A Recyclable Polypropylene Multilayer Film Maintaining the Quality and the Aroma of Coffee Pods during Their Shelf Life
Abstract
1. Introduction
2. Results and Discussion
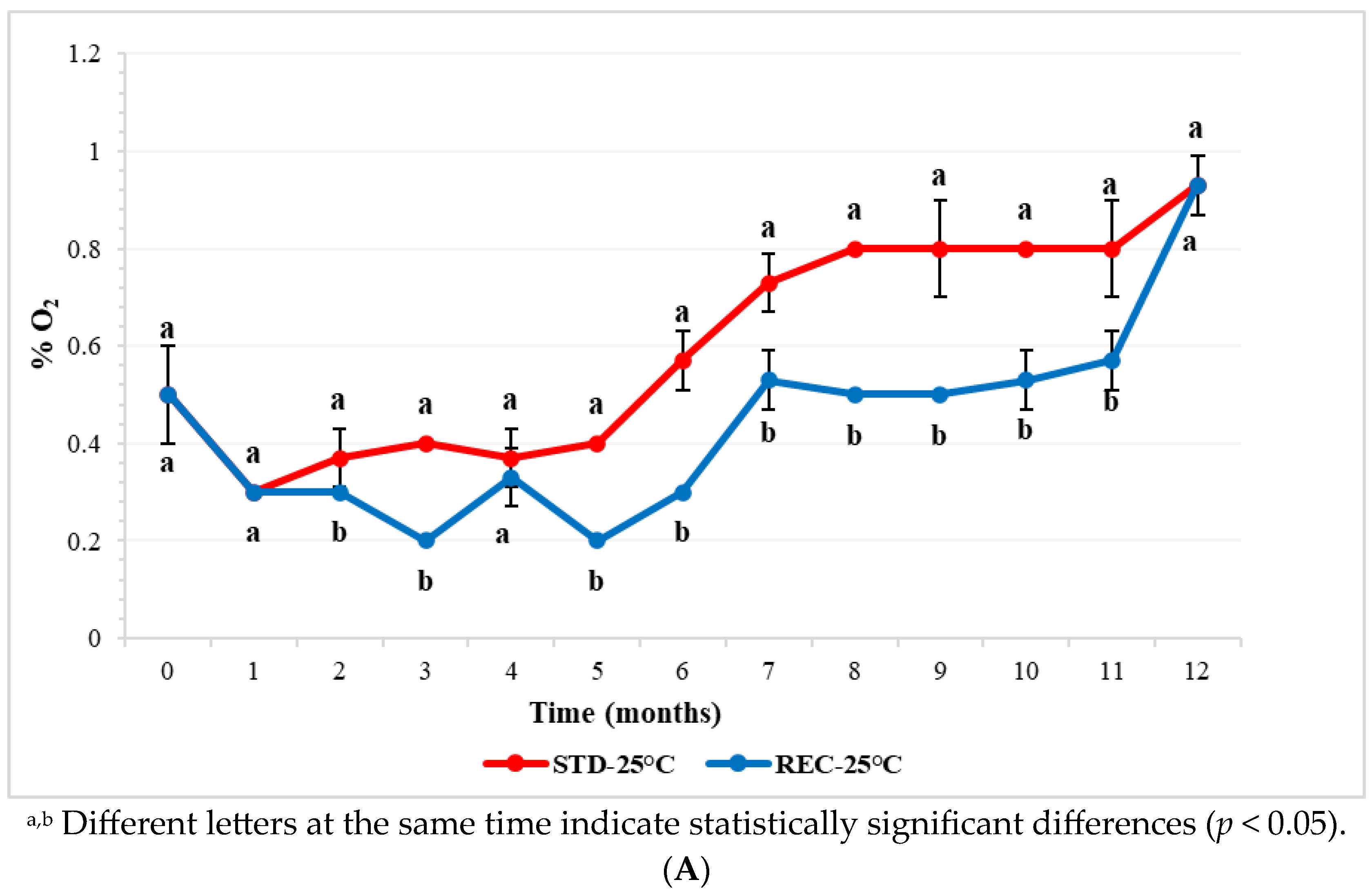
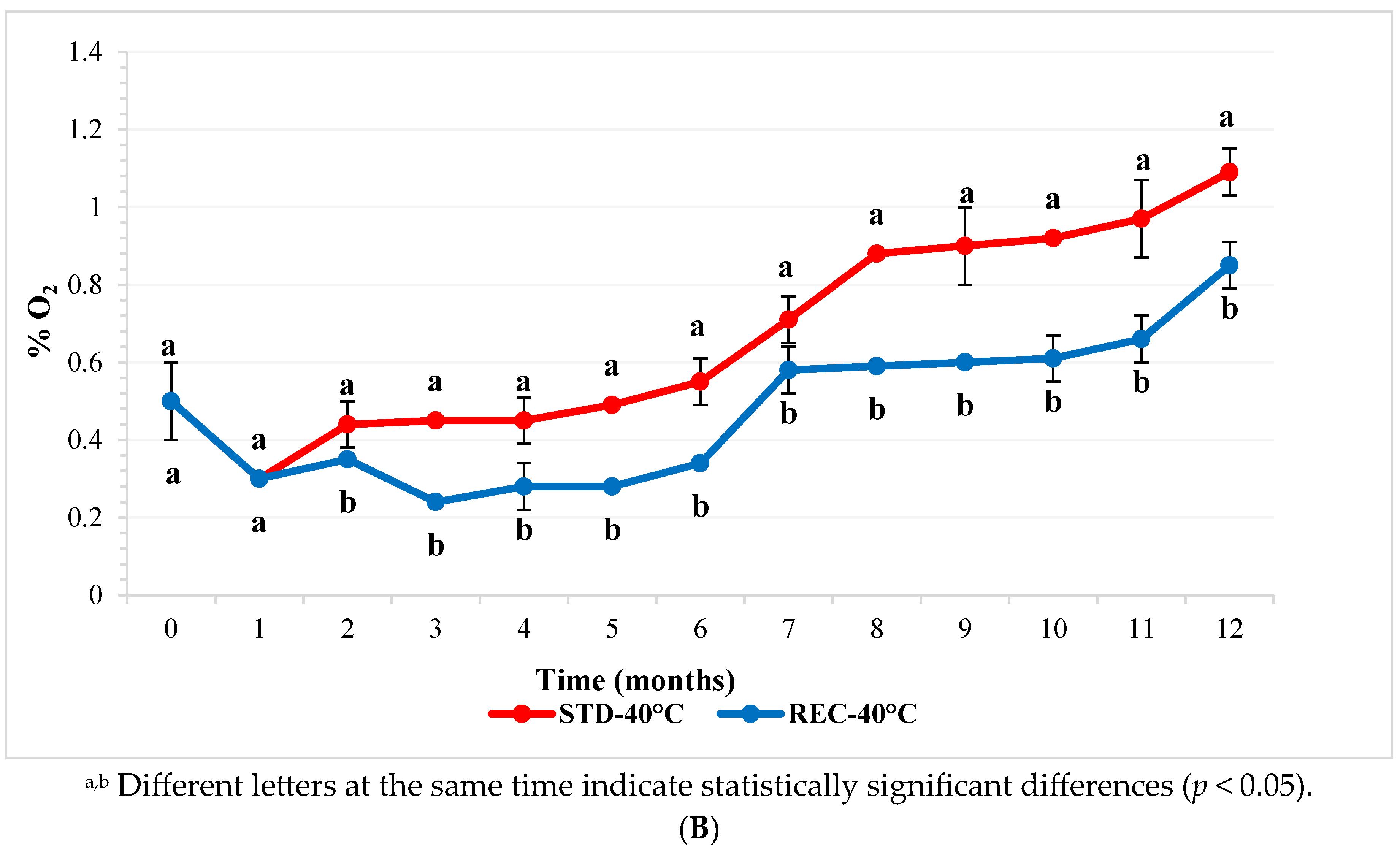
2.1. Oxygen in Coffee Pod Headspace
2.2. Moisture in Coffee Pods
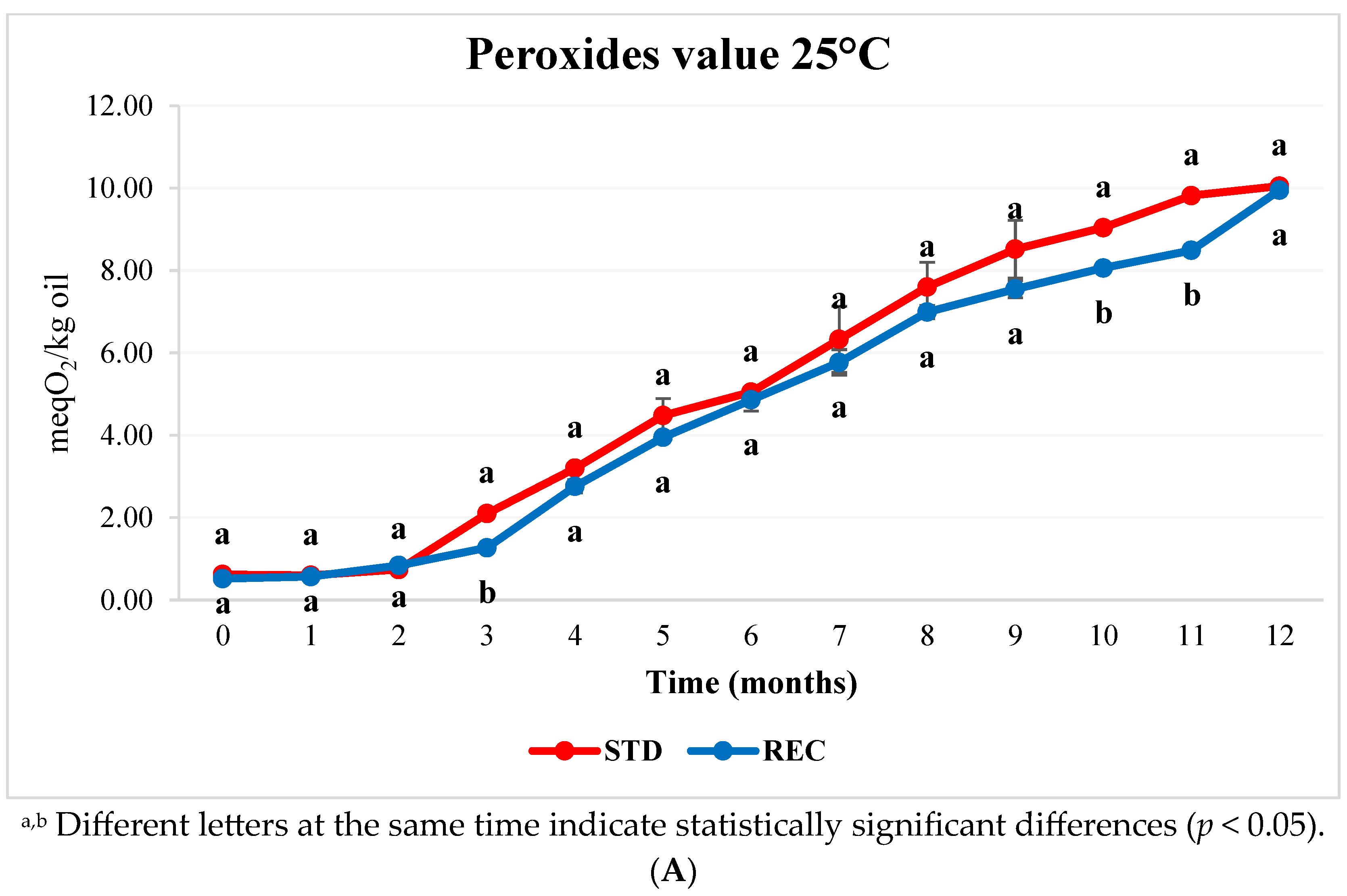
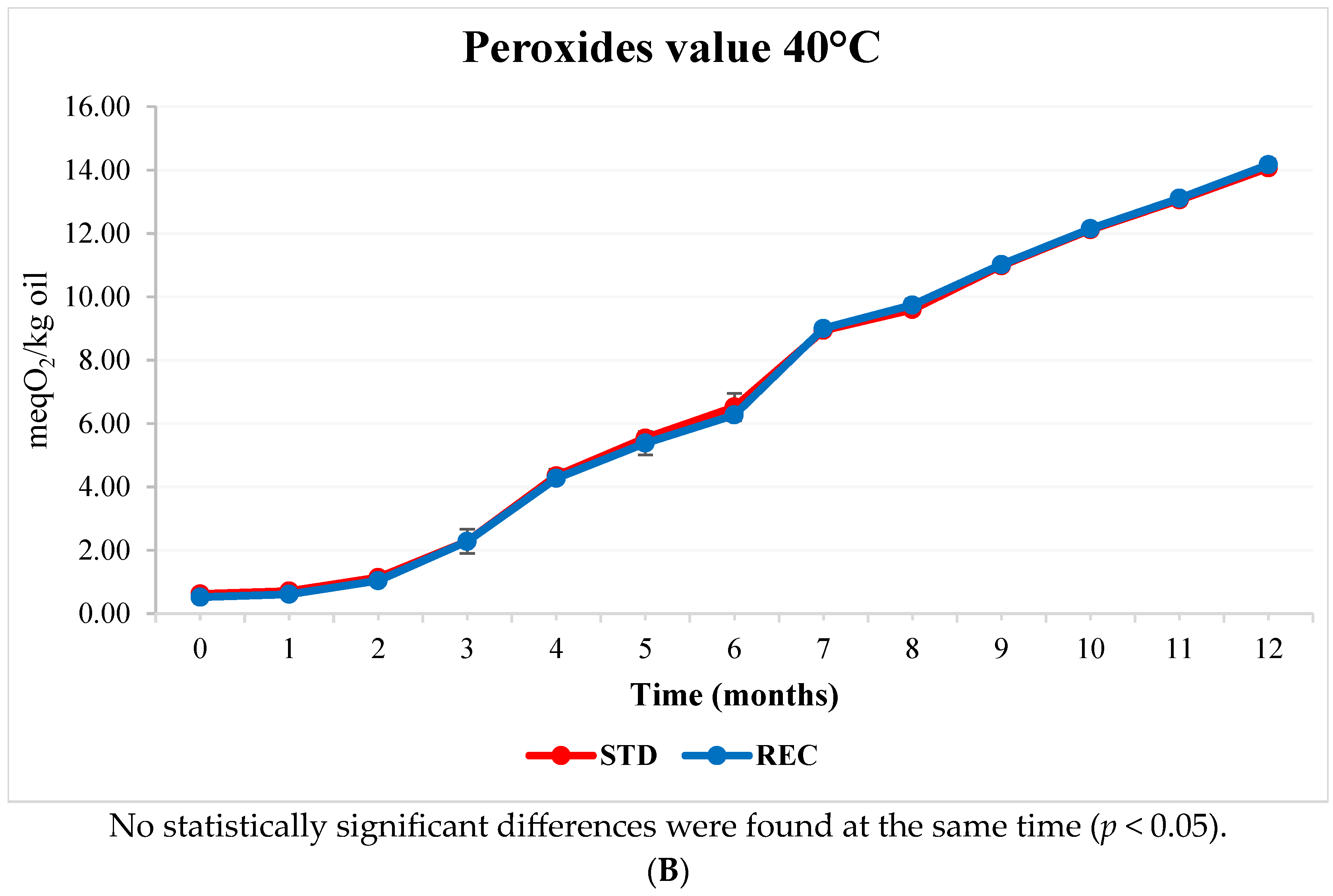
2.3. Fat and Peroxide Values in Coffee Pods
2.4. Volatile Organic Compounds in Coffee Pods
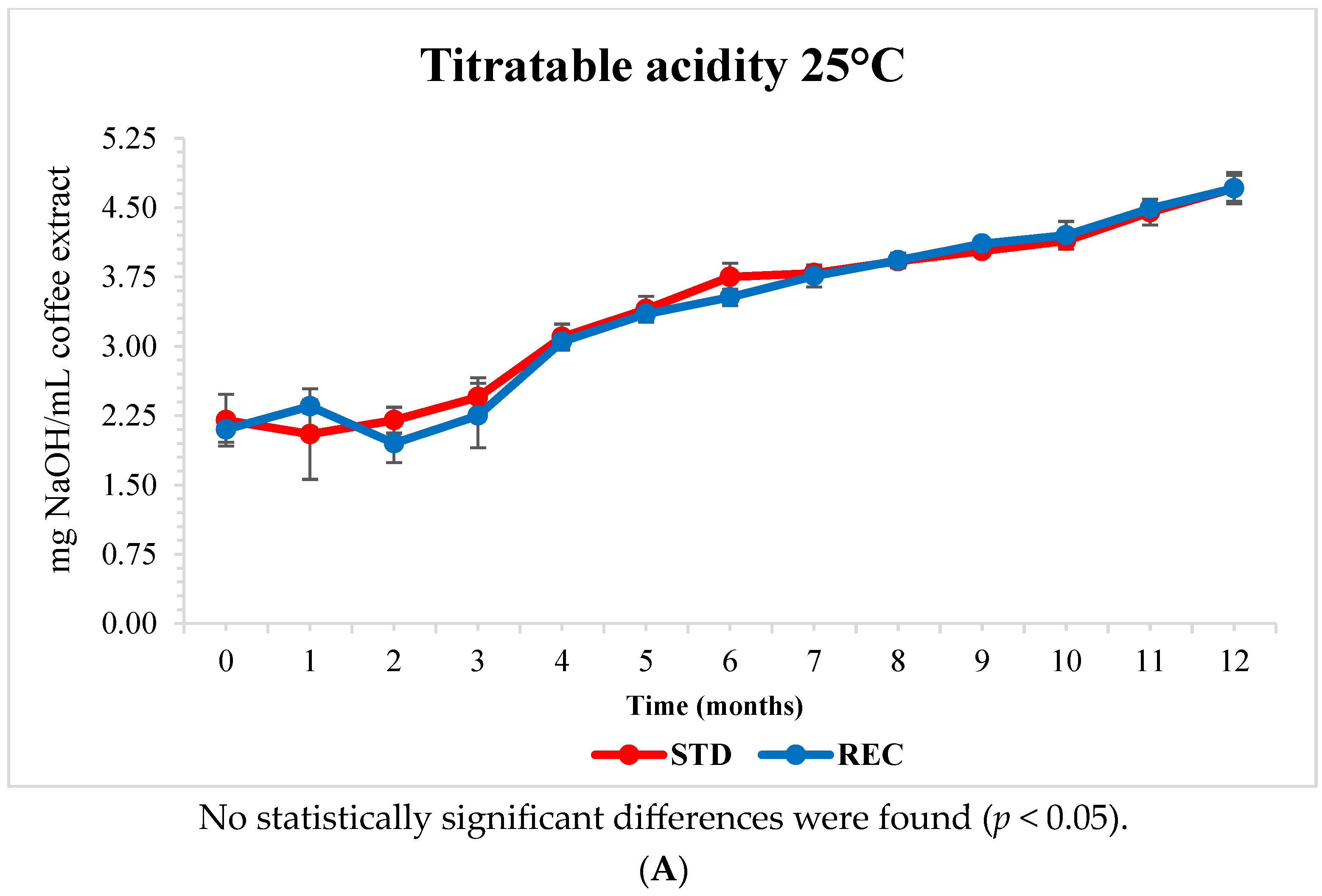
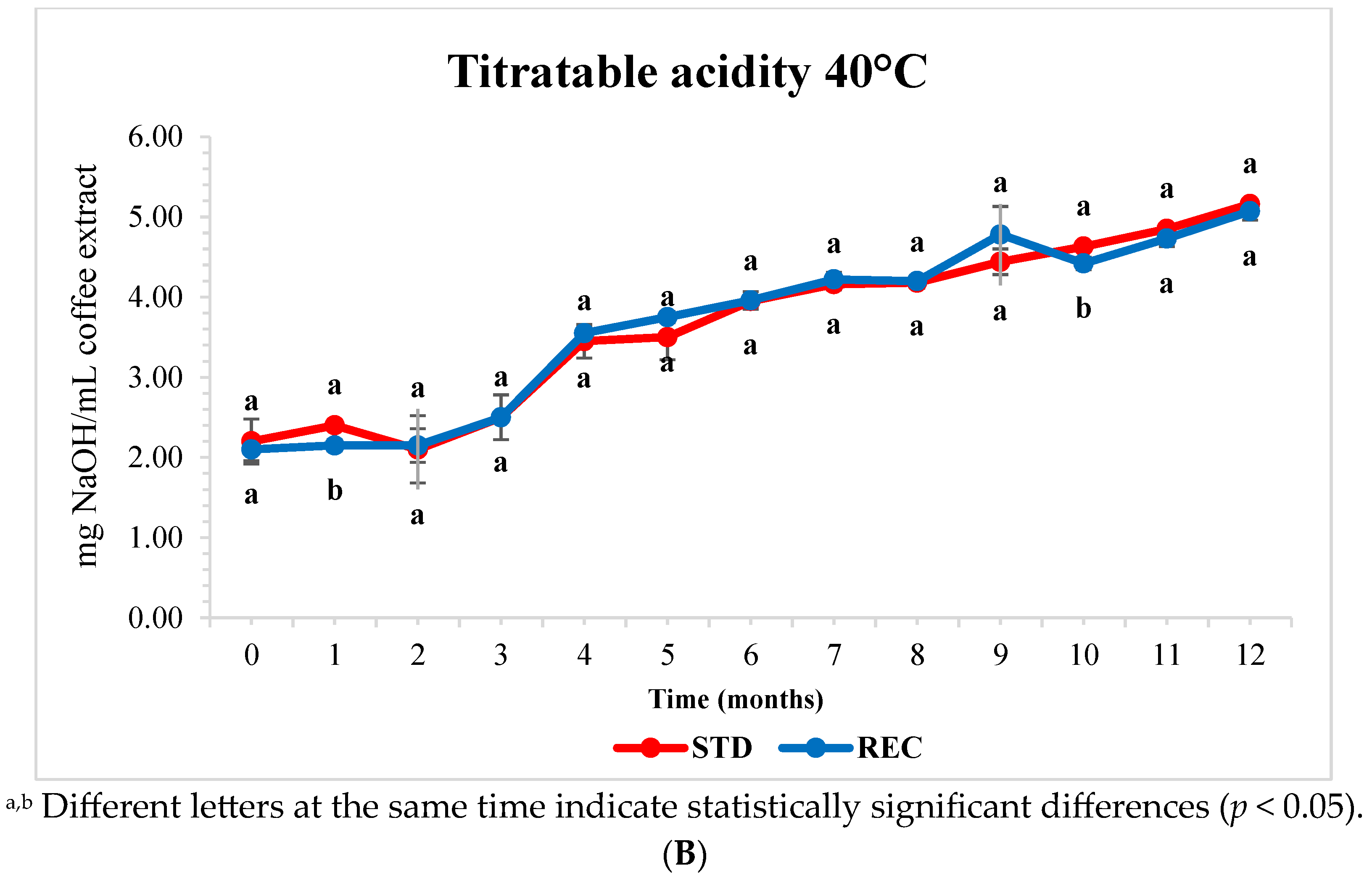
2.5. pH and Titratable Acidity of Extracted Coffee
2.6. Acceptability of Extracted Coffee
3. Materials and Methods
3.1. Chemicals
3.2. Samples
3.3. Percentage of Oxygen during Shelf Life
3.4. Moisture Determination
3.5. Fat Extraction
3.6. Determination of Peroxide Value (PV)
3.7. Volatile Organic Compounds (VOCs)
3.8. pH and Titratable Acidity in the Extracted Coffee
3.9. Sensory Evaluation of Extracted Coffee
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melese, Y.Y.; Kolech, S.A. Coffee (Coffea arabica L.): Methods, Objectives, and Future Strategies of Breeding in Ethiopia—Review. Sustainability 2021, 13, 10814. [Google Scholar] [CrossRef]
- van der Vossen, H.; Bertrand, B.; Charrier, A. Next Generation Variety Development for Sustainable Production of Arabica Coffee (Coffea arabica L.): A Review. Euphytica 2015, 204, 243–256. [Google Scholar] [CrossRef]
- Visser, R.; Dlamini, S. Green Purchasing Behaviour towards Compostable Coffee Pods. Sustainability 2021, 13, 6558. [Google Scholar] [CrossRef]
- Li, J. Comparative Life Cycle Assessment of Single-Serve Coffee Packaging in Ontario. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2018. [Google Scholar]
- Thoden van Velzen, E.U.; Goyal, B.; Barouta, D.; Brouwer, M.T.; Smeding, I.W. Sustainability Assessment of Different Types of Coffee Capsules; Wageningen Food & Biobased Research: Wageningen, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Tonelli, A.; Vignali, G.; Mosna, D. Comparative Life Cycle Assessment of Different Packaging Systems for Coffee Capsules; University of Parma: Parma, Italy, 2019. [Google Scholar] [CrossRef]
- Kooduvalli, K.; Vaidya, U.K.; Ozcan, S. Life Cycle Assessment of Compostable Coffee Pods: A US University Based Case Study. Sci. Rep. 2020, 10, 9158. [Google Scholar] [CrossRef] [PubMed]
- Allahvaisi, S. Polypropylene in the Industry of Food Packaging. In Polypropylene; IntechOpen: London, UK, 2012; ISBN 978-953-51-0636-4. [Google Scholar]
