A Novel Intumescent MCA-Modified Sodium Silicate/Acrylic Flame-Retardant Coating to Improve the Flame Retardancy of Wood
Abstract
:1. Introduction
2. Results and Discussion
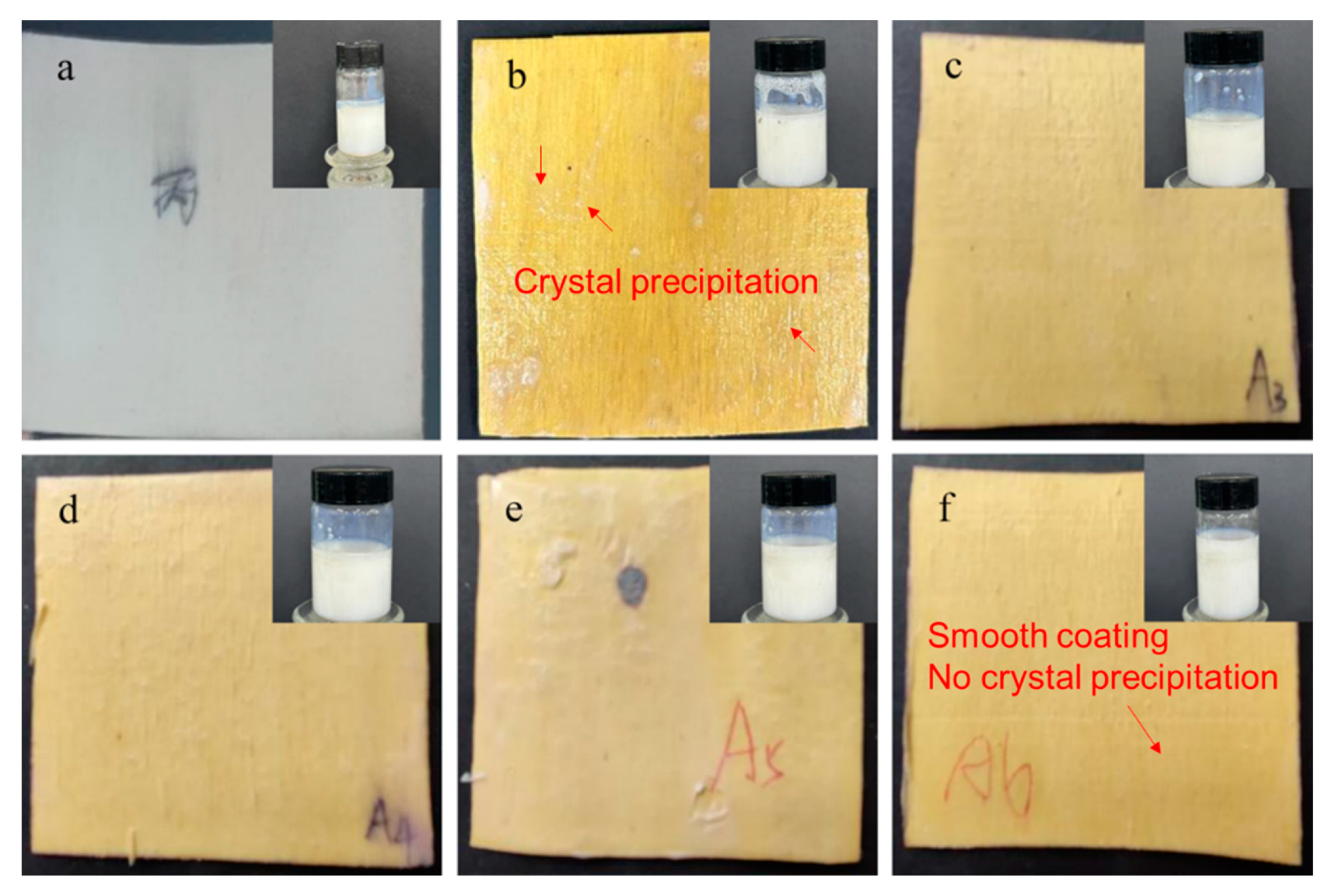
2.1. Appearance of Sodium Silicate/MCA/Waterborne Acrylic Flame-Retardant Coating
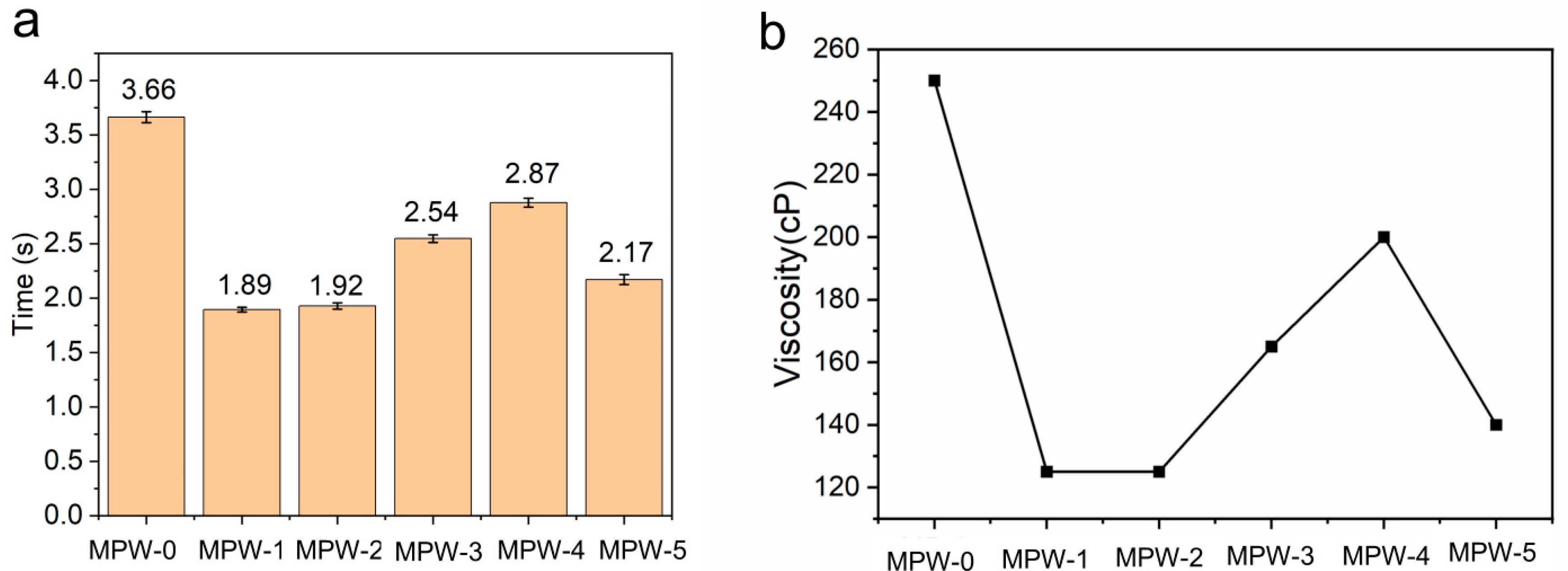
2.2. Viscosity and Film Adhesion of Sodium Silicate/MCA/Waterborne Acrylic Flame-Retardant Coating
2.3. Ignition Testing of Flame-Retardant Veneer
2.4. Limiting Oxygen Index (LOI) Analysis
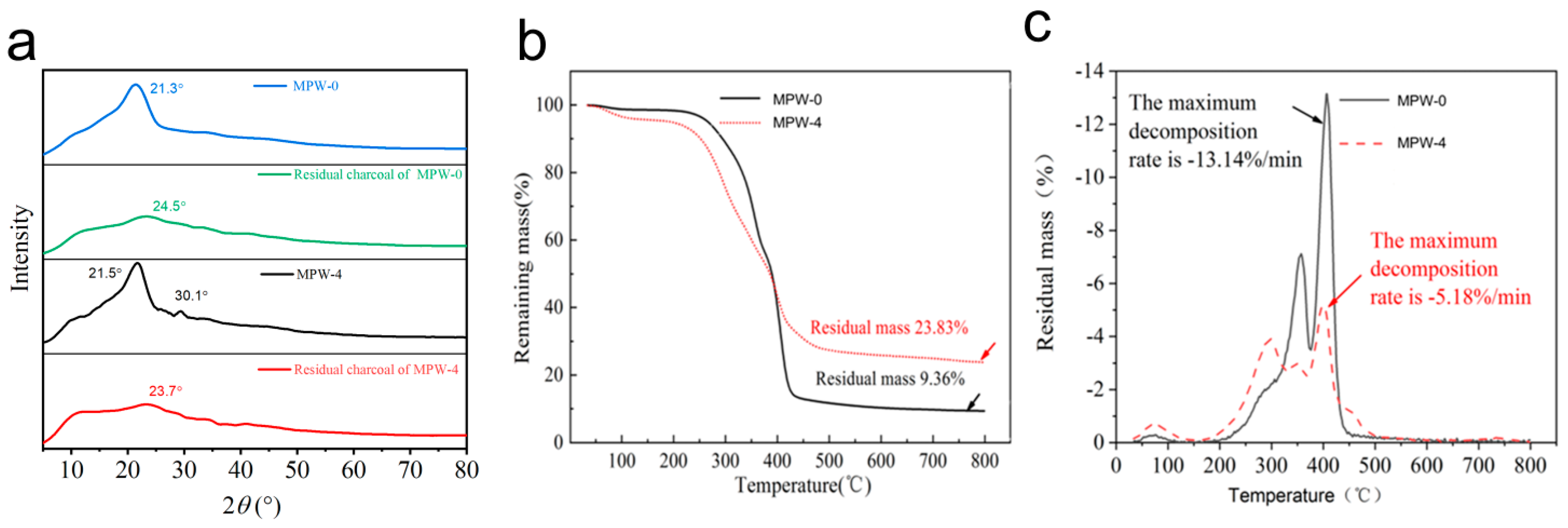
2.5. Characterization of Flame-Retardant Coatings
2.6. Flame-Retardant Properties of Flame-Retardant Poplar Veneer Analysis
2.7. The Mechanism of Sodium Silicate/MCA/Waterborne Acrylic Acid Flame-Retardant Coating
3. Materials and Methods
3.1. Materials
3.2. Preparation of Poplar Veneer
3.3. Preparation of Sodium Silicate/MCA/Waterborne Acrylic Flame Retardant Coating
3.4. Preparation of Flame-Retardant Poplar Veneers
3.5. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Z.X.; Du, C.G.; Wei, J.G.; Yu, H.L. Review on Researches on Fire Retardant of Bamboo-based Material. Zhejiang For. Technol. 2016, 6, 71–77. [Google Scholar]
- Du, C.G.; Wei, J.G.; Jin, C.D. Fire retardant treatment process of bamboo scrimber. J. For. Eng. 2016, 1, 51–54. [Google Scholar]
- Carmen, M.P.; Pfriem, A. Treatments and modification to improve the reaction to fire of wood and wood based products—An overview. Fire Mater. 2020, 44, 100–111. [Google Scholar]
