Abstract
The thermal stability of oxyfluorotellurite glass systems, (65-x)TeO2-20ZnF2-12PbO-3Nb2O5-xPr2O3, doped with praseodymium was examined. The different concentrations of praseodymium oxide (x = 0.5 and 2 mol%) were applied to verify the thermal, optical and luminescence properties of the materials under study. The relatively high values of the Dietzel (ΔT) and Saad–Poulain (S or H′) thermal stability factors determined using a differential thermal analysis (DTA) indicate the good thermal stability of the glass matrix, which gradually improves with the content of the active dopant. The temperature dependence of optical spectra in the temperature range 300–675 K for the VIS–NIR region was investigated. The involved Pr3+ optical transition intensities and relaxation dynamic of the praseodymium luminescent level were determined. The ultrashort femtosecond pulses were utilized to examine a dynamic relaxation of the praseodymium luminescent levels. Although the measured emission of the Pr3+ active ions in the studied glass encompasses the quite broad spectral region, the observed luminescence may only be attributed to 3PJ excited states. As a result, the observed decrease in the experimental lifetime for the 3P0 level along with the increasing activator content was identified as an intensification of the Pr–Pr interplay and the associated self-quenching process. The maximum relative sensitivities (Sr) estimated over a relatively wide temperature range are ~0.46% K−1 (at 300 K) for FIR (I530/I497) and 0.20% K−1 (at 600 K) for FIR (I630/I497), which seems to confirm the possibility of using investigated glasses in optical temperature sensors.
1. Introduction
Amorphous glass structures doped with rare earth (RE) ions attract the attention of scientists due to their wide application possibilities in optoelectronics, photonics, telecommunications, thermoelectrics, medicine and environmental protection [1,2,3,4,5,6,7,8,9]. Studies in the literature show that glasses, ceramics, fibers or crystals doped with rare earth elements can be used in various optical equipment such as solid-state lasers, broadband amplifiers, temperature sensors and fibers [10,11,12,13,14,15,16,17,18].
Moreover, in many cases, due to their unique properties as a host material, RE-doped glasses seem to be even more suitable than crystals. Glass production is easy and cheap compared to crystal. In addition, the glasses are more transparent, characterized by a high refractive index, good RE solubility, thermal stability, low phonon energy, good mechanical strength and proper non-optical linearity. They allow a relatively free selection of the chemical composition and/or its modification by various types of admixtures. This gives the possibility to influence the optical, fluorescence and melting point properties and the immediate surroundings of RE ions to a certain grade. From this point of view, it is known that the luminescence energy efficiency of rare earth ions increases if the glass matrix has low phonon energy, which is the case with tellurium glasses ≤ 800 cm−1 or fluoride glasses at ~500 cm−1 [6,19]. The main purpose is therefore to create a glass matrix whose structure will provide an appropriate environment for rare earth element admixtures to obtain the best optical output of materials depending on their applications. In this context, fluorotellurite glass combines the advantages of fluoride glass (low-energy phonon environments) with the advantages of tellurite glass (chemical durability, thermal stability and mechanical strength) [6,20,21,22].
Amongst the rare earth elements, praseodymium is a relatively alluring optical activator due to the amount of available energy levels in the UV-VIS-NIR range. Metastable states 1D2 and 3P0, when stimulated, can emit red, green and blue light simultaneously for laser operation in both crystals [10,23,24,25] and glass hosts [2,26,27,28]. And due to the 1D2 → 1G4 transition, Pr3+-doped glasses can also exhibit another interesting near-infrared (NIR) emission [29]. Although many spectroscopic studies for various matrices/hosts (crystals, glasses, glass ceramics) activated with Pr3+ ions have already been published, and optical enhancement based on 1G4 → 3H5 and 3F3,4 → 3H4 transitions in the NIR has been demonstrated, a description of the optical properties of Pr3+ single-doped fluorotellurite glass is still relatively rare.
Therefore, this work characterizes the variability in the spectroscopic properties of oxyfluorotellurite glass (65-x)TeO2-20ZnF2-12PbO-3Nb2O5-xPr2O3 under changes in the concentration (x = 0.5 and 2 mol%) of Pr3+ ions and temperature (300–675 K). The designation TZPN was adopted for the base undoped glass 65TeO2-20ZnF2-12PbO-3Nb2O5. For glasses doped with praseodymium, the designations TZPN:0.5%Pr and TZPN:2%Pr were adopted, respectively. To the best of our knowledge, the glass composition that we propose, namely (65-x)TeO2-20ZnF2-12PbO-3Nb2O5-xPr2O3 doped with praseodymium, has not yet been synthesized and/or examined. This type of amorphous material may be highly relevant owing to its still moderate red component of luminophores utilized in the light sources. The intentions of the present study are to (a) examine the impact of Pr2O3 on the oxyfluoride glass thermal properties, (b) estimate the radiative transition rates based on the modified Judd–Ofelt phenomenological theory, and (c) determine the effect of temperature on absorption and emission spectra, eventually determining the related glass thermographic qualities. Moreover, the interionic peculiarities and dynamic relaxation of the luminescent excited state were studied by employing femtosecond laser pulses.
2. Results and Discussion
2.1. Thermal Features
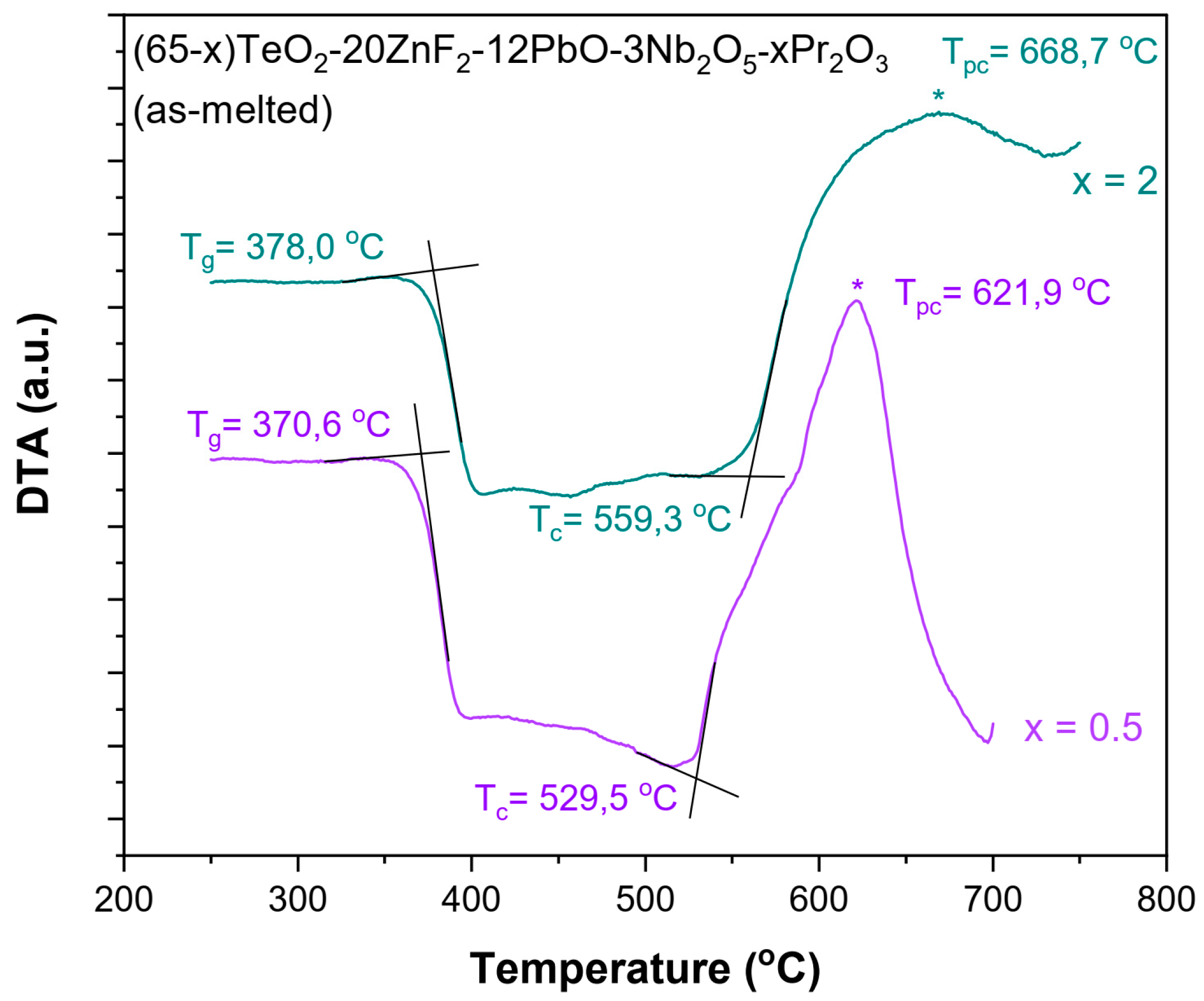
In the initial phase of the research, the thermogravimetric measurements were carried out. DTA curves were recorded for the TZPN:0.5%Pr and TZPN:2%Pr samples (Figure 1) and using the method of Keavney and Eberlin [30], characteristic temperatures were determined, such as the glass transition temperature (Tg) and onset of crystallization temperatures (Tc), in order to determine the thermal stability of the glasses. From the estimated data, it can be seen that the value of Tg indicating the initiation of the glass softening increases slightly with the concentration of Pr2O3 from 370.6 °C to 378.0 °C for samples containing 0.5 and 2 mol% Pr2O3, respectively. The Tg value for TZPN undoped glass is even lower and equals 365.3 °C [31]. In the case of the glass crystallization temperature, we also observe an increase in Tc with the Pr2O3 concentration rising. As it is seen in Figure 1, there is a shift from 529.5 °C with a 0.5 mole ratio of Pr2O3 to 559.3 °C with a 2 mole ratio of Pr2O3.