- Gahleitner, M.; Paulik, C. Chapter 11—Polypropylene and Other Polyolefins. In Brydson’s Plastics Materials, 8th ed.; Gilbert, M., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 279–309. ISBN 978-0-323-35824-8. [Google Scholar]
- Maddah, H.A. Polypropylene as a Promising Plastic: A Review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar]
- Ward, I.M. Structure and Properties of Oriented Polymers; Springer Science & Business Media: Berlin, Germany, 2012; ISBN 978-94-011-5844-2. [Google Scholar]
- Alcock, B.; Cabrera, N.O.; Barkoula, N.-M.; Wang, Z.; Peijs, T. The Effect of Temperature and Strain Rate on the Impact Performance of Recyclable All-Polypropylene Composites. Compos. Part B Eng. 2008, 39, 537–547. [Google Scholar] [CrossRef]
- Cozzolino, C.A.; Pozzoli, S.; La Vecchia, S.; Piergiovanni, L.; Farris, S. An Alternative Approach to Control the Oxygen Permeation across Single-Dose Coffee Capsules. Food Packag. Shelf Life 2015, 4, 19–25. [Google Scholar] [CrossRef]
- Yeretzian, C.; Blank, I.; Wyser, Y. Chapter 14—Protecting the Flavors—Freshness as a Key to Quality. In The Craft and Science of Coffee; Folmer, B., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 329–353. ISBN 978-0-12-803520-7. [Google Scholar]
- Yeretzian, C.; Jordan, A.; Badoud, R.; Lindinger, W. From the Green Bean to the Cup of Coffee: Investigating Coffee Roasting by on-Line Monitoring of Volatiles. Eur. Food Res. Technol. 2002, 214, 92–104. [Google Scholar] [CrossRef]
- Schenker, S.; Heinemann, C.; Huber, M.; Pompizzi, R.; Perren, R.; Escher, R. Impact of Roasting Conditions on the Formation of Aroma Compounds in Coffee Beans. J. Food Sci. 2002, 67, 60–66. [Google Scholar] [CrossRef]
- Leelaphiwat, P.; Auras, R.A.; Harte, J.B.; Ong, P.K.C.; Chonhenchob, V. Barrier Properties of Polymeric Packaging Materials to Major Aroma Volatiles in Herbs. MATEC Web Conf. 2016, 67, 06100. [Google Scholar] [CrossRef]
- Trenzová, K.; Gross, M.; Vítová, E.; Pořízka, J.; Diviš, P. Exploring the Impact of Different Packaging Types and Repeated Package Opening on Volatile Compound Changes in Ground Roasted Coffee. J. Microbiol. Biotechnol. Food Sci. 2024, e11022. [Google Scholar] [CrossRef]
- Gloess, A.; Schönbächler, B.; Rast, M.; Deuber, L.; Yeretzian, C. Freshness Indices of Roasted Coffee: Monitoring the Loss of Freshness for Single Serve Capsules and Roasted Whole Beans in Different Packaging. Chimia 2014, 68, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Toci, A.T.; Neto, V.J.M.F.; Torres, A.G.; Farah, A. Changes in Triacylglycerols and Free Fatty Acids Composition during Storage of Roasted Coffee. LWT—Food Sci. Technol. 2013, 50, 581–590. [Google Scholar] [CrossRef]
