Natural Bioactive Compounds in the Management of Periodontal Diseases: A Comprehensive Review
Abstract
1. Introduction
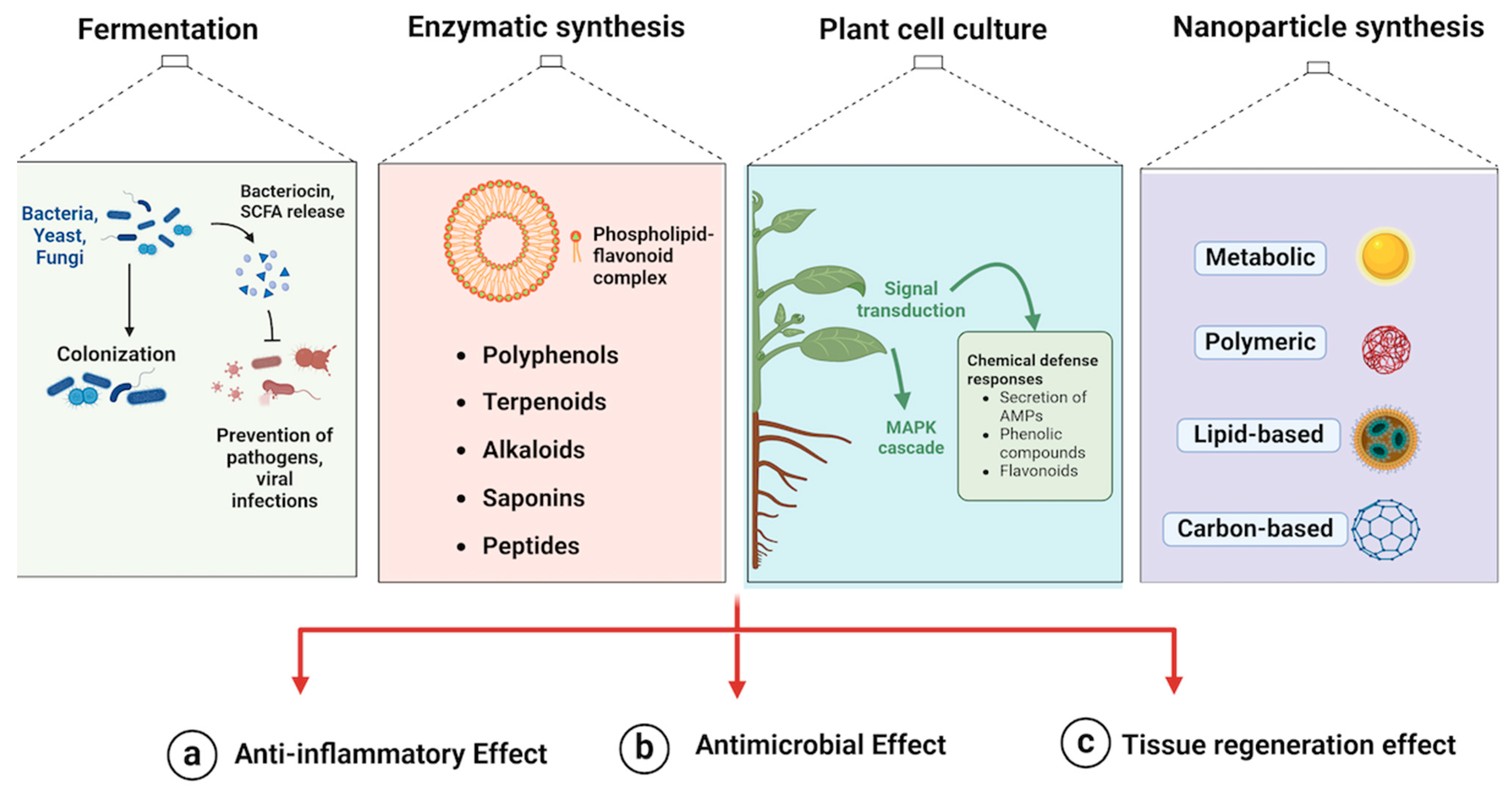
2. Biomanufacturing Processes
2.1. Fermentation
2.2. Enzymatic Synthesis
2.3. Plant Cell Cultures
2.4. Nanoparticle Synthesis
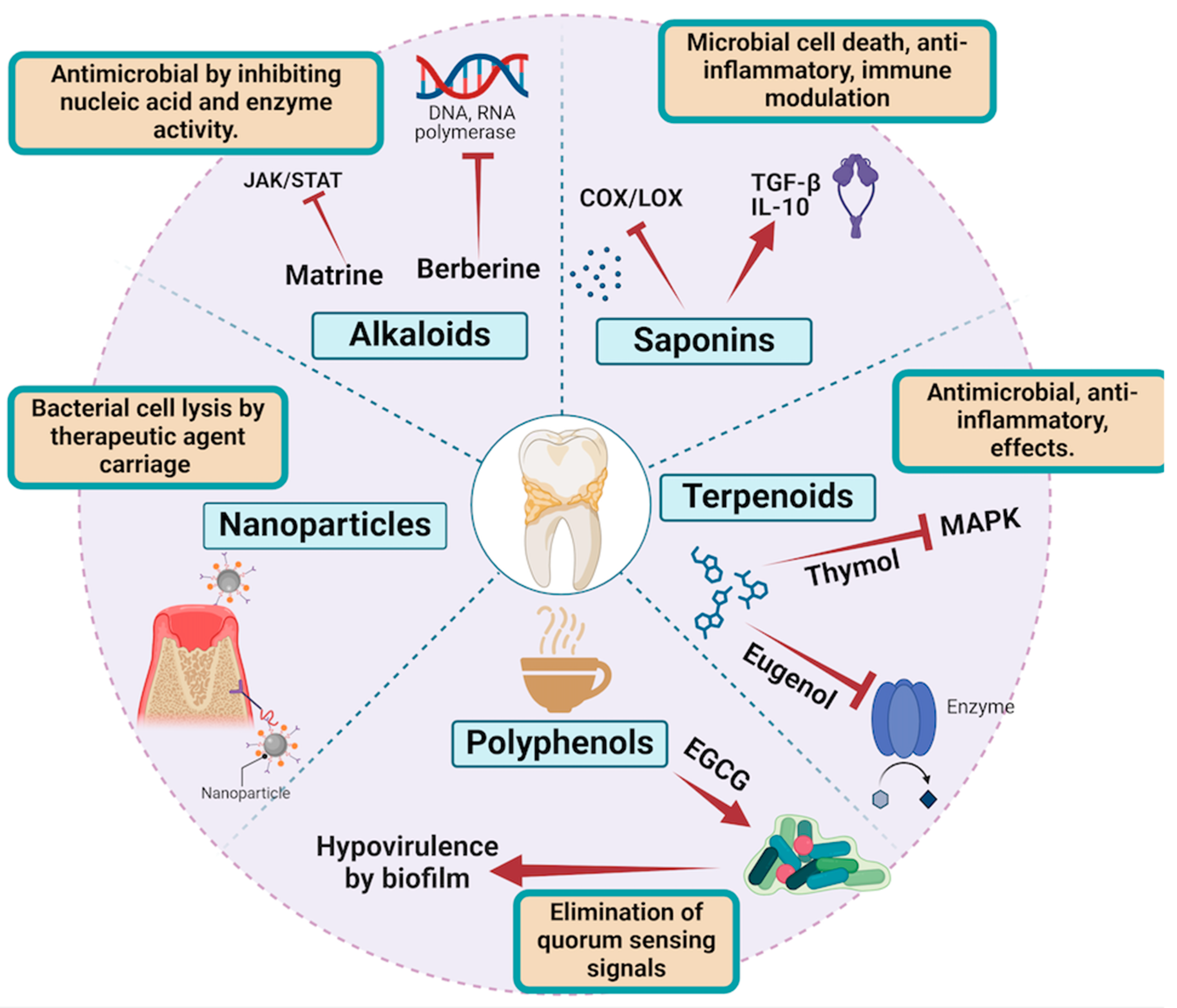
3. Mechanisms of Action of Bioactive Compounds
Molecular Mechanism of Bioactive Compounds in the Management of Periodontal Diseases
- Polyphenols
- Terpenoids
- Alkaloids
- Saponins
- Nanoparticles
4. Clinical Applications of Bioactive Compounds
4.1. Topical Applications
4.2. Systemic Administration
5. The Role of Bioactive Compounds and Nanoparticles in Periodontal Therapy
6. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Abdulkareem, A.A.; Al-Taweel, F.B.; Al-Sharqi, A.J.B.; Gul, S.S.; Sha, A.; Chapple, I.L.C. Current concepts in the pathogenesis of periodontitis: From symbiosis to dysbiosis. J. Oral Microbiol. 2023, 15, 2197779. [Google Scholar] [CrossRef] [PubMed]
- Aral, K.; Milward, M.R.; Kapila, Y.; Berdeli, A.; Cooper, P.R. Inflammasomes and their regulation in periodontal disease: A review. J. Periodontal Res. 2020, 55, 473–487. [Google Scholar] [CrossRef]
- Mills, A.; Berlin-Broner, Y.; Levin, L. Improving Patient Well-Being as a Broader Perspective in Dentistry. Int. Dent. J. 2023, 73, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Nasseh, K.; Vujicic, M.; Glick, M. The relationship between periodontal interventions and healthcare costs and utilization. Evidence from an integrated dental, medical, and pharmacy commercial claims database. Health Econ. 2017, 26, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.E. Nonsurgical approaches for the treatment of periodontal diseases. Dent. Clin. N. Am. 2005, 49, 611–636. [Google Scholar] [CrossRef] [PubMed]
- Serino, G.; Rosling, B.; Ramberg, P.; Socransky, S.S.; Lindhe, J. Initial outcome and long-term effect of surgical and nonsurgical treatment of advanced periodontal disease. J. Clin. Periodontol. 2001, 28, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Deporter, D.A. Periodontal disease part II: Overview of treatment modalities. Can. Fam. Physician 1988, 34, 1391–1392. [Google Scholar]
- Slots, J.; Rams, T.E. Antibiotics in periodontal therapy: Advantages and disadvantages. J. Clin. Periodontol. 1990, 17, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.M.; Yerex, K.; Kelekis-Cholakis, A.; Duan, K. Advances in novel therapeutic approaches for periodontal diseases. BMC Oral Health 2022, 22, 492. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-H.; Chen, J.-Y.; Suo, W.-H.; Shao, W.-R.; Huang, C.-Y.; Li, M.-T.; Li, Y.-Y.; Li, Y.-H.; Liang, E.-L.; Chen, Y.-H.; et al. Unlocking the Future of Periodontal Regeneration: An Interdisciplinary Approach to Tissue Engineering and Advanced Therapeutics. Biomedicines 2024, 12, 1090. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Patil, P.C.; Luzzio, F.A.; Demuth, D.R. In Vitro and In Vivo Activity of Peptidomimetic Compounds That Target the Periodontal Pathogen Porphyromonas gingivalis. Antimicrob. Agents Chemother. 2018, 62, e00057-18. [Google Scholar] [CrossRef] [PubMed]