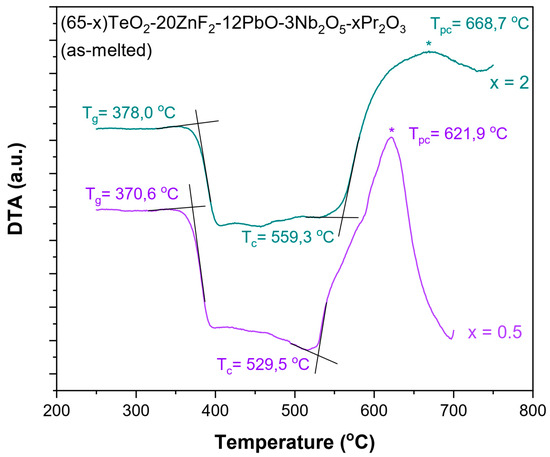
Figure 1.
DTA curves recorded for (65-x)TeO2-20ZnF2-12PbO-3Nb2O5-xPr2O3 glasses doped with x = 0.5 and 2 mol % (asterisks indicate the maximum of peak crystallization Tpc).
It should be noted that the exothermic crystallization peak (Tpc) of both samples occurs in the range of 600–700 °C. Furthermore, in this case, we observe an increase in the Tpc with the content of the active component. A similar effect has been observed in other glass systems [9,21,32,33,34]. In the case of undoped glass 65TeO2-20ZnF2-12PbO-3Nb2O5, values of the characteristic crystallization temperatures are Tc = 552.3 °C and Tpc = 588.5 °C, respectively [31]. All this affects the ability to form the glass and its thermal stability, which can be qualitatively determined by the criteria of thermal stability:
Higher values of the parameters ΔT [30], H′ [35], and S [35] mean better thermal stability of the glass and in the case of the investigated samples, their values are, respectively,
TZPN:0.5%Pr (ΔT = 158.9 °C; H′ = 0.43; S = 39.62 °C);
TZPN:2%Pr (ΔT = 181.3 °C; H′ = 0.48; S = 52.47 °C).
As it can be seen, thermal stability of the TZPN:Pr glasses improves with the content of Pr2O3. It should be noted that the estimation of the maximum Tpc value may be subject to a certain (difficult to eliminate) error due to the asymmetric shape of the crystallization bands (a large number of physicochemical processes take place in this temperature range), dependence location of the crystallization peak of the technical parameters of the experiment (sample mass, heating rate) and constructional features of the apparatus [36]. However, as shown in the obtained results, it does not have a major impact on the nature of changes in the thermal stability parameter S of the tested glass (it is the same trend as in the case of the parameters ΔT and H′).
2.2. Absorption Spectra and Modified Judd–Ofelt Analysis
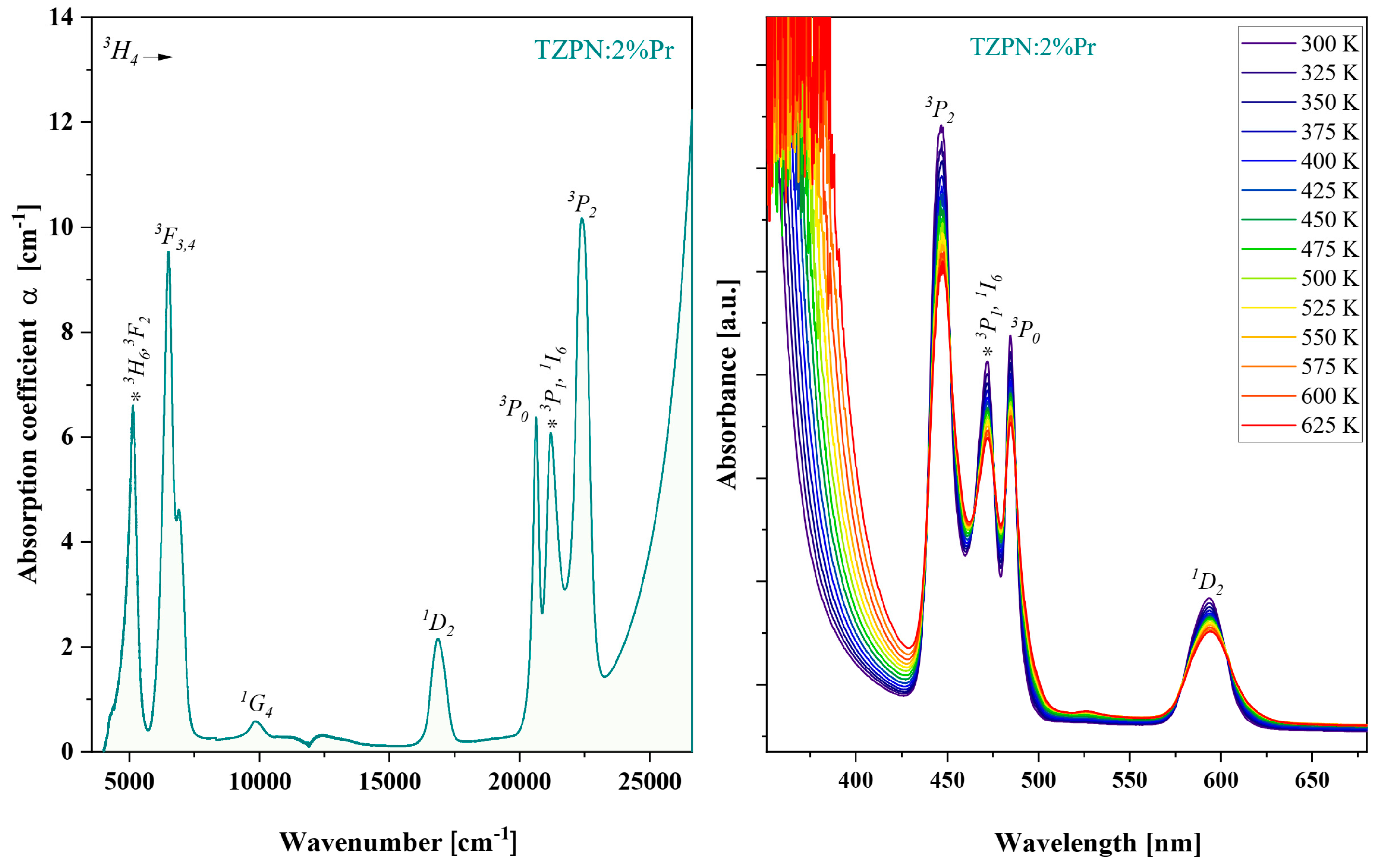
Absorption spectra of TZPN:Pr glass samples were investigated in the wide UV-VIS-NIR range. Figure 2 shows the absorption spectrum taken at room temperature for the 63TeO2-20ZnF2-12PbO-3Nb2O5-xPr2O3 glass sample in the wavenumber spectral range from 5000 to 25,000 cm−1. The visible absorption bands on the left part of Figure 2 are related to the electron transitions of praseodymium from the ground state 3H4 to respective excited states: 3H6 and 3F2 (5133 cm−1); 3F3,4 (6595 cm−1); 1G4 (9851 cm−1); 1D2 (16,882 cm−1) and groups of bands 3P0,1,2 and 1I6 (21,773 cm−1).