- Yan, L.; Xu, Z.H.; Deng, N. Effects of polyethylene glycol borate on the flame retardancy and smoke suppression performance of transparent flame retardant coatings applied on wood substrates. Prog. Org. Coat. 2019, 135, 123–134. [Google Scholar] [CrossRef]
- Wang, T.S.; Liu, T.; Ma, T.T.; Li, L.P.; Wang, Q.W.; Guo, C.G. Study on degradation of phosphorus and nitrogen composite UV-cured flame retardant coating on wood surface. Prog. Org. Coat. 2018, 124, 240–248. [Google Scholar] [CrossRef]
- Wang, T.S.; Li, L.P.; Cao, Y.j.; Wang, Q.W.; Guo, C.G. Preparation and flame retardancy of castor oil based UV-cured flame retardant coating containing P/Si/S on wood surface. Ind. Crops Prod. 2019, 130, 562–570. [Google Scholar] [CrossRef]
- Sohbatzadeh, F.; Shabannejad, A.; Ghasemi, M.; Mahmoudsani, M. Deposition of halogen-free flame retardant and water-repellent coatings on firwood surfaces using the new version of DBD. Prog. Org. Coat. 2020, 151, 106070. [Google Scholar] [CrossRef]
- Wei, X.Y.; Wang, Y.Y.; Wang, X.R. New Progress in Research on Fire Retardant Coatings. Shandong Chem. Ind. 2019, 20, 83–84. [Google Scholar]
- Hu, G.W.; Shen, H.F.; Lan, R.H.; Chen, H.Q. Coating and Protection. Progress in Fire Retardant Coatings: Jiangsu, China, 2006. [Google Scholar]
- Li, S.; Wang, X.; Xu, M.; Liu, L.; Wang, W.; Gao, S.; Li, B. Effect of a biomass based waterborne fire retardant coating on the flame retardancy for wood. Polym. Adv. Technol. 2021, 32, 4805–4814. [Google Scholar] [CrossRef]
