hemostasis (%) | |
|---|---|---|---|
| C/SA | 140 ± 8.94 a | 1.24 ± 0.04 a | 100 |
| EDC/NHS cross-linked | 132.5 ± 12.82 a | 1.11 ± 0.07 a | 100 |
| C/OSA | 138.75 ± 8.35 a | 1.03 ± 0.08 a | 100 |
| Beiling | 165.71 ± 12.72 b | 1.38 ± 0.07 a | 100 |
| Gauze | 251.43 ± 10.69 c | 1.78 ± 0.08 b | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Shen, Y.; Li, M.; Wang, Q.; Li, R.; Gong, S. Comprehensive Assessment of Collagen/Sodium Alginate-Based Sponges as Hemostatic Dressings. Molecules 2024, 29, 2999. https://doi.org/10.3390/molecules29132999
Sun L, Shen Y, Li M, Wang Q, Li R, Gong S. Comprehensive Assessment of Collagen/Sodium Alginate-Based Sponges as Hemostatic Dressings. Molecules. 2024; 29(13):2999. https://doi.org/10.3390/molecules29132999
Chicago/Turabian StyleSun, Leilei, Yanyan Shen, Mingbo Li, Qiuting Wang, Ruimin Li, and Shunmin Gong. 2024. "Comprehensive Assessment of Collagen/Sodium Alginate-Based Sponges as Hemostatic Dressings" Molecules 29, no. 13: 2999. https://doi.org/10.3390/molecules29132999