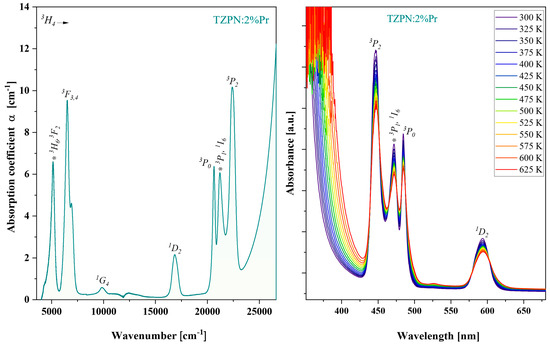
Figure 2.
Absorption spectrum of oxyfluorotellurite glasses doped with 2%Pr (left). Impact of temperature, 300–625 K, on TZPN:2%Pr absorption spectra (right).
The presented absorption spectrum was used to calculate the intensity of transitions based on the Judd–Ofelt theory [37,38]. The application of the J-O theory in the case of the Pr3+ ion as an optical activator is not so simple and depends on the type of host matrix [10]. The absorption transitions satisfying the selection rules ΔS = 0, ΔL ≤ ±2 and ΔJ ≤ ±2 are the so-called hypersensitive transitions characterized by high values of experimental oscillator strengths [28]. The values of experimental oscillator strengths presented in Table 1 indicate that the 3H4 → 3P2 transition is hypersensitive.

Table 1.
Oscillator strengths of Pr3+ f-f transitions in 63TeO2-20ZnF2-12Pb2O5-3Nb2O5-2Pr2O3 at 300 K.
Moreover, the small energy difference between the ground state configuration 4f2 and the first opposite parity excited configuration 4f15d1 results in a large deviation between the measured and calculated oscillator strengths and causes some problems for fitting 3H4 → 3P2 hypersensitive transition [6]. To overcome these problems, a modified J-O theory should be used to estimate the phenomenological parameters Ωt.
Following the standard J-O procedure, the theoretical oscillator strengths can be calculated using the following formula:
where m is the electron mass, c is the speed of light, h is Planck’s constant, λ is the mean wavelength of transition, (2J + 1) is the degeneracy of the ground state of the lanthanide ion, are double reduced matrix elements of the unit tensor and n is the refractive index.
On the other hand, the experimental oscillator strengths were estimated from the absorption spectrum using the relationship
where ε(ν) denotes the molar extinction and ν denotes the energy expressed in wavenumbers. Of course, the contribution of magnetic dipole transitions should also be taken into account and be subtracted from Pexp before the Judd–Ofelt treatment. In the case of Pr3+ ions, these are 3H4 → 3F3,4 and 3H4 → 1G4 transitions, respectively. Ultimately, using the least squares fitting method, this leads to the estimation of the three phenomenological parameters Ω2,4,6 and after obtaining them, it leads to calculating the transition rates between any given states:
where e is the charge of the electron and all other variables have the same meaning as in the previous equations. Next, one can obtain the values of luminescence branching coefficients and radiative lifetimes using the appropriate equations:
Pexp = 4.318 × 10−9∫ε(ν)dν
Unfortunately, the proposed approach in the case of praseodymium quite often, and regardless of the type of host matrix, leads to a negative value of Ω2 [6,10,28,39,40,41]. This requires a nonstandard approach and a certain modification of the J-O theory proposed by Kornienko [42] involving the following formula to calculate the intensity of electric dipoles:
where α = ½ × [E4f5d − Ef0] is called the fitting parameter, which in the case of Pr3+ ions in any glass matrix is of the order of 1.0 × 10−5 cm−1; P′cal is the oscillator force calculated from Equation (9); EJ is the energy of the initial state; EJ′ is the energy of the final state; and Ef0 is the energy corresponding to the center of gravity of the configuration, which is ~9940 cm−1.
P′cal = Pcal × [1 + 2α (EJ + EJ′ − 2Ef0)]
In the modified J-O model, the host-insensitive double reduced matrix elements (ǁUtǁ2) from standard J-O theory were multiplied by the fitting parameter α. The theoretical oscillator strengths calculated in this way (P′cal) and experimentally determined (Pexp) are listed in Table 1. The estimated phenomenological J-O intensity parameters (Ω2,4,6) are following:
Ω2 = 9.30 × 10−20 [cm2], Ω4 = 13.24 × 10−20 [cm2], Ω6 = 10.01 × 10−20 [cm2]
ΔΩ2 = 2.90 × 10−20 [cm2], ΔΩ4 = 0.56 × 10−20 [cm2], ΔΩ6 = 0.82 × 10−20 [cm2]
RMS = 1.34 × 10−6
The relatively small root mean square deviation (RMS = 1.34 × 10−6) indicates a good fit of P′cal with Pexp and the optimal set of obtained intensity parameters: Ω2 = 9.30 × 10−20 cm2, Ω4 = 13.24 × 10−20 cm2 and Ω6 = 10.01 × 10−20 cm2. As it is known, there is a certain relationship between the magnitude of the phenomenological parameters Ω2,4,6 and the covalence of chemical bonds, structural changes in the vicinity of the incorporated rare earth ions (RE) and the bulk properties of the glass matrix [43]. There is a higher value of the Ω2 parameter than the greater degree of asymmetry around the Pr3+ ions and stronger covalence of the Pr–O bonds [6]. In turn, higher values of Ω4 and Ω6 mean lower rigidity and viscosity of the host material [10]. The trend of J-O intensity parameters observed in the TZPN:Pr glass system is Ω2 < Ω6 < Ω4 and is the same as in the case of other tellurite glasses doped with praseodymium [44,45,46,47]. These values are significantly higher, indicating a more asymmetric and covalent environment around Pr3+ ions in the tested system. In turn, the value of Ω6 depends more on the overlap integrals of the 4f and 5d orbits than on the environment in which the Pr3+ ions are located [6]. From the absorption spectra (Figure 2—right) one can notice that as the temperature increases, the absorbance of the observed absorption transitions slightly decreases without changing their position. This indicates the homogeneous distribution of Pr3+ ions in the glass TZPN:2%Pr and consequently, the excitation efficiency of the material under study may be insignificantly affected by the temperature elevation.
2.3. Emission Spectra, Radiative Properties and Color Perception
Using J-O parameters Ω2,4,6, the radiative properties of fluorescent transitions from 1G4, 1D2 and 3P0 levels of the studied glasses are determined. The emission performance such as radiative transition probabilities (Wr), luminescence branching ratios (β) and radiative lifetimes (τrad) of the mentioned excited levels for the TZPN:2%Pr glass was estimated and collected in Table 2.

Table 2.
Calculated values of the radiative transition rates Wr, luminescence branching ratios β and total radiative lifetimes τrad for excited states of Pr3+ in 63TeO2-20ZnF2-12PbO-3Nb2O5-2Pr2O3.
The results of the J-O theory analysis fully confirm the emission spectra recorded at room temperature for TZPN:0.5%Pr and TZPN:2%Pr glass samples. Typically, with 450 nm excitation, the Pr3+ ions from the 3H4 ground level are excited to a higher-energy 3P2 excited state but as a result of non-radiative relaxation, a transition to the lower excited energy level 3P0 occurs. The excitation wavelength 450 nm related to a prominent 3H4 → 3P2 absorption line was selected to avoid a potential effect of the competitive processes, which might be harmful for the desired praseodymium luminescence.