- Illy, A.; Viani, R. Espresso Coffee: The Science of Quality; Academic Press: San Diego, CA, USA, 2005; ISBN 978-0-12-370371-2. [Google Scholar]
- Nicoli, M.C.; Manzocco, L.; Calligaris, S. Packaging and Shelflife of Coffee|PDF|Coffee|Shelf Life. Available online: https://www.scribd.com/document/534164323/Packaging-and-shelflife-of-coffee (accessed on 20 May 2024).
- Baggenstoss, J.; Perren, R.; Escher, F. Water Content of Roasted Coffee: Impact on Grinding Behaviour, Extraction, and Aroma Retention. Eur. Food Res. Technol. 2008, 227, 1357–1365. [Google Scholar] [CrossRef][Green Version]
- Agustini, S.; Yusya, M.K. The Effect of Packaging Materials on the Physicochemical Stability of Ground Roasted Coffee. Curr. Res. Biosci. Biotechnol. 2020, 1, 66–70. [Google Scholar] [CrossRef]
- International Coffee Organization—International Coffee Council (2017/18)/Council-17-18-e.Asp. Available online: https://www.ico.org/Council-17-18-e.asp (accessed on 20 May 2024).
- Rubayiza, A.B.; Meurens, M. Chemical Discrimination of Arabica and Robusta Coffees by Fourier Transform Raman Spectroscopy. J. Agric. Food Chem. 2005, 53, 4654–4659. [Google Scholar] [CrossRef]
- Cong, S.; Dong, W.; Zhao, J.; Hu, R.; Long, Y.; Chi, X. Characterization of the Lipid Oxidation Process of Robusta Green Coffee Beans and Shelf Life Prediction during Accelerated Storage. Molecules 2020, 25, 1157. [Google Scholar] [CrossRef]
- Basile, G.; De Luca, L.; Calabrese, M.; Lambiase, G.; Pizzolongo, F.; Romano, R. The Lipidic and Volatile Components of Coffee Pods and Capsules Packaged in an Alternative Multilayer Film. Foods 2024, 13, 759. [Google Scholar] [CrossRef]
- Gruczyńska, E.; Kowalska, D.; Kozłowska, M.; Majewska, E.; Tarnowska, K. Furan in Roasted, Ground and Brewed Coffee. Rocz. Panstw. Zakl. Hig. 2018, 69, 111–118. [Google Scholar]
- Guenther, H.; Hoenicke, K.; Biesterveld, S.; Gerhard-Rieben, E.; Lantz, I. Furan in Coffee: Pilot Studies on Formation during Roasting and Losses during Production Steps and Consumer Handling. Food Addit. Contam. Part A 2010, 27, 283–290. [Google Scholar] [CrossRef]
- Starowicz, M.; Zieliński, H. How Maillard Reaction Influences Sensorial Properties (Color, Flavor and Texture) of Food Products? Food Rev. Int. 2019, 35, 707–725. [Google Scholar] [CrossRef]