- Bunte, K.; Hensel, A.; Beikler, T. Polyphenols in the prevention and treatment of periodontal disease: A systematic review of in vivo, ex vivo and in vitro studies. Fitoterapia 2019, 132, 30–39. [Google Scholar] [CrossRef] [PubMed]
- López-Valverde, N.; López-Valverde, A.; Montero, J.; Rodríguez, C.; Macedo de Sousa, B.; Aragoneses, J.M. Antioxidant, anti-inflammatory and antimicrobial activity of natural products in periodontal disease: A comprehensive review. Front. Bioeng. Biotechnol. 2023, 11, 1226907. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Erol, Z.; Rugji, J.; Taşçı, F.; Kahraman, H.A.; Toppi, V.; Musa, L.; Di Giacinto, G.; Bahmid, N.A.; Mehdizadeh, M.; et al. An overview of fermentation in the food industry-looking back from a new perspective. Bioresour. Bioprocess. 2023, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Maicas, S. The Role of Yeasts in Fermentation Processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G. Probiotics and periodontal health. J. Med. Life 2011, 4, 387–394. [Google Scholar] [PubMed]
- Holt, S.C.; Ebersole, J.L. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: The ‘red complex’, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol. 2000 2005, 38, 72–122. [Google Scholar] [CrossRef] [PubMed]
- Gungor, O.E.; Kirzioglu, Z.; Kivanc, M. Probiotics: Can they be used to improve oral health? Benef. Microbes 2015, 6, 647–656. [Google Scholar] [CrossRef]
- Yang, S.C.; Lin, C.H.; Sung, C.T.; Fang, J.Y. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front. Microbiol. 2014, 5, 241. [Google Scholar]
- Joerger, R.D. Alternatives to antibiotics: Bacteriocins, antimicrobial peptides and bacteriophages. Poult. Sci. 2003, 82, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, M.; Trivedi, H.M.; Chen, D.; Gajendrareddy, P.; Chen, L. Recent Development of Active Ingredients in Mouthwashes and Toothpastes for Periodontal Diseases. Molecules 2021, 26, 2001. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, I.; Varriale, S.; Topakas, E.; Rova, U.; Christakopoulos, P.; Faraco, V. Enzymatic synthesis of bioactive compounds with high potential for cosmeceutical application. Appl. Microbiol. Biotechnol. 2016, 100, 6519–6543. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Li, R.; Xu, W.; Sun, H.; Li, Q.; Wang, D.; Dong, S.; Ding, J. Functional biomaterials for comprehensive periodontitis therapy. Acta Pharm. Sin. B 2023, 13, 2310–2333. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Twarowski, B.; Fecka, I.; Tuberoso, C.I.G.; Jerković, I. Thymol as a Component of Chitosan Systems-Several New Applications in Medicine: A Comprehensive Review. Plants 2024, 13, 362. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rojas, B.; Gutiérrez-Venegas, G. Flavonoids exert multiple periodontic benefits including anti-inflammatory, periodontal ligament-supporting, and alveolar bone-preserving effects. Life Sci. 2018, 209, 72–122. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Asif, M. Green Synthesis, Green Chemistry, and Environmental Sustainability: An Overview on Recent and Future Perspectives of Green Chemistry In Pharmaceuticals. Green Chem. Technol. Lett. 2021, 7, 18–27. [Google Scholar] [CrossRef]