- Gu, J.Y. Adhesives and Coatings; China Forestry Publishing House: Beijing, China, 2012. [Google Scholar]
- Xie, F.Z.; Zhang, X.B.; Gong, H.L.; Zhang, Y.B. Research Status and Application of Waterborn Coatings in Aeronautical Field. Mod. Paint Finish. 2019, 5, 20–22. [Google Scholar]
- Athawale, V.D.; Nimbalkar, R.V. Waterborne coatings based on renewable oil resources: An overview. J. Am. Oil. Chem. Soc. 2011, 88, 159–185. [Google Scholar] [CrossRef]
- Omel’chenko, S.I.; Tryhub, S.O.; Laskovenko, N.M. Prospects of investigation and production of waterborne paintwork materials. Mater. Sci. 2001, 37, 790–801. [Google Scholar] [CrossRef]
- Cheng, L.S.; Ren, S.B.; Lu, X.N. Application of eco-friendly waterborne polyurethane composite coating incorporated with nano cellulose crystalline and silver nano particles on wood antibacterial board. Polymers 2020, 12, 407. [Google Scholar] [CrossRef]
- Fang, G.Z. Functional Improvement of Wood; Chemical Industry Press: Beijing, China, 2008. [Google Scholar]
- He, M.F.; Li, X.Y.; Lia, H.L.; Wei, X. Research Progress in Wood Fire Retardant Technology. For. Mach. Woodwork. Equip. 2022, 3, 21–29+36. [Google Scholar]
- Saara, H.; Altgen, M.; Altgen, D. The effect of diammonium phosphate and sodium silicate on the adhesion and fire performance of birch veneer. Holzforschung 2019, 4, 59–74. [Google Scholar]