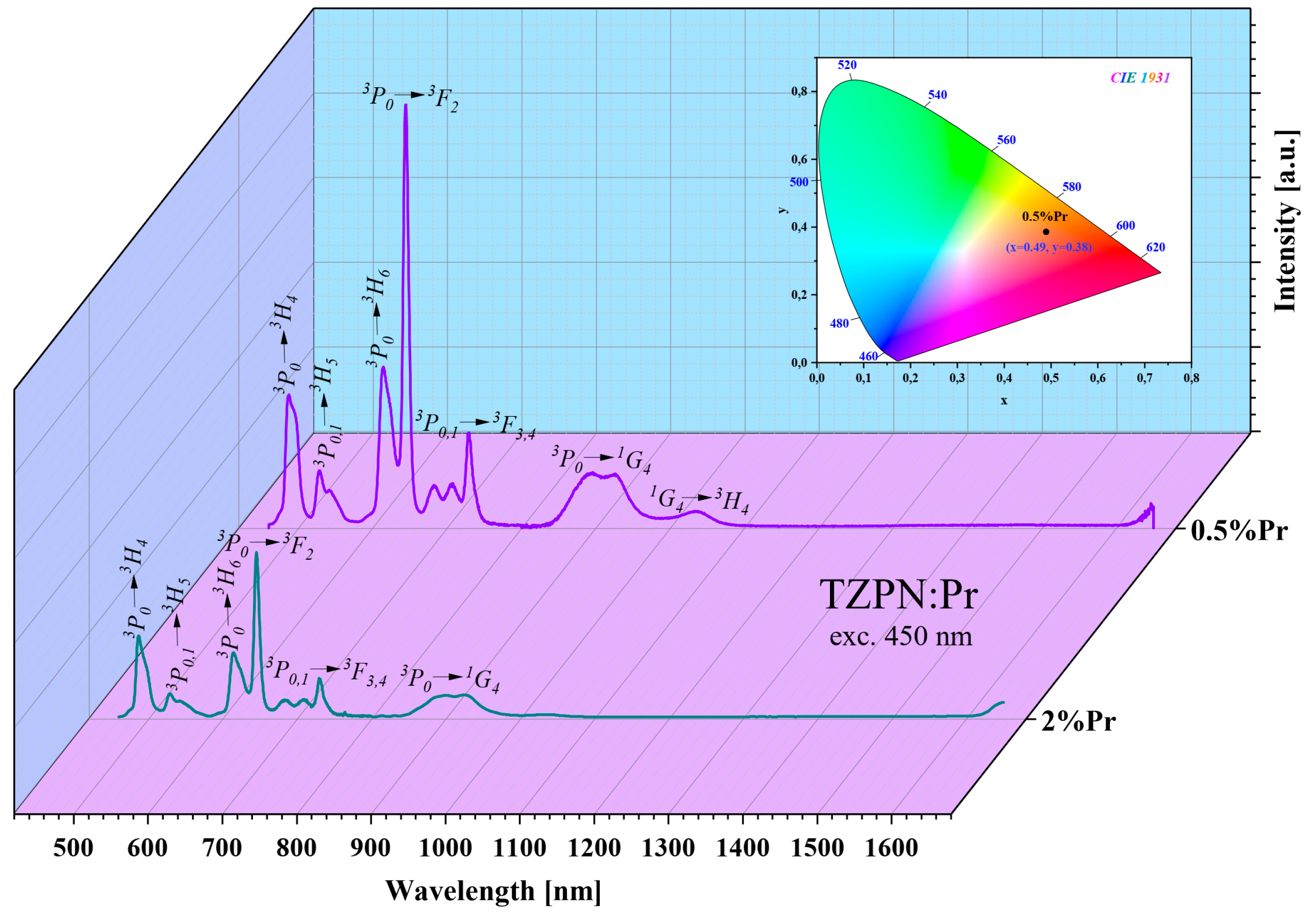
Due to a small energy difference between the adjacent luminescent states 3P0 and 3P1, they undergo thermalization and radiative relaxation has its source in both of these excited states (3P0,1). Therefore, the emission spectra presented in Figure 3 contain bands originating mainly from the 3P0 and 3P1 excited states. In the wavelength range from 450 nm to 1100 nm, 6–7 prominent bands can be seen, where the most intense are two bands corresponding to the transitions 3P0 → 3H4 (489 nm) and 3P0 → 3F2 (645 nm).
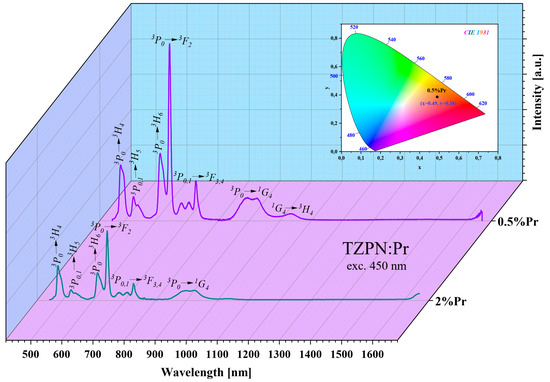
Figure 3.
Emission spectra of TZPN:0.5%Pr and TZPN:2%Pr glasses excited at 450 nm. The inset shows a chromaticity diagram of TZPN:0.5%Pr glass luminescence.
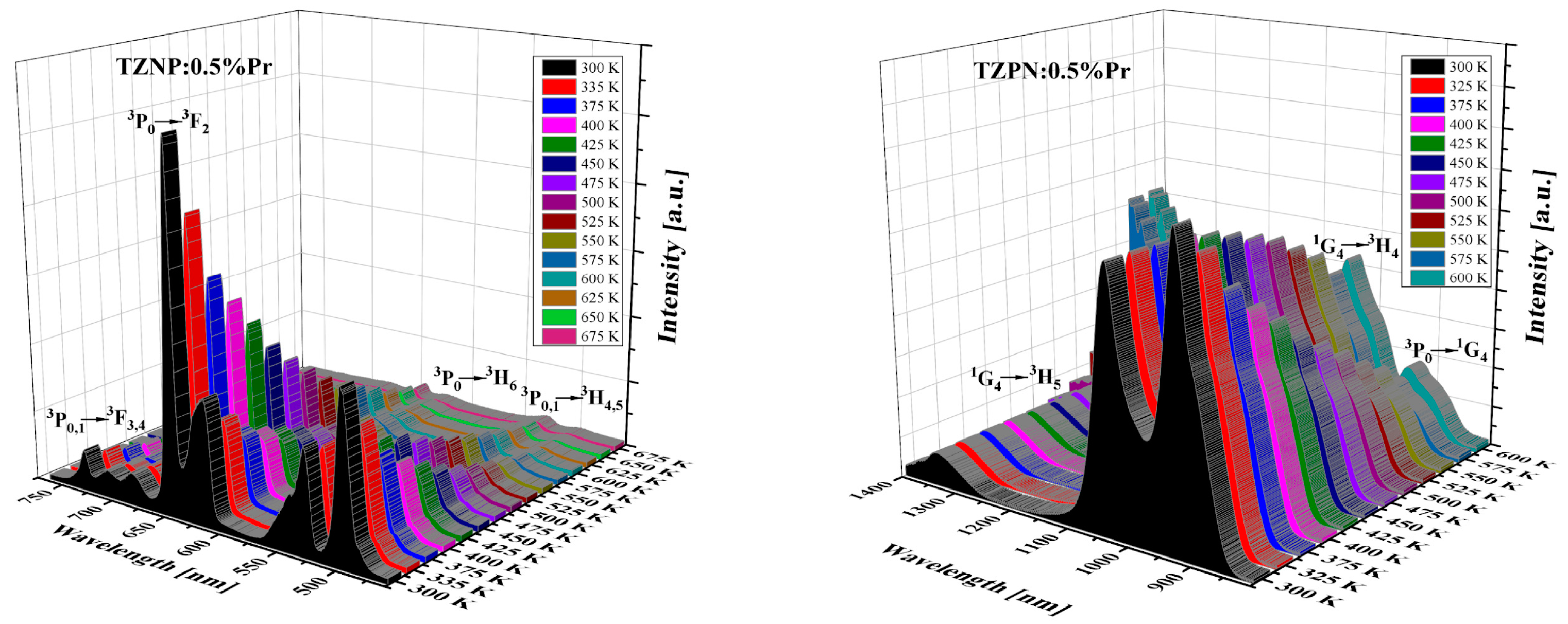
The influence of temperature on the emission spectrum of TZPN:0.5%Pr glass in the visible and near-infrared range was also examined (see Figure 4). The emission intensity of all bands decreases with increasing temperature but the spectral shape and peaks’ position are unaffected. The exception is that the band at 1327 nm corresponding to the 1G4 → 3H5 transition for that emission intensity diminishes with temperature increasing from 300 K to 600 K. The NIR luminescence at ~1.3 µm is widely used in telecommunications technology in the case of low-loss optical amplifiers operating in the O-band (1260–1360 nm) [48]. The attenuation of conventional O-band optical fiber is relatively large, for example, almost twice that of the C-band (1530–1565 nm) [49]. To compensate for this attenuation and effectively amplify light in the O-band, a praseodymium-doped fiber amplifier (PDFA) can be used based on a low-phonon-energy host glass and as one can see, the studied TZPN oxyfluorotellurite glass meets these requirements.
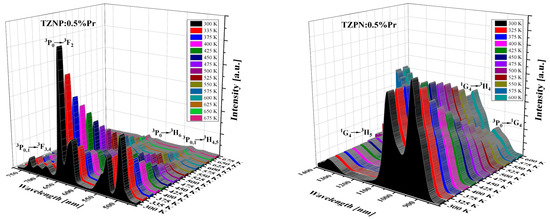
Figure 4.
Effect of temperature on visible (left) and near-infrared (right) emission spectra of TZPN:0.5%Pr glass.