- Chen, X.; Wu, G.; Feng, Z.; Dong, Y.; Zhou, W.; Li, B.; Bai, S.; Zhao, Y. Advanced biomaterials and their potential applications in the treatment of periodontal disease. Crit. Rev. Biotechnol. 2016, 36, 760–775. [Google Scholar] [CrossRef]
- Anjali; Kumar, S.; Korra, T.; Thakur, R.; Arutselvan, R.; Kashyap, A.S.; Nehela, Y.; Chaplygin, V.; Minkina, T.; Keswani, C. Role of plant secondary metabolites in defence and transcriptional regulation in response to biotic stress. Plant Stress 2023, 8, 100154. [Google Scholar] [CrossRef]
- Bapat, V.A.; Kavi Kishor, P.B.; Jalaja, N.; Jain, S.M.; Penna, S. Plant Cell Cultures: Biofactories for the Production of Bioactive Compounds. Agronomy 2023, 13, 858. [Google Scholar] [CrossRef]
- Abdulhafiz, F. Plant Cell Culture Technologies: A Promising Alternative to Produce High-Value Secondary Metabolites. Arab. J. Chem. 2022, 15, 104161. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, V.; Slavov, A.; Vasileva, I.; Pavlov, A. Plant cell culture as emerging technology for production of active cosmetic ingredients. Eng. Life Sci. 2018, 18, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhao, J.; Zeng, Z.; Wan, D.; Yu, P.; Cheng, D.; Gong, D.; Deng, S. Antibacterial activity and membrane-disrupting mechanism of monocaprin against Escherichia coli and its application in apple and carrot juices. LWT 2020, 131, 109794. [Google Scholar] [CrossRef]
- Williams, D.A.; Pradhan, K.; Paul, A.; Olin, I.R.; Tuck, O.T.; Moulton, K.D.; Kulkarni, S.S.; Dube, D.H. Metabolic inhibitors of bacterial glycan biosynthesis. Chem. Sci. 2020, 11, 1761–1774. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Zhong, R.; Miao, L.; Zhang, H.; Tan, L.; Zhao, Y.; Tu, Y.; Angel Prieto, M.; Simal-Gandara, J.; Chen, L.; He, C.; et al. Anti-inflammatory activity of flavonols via inhibiting MAPK and NF-κB signaling pathways in RAW264.7 macrophages. Curr. Res. Food Sci. 2022, 5, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Xu, Z.Z.; Strichartz, G.; Serhan, C.N. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci. 2011, 34, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Varesi, A.; Chirumbolo, S.; Campagnoli, L.I.M.; Pierella, E.; Piccini, G.B.; Carrara, A.; Ricevuti, G.; Scassellati, C.; Bonvicini, C.; Pascale, A. The Role of Antioxidants in the Interplay between Oxidative Stress and Senescence. Antioxidants 2022, 11, 1224. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.B.; Styring, A.K.; McCullagh, J.S.O. Polyphenols: Bioavailability, Microbiome Interactions and Cellular Effects on Health in Humans and Animals. Pathogens 2022, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic Effects of Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate (EGCG) in Relation to Molecular Pathways Controlling Inflammation, Oxidative Stress, and Apoptosis. Int. J. Mol. Sci. 2022, 24, 340. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.P.; Ramachandra, S.S. Quorum Sensing and Quorum Quenching with a Focus on Cariogenic and Periodontopathic Oral Biofilms. Microorganisms 2022, 10, 1783. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, S.P.; Reddy, A.T.; Kodidhela, L.D.; Varadacharyulu, N.C. The tea catechin epigallocatechin gallate inhibits NF-κB-mediated transcriptional activation by covalent modification. Arch. Biochem. Biophys. 2020, 695, 108620. [Google Scholar] [CrossRef] [PubMed]
- Miralda, I.; Uriarte, S.M. Periodontal Pathogens’ strategies disarm neutrophils to promote dysregulated inflammation. Mol. Oral Microbiol. 2021, 36, 103–120. [Google Scholar] [CrossRef]
- Hosseini Hooshiar, M.; Badkoobeh, A.; Kolahdouz, S.; Tadayonfard, A.; Mozaffari, A.; Nasiri, K.; Salari, S.; Safaralizadeh, R.; Yasamineh, S. The potential use of nanozymes as antibacterial agents in oral infection, periodontitis, and peri-implantitis. J. Nanobiotechnol. 2024, 22, 207. [Google Scholar] [CrossRef]
- Karunaweera, N.; Raju, R.; Gyengesi, E.; Münch, G. Plant polyphenols as inhibitors of NF-κB induced cytokine production-a potential anti-inflammatory treatment for Alzheimer’s disease? Front. Mol. Neurosci. 2015, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Z.; Cao, X.; Chen, X.; Zou, T.; You, J. Plant-Derived Polyphenols as Nrf2 Activators to Counteract Oxidative Stress and Intestinal Toxicity Induced by Deoxynivalenol in Swine: An Emerging Research Direction. Antioxidants 2022, 11, 2379. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, J.; Shin, D.W. The Molecular Mechanism of Polyphenols with Anti-Aging Activity in Aged Human Dermal Fibroblasts. Molecules 2022, 27, 4351. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Chae, S.W.; Im, G.J.; Chung, J.W.; Song, J.J. Eugenol: A phyto-compound effective against methicillin-resistant and methicillin-sensitive Staphylococcus aureus clinical strain biofilms. PLoS ONE 2015, 10, e0119564. [Google Scholar] [CrossRef] [PubMed]
- Miguel, V.; Villarreal, M.A.; García, D.A. Effects of gabergic phenols on the dynamic and structure of lipid bilayers: A molecular dynamic simulation approach. PLoS ONE 2019, 14, e0218042. [Google Scholar] [CrossRef] [PubMed]
- Das Chagas Pereira de Andrade, F.; Mendes, A.N. Computational analysis of eugenol inhibitory activity in lipoxygenase and cyclooxygenase pathways. Sci. Rep. 2020, 10, 16204. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Li, F.; Fu, Y.; Cao, Y.; Song, X.; Wang, T.; Wang, W.; Guo, M.; Zhou, E.; Li, D.; et al. Thymol inhibits LPS-stimulated inflammatory response via down-regulation of NF-κB and MAPK signaling pathways in mouse mammary epithelial cells. Inflammation 2014, 37, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Potocka, W.; Assy, Z.; Bikker, F.J.; Laine, M.L. Current and Potential Applications of Monoterpenes and Their Derivatives in Oral Health Care. Molecules 2023, 28, 7178. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutierrez-Grijalva, E.P.; Ambriz-Perez, D.L.; Heredia, J.B. Flavonoids as Cytokine Modulators: A Possible Therapy for Inflammation-Related Diseases. Int. J. Mol. Sci. 2016, 17, 921. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Chen, X.; Bi, L. Research Progress on Anti-Inflammatory Effects and Mechanisms of Alkaloids from Chinese Medical Herbs. Evid. Based Complement. Alternat. Med. 2020, 2020, 1303524. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian Haftcheshmeh, S.; Momtazi-Borojeni, A.A. Berberine as a promising natural compound for the treatment of periodontal disease: A focus on anti-inflammatory properties. J. Cell. Mol. Med. 2021, 25, 11333–11337. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.Y.; Tang, C.H.; Chen, Y.H.; Wei, I.H. Berberine suppresses neuroinflammatory responses through AMP-activated protein kinase activation in BV-2 microglia. J. Cell. Biochem. 2010, 110, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.Y.; Chung, S.W.; Cho, D.; Kim, T.S. Involvement of p38 mitogen-activated protein kinase in the induction of interleukin-12 p40 production in mouse macrophages by berberine, a benzodioxoloquinolizine alkaloid. Biochem. Pharmacol. 2002, 63, 1901–1910. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chan, C.C.; Huang, W.C.; Kuo, M.L. Berberine Inhibits Pro-inflammatory Cytokine-induced IL-6 and CCL11 Production via Modulation of STAT6 Pathway in Human Bronchial Epithelial Cells. Int. J. Med. Sci. 2020, 17, 1464–1473. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; He, F.; Wu, L.; Xu, Y.; Du, Q. Matrine Exerts Pharmacological Effects Through Multiple Signaling Pathways: A Comprehensive Review. Drug Des. Devel. Ther. 2022, 16, 533–569. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Karima, G.; Khan, M.Z.; Shin, J.H.; Kim, J.D. Therapeutic Effects of Saponins for the Prevention and Treatment of Cancer by Ameliorating Inflammation and Angiogenesis and Inducing Antioxidant and Apoptotic Effects in Human Cells. Int. J. Mol. Sci. 2022, 23, 10665. [Google Scholar] [CrossRef] [PubMed]
- Tatli Cankaya, I.I.; Somuncuoglu, E.I. Potential and Prophylactic Use of Plants Containing Saponin-Type Compounds as Antibiofilm Agents against Respiratory Tract Infections. Evid. Based Complement. Alternat. Med. 2021, 2021, 6814215. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Siddiqui, A.J.; Ashraf, S.A.; Ashraf, M.S.; Alomrani, S.O.; Alreshidi, M.; Tepe, B.; Sachidanandan, M.; Danciu, C.; Patel, M. Saponin-Derived Silver Nanoparticles from Phoenix dactylifera (Ajwa Dates) Exhibit Broad-Spectrum Bioactivities Combating Bacterial Infections. Antibiotics 2023, 12, 1415. [Google Scholar] [CrossRef] [PubMed]