- Ping, L.; Zhang, Y.; Zuo, Y.F.; Lu, J.X.; Yuan, G.M.; Wu, Y.Q. Preparation and characterization of sodium silicate impregnated Chinese fir wood with high strength, water resistance, flame retardant and smoke suppression. Prog. Artif. Intell. 2020, 1, 1043–1053. [Google Scholar]
- Skachkova, V.K.; Grachev, A.V.; Shaulov, A.Y.; Berlin, A.A. Inorganic Polymers Using Sodium Silicate Liquid Glass. Features of Silicate Polycondensation. Russ. J. Phys. Chem. B 2019, 13, 849–852. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef]
- Truby, R.L.; Lewis, J.A. Printing soft matter in three dimensions. Nature 2016, 540, 371–378. [Google Scholar] [CrossRef]
- Yu, Y.; Mu, Z.; Jin, B. Organic–Inorganic Copolymerization for a Homogenous Composite without an Interphase Boundary. Angew. Chem. 2020, 5, 2071–2075. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, P.; Ma, X.; Li, P.; Sun, Z.; Li, X.; Zuo, Y. Study on the Effect of Acrylic Acid Emulsion on the Properties of Poplar Wood Modified by Sodium Silicate Impregnation. Forests 2023, 14, 1221. [Google Scholar] [CrossRef]
- Dong, B.; Li, Z.; Liu, J. Preparation of SiO2 antireflection film with high hardness and adhesion by mPEG. React. Funct. Polym. 2022, 171, 105176. [Google Scholar] [CrossRef]
- Chicot, D.; Lesage, J. Absolute hardness of films and coatings. Thin Solid Film. 1995, 2, 123–130. [Google Scholar] [CrossRef]
- ASTM D3359-02; Standard Test Methods for Measuring Adhesion by Tape Test. American Society for Testing and Materials(ASTM) International: West Conshohocken, PA, USA, 2002.