To determine the chromaticity of the emitted luminescence, the emission spectra profiles of TZPN glass doped with Pr3+ were used. The chromaticity color coordinates estimated for the TZPN:0.5%Pr glass sample excited at 450 nm are x = 0.490 and y = 0.385, respectively, and lie in the orange-red region of the chromaticity diagram of Commission Internationale de l’Eclairage (CIE) 1931 (see inset Figure 3).
The color temperature (CCT) correlated with them, determined based on the empirical McCamy formula [50]:
where n = (x − xe)/(y − ye) is the inverse slope line and xe = 0.3320 and ye = 0.1858 is the epicenter estimated based on the chromaticity coordinates. The CCT of TZPN:Pr oxyfluorotellurite glass at λexc = 450 nm was found to be 2128.83 K and therefore may be a promising candidate for the production of various types of photonic equipment (LEDs, solid-state lasers, displays and devices) emitting in the orange-red range of visible light [51,52].
CCT = −449n3 + 3525n2 − 6823n + 5520.33
Another important parameter due to possible luminescent applications is color purity (CP), which determines how monochromatic/pure the emitted light is and was calculated according to the relationship
where (x, y) are the estimated chromaticity color coordinates, (xs, ys) are the standard coordinates of white light and (xd, yd) are the coordinates of the dominant wavelength. The color purity for the glass sample TZPN:0.5%Pr reached an effective value of 45.85%. Similar CP values are reported by Poojha et al. for Pr3+-doped lead boro-tellurite glasses [52].
2.4. Photoluminescence Decay Analysis
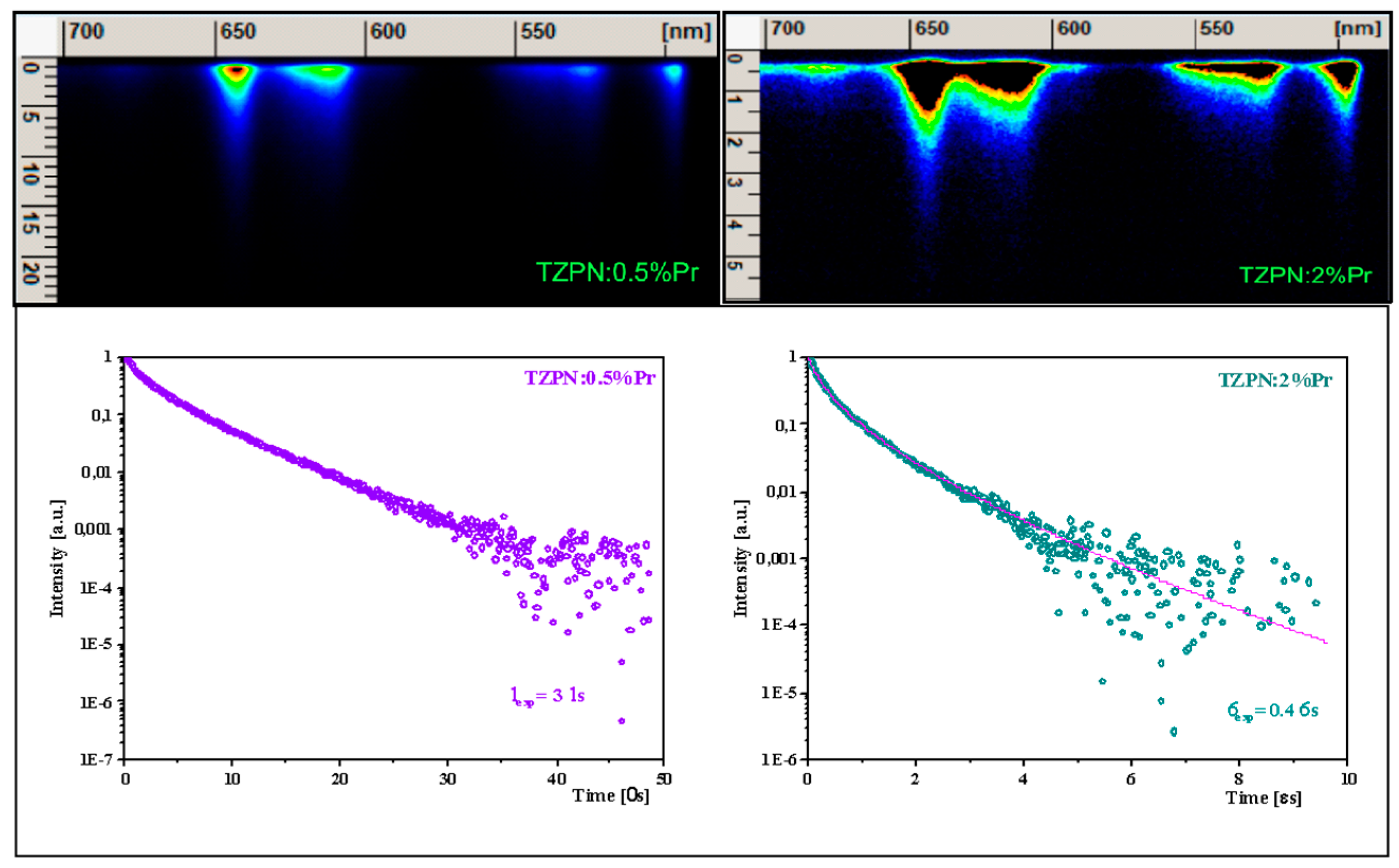
The images taken from the streak camera shown in the upper part of Figure 5 consist of luminescence originating in the 3P0 excited state.
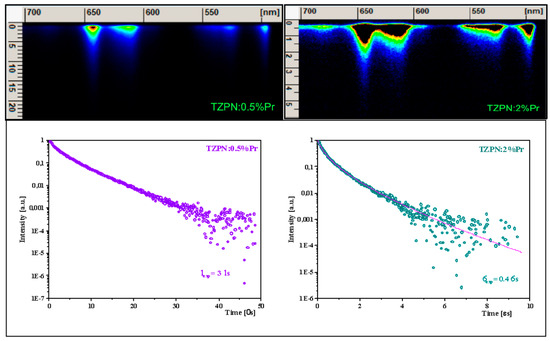
Figure 5.
Streak camera images related to praseodymium emission in TZPN:0.5%Pr and TZPN:2%Pr glasses (upper) and the adequate decay curves of 3P0 luminescence (bottom).
Examinations of photoluminescence (PL) decay curves for the 3P0 excited level (see lower part of Figure 5) show a partly single exponential character of the decay curve for the TZPN:0.5%Pr glass sample with a lifetime of 3 μs. When the concentration of Pr3+ ions increases to 2 mol%, the decay becomes faster (τavg = 0.4 μs) and the decay curve deviates from a single exponential time dependence. Consequently, we follow a commonly applied approximation and determine the so-called mean lifetime value expressed as follows [53]:
where t represents time and I is the intensity of luminescence.
The decrease in the experimental lifetime with a higher sample concentration is related to the self-quenching effect of Pr3+ luminescence. This happens due to the reduced distance between Pr3+ ions, which in turn results in the share of non-radiative energy transfer increases through cross-relaxation and energy migration processes.
At the same time, the obtained lifetimes of the excited state 3P0 are relatively shorter than a radiative lifetime obtained by the Judd–Ofelt calculation (see Table 2). The quantum efficiency (η) of the tested material estimated at over 62% results from the relationship
and in many cases is higher or comparable to the corresponding data obtained for other praseodymium-doped glasses [6,10,27,28,52,54].
The Inokuti–Hirayama model can be applied to investigate the non-radiative Pr–Pr energy transfer in material under study [55]. In the case of much slower energy migration in relation to interionic energy transfer, the time evolution of donor emission intensity may be defined as
where Φ(t) is the emission intensity after pulse excitation, A is constant, S = 6 for dipole–dipole interactions, τ0 is the intrinsic decay probability of the donor-involved excited state when the acceptor is absent and α is a parameter expressed as
where Na denotes the acceptor concentration (Na = 2.36 × 1021), R0 is the critical Pr–Pr energy transfer distance and Γ = 1.77 (for S = 6) is Euler’s function. In fact, the recorded 3P0 decay curve for TZPN:2%Pr glass is non-exponential and as a result of the appropriate fitting, we acquired the reasonable results for S = 6 and α = 5.64. Consequently, the critical energy transfer distance was estimated to be R0 = 6.85 Å. An interaction parameter between praseodymium ions can be expressed as Cda = R06∙τ0−1 and the related value of 2.29 × 10−38 cm6s−1 was estimated for our glass. Based on the formula Wda = Cda∙R0−6, the donor–acceptor energy transfer rate was found to be 2.49 × 106 s−1.