- Lončarić, M.; Strelec, I.; Moslavac, T.; Šubarić, D.; Pavić, V.; Molnar, M. Lipoxygenase Inhibition by Plant Extracts. Biomolecules 2021, 11, 152. [Google Scholar] [CrossRef]
- Shen, L.; Luo, H.; Fan, L.; Tian, X.; Tang, A.; Wu, X.; Dong, K.; Su, Z. Potential Immunoregulatory Mechanism of Plant Saponins: A Review. Molecules 2023, 29, 113. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Juang, Y.P.; Liang, P.H. Biological and Pharmacological Effects of Synthetic Saponins. Molecules 2020, 25, 4974. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.; Yu, T.; Song, G.; Xu, T.; Xin, T.; Lin, Y.; Han, B. Nano-Based Drug Delivery Systems for Periodontal Tissue Regeneration. Pharmaceutics 2022, 14, 2250. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomedicine 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Thambirajoo, M.; Maarof, M.; Lokanathan, Y.; Katas, H.; Ghazalli, N.F.; Tabata, Y.; Fauzi, M.B. Potential of Nanoparticles Integrated with Antibacterial Properties in Preventing Biofilm and Antibiotic Resistance. Antibiotics 2021, 10, 1338. [Google Scholar] [CrossRef] [PubMed]
- Sahli, C.; Moya, S.E.; Lomas, J.S.; Gravier-Pelletier, C.; Briandet, R.; Hémadi, M. Recent advances in nanotechnology for eradicating bacterial biofilm. Theranostics 2022, 12, 2383–2405. [Google Scholar] [CrossRef]
- Niu, J.; Wang, K.; Kolattukudy, P.E. Cerium oxide nanoparticles inhibit oxidative stress and nuclear factor-κB activation in H9c2 cardiomyocytes exposed to cigarette smoke extract. J. Pharmacol. Exp. Ther. 2011, 338, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xia, X.; Niu, Z.; Wang, K.; Liu, J.; Li, X. hCeO2@ Cu5.4O nanoparticle alleviates inflammatory responses by regulating the CTSB-NLRP3 signaling pathway. Front. Immunol. 2024, 15, 1344098. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, U.; Gittess, D.; Sakthivel, T.S.; Babu, B.; Seal, S. Cerium oxide nanomaterial with dual antioxidative scavenging potential: Synthesis and characterization. J. Biomater. Appl. 2021, 36, 834–842. [Google Scholar] [CrossRef]
- Tsamesidis, I.; Gkiliopoulos, D.; Pouroutzidou, G.K.; Lymperaki, E.; Papoulia, C.; Reybier, K.; Perio, P.; Paraskevopoulos, K.M.; Kontonasaki, E.; Theocharidou, A. Effect of artemisinin-loaded mesoporous cerium-doped calcium silicate nanopowder on cell proliferation of human periodontal ligament fibroblasts. Nanomaterials 2021, 11, 2189. [Google Scholar] [CrossRef] [PubMed]
- Cannillo, V.; Salvatori, R.; Bergamini, S.; Bellucci, D.; Bertoldi, C. Bioactive Glasses in Periodontal Regeneration: Existing Strategies and Future Prospects-A Literature Review. Materials 2022, 15, 2194. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, Z.; Liu, L.; Fan, C.; Wang, J.; Yang, J.; Hao, Y.; Mei, L.; Su, W.; Xu, Q. Fucoidan-hybrid hydroxyapatite nanoparticles promote the osteogenic differentiation of human periodontal ligament stem cells under inflammatory condition. Int. J. Biol. Macromol. 2024, 270, 132416. [Google Scholar] [CrossRef] [PubMed]