- GB 8624-2012; Standard for Classification of Combustibility of Building Materials and Products. China Architecture & Building Press: Beijing, China, 2012.
- Hai, P.; Jiang, M.S. Flame suppression mechanism of aluminum dust cloud by melamine cyanurate and melamine polyphosphate. J. Hazard. Mater. 2019, 2, 797–810. [Google Scholar]
- Bi, X.; Zhang, Y.; Li, P. Building bridging structures and crystallization reinforcement in sodium silicate-modified poplar by dimethylol dihydroxyethylene urea. Wood Sci. Technol. 2022, 56, 1487–1508. [Google Scholar] [CrossRef]
- Filippi, S.; Cappello, M.; Polacco, G. Limiting oxygen index reduction in bitumen modified with nanoclays. Fire Saf. J. 2020, 111, 102929. [Google Scholar] [CrossRef]
- ISO 4589-2; Plastics-Determination of Burning Behaviour by Oxygen Index—Part 2: Ambient-Temperature Test. International Organization for Standardization: Geneva, Switzerland, 2017.
- Musyarofah; Soontaranon, S.; Limphirat, W. XRD, WAXS, FTIR, and XANES studies of silica-zirconia systems. Ceram. Int. 2019, 12, 15660–15670. [Google Scholar]
- Zhang, L.; Liu, X.; Fang, L. Synthesis, characterization and performance of δ-layered sodium silicate from alumina-extracted slag of coal fly ash. Inorg. Chem. Ind. 2018, 50, 54–59. [Google Scholar]
- He, L.; Liu, Y.; Lin, M. A new approach to measure melamine, cyanuric acid, and melamine cyanurate using surface enhanced Raman spectroscopy coupled with gold nanosubstrates. Sens. Instrum. Food Qual. Saf. 2008, 2, 66–71. [Google Scholar]
- Wang, Y.; Kou, X.; Zhao, J. Three sources-in-one bio-MOFs doped silicone acrylic emulsion-based waterborne flame-retarding coatings with formaldehyde adsorption property. Prog. Org. Coat. 2023, 175, 107329. [Google Scholar] [CrossRef]
- Wu, D.C.; Chen, C.; Hou, X.L.; Sun, K. Effect of pyrolysis temperature on structures of chars forming froming from cellulose and lignin. Biomass Chem. Eng. 2021, 55, 1–9. [Google Scholar]
- Yuan, W.C.; Wei, S.S.; Zhang, X.Y. Preparation of MCA flame retardants and its application in PA6. Packag. Eng. 2020, 41, 167–172. [Google Scholar]
- Yan, F.L.; Xiao, Q.M. Thermogravimetric analysis of the co-combustion of coal and paper mill sludge. Appl. Energy 2010, 11, 3526–3532. [Google Scholar] [CrossRef]
- Long, Y.; Li, Q.; Zhou, H. A grey-relation-based method (GRM) for thermogravimetric (TG) data analysis. J. Mater. Cycles Waste Manag. 2017, 20, 1026–1035. [Google Scholar] [CrossRef]
- Zhou, K.; Gui, Z.; Hu, Y. The influence of graphene based smoke suppression agents on reduced fire hazards of polystyrene composites. Compos. Part A Appl. Sci. Manuf. 2016, 80, 217–227. [Google Scholar] [CrossRef]
- Jiao, C.; Zhao, X.; Song, W.; Chen, X.L. Synergistic flame retardant and smoke suppression effects of ferrous powder with ammonium polyphosphate in thermoplastic polyurethane composites. Therm. Anal. Calorim. 2015, 120, 1173–1181. [Google Scholar] [CrossRef]