2.5. Temperature Sensor Applications
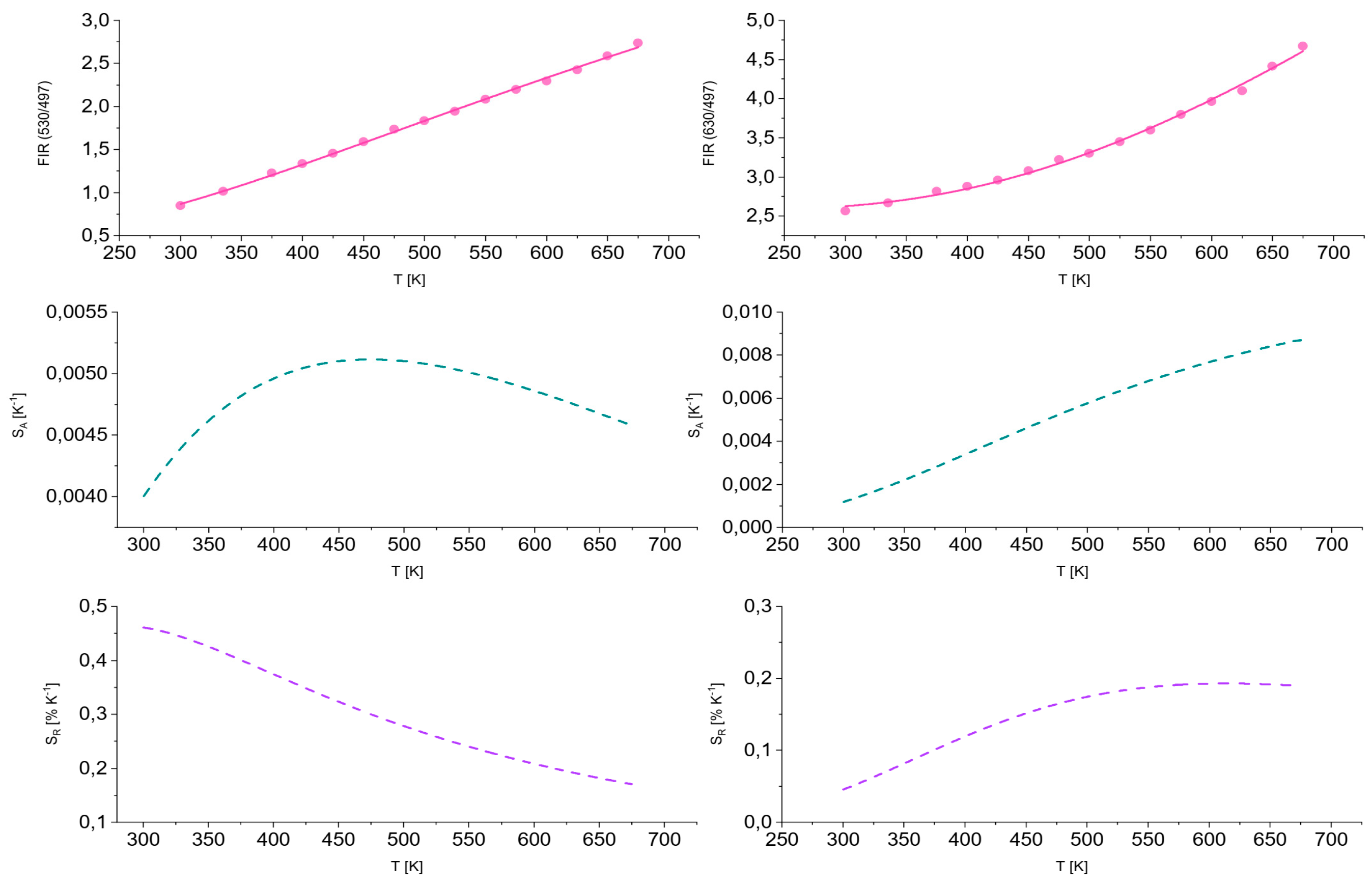
In order to assess the suitability of the studied materials for applications in optical sensing thermometry, we examined changes in the fluorescence intensity ratio (FIR) as a function of temperature (in the range of 250–700 K) and corresponding absolute (SA) and relative (SR) thermal sensitivities for TZPN:0.5%Pr glass.
The obtained results are presented in Figure 6 in the form of temperature dependence curves of fluorescence intensity ratios FIR (I530/I497) (on the left) and FIR (I630/I497) (on the right) by fitting with the following relationship:
where ∆E is the energy difference between the thermalized levels, kB is the Boltzmann constant, T is the temperature expressed in the absolute scale [K] and A and B are constants. In the case of FIR (I530/I497), we observe a linear increase in the fluorescence intensity ratio with a maximum absolute temperature sensitivity value of SA = 5.1 × 10−3 K−1 at T = 460 K and approximately 0.46% K−1 relative temperature sensitivity for T = 300 K. The values of the fluorescence intensity ratios FIR (I630/I497) increase exponentially with temperature and the maximum absolute temperature sensitivity is reached for T = 675 K and amounted to 8.7 × 10−3 K−1, which translates into SR = 0.20% K−1 of relative temperature sensitivity at about T = 460 K. All absolute and relative curves of temperature sensitivity for TZPN:0.5%Pr glass have a non-linear way with a different tendency. For a comparison, A.S. Rao examined the impact of temperature on four different emission bands of praseodymium [56]. Some fluorescence intensity ratio (FIR) models based on the relationships between different emission peaks were studied to estimate a maximum relative sensitivity, 1.03% K−1. Moreover, other Pr-doped inorganic phosphors were investigated by Jiawen Wang et al. [57] and a comparable evaluation approach gave rise to quite high relative sensitivity (1~3.25% K−1) and low temperature uncertainty (0.15–0.5 K). These reported sensitivities are higher in relation to TZPN:Pr estimations but regardless, our results are attributed to a wide temperature range up to 675 K.
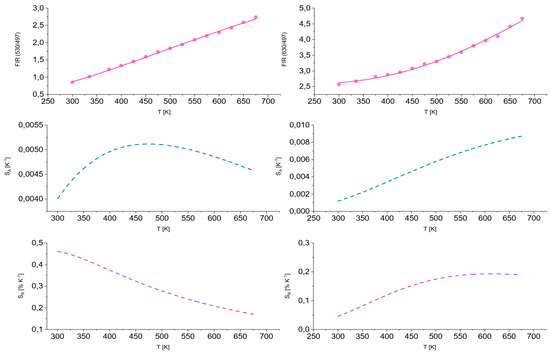
Figure 6.
Fluorescence intensity ratios attributed to praseodymium luminescence as well as corresponding absolute and relative thermal sensitivities estimated for FIR (I530/I497) and FIR (I630/I497) for TZPN:0.5%Pr glass.
3. Materials and Methods
A traditional melt quenching method was employed to manufacture TeO2-ZnF2-PbO-Nb2O5 oxyfluoride glasses activated with 0.5 and 2 mol% of Pr2O3. Tellurium oxide (5N), zinc fluoride (5N), lead oxide (4N), niobium oxide (Nb2O3) and praseodymium oxide (5N) were thoroughly mixed, ground and incorporated in a corundum crucible. The melting process in the ambient atmosphere took 30 min at 830 °C. The glasses were poured into preheated brass molds and then annealed for a few hours at 350 °C in order to reduce the internal stresses. The resulting glass samples were transparent and homogeneous.
The specimens were characterized by the DTA (differential thermal analysis) technique applying the normal pressure and atmosphere. The DSC 404/3/F calorimeter (Erich NETZSCH B.V. & Co. Holding KG Gebrüder-Netzsch, Selb, Germany), platinum crucibles and reference holders were adequately employed. The same heating rates (10 K/min) were realized for all studied glasses.
The spectrophotometer Agilent Cary 5000 (Agilent, Santa Clara, CA, USA) was used to measure the survey absorption spectra within UV-VIS-NIR spectral ranges. Luminescence experiments were carried out in the visible and near-infrared spectral regions as well. An FLS1000 Spectrofluorimeter (Edinburgh Instruments Ltd., Livingston, UK) was utilized to record the emission spectra. The Linkam THMS 600 Heating/Freezing Stage (Linkam Scientific Instruments Ltd., Redhill, UK) was used to perform temperature-dependent measurements. To record luminescence decay curves, the glass samples were excited by a femtosecond LIBRA Ti:sapphire laser (Coherent Inc., Santa Clara, CA, USA) coupled with optical parametric amplifier “Opera” (OPO).