- Shariati, A.; Chegini, Z.; Ghaznavi-Rad, E.; Zare, E.N.; Hosseini, S.M. PLGA-Based Nanoplatforms in Drug Delivery for Inhibition and Destruction of Microbial Biofilm. Front. Cell. Infect. Microbiol. 2022, 12, 926363. [Google Scholar] [CrossRef] [PubMed]
- Shakya, A.K.; Al-Sulaibi, M.; Naik, R.R.; Nsairat, H.; Suboh, S.; Abulaila, A. Review on PLGA Polymer Based Nanoparticles with Antimicrobial Properties and Their Application in Various Medical Conditions or Infections. Polymers 2023, 15, 3597. [Google Scholar] [CrossRef] [PubMed]
- Basudan, A.M. Nanoparticle-based periodontal drug delivery-A review on current trends and future perspectives. Saudi Dent. J. 2022, 34, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Basilicata, M.; Di Lauro, M.; Campolattano, V.; Marrone, G.; Celotto, R.; Mitterhofer, A.P.; Bollero, P.; Di Daniele, N.; Noce, A. Natural Bioactive Compounds in the Management of Oral Diseases in Nephropathic Patients. Int. J. Environ. Res. Public Health 2022, 19, 1665. [Google Scholar] [CrossRef] [PubMed]
- Jayusman, P.A.; Nasruddin, N.S.; Mahamad Apandi, N.I.; Ibrahim, N.; Budin, S.B. Therapeutic Potential of Polyphenol and Nanoparticles Mediated Delivery in Periodontal Inflammation: A Review of Current Trends and Future Perspectives. Front. Pharmacol. 2022, 13, 847702. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pacheco, C.G.; Fernandes, N.A.R.; Primo, F.L.; Tedesco, A.C.; Bellile, E.; Retamal-Valdes, B.; Feres, M.; Guimarães-Stabili, M.R.; Rossa, C., Jr. Local application of curcumin-loaded nanoparticles as an adjunct to scaling and root planing in periodontitis: Randomized, placebo-controlled, double-blind split-mouth clinical trial. Clin. Oral Investig. 2021, 25, 3217–3227. [Google Scholar] [CrossRef] [PubMed]
- Sah, A.K.; Dewangan, M.; Suresh, P.K. Potential of chitosan-based carrier for periodontal drug delivery. Colloids Surf. B Biointerfaces 2019, 178, 185–198. [Google Scholar] [CrossRef]
- Griauzdyte, V.; Jagelaviciene, E. Antimicrobial Activity of Zinc against Periodontal Pathogens: A Systematic Review of In Vitro Studies. Medicina 2023, 59, 2088. [Google Scholar] [CrossRef]
- Kadama, P.; Mahalea, S.; Sonara, P.; Chaudharia, D.; Shimpia, S.; Kathurwar, A. Efficacy of silver nanoparticles in chronic periodontitis patients: A clinical-microbiological study. Iberoam. J. Med. 2020, 3, 142–147. [Google Scholar] [CrossRef]
- Gadagi, J.S.; Chava, V.K.; Reddy, V.R. Green tea extract as a local drug therapy on periodontitis patients with diabetes mellitus: A randomized case-control study. J. Indian Soc. Periodontol. 2013, 17, 198–203. [Google Scholar]
- Huangfu, H.; Du, S.; Zhang, H.; Wang, H.; Zhang, Y.; Yang, Z.; Zhang, X.; Ren, S.; Chen, S.; Wang, C.; et al. Facile engineering of resveratrol nanoparticles loaded with 20(S)-protopanaxadiol for the treatment of periodontitis by regulating the macrophage phenotype. Nanoscale 2023, 15, 7894–7908. [Google Scholar] [CrossRef]
- Shaheen, M.Y. Nanocrystalline hydroxyapatite in periodontal bone regeneration: A systematic review. Saudi Dent. J. 2022, 34, 647–660. [Google Scholar] [CrossRef]