- Zhou, L.L.; Li, W.X.; Zhao, H.B.; Zhao, B. Comparative Study of M(Ⅱ)Al (M=Co, Ni) Layered Double Hydroxides for Silicone Foam: Characterization, Flame Retardancy, and Smoke Suppression. Int. J. Mol. Sci. 2022, 23, 11049. [Google Scholar] [CrossRef]
- Salasinska, K.; Celinski, M.; Mizera, K.; Kozikowski, P.; Leszczynski, M.K.; Gajek, A. Synergistic effect between histidine phosphate complex and hazelnut shell for flammability reduction of low-smoke emission epoxy resin. Polym. Degrad. Stab. 2020, 181, 109292. [Google Scholar] [CrossRef]
- Xiong, J.Q.; Kim, S.J.; Kurade, M.B.; Govindwar, S.; Abou-Shanab, R.A.; Kim, J.R.; Jeon, B.H. Smoke suppression properties of ferrite yellow on flame retardant thermoplastic polyu-rethane based on ammonium polyphosphate. Hazard. Mater. 2014, 266, 114–121. [Google Scholar]
- Guo, W.; Liu, J.; Zhang, P.; Song, L.; Wang, X.; Hu, Y. Multi-functional hydroxyapatite/polyvinyl alcohol composite aerogels with self-cleaning, supe-rior fire resistance and low thermal conductivity. Compos. Sci. Technol. 2018, 158, 128–136. [Google Scholar] [CrossRef]
- Ran, S.; Fang, F.; Guo, Z.; Song, P.G.; Cai, Y.F.; Fang, Z.P.; Wang, H. Synthesis of decorated graphene with, P., N-containing compounds and its flame retardancy and smoke suppression effects on polylactic acid. Compos. B Eng. 2019, 170, 41–50. [Google Scholar] [CrossRef]
- Qian, Y.; Qiao, P.; Li, L.; Han, H.Y.; Zhang, H.M.; Chang, G.Z. Hydrothermal Synthesis of Lanthanum-Doped MgAl-Layered Double Hydroxide/Graphene Oxide Hybrid and Its Application as Flame Retardant for Thermoplastic Polyurethane. Adv. Polym. Technol. 2020, 10, 9078731. [Google Scholar] [CrossRef]
- Wei, Y.; Ding, A.; Luo, F. Comparison of polysilicic acid (PSiA) and magnesium sulfate modified polysilicic acid (PMSiS) for effective removal of Congo red from simulated wastewater. Korean J. Chem. Eng. 2020, 6, 978–984. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/Silica Nanocomposites: Preparation, Characterization, Properties, and Applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef]
- Bian, R.; Li, L.; Bao, D. Cd immobilization in a contaminated rice paddy by inorganic stabilizers of calcium hydroxide and silicon slag and by organic stabilizer of biochar. Environ. Sci. Pollut. Res. Int. 2016, 10, 10028–10036. [Google Scholar] [CrossRef]
- Zhang, L.; Cui, T.; Cao, X.; Zhao, C.; Chen, Q.; Wu, L.; Li, H. Inorganic-Macroion-Induced Formation of Bicontinuous Block Copolymer Nanocomposites with Enhanced Conductivity and Modulus. Angew. Chem. Int. Edit. 2017, 56, 9013–9017. [Google Scholar] [CrossRef]
- Zhang, S.; Cheng, R.; Dong, Q.; He, X.; Wang, Q.; Tang, Y.; Yu, Y.; Xie, K.; Da, J.; Terano, M.; et al. Ethylene/1-Hexene Copolymerization with A Novel SiO2-Supported Inorganic and Organic Hybrid Chromium-based Catalyst. Macromol. React. Eng. 2013, 6, 254–266. [Google Scholar] [CrossRef]