- Hashim, L.; Chaveron, H. Use of Methylpyrazine Ratios to Monitor the Coffee Roasting. Food Res. Int. 1995, 28, 619–623. [Google Scholar] [CrossRef]
- Mahmud, M.M.C.; Keast, R.; Mohebbi, M.; Shellie, R.A. Identifying Aroma-Active Compounds in Coffee-Flavored Dairy Beverages. J. Food Sci. 2022, 87, 982–997. [Google Scholar] [CrossRef]
- Moon, J.-K.; Yoo, H.S.; Shibamoto, T. Role of Roasting Conditions in the Level of Chlorogenic Acid Content in Coffee Beans: Correlation with Coffee Acidity. J. Agric. Food Chem. 2009, 57, 5365–5369. [Google Scholar] [CrossRef]
- Gancarz, M.; Dobrzański, B.; Malaga-Toboła, U.; Tabor, S.; Combrzyński, M.; Ćwikła, D.; Strobel, W.R.; Oniszczuk, A.; Karami, H.; Darvishi, Y.; et al. Impact of Coffee Bean Roasting on the Content of Pyridines Determined by Analysis of Volatile Organic Compounds. Molecules 2022, 27, 1559. [Google Scholar] [CrossRef]
- Toledo, P.R.A.B.; Pezza, L.; Pezza, H.R.; Toci, A.T. Relationship Between the Different Aspects Related to Coffee Quality and Their Volatile Compounds. Compr. Rev. Food Sci. Food Saf. 2016, 15, 705–719. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Magalhães Júnior, A.I.; Vásquez, Z.S.; Medeiros, A.B.P.; Vandenberghe, L.P.S.; Soccol, C.R. Exploring the Impacts of Postharvest Processing on the Aroma Formation of Coffee Beans—A Review. Food Chem. 2019, 272, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Zakidou, P.; Plati, F.; Matsakidou, A.; Varka, E.-M.; Blekas, G.; Paraskevopoulou, A. Single Origin Coffee Aroma: From Optimized Flavor Protocols and Coffee Customization to Instrumental Volatile Characterization and Chemometrics. Molecules 2021, 26, 4609. [Google Scholar] [CrossRef]
- Rodrigues, C.I.; Marta, L.; Maia, R.; Miranda, M.; Ribeirinho, M.; Máguas, C. Application of Solid-Phase Extraction to Brewed Coffee Caffeine and Organic Acid Determination by UV/HPLC. J. Food Compos. Anal. 2007, 20, 440–448. [Google Scholar] [CrossRef]
- Yeager, S.E.; Batali, M.E.; Guinard, J.-X.; Ristenpart, W.D. Acids in Coffee: A Review of Sensory Measurements and Meta-Analysis of Chemical Composition. Crit. Rev. Food Sci. Nutr. 2023, 63, 1010–1036. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Martínez, M.; Caemmerer, B.; De Peña, M.P.; Cid, C.; Kroh, L.W. Influence of Brewing Method and Acidity Regulators on the Antioxidant Capacity of Coffee Brews. J. Agric. Food Chem. 2010, 58, 2958–2965. [Google Scholar] [CrossRef] [PubMed]
- AOAC 965.33 Peroxide Value|PDF|Titration|Chemistry. Available online: https://it.scribd.com/document/501494648/AOAC-965-33-Peroxide-Value (accessed on 6 March 2024).