4. Conclusions
It was found that TZPN glass thermal stability increases for a higher concentration of optically active ions. The impact of temperature on the absorption and variation of fluorescence intensity in the VIS-NIR region was monitored in the temperature range of 300–675 K. The observed luminescence can be mainly attributed to transitions originating from two closely located, thermally coupled levels (3P0 and 3P1). The values of the radiative transition probabilities Wr, branching ratios β and the fluorescence intensity ratio at different temperatures for the selected praseodymium transitions were adequately investigated. The branching ratio of praseodymium luminescence in material under study is related to a wide spectral region; hence, its various useful potential applications can be considered. The CIE coordinates for the TZPN:Pr glasses are in the orange-red region. The maximum lifetime of the 3P0 level was observed for a sample with a lower concentration of active ions. This is explained by the self-quenching effect—the experimental lifetime decreases with increasing Pr3+ concentration. Application potential of the investigated material in optical sensor thermometry was evaluated as well.
Author Contributions
Conceptualization, B.K. and R.L.; methodology, B.K. and R.L.; validation, B.K.; formal analysis, B.K., R.L. and W.R.-R.; investigation, B.K., R.L. and W.R.-R.; resources, B.K.; data curation, B.K. and R.L.; writing—original draft preparation, B.K.; writing—review and editing, B.K. and R.L., visualization, B.K. and R.L.; supervision, B.K. and W.R.-R.; project administration, B.K.; funding acquisition, B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Arshad, M.; Saqib, N.U.; Rooh, G.; Chanithima, N.; Zaman, F.; Asif, M.; Kim, H.J.; Kothan, S.; Haq, S.U.; Kaewkhao, J. Spectroscopic and photoluminescence properties of praseodymium doped potassium aluminum phosphate (P2O5-K2O-Al2O3) glasses for optoelectronics applications. J. Non-Cryst. Solids 2022, 586, 121570. [Google Scholar] [CrossRef]
- Mao, S.; Ta, C.; Wen, H.; Talewar, R.A. Talewar, Optical properties of 40ZnO-40P2O5-x(10Li2O-10Nb2O5-0.2Pr3+) glass. Results Opt. 2023, 12, 100429. [Google Scholar] [CrossRef]
- Wantana, N.; Kaewnuam, E.; Chanthima, N.; Kim, H.J.; Kaewkhao, J. Tuneable luminescence of Pr3+-doped sodium aluminium gadolinium phosphate glasses for photonics applications. Optik 2022, 267, 169668. [Google Scholar] [CrossRef]
- Nazabal, V.; Adam, J. (INVITED) Infrared luminescence of chalcogenide glasses doped with rare earth ions and their potential applications. Opt. Mater. X. 2022, 15, 100168. [Google Scholar] [CrossRef]
- Kolavekar, S.B.; Ayachit, N.H. Synthesis of praseodymium trioxide doped lead-boro-tellurite glasses and their optical and physical properties. J. Mater. 2019, 5, 455–462. [Google Scholar] [CrossRef]
- Zhang, F.; Bi, Z.; Huang, A.; Xiao, Z. Luminescence and Judd–Ofelt analysis of the Pr3+ doped fluorotellurite glass. J. Lumin. 2015, 160, 85–89. [Google Scholar] [CrossRef]
- Nazabal, V.; Poulain, M.; Olivier, M.; Pirasteh, P.; Camy, P.; Doualan, D.-L.; Guy, S.; Djouama, T.; Boutarfaia, A.; Adam, J.L. Fluoride and oxyfluoride glasses for optical applications. J. Fluor. Chem. 2012, 134, 18–23. [Google Scholar] [CrossRef]
- Liao, G.; Chen, Q.; Xing, J.; Gebavi, H.; Milanese, D.; Fokine, M.; Ferraris, M. Preparation and characterization of new fluorotellurite glasses for photonics application. J. Non-Cryst. Solids 2019, 355, 447–452. [Google Scholar] [CrossRef]
- El-Okr, M.; Ibrahem, M.; Farouk, M. Structure and properties of rare-earth-doped glassy systems. J. Phys. Chem. Solids 2008, 69, 2564–2567. [Google Scholar] [CrossRef]
- Maheshwari, K.; Aman Prasad, R.; Tayal, Y.; Rao, A.S. Spectroscopic studies of Pr3+ doped red-emitting BaO–ZnO–Li2O–P2O5 glasses for luminescent devices applications. Opt. Mater. 2023, 140, 113910. [Google Scholar] [CrossRef]
- de Araújo, C.B.; Kassab, L.R.; da Silva, D.M. Optical properties of glasses and glass-ceramics for optical amplifiers, photovoltaic devices, color displays, optical limiters, and Random Lasers. Opt. Mater. 2022, 131, 112648. [Google Scholar] [CrossRef]
- Tanaka, H.; Kalusniak, S.; Badtke, M.; Demesh, M.; Kuleshov, N.V.; Kannari, F.; Kränkel, C. Visible solid-state lasers based on Pr3+ and Tb3+. Prog. Quantum Electron. 2022, 84, 100411. [Google Scholar] [CrossRef]
- Kumar, M.; Vijayalakshmi, R.P.; Ratnakaram, Y.C. Investigation of structural and optical properties of Pr3+ doped and Pr3+/Dy3+ co-doped multicomponent bismuth phosphate glasses for visible light applications. J. Mol. Struct. 2022, 1265, 133333. [Google Scholar] [CrossRef]
- Bian, Z.P.; Li, D.S.; Zhao, X.; Lin, H. Multi-peak emissions of Pr3+-doped heavy metal tellurite glasses for laser-driven illumination. Radiat. Phys. Chem. 2018, 151, 126–132. [Google Scholar] [CrossRef]
- Belançon, M.P.; Marconi, J.D.; Ando, M.F.; Barbosa, L.C. Near-IR emission in Pr3+ single doped and tunable near-IR emission in Pr3+/Yb3+ codoped tellurite tungstate glasses for broadband optical amplifiers. Opt. Mater. 2014, 36, 1020–1026. [Google Scholar] [CrossRef]
- Jacquier, B.; Lebrasseur, E.; Guy, S.; Belarouci, A.; Menchini, F. Rare earth-doped confined structures for amplifiers and lasers. J. Alloys Compd. 2000, 303–304, 207–213. [Google Scholar] [CrossRef]
- Kwaśny, M.; Mierczyk, Z.; Stȩpień, R.; Jȩdrzejewski, K. Nd3+-, Er3+- and Pr3+-doped fluoride glasses for laser applications. J. Alloys Compd. 2000, 300–301, 341–347. [Google Scholar] [CrossRef]
- Kumar Rai, V.; Rai, D.K.; Rai, S.B. Pr3+ doped lithium tellurite glass as a temperature sensor. Sens. Actuators A Phys. 2006, 128, 14–17. [Google Scholar] [CrossRef]
- Kumar Rai, V.; Kumar, K.; Rai, S.B. Upconversion in Pr3+ doped tellurite glass. Opt. Mater. 2007, 29, 873–878. [Google Scholar] [CrossRef]
- Lalla, E.A.; Konstantinidis, M.; De Souza, I.; Daly, M.G.; Martín, I.R.; Lavín, V.; Rodríguez-Mendoza, U.R. Judd-Ofelt parameters of RE3+-doped fluorotellurite glass (RE3+= Pr3+, Nd3+, Sm3+, Tb3+, Dy3+, Ho3+, Er3+, and Tm3+). J. Alloys Compd. 2020, 845, 156028. [Google Scholar] [CrossRef]
- Algarni, H.; Reben, M.; Yousef, E. Optical features of novel fluorotellurite glasses based on TeO2- LiNbO3- BaF2- La2O3- (Nb2O5 or TiO2). Optik 2018, 156, 720–727. [Google Scholar] [CrossRef]
- Génova, R.T.; Martín, I.R.; Rodríguez-Mendoza, U.R.; Lahoz, F.; Lozano-Gorrín, A.D.; Núñez, P.; González-Platas, J.; Lavín, V. Optical intensities of Pr3+ ions in transparent oxyfluoride glass and glass–ceramic. Applications of the standard and modified Judd–Ofelt theories. J. Alloys Compd. 2004, 380, 167–172. [Google Scholar] [CrossRef]
- Xu, J.; Guyot, Y.; Lebbou, K.; Xu, X.; Liu, J.; Xu, J.; Moncorgé, R. Luminescence properties of Pr3+ doped Bi4Ge3O12 crystal fibers grown by the micro-pulling down technique. J. Lumin. 2023, 260, 119882. [Google Scholar] [CrossRef]