- Adel, M.; Soumia, A.; Amal, D.; Amina, S.; Bouhadjar, B.; Mohamed, S.; Abdelkader, B. Adsorption behavior of cationic and anionic dyes on magadiite-chitosan composite beads. Carbohydr. Polym. 2023, 229, 115399. [Google Scholar]




| Name | MPW-0 | MPW-1 | MPW-2 | MPW-3 | MPW-4 | MPW-5 |
|---|---|---|---|---|---|---|
| Paint film hardness | HB | HB | H | HB | HB | HB |
| Paint film adhesion | 4B | 4B | 4B | 4B | 4B | 4B |
| Name | Poplar Veneer | MPW-0 | MPW-1 | MPW-2 | MPW-3 | MPW-4 | MPW-5 |
|---|---|---|---|---|---|---|---|
| LOI (%) | 20.7 | 20.0 | 27.0 | 27.9 | 28.0 | 28.6 | 26.8 |
| Name | Mean | ||||||
|---|---|---|---|---|---|---|---|
| Time to Ignition (s) | HRR (kW/m2) | THR (MJ/m2) | MLR (g/s) | TSP (m2) | TSR (m2/m2) | SEA (m2/kg) | |
| MPW-0 | 19 ± 1 | 634.12 ± 3.98 | 24.48 ± 0.65 | 0.49 ± 0.04 | 1.52 ± 0.07 | 143.05 ± 13.64 | 382.46 ± 10.53 |
| MPW-1 | 24 ± 0 | 624.77 ± 4.14 | 24.38 ± 0.49 | 0.41 ± 0.03 | 1.12 ± 0.02 | 120.87 ± 11.83 | 357.29 ± 16.23 |
| MPW-4 | 28 ± 1 | 508.82 ± 9.11 | 21.46 ± 0.77 | 0.36 ± 0.03 | 1.09 ± 0.03 | 109.02 ± 4.4 | 336.41 ± 10.79 |
| Sample Number | Sodium Silicate/MCA/Waterborne Acrylic Flame-Retardant Coating | Flame-Retardant Poplar Veneer | |
|---|---|---|---|
| MCA/(MCA+ Sodium Silicate) | Sodium Silicate/(MCA+ Sodium Silicate) | ||
| MP-0 | 0 | 0 | - |
| MP-1 | 0 | 100% | - |
| MP-2 | 5% | 95% | - |
| MP-3 | 7% | 92% | - |
| MP-4 | 9% | 91% | - |
| MP-5 | 11% | 89% | - |
| MPW-0 | - | - | Poplar veneer treated with MP-0 |
| MPW-1 | - | - | Poplar veneer treated with MP-1 |
| MPW-2 | - | - | Poplar veneer treated with MP-2 |
| MPW-3 | - | - | Poplar veneer treated with MP-3 |
| MPW-4 | - | - | Poplar veneer treated with MP-4 |
| MPW-5 | - | - | Poplar veneer treated with MP-5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Ran, Y.; Shao, Y.; Zhu, J.; Du, C.; Yang, F.; Bao, Q.; Shan, Y.; Zhang, W. A Novel Intumescent MCA-Modified Sodium Silicate/Acrylic Flame-Retardant Coating to Improve the Flame Retardancy of Wood. Molecules 2024, 29, 3021. https://doi.org/10.3390/molecules29133021
Wang Y, Ran Y, Shao Y, Zhu J, Du C, Yang F, Bao Q, Shan Y, Zhang W. A Novel Intumescent MCA-Modified Sodium Silicate/Acrylic Flame-Retardant Coating to Improve the Flame Retardancy of Wood. Molecules. 2024; 29(13):3021. https://doi.org/10.3390/molecules29133021
Chicago/Turabian StyleWang, Yuting, Ying Ran, Yuran Shao, Jiawei Zhu, Chungui Du, Fei Yang, Qichao Bao, Yingying Shan, and Weigang Zhang. 2024. "A Novel Intumescent MCA-Modified Sodium Silicate/Acrylic Flame-Retardant Coating to Improve the Flame Retardancy of Wood" Molecules 29, no. 13: 3021. https://doi.org/10.3390/molecules29133021
APA StyleWang, Y., Ran, Y., Shao, Y., Zhu, J., Du, C., Yang, F., Bao, Q., Shan, Y., & Zhang, W. (2024). A Novel Intumescent MCA-Modified Sodium Silicate/Acrylic Flame-Retardant Coating to Improve the Flame Retardancy of Wood. Molecules, 29(13), 3021. https://doi.org/10.3390/molecules29133021