| Authors | Year | Bioactive Compound/Nanoparticle | Main Findings/Outcomes | Reference |
|---|---|---|---|---|
| Pérez-Pacheco, C.G., et al. | 2021 | Curcumin Nanoparticles | Reduced inflammation and improved periodontal healing compared to control. | [89] |
| Abhishek, K. Sah, et al. | 2019 | Chitosan-Based Nanoparticles | Chitosan-based drug delivery systems represent an attractive strategy for achieving the therapeutic concentration of drugs in periodontal pocket. | [90] |
| Griauzdyte, V.; Jagelaviciene, E. | 2023 | ZnO | The analysis of the literature confirms the antibacterial action of zinc against periodontal pathogenic bacteria. At low concentrations, these substances do not exhibit cytotoxic effects on fibroblasts. | [91] |
| Pooja K., et al. | 2020 | Silver nanoparticles | Silver nanoparticles gel with scaling and root planing gives promising results, and it can definitely aid in periodontal diseases | [92] |
| Gadagi, J.S., et al. | 2013 | Green tea extract | Local drug delivery using green tea extract could be used as an adjunct in the treatment of chronic periodontitis in diabetic and non-diabetic individuals. | [93] |
| Huangfu, H., et al. | 2023 | RES@PPD nanoparticles | RES@PPD NPs can remarkably decrease the level of pro-inflammatory cytokines, upregulate the anti-inflammatory cytokines, and exhibit a profound therapeutic effect on local inflammation. | [94] |
| Shaheen, M.Y. | 2022 | Nanocrystalline hydroxyapatite (NCHA) | NCHA is a suitable bone substitute material for periodontal bone regeneration, with outcomes comparable to that of conventionally used graft materials, such as bovine xenograft and other synthetic alloplastic materials. | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashim, N.T.; Babiker, R.; Rahman, M.M.; Mohamed, R.; Priya, S.P.; Chaitanya, N.C.; Islam, M.S.; Gobara, B. Natural Bioactive Compounds in the Management of Periodontal Diseases: A Comprehensive Review. Molecules 2024, 29, 3044. https://doi.org/10.3390/molecules29133044
Hashim NT, Babiker R, Rahman MM, Mohamed R, Priya SP, Chaitanya NC, Islam MS, Gobara B. Natural Bioactive Compounds in the Management of Periodontal Diseases: A Comprehensive Review. Molecules. 2024; 29(13):3044. https://doi.org/10.3390/molecules29133044
Chicago/Turabian StyleHashim, Nada Tawfig, Rasha Babiker, Muhammed Mustahsen Rahman, Riham Mohamed, Sivan Padma Priya, Nallan CSK Chaitanya, Md Sofiqul Islam, and Bakri Gobara. 2024. "Natural Bioactive Compounds in the Management of Periodontal Diseases: A Comprehensive Review" Molecules 29, no. 13: 3044. https://doi.org/10.3390/molecules29133044
APA StyleHashim, N. T., Babiker, R., Rahman, M. M., Mohamed, R., Priya, S. P., Chaitanya, N. C., Islam, M. S., & Gobara, B. (2024). Natural Bioactive Compounds in the Management of Periodontal Diseases: A Comprehensive Review. Molecules, 29(13), 3044. https://doi.org/10.3390/molecules29133044