- Bertrand, B.; Boulanger, R.; Dussert, S.; Ribeyre, F.; Berthiot, L.; Descroix, F.; Joët, T. Climatic Factors Directly Impact the Volatile Organic Compound Fingerprint in Green Arabica Coffee Bean as Well as Coffee Beverage Quality. Food Chem. 2012, 135, 2575–2583. [Google Scholar] [CrossRef]
- Gloess, A.N.; Schönbächler, B.; Klopprogge, B.; D`Ambrosio, L.; Chatelain, K.; Bongartz, A.; Strittmatter, A.; Rast, M.; Yeretzian, C. Comparison of Nine Common Coffee Extraction Methods: Instrumental and Sensory Analysis. Eur. Food Res. Technol. 2013, 236, 607–627. [Google Scholar] [CrossRef]
- Stokes, C.N.; O’Sullivan, M.G.; Kerry, J.P. Hedonic and Descriptive Sensory Evaluation of Instant and Fresh Coffee Products. Eur. Food Res. Technol. 2017, 243, 331–340. [Google Scholar] [CrossRef]
| 25 °C | 40 °C | |||
|---|---|---|---|---|
| Time (Months) | STD | REC | STD | REC |
| 0 | 1.15 ± 0.09 | 1.21 ± 0.10 | 1.18 ± 0.07 | 1.17 ± 0.11 |
| 3 | 1.75 ± 0.11 | 1.68 ± 0.09 | 1.64 ± 0.09 | 1.56 ± 0.10 |
| 6 | 2.05 ± 0.13 | 1.99 ± 0.08 | 1.85 ± 0.11 | 1.81 ± 0.07 |
| 9 | 2.33 ± 0.09 | 2.15 ± 0.11 | 2.01 ± 0.06 | 1.98 ± 0.09 |
| 12 | 2.51 ± 0.10 | 2.37 ± 0.12 | 2.14 ± 0.10 | 2.11 ± 0.08 |
| Time (Months) | 0 | 0 | 4 | 4 | 8 | 8 | 12 | 12 |
|---|---|---|---|---|---|---|---|---|
| STD | REC | STD | REC | STD | REC | |||
| Σ Furans | 43.36 ± 0.29 a | 43.38 ± 1.03 a | 41.85 ± 0.34 a | 37.14 ± 1.04 b | 35.61 ± 0.93 a | 32.65 ± 2.54 b | 37.03 ± 0.56 a | 35.44 ± 0.74 b |
| 2-Furanmethanol | 28.66 ± 0.19 a | 28.25 ± 0.97 a | 27.97 ± 0.30 a | 21.80 ± 1.32 b | 23.40 ± 0.11 a | 21.30 ± 0.25 b | 23.47 ± 0.55 a | 21.54 ± 0.75 a |
| 2-Furanmethanol acetate | 5.18 ± 0.10 a | 5.32 ± 0.05 a | 5.13 ± 0.03 c | 6.43 ± 0.09 a | 4.69 ± 0.44 a | 5.16 ± 0.14 a | 5.2 ± 0.07 a | 4.12 ± 0.10 b |
| Furfural | 3.03 ± 0.03 b | 3.63 ± 0.01 a | 2.91 ± 0.26 a | 2.69 ± 0.03 a | 2.77 ± 0.14 a | 3.09 ± 0.06 a | 2.67 ± 0.08 b | 3.82 ± 0.02 a |
| Dihydro-2-methyl-3-furanone | 0.38 ± 0.01 a | 0.39 ± 0.02 a | 0.48 ± 0.14 a | 0.73 ± 0.06 a | 0.39 ± 0.06 a | 0.52 ± 0.11 a | 0.56 ± 0.02 a | 0.53 ± 0.04 a |
| 5-methyl-Furfural | 3.10 ± 0.02 a | 3.10 ± 0.03 a | 3.11 ± 0.13 a | 3.12 ± 0.02 a | 2.24 ± 0.83 a | 0.34 ± 0.02 a | 3.17 ± 0.06 a | 2.51 ± 0.06 b |
| 2,2′-Methylenebisfuran | 2.34 ± 0.01 a | 2.07 ± 0.03 b | 1.51 ± 0.06 b | 1.71 ± 0.21 b | 1.26 ± 0.01 a | 1.38 ± 0.14 a | 1.50 ± 0.05 b | 2.31 ± 0.26 a |
| 2-Furancarboxylic acid | 0.39 ± 0.02 b | 0.46 ± 0.02 a | 0.30 ± 0.02 a | 0.37 ± 0.06 a | 0.39 ± 0.13 a | 0.52 ± 0.09 a | 0.30 ± 0.02 a | 0.34 ± 0.01 a |
| Furan, 2-(methoxymethyl)- | 0.28 ± 0.03 a | 0.16 ± 0.01 b | 0.44 ± 0.10 a | 0.29 ± 0.01 a | 0.47 ± 0.11 a | 0.34 ± 0.18 a | 0.16 ± 0.02 a | 0.27 ± 0.07 a |
| Σ Pyrazines | 30.10 ± 0.24 a | 30.34 ± 0.83 a | 31.25 ± 1.43 a | 33.19 ± 0.51 a | 33.42 ± 0.22 a | 33.25 ± 0.59 a | 31.22 ± 0.47 a | 30.12 ± 0.52 a |
| 2-Methylpyrazine | 4.95 ± 0.04 b | 5.54 ± 0.07 a | 5.91 ± 1.43 a | 6.00 ± 0.10 a | 6.60 ± 0.08 a | 5.53 ± 0.11 c | 5.71 ± 1.71 a | 4.49 ± 0.49 a |
| 2,5 dimethylpyrazine | 8.33 ± 0.02 b | 9.34 ± 0.10 a | 8.42 ± 0.23 b | 9.95 ± 0.40 a | 8.78 ± 0.17 a | 9.42 ± 0.27 a | 9.57 ± 0.01 a | 8.6 ± 0.25 b |
| ethyl pyrazine | 3.43 ± 0.40 a | 3.34 ± 0.24 a | 3.64 ± 0.13 a | 3.56 ± 0.14 a | 3.42 ± 0.40 a | 3.12 ± 0.08 a | 3.64 ± 0.13 a | 3.31 ± 0.23 a |
| 3,5 dimethylpyrazine | 0.60 ± 0.07 a | 0.39 ± 0.08 a | 0.72 ± 0.09 a | 0.72 ± 0.10 a | 0.82 ± 0.01 a | 0.81 ± 0.01 a | 0.84 ± 0.07 a | 0.83 ± 0.08 a |
| 2-Ethyl-6-methylpyrazine | 2.83 ± 0.09 a | 2.84 ± 0.01 a | 2.65 ± 0.34 b | 3.84 ± 0.04 a | 3.17 ± 0.54 a | 3.79 ± 0.34 a | 3.01 ± 0.85 a | 2.53 ± 0.11 a |
| 2,3,5-Trimethylpyrazine | 4.08 ± 0.12 a | 2.97 ± 0.46 a | 4.73 ± 0.23 a | 3.42 ± 0.13 b | 4.96 ± 0.10 a | 5.07 ± 0.06 a | 3.26 ± 0.10 b | 4.71 ± 0.12 a |
| 3-Methoxy-2-isopropylpyrazine | 1.26 ± 0.02 a | 1.27 ± 0.07 a | 1.20 ± 0.11 a | 0.86 ± 0.06 b | 1.06 ± 0.07 ab | 1.01 ± 0.01 b | 1.07 ± 0.06 a | 1.17 ± 0.06 a |
| 2,5-Dimethyl-3-ethylpyrazine | 2.80 ± 0.02 b | 2.93 ± 0.01 a | 2.76 ± 0.05 a | 3.21 ± 0.21 a | 2.91 ± 0.26 a | 2.87 ± 0.18 a | 2.68 ± 0.07 a | 2.76 ± 0.08 a |
| Isopropenylpyrazine | 0.82 ± 0.05 a | 0.82 ± 0.01 a | 0.74 ± 0.08 a | 0.83 ± 0.11 a | 0.80 ± 0.03 a | 0.91 ± 0.09 a | 0.97 ± 0.41 a | 0.74 ± 0.08 a |
| 5-Methyl-6,7dihydro5-Hcyclopentapyrazine | 0.63 ± 0.03 a | 0.63 ± 0.01 a | 0.29 ± 0.06 a | 0.45 ± 0.15 a | 0.41 ± 0.02 a | 0.39 ± 0.12 a | 0.28 ± 0.04 b | 0.41 ± 0.03 a |