- Macalik, B.; Lisiecki, R.; Kowalski, R.M.; Ryba-Romanowski, W. Down- and up-conversion of femtosecond light pulses into Pr3+ luminescence in LiTaO3:Pr3+ single crystal. J. Lumin. 2020, 224, 117294. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Kaczkan, M.; Majchrowski, A.; Malinowski, M. Spectroscopic characterization of orthorhombic δ-BiB3O6 phase nonlinear single crystal doped with Pr3+ ions. J. Lumin. 2019, 207, 251–257. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.; Han, L.; Liu, C.; You, W.; Ye, X.; Zhang, L. Composition dependence visible fluorescence from praseodymium ions doped Al2O3–CaO-M(M=B2O3, GeO2, Ga2O3) glasses. Phys. B 2023, 658, 414842. [Google Scholar] [CrossRef]
- Himamaheswara Rao, V.; Syam Prasad, P.; Sowri Babu, K. Visible luminescence characteristics of Pr3+ ions in TeO2–Sb2O3–WO3 glasses. Opt. Mater. 2020, 101, 109740. [Google Scholar] [CrossRef]
- Jamalaiah, B.C.; Viswanadha, G.; Venkata Rao, K. Rich reddish-orange emitting PBTNAPr glasses for laser applications. Opt. Mater. 2019, 96, 109340. [Google Scholar] [CrossRef]
- Yang, J.; Shen, L.; Yue, E.; Pun, B.; Lin, H. Superiority of shortwave transparent glasses with moderate phonon energy in achieving effective radiations from 1D2 level of Pr3+. J. Lumin. 2019, 213, 51–60. [Google Scholar] [CrossRef]
- Wold, A. Chemistry of glasses. Mater. Res. Bull. 1991, 26, 836–837. [Google Scholar] [CrossRef]
- Klimesz, B.; Lisiecki, R.; Ryba-Romanowski, W. Oxyfluorotellurite glasses doped with neodymium and ytterbium-thermal and spectroscopic properties as well as energy transfer phenomena. J. Lumin. 2018, 199, 310–318. [Google Scholar] [CrossRef]
- Ramakrishna, P.; Padhi, R.; Parida, S.K.; Mohapatra, D.; Jena, H.; Panigrahi, B. Effect of U on the photoluminescence of Pr and structural properties of U/Pr doped and co-doped Li2O–ZnO–SrO borophosphate glass. Opt. Mater. 2022, 134, 113121. [Google Scholar] [CrossRef]
- Mohamed, N.; Hassan, J.; Matori, K.M.; Azis, R.S.; Wahab, Z.A.; Ismail, Z.M.M.; Baharuddin, N.; Rashid, S.S.A. Influence of Pr doping on the thermal, structural and optical properties of novel SLS-ZnO glasses for red phosphor. Results Phys. 2017, 7, 1202–1206. [Google Scholar] [CrossRef]
- Vighnesh, K.R.; Ramya, B.; Nimitha, S.; Wagh, A.; Sayyed, M.I.; Sakar, E.; Yakout, H.A.; Dahshan, A.; Kamath, S.D. Structural, optical, thermal, mechanical, morphological & radiation shielding parameters of Pr3+ doped ZAlFB glass systems. Opt. Mater. 2020, 99, 109512. [Google Scholar] [CrossRef]
- Saad, M.; Poulain, M. Glass Forming Ability Criterion. Mater. Sci. Forum. 1987, 19–20, 11–18. [Google Scholar] [CrossRef]
- Klimesz, B.; Lisiecki, R.; Ryba-Romanowski, W. Oxyfluorotellurite glasses doped by dysprosium ions. Thermal and optical properties. Opt. Mater. 2015, 42, 538–543. [Google Scholar] [CrossRef]
- Judd, B.R. Optical Absorption Intensities of Rare-Earth Ions. Phys. Rev. 1962, 127, 750–761. [Google Scholar] [CrossRef]
- Ofelt, G.S. Intensities of Crystal Spectra of Rare-Earth Ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Medeiros Neto, J.A.; Hewak, D.W.; Tate, H. Application of a modified Judd-Ofelt theory to praseodymium-doped fluoride glasses. J. Non-Cryst. Solids 1995, 183, 201–207. [Google Scholar] [CrossRef]
- Jose, G.; Thomas, V.; Jose, G.; Paulose, P.I.; Unnikrishnan, N.V. Application of a modified Judd–Ofelt theory to Pr3+ doped phosphate glasses and the evaluation of radiative properties. J. Non-Cryst. Solids 2003, 319, 89–94. [Google Scholar] [CrossRef]
- Kumar, M.V.V.; Gopal, K.R.; Reddy, R.R.; Reddy, G.V.L.; Hussain, N.S.; Jamalaiah, B.C. Application of modified Judd–Ofelt theory and the evaluation of radiative properties of Pr3+-doped lead telluroborate glasses for laser applications. J. Non-Cryst. Solids 2013, 364, 20–27. [Google Scholar] [CrossRef]
- Kornienko, A.A.; Kaminskii, A.A.; Dunina, E.B. Dependence of the line strength of f–f transitions on the manifold energy. II. Analysis of Pr3+ in KPrP4O12. Phys. Status Solidi B 1990, 157, 267–273. [Google Scholar] [CrossRef]
- Anjaiah, J.; Laxmikanth, C.; Veeraiah, N.; Kistaiah, P. Luminescence properties of Pr3+ doped Li2O–MO–B2O3 glasses. J. Lumin. 2015, 161, 147–153. [Google Scholar] [CrossRef]
- Hormadaly, J.; Reisfeld, R. Intensity parameters and laser analysis of Pr3+ and Dy3+ in oxide glasses. J. Non-Cryst. Solids 1979, 30, 337–348. [Google Scholar] [CrossRef]
- Man, S.Q.; Pun, E.Y.B.; Chung, P.S. Tellurite glasses for 1.3 μm optical amplifiers. Opt. Commun. 1999, 168, 369–373. [Google Scholar] [CrossRef]
- Kumar Rai, V.; Rai, S.B.; Rai, D.K. Spectroscopic properties of Pr3+ doped in tellurite glass. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 62, 302–306. [Google Scholar] [CrossRef]
- Marek, Ł.; Sobczyk, M. Spectroscopic investigations of Pr3+ ions in Na2O-La2O3-ZnO-TeO2 glasses. J. Non-Cryst. Solids 2018, 487, 96–103. [Google Scholar] [CrossRef]
- Wei, D.; Seo, H.J.; Liu, Y.; Yang, X. Broadband infrared emission of Pr3+-doped BiLa2O4.5 phosphor for optical amplifier applications. J. Lumin. 2023, 253, 119488. [Google Scholar] [CrossRef]
- Ohishi, Y.; Kanamori, T.; Kitagawa, T.; Takahashi, T.; Snitzer, E.; Sigel, G.H. Pr3+-doped fluoride fiber amplifier operating at 1.31 μm. Opt. Lett. 1991, 16, 1747–1749. [Google Scholar] [CrossRef]
- McCamy, C.S. Correlated color temperature as an explicit function of chromaticity coordinates. Color. Res. Appl. 1992, 17, 142–144. [Google Scholar] [CrossRef]
- Mariselvam, K.; Liu, J. Investigations on the luminescence and gamma ray shielding features of Pr3+: BLCZFB glass for orange-red laser and radiation applications. Phys. B 2021, 614, 413024. [Google Scholar] [CrossRef]
- Komal Poojha, M.K.; Naseer, K.A.; Matheswaran, P.; Marimuthu, K.; El Shiekh, E. Effect of alkali/alkaline modifiers on Pr3+ ions doped lead boro-tellurite glasses for lasing material applications. Opt. Mater. 2023, 143, 114289. [Google Scholar] [CrossRef]
- Zatryb, G.; Klak, M. On the choice of proper average lifetime formula for an ensemble of emitters showing non-single exponential photoluminescence decay. J. Phys. Condens. Matter 2020, 32, 415902. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.N.A.; Siva, B.V.; Neeraja, K.; Mohan, N.K.; Rojas, J.I. Effect of modifier oxides on spectroscopic and optical properties of Pr3+ doped PbO–Ro2O3–WO3–B2O3 glasses (with Ro2O = Sb2O3, Al2O3, and Bi2O3). J. Lumin. 2021, 230, 117666. [Google Scholar] [CrossRef]
- Inokuti, M.; Hirayama, F. Influence of Energy Transfer by the Exchange Mechanism on Donor Luminescence. J. Chem. Phys. 1965, 43, 1978. [Google Scholar] [CrossRef]
- Rao, A.A.S. Luminescence and optical thermometry strategy based on emission spectra of Li2Ba5W3O15:Pr3+ phosphors. Opt. Mater. 2023, 145, 114476. [Google Scholar] [CrossRef]
- Wang, J.; Chen, N.; Li, J.; Feng, Q.; Lei, R.; Wang, H.; Xu, S. A novel high-sensitive optical thermometer based on the multi-color emission in Pr3+ doped LiLaMgWO6 phosphors. J. Lumin. 2021, 238, 118240. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).