| 2-methyl-5-(1-propenyl) Pyrazine | 0.37 ± 0.04 ab | 0.27 ± 0.01 b | 0.19 ± 0.04 a | 0.35 ± 0.07 a | 0.49 ± 0.16 a | 0.33 ± 0.16 a | 0.19 ± 0.03 b | 0.565 ± 0.05 a |
| Σ Pyridines | 4.86 ± 0.14 b | 5.62 ± 0.11 a | 6.32 ± 0.10 a | 5.32 ± 0.43 b | 6.28 ± 0.12 a | 5.52 ± 0.29 a | 6.08 ± 0.45 a | 5.39 ± 0.13 a |
| Pyridine | 3.60 ± 0.16 b | 4.30 ± 0.12 a | 5.53 ± 0.55 a | 4.62 ± 0.09 a | 5.18 ± 0.02 a | 4.48 ± 0.24 b | 5.52 ± 0.56 a | 4.59 ± 0.26 a |
| Pyridine, 1,2,3,6-tetrahydro-1-methyl- | 0.95 ± 0.01 c | 1.00 ± 0.01 b | 0.58 ± 0.45 a | 0.39 ± 0.08 a | 0.72 ± 0.04 a | 0.62 ± 0.11 a | 0.36 ± 0.13 a | 0.48 ± 0.11 a |
| Pyridine, 3-ethyl | 0.31 ± 0.08 a | 0.32 ± 0.01 a | 0.21 ± 0.01 b | 0.31 ± 0.03 a | 0.38 ± 0.10 a | 0.42 ± 0.05 a | 0.2 ± 0.01 b | 0.32 ± 0.02 a |
| Σ Ketones | 5.82 ± 0.49 a | 6.56 ± 0.28 a | 5.55 ± 0.34 b | 7.23 ± 0.39 a | 6.02 ± 0.65 a | 7.16 ± 0.89 a | 7.08 ± 0.49 a | 6.97 ± 0.22 a |
| Acetone | 0.66 ± 0.01 b | 1.11 ± 0.01 a | 0.89 ± 0.04 a | 0.89 ± 0.08 a | 1.17 ± 0.01 a | 1.18 ± 0.06 a | 1.33 ± 0.01 b | 1.48 ± 0.06 a |
| 1-(Acetyloxy)-2-propanone | 3.42 ± 0.23 a | 3.92 ± 0.01 a | 3.23 ± 0.06 b | 5.06 ± 0.20 a | 3.96 ± 0.57 a | 4.57 ± 0.32 a | 4.16 ± 0.04 a | 4.03 ± 0.04 a |
| 2-Hydroxy-3-methyl-2- cyclopenten-1-one | 0.49 ± 0.01 b | 0.47 ± 0.03 a | 0.52 ± 0.11 a | 0.34 ± 0.02 a | 0.40 ± 0.06 a | 0.49 ± 0.19 a | 0.49 ± 0.08 a | 0.46 ± 0.06 a |
| 3-Cyclobutene-1,2-dione, 3,4-dimethyl- | 0.66 ± 0.03 a | 0.55 ± 0.03 b | 0.41 ± 0.06 a | 0.42 ± 0.05 a | 0.36 ± 0.01 b | 0.43 ± 0.10 ab | 0.35 ± 0.02 b | 0.65 ± 0.08 a |
| 3-Ethyl-2-hydroxy-2- Cyclopenten-1-one | 0.59 ± 0.02 ab | 0.51 ± 0.01 a | 0.50 ± 0.08 a | 0.52 ± 0.09 a | 0.40 ± 0.03 a | 0.49 ± 0.10 a | 0.75 ± 0.42 a | 0.35 ± 0.07 a |
| Σ Phenols | 7.97 ± 0.45 a | 5.69 ± 0.19 b | 6.78 ± 0.38 a | 6.34 ± 0.55 a | 7.95 ± 0.13 b | 9.06 ± 0.25 a | 5.64 ± 1.13 a | 6.29 ± 0.17 a |
| Phenol | 0.25 ± 0.01 a | 0.29 ± 0.03 a | 0.29 ± 0.04 b | 0.77 ± 0.18 a | 0.50 ± 0.22 a | 1.37 ± 0.40 a | 0.30 ± 0.02 ab | 0.43 ± 0.04 a |
| 2-methoxyphenol | 3.40 ± 0.36 a | 1.65 ± 0.01 b | 3.63 ± 0.19 a | 1.84 ± 0.13 b | 3.33 ± 0.01 a | 3.82 ± 0.11 a | 2.32 ± 0.77 a | 3.75 ± 0.06 a |
| 4-ethyl-2-methoxyphenol | 1.67 ± 0.06 ab | 1.46 ± 0.11 b | 1.18 ± 0.10 a | 1.61 ± 0.17 a | 1.36 ± 0.25 a | 1.68 ± 0.29 a | 1.24 ± 0.10 a | 1.04 ± 0.04 a |
| 4-Vinylphenol | 2.65 ± 0.01 b | 2.29 ± 0.06 c | 1.68 ± 0.13 a | 2.12 ± 0.07 a | 2.76 ± 0.16 a | 2.19 ± 0.06 a | 1.78 ± 0.28 a | 1.07 ± 0.07 b |
| Σ Pyrroles | 2.54 ± 0.11 a | 2.32 ± 0.09 b | 2.28 ± 0.23 a | 3.00 ± 0.09 a | 2.60 ± 0.09 a | 2.65 ± 0.10 a | 2.22 ± 0.14 a | 2.25 ± 0.15 a |
| 2-Acetylpyrrole | 0.55 ± 0.02 a | 0.59 ± 0.01 a | 0.68 ± 0.02 c | 1.07 ± 0.02 a | 0.70 ± 0.05 a | 0.68 ± 0.09 a | 0.72 ± 0.08 a | 0.79 ± 0.05 a |
| 2-Acetyl-1-methylpyrrole | 0.72 ± 0.02 a | 0.66 ± 0.01 a | 0.61 ± 0.04 a | 0.72 ± 0.07 a | 0.61 ± 0.06 a | 0.65 ± 0.01 a | 0.61 ± 0.05 a | 0.75 ± 0.06 a |
| 1H-Pyrrole, 1-(2-furanylmethyl)- | 1.27 ± 0.03 a | 1.07 ± 0.01 b | 0.99 ± 0.16 a | 1.21 ± 0.04 a | 1.29 ± 0.02 a | 1.32 ± 0.01 a | 0.89 ± 0.06 a | 0.705 ± 0.26 a |
| Σ Aldehydes | 0.32 ± 0.04 b | 0.40 ± 0.01 a | 0.32 ± 0.04 a | 0.46 ± 0.15 a | 0.44 ± 0.02 a | 0.41 ± 0.16 a | 0.32 ± 0.04 b | 0.66 ± 0.06 a |
| 3-Methyl-p-anisaldehyde | 0.32 ± 0.04 b | 0.40 ± 0.01 a | 0.32 ± 0.04 a | 0.46 ± 0.15 a | 0.44 ± 0.02 a | 0.41 ± 0.16 a | 0.32 ± 0.04 b | 0.66 ± 0.06 a |
| Σ Organic Acid | 5.03 ± 0.11 b | 5.69 ± 0.05 a | 5.65 ± 0.28 b | 7.32 ± 0.11 a | 7.68 ± 0.11 b | 9.30 ± 0.16 a | 10.41 ± 0.25 b | 12.89 ± 0.11 a |
| Acetic acid | 4.51 ± 0.14 b | 5.27 ± 0.04 a | 5.32 ± 0.25 b | 6.90 ± 0.05 a | 7.25 ± 0.15 b | 8.97 ± 0.25 a | 10.12 ± 0.22 b | 12.57 ± 0.13 a |
| 3-Methylbutanoic acid | 0.52 ± 0.05 a | 0.42 ± 0.01 b | 0.33 ± 0.09 a | 0.42 ± 0.16 a | 0.43 ± 0.04 a | 0.33 ± 0.09 a | 0.29 ± 0.03 a | 0.315 ± 0.02 a |
| Time (Months) | 0 | 0 | 4 | 4 | 8 | 8 | 12 | 12 |
|---|---|---|---|---|---|---|---|---|
| STD | REC | STD | REC | STD | REC | |||
| Σ Furans | 43.22 ± 1.88 a | 43.38 ± 1.03 a | 41.88 ± 0.79 a | 38.71 ± 2.50 a | 35.35 ± 0.30 a | 35.58 ± 0.16 a | 36.15 ± 0.70 a | 33.17 ± 0.47 b |
| 2-Furanmethanol | 28.50 ± 1.22 a | 28.12 ± 0.97 a | 25.83 ± 1.07 a | 23.30 ± 2.29 a | 23.00 ± 1.19 a | 22.33 ± 0.13 ab | 22.30 ± 0.76 a | 20.29 ± 0.21 a |
| 2-Furanmethanol acetate | 5.18 ± 0.38 a | 5.32 ± 0.05 a | 4.71 ± 0.35 a | 5.11 ± 0.22 a | 4.69 ± 0.44 a | 4.51 ± 0.19 a | 5.20 ± 0.07 a | 4.41 ± 0.15 b |
| Furfural | 3.04 ± 0.14 c | 3.63 ± 0.01 a | 2.02 ± 0.09 a | 4.08 ± 0.13 a | 2.77 ± 0.46 a | 3.03 ± 0.19 a | 2.67 ± 0.08 a | 2.50 ± 0.16 a |
| Dihydro-2-methyl-3-furanone | 0.38 ± 0.01 a | 0.39 ± 0.02 a | 0.60 ± 0.02 b | 0.66 ± 0.15 a | 0.39 ± 0.02 a | 0.40 ± 0.08 a | 0.56 ± 0.02 a | 0.50 ± 0.07 a |
| 5-methyl-Furfural | 3.11 ± 0.11 a | 3.10 ± 0.03 a | 5.11 ± 0.31 a | 3.19 ± 0.31 b | 2.24 ± 1.01 a | 2.51 ± 0.01 a | 3.17 ± 0.06 a | 2.71 ± 0.13 b |
| 2,2′-Methylenebisfuran | 2.34 ± 0.07 a | 2.07 ± 0.03 b | 2.61 ± 0.08 a | 1.59 ± 0.06 b | 1.26 ± 0.02 a | 1.78 ± 0.74 a | 1.50 ± 0.21 a | 2.02 ± 0.67 a |
| 2-Furancarboxylic acid | 0.40 ± 0.01 ab | 0.46 ± 0.02 a | 0.66 ± 0.03 a | 0.27 ± 0.04 a | 0.39 ± 0.13 a | 0.32 ± 0.02 a | 0.30 ± 0.02 a | 0.34 ± 0.06 a |
| Furan, 2-(methoxymethyl)- | 0.28 ± 0.02 a | 0.16 ± 0.01 b | 0.35 ± 0.00 a | 0.35 ± 0.02 a | 0.39 ± 0.06 a | 0.55 ± 0.15 a | 0.37 ± 0.04 a | 0.30 ± 0.01 b |
| Σ Pyrazines | 30.15 ± 0.55 a | 30.34 ± 0.83 a | 28.21 ± 0.25 b | 31.35 ± 0.47 a | 32.86 ± 0.17 a | 32.42 ± 0.44 a | 31.87 ± 0.78 a | 29.02 ± 0.22 b |
| 2-Methylpyrazine | 4.95 ± 0.12 b | 5.54 ± 0.07 a | 5.37 ± 0.24 a | 5.92 ± 0.65 a | 6.60 ± 0.28 a | 5.68 ± 0.75 a | 5.71 ± 0.71 a | 4.31 ± 0.26 a |
| 2,5 dimethylpyrazine | 8.34 ± 0.34 b | 9.34 ± 0.10 a | 8.11 ± 0.51 b | 9.28 ± 0.41 a | 8.40 ± 0.54 a | 9.55 ± 0.08 a | 9.32 ± 0.35 a | 9.27 ± 0.24 a |
| ethyl pyrazine | 3.43 ± 0.09 a | 3.34 ± 0.24 a | 3.47 ± 0.11 a | 4.12 ± 0.54 a | 3.42 ± 0.33 a | 3.14 ± 0.03 a | 3.64 ± 0.13 a | 3.71 ± 0.06 a |
| 3,5 dimethylpyrazine | 0.61 ± 0.03 a | 0.39 ± 0.08 a | 0.27 ± 0.01 a | 0.39 ± 0.06 a | 0.82 ± 0.09 a | 0.45 ± 0.01 b | 0.84 ± 0.07 a | 0.79 ± 0.01 a |
| 2-Ethyl-6-methylpyrazine | 2.83 ± 0.09 a | 2.84 ± 0.01 a | 2.86 ± 0.08 b | 3.03 ± 0.01 b | 3.17 ± 0.54 a | 2.90 ± 0.29 a | 3.36 ± 0.35 a | 2.32 ± 0.15 b |
| 2,3,5-Trimethylpyrazine | 4.08 ± 0.21 a | 2.97 ± 0.46 a | 3.49 ± 0.13 b | 3.85 ± 0.48 a | 4.96 ± 0.20 a | 4.69 ± 0.54 a | 3.26 ± 0.10 a | 3.10 ± 0.66 a |
| 3-Methoxy-2-isopropylpyrazine | 1.26 ± 0.05 a | 1.27 ± 0.07 a | 0.98 ± 0.04 a | 1.12 ± 0.17 a | 1.06 ± 0.11 a | 1.20 ± 0.23 a | 1.07 ± 0.06 a | 1.20 ± 0.11 a |
| 2,5-Dimethyl-3-ethylpyrazine | 2.81 ± 0.12 b | 2.93 ± 0.01 a | 1.94 ± 0.06 a | 2.09 ± 0.03 a | 2.91 ± 0.20 a | 3.05 ± 0.07 a | 2.68 ± 0.77 a | 2.63 ± 0.27 a |
| Isopropenylpyrazine | 0.82 ± 0.03 a | 0.82 ± 0.01 a | 0.45 ± 0.02 a | 0.67 ± 0.09 a | 0.80 ± 0.29 a | 0.85 ± 0.05 a | 0.97 ± 0.06 a | 0.80 ± 0.01 a |
| 5-Methyl-6,7dihydro5- Hcyclopentapyrazine | 0.64 ± 0.02 a | 0.63 ± 0.01 a | 0.87 ± 0.03 a | 0.39 ± 0.05 b | 0.29 ± 0.14 a | 0.47 ± 0.14 a | 0.73 ± 0.06 a | 0.41 ± 0.13 b |
| 2-methyl-5-(1-propenyl) Pyrazine | 0.38 ± 0.01 ab | 0.27 ± 0.01 b | 0.41 ± 0.01 a | 0.49 ± 0.11 a | 0.43 ± 0.07 a | 0.44 ± 0.07 a | 0.29 ± 0.06 a | 0.48 ± 0.07 a |
| Σ Pyridines | 4.88 ± 0.15 b | 5.62 ± 0.11 a | 5.99 ± 0.21 a | 3.68 ± 0.02 b | 6.28 ± 0.18 a | 6.45 ± 0.69 a | 6.08 ± 0.45 a | 5.33 ± 0.54 a |
| Pyridine | 3.61 ± 0.05 b | 4.30 ± 0.12 a | 4.84 ± 0.12 a | 2.59 ± 0.04 b | 5.18 ± 0.02 a | 5.25 ± 0.57 a | 5.52 ± 0.56 a | 4.51 ± 0.54 a |
| Pyridine, 1,2,3,6-tetrahydro-1-methyl- | 0.95 ± 0.04 b | 1.00 ± 0.01 b | 0.45 ± 0.01 a | 0.59 ± 0.15 a | 0.72 ± 0.06 a | 0.82 ± 0.09 a | 0.36 ± 0.13 a | 0.50 ± 0.08 a |
| Pyridine, 3-ethyl | 0.32 ± 0.01 a | 0.32 ± 0.01 a | 0.70 ± 0.02 a | 0.50 ± 0.06 ab | 0.38 ± 0.11 a | 0.38 ± 0.03 a | 0.20 ± 0.01 a | 0.32 ± 0.08 a |
| Σ Ketones | 5.85 ± 0.32 a | 6.56 ± 0.08 a | 6.86 ± 0.44 a | 7.82 ± 0.18 a | 6.30 ± 0.24 a | 6.41 ± 0.92 a | 6.61 ± 0.11 b | 6.94 ± 0.18 a |
| Acetone | 0.66 ± 0.03 b | 1.11 ± 0.01 a | 0.72 ± 0.03 a | 0.73 ± 0.03 a | 1.17 ± 0.01 a | 1.19 ± 0.01 a | 1.33 ± 0.01 b | 1.55 ± 0.07 a |
| 1-(Acetyloxy)-2-propanone | 3.42 ± 0.12 a | 3.92 ± 0.01 a | 4.01 ± 0.25 b | 5.09 ± 0.05 a | 3.96 ± 0.44 a | 3.90 ± 0.15 a | 4.16 ± 0.04 a | 3.86 ± 0.40 ab |
| 2-Hydroxy-3-methyl-2- cyclopenten-1-one | 0.50 ± 0.01 b | 0.47 ± 0.03 a | 0.61 ± 0.03 a | 0.68 ± 0.03 a | 0.40 ± 0.01 a | 0.46 ± 0.17 a | 0.49 ± 0.08 a | 0.51 ± 0.13 a |
| 3-Cyclobutene-1,2-dione, 3,4-dimethyl- | 0.67 ± 0.02 a | 0.55 ± 0.03 b | 0.90 ± 0.04 a | 0.66 ± 0.04 b | 0.36 ± 0.10 a | 0.45 ± 0.18 a | 0.35 ± 0.02 a | 0.53 ± 0.10 a |
| 3-Ethyl-2-hydroxy-2- Cyclopenten-1-one | 0.60 ± 0.02 a | 0.51 ± 0.01 b | 0.63 ± 0.01 a | 0.66 ± 0.16 a | 0.41 ± 0.01 a | 0.41 ± 0.01 a | 0.28 ± 0.04 a | 0.49 ± 0.13 a |
| Σ Phenols | 7.99 ± 0.43 a | 5.69 ± 0.09 b | 7.95 ± 0.51 a | 6.75 ± 1.20 a | 8.30 ± 0.66 a | 6.14 ± 0.27 b | 6.24 ± 0.47 a | 7.31 ± 0.38 a |
| Phenol | 0.26 ± 0.00 a | 0.29 ± 0.03 a | 0.48 ± 0.03 a | 0.96 ± 0.07 a | 0.50 ± 0.22 a | 0.36 ± 0.04 a | 0.30 ± 0.02 b | 0.42 ± 0.04 a |
| 2-methoxyphenol | 3.41 ± 0.15 a | 1.65 ± 0.01 b | 2.86 ± 0.07 a | 2.30 ± 0.18 a | 3.33 ± 1.00 a | 3.24 ± 0.13 a | 2.32 ± 0.04 a | 3.78 ± 0.02 a |
| 4-ethyl-2-methoxyphenol | 1.68 ± 0.08 ab | 1.46 ± 0.11 b | 1.51 ± 0.11 a | 1.85 ± 0.19 a | 2.21 ± 0.40 a | 1.18 ± 0.05 b | 1.31 ± 0.14 a | 1.58 ± 0.11 a |
| 4-Vinylphenol | 2.65 ± 0.14 a | 2.29 ± 0.06 b | 3.11 ± 0.28 a | 1.64 ± 0.38 b | 2.26 ± 0.27 a | 1.36 ± 0.05 b | 2.31 ± 0.12 a | 1.53 ± 0.33 a |
| Σ Pyrroles | 2.56 ± 0.08 a | 2.32 ± 0.01 b | 2.85 ± 0.15 a | 3.28 ± 0.06 a | 2.79 ± 0.06 a | 2.76 ± 0.14 a | 2.49 ± 0.19 a | 2.23 ± 0.13 a |
| 2-Acetylpyrrole | 0.55 ± 0.02 a | 0.59 ± 0.01 a | 0.33 ± 0.02 b | 0.78 ± 0.04 a | 0.70 ± 0.08 a | 0.66 ± 0.08 a | 0.72 ± 0.08 a | 0.76 ± 0.09 a |
| 2-Acetyl-1-methylpyrrole | 0.73 ± 0.03 a | 0.66 ± 0.01 a | 1.26 ± 0.09 a | 0.86 ± 0.01 b | 0.61 ± 0.11 a | 0.72 ± 0.10 a | 0.61 ± 0.05 a | 0.72 ± 0.11 a |
| 1H-Pyrrole, 1-(2-furanylmethyl) - | 1.28 ± 0.06 a | 1.07 ± 0.01 b | 1.26 ± 0.08 a | 1.64 ± 0.61 a | 1.48 ± 0.08 a | 1.38 ± 0.08 a | 1.16 ± 0.01 a | 0.75 ± 0.23 a |
| Σ Aldehydes | 0.33 ± 0.02 b | 0.40 ± 0.01 a | 0.48 ± 0.03 a | 0.62 ± 0.03 a | 0.49 ± 0.04 a | 0.38 ± 0.06 a | 0.19 ± 0.04 a | 0.53 ± 0.23 a |
| 3-Methyl-p-anisaldehyde | 0.33 ± 0.01 b | 0.40 ± 0.01 a | 0.48 ± 0.02 a | 0.62 ± 0.04 a | 0.49 ± 0.04 a | 0.38 ± 0.04 a | 0.19 ± 0.03 a | 0.53 ± 0.01 a |
| Σ Organic Acid | 5.03 ± 0.19 b | 5.69 ± 0.05 a | 5.79 ± 0.26 b | 7.79 ± 0.03 a | 7.63 ± 0.22 b | 9.86 ± 0.32 a | 10.37 ± 0.28 b | 15.47 ± 0.39 a |
| Acetic acid | 4.51 ± 0.18 b | 5.27 ± 0.04 a | 5.32 ± 0.38 b | 7.32 ± 0.03 a | 7.25 ± 0.15 b | 9.34 ± 0.28 a | 10.12 ± 0.22 b | 15.10 ± 0.42 a |
| 3-Methylbutanoic acid | 0.52 ± 0.04 a | 0.42 ± 0.01 b | 0.47 ± 0.01 a | 0.47 ± 0.11 a | 0.38 ± 0.03 a | 0.52 ± 0.04 a | 0.25 ± 0.11 a | 0.37 ± 0.04 a |
| 25 °C | 40 °C | |||
|---|---|---|---|---|
| Time (Months) | STD | REC | STD | REC |
| 0 | 5.66 ± 0.02 a | 5.62 ± 0.02 b | 5.66 ± 0.02 a | 5.62 ± 0.02 b |
| 1 | 5.67 ± 0.03 a | 5.68 ± 0.02 a | 5.64 ± 0.02 a | 5.63 ± 0.02 a |
| 2 | 5.65 ± 0.04 a | 5.50 ± 0.02 b | 5.65 ± 0.02 a | 5.54 ± 0.03 b |
| 3 | 5.63 ± 0.02 a | 5.61 ± 0.02 a | 5.62 ± 0.02 a | 5.52 ± 0.05 b |
| 4 | 5.55 ± 0.02 a | 5.59 ± 0.04 a | 5.54 ± 0.02 a | 5.48 ± 0.02 b |
| 5 | 5.54 ± 0.02 a | 5.55 ± 0.03 a | 5.46 ± 0.02 a | 5.40 ± 0.02 b |
| 6 | 5.53 ± 0.02 a | 5.52 ± 0.03 a | 5.40 ± 0.02 a | 5.37 ± 0.02 a |
| 7 | 5.48 ± 0.02 a | 5.47 ± 0.02 a | 5.36 ± 0.02 a | 5.35 ± 0.02 a |
| 8 | 5.48 ± 0.02 a | 5.46 ± 0.02 a | 5.31 ± 0.02 a | 5.32 ± 0.02 a |
| 9 | 5.46 ± 0.02 a | 5.44 ± 0.02 a | 5.29 ± 0.05 a | 5.31 ± 0.02 a |
| 10 | 5.44 ± 0.02 a | 5.44 ± 0.02 a | 5.29 ± 0.04 a | 5.29 ± 0.03 a |
| 11 | 5.39 ± 0.02 a | 5.41 ± 0.02 a | 5.30 ± 0.02 a | 5.30 ± 0.02 a |
| 12 | 5.37 ± 0.02 a | 5.32 ± 0.02 a | 5.29 ± 0.02 a | 5.28 ± 0.02 a |
| STD | REC | STD | REC | STD | REC | STD | REC | |
|---|---|---|---|---|---|---|---|---|
| Time (Months) | 0 | 0 | 4 | 4 | 8 | 8 | 12 | 12 |
| Smell acceptability | 8.5 ± 0.5 a | 8.5 ± 0.5 a | 8.5 ± 0.5 a | 8.5 ± 0.5 a | 8.0 ± 0.5 a | 8.0 ± 0.5 a | 7.5 ± 0.5 b | 7.5 ± 0.5 b |
| Taste acceptability | 8.5 ± 0.5 a | 8.5 ± 0.5 a | 8.5 ± 0.5 a | 8.5 ± 0.5 a | 8.0 ± 0.5 a | 8.0 ± 0.5 a | 7.5 ± 0.5 b | 7.5 ± 0.5 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrese, M.; De Luca, L.; Basile, G.; Lambiase, G.; Romano, R.; Pizzolongo, F. A Recyclable Polypropylene Multilayer Film Maintaining the Quality and the Aroma of Coffee Pods during Their Shelf Life. Molecules 2024, 29, 3006. https://doi.org/10.3390/molecules29133006
Calabrese M, De Luca L, Basile G, Lambiase G, Romano R, Pizzolongo F. A Recyclable Polypropylene Multilayer Film Maintaining the Quality and the Aroma of Coffee Pods during Their Shelf Life. Molecules. 2024; 29(13):3006. https://doi.org/10.3390/molecules29133006
Chicago/Turabian StyleCalabrese, Martina, Lucia De Luca, Giulia Basile, Gianfranco Lambiase, Raffaele Romano, and Fabiana Pizzolongo. 2024. "A Recyclable Polypropylene Multilayer Film Maintaining the Quality and the Aroma of Coffee Pods during Their Shelf Life" Molecules 29, no. 13: 3006. https://doi.org/10.3390/molecules29133006
APA StyleCalabrese, M., De Luca, L., Basile, G., Lambiase, G., Romano, R., & Pizzolongo, F. (2024). A Recyclable Polypropylene Multilayer Film Maintaining the Quality and the Aroma of Coffee Pods during Their Shelf Life. Molecules, 29(13), 3006. https://doi.org/10.3390/molecules29133006












