Rational Design of Flexible, Self-Supporting, and Binder-Free Prussian White/KetjenBlack/MXene Composite Electrode for Sodium-Ion Batteries with Boosted Electrochemical Performance
Abstract
1. Introduction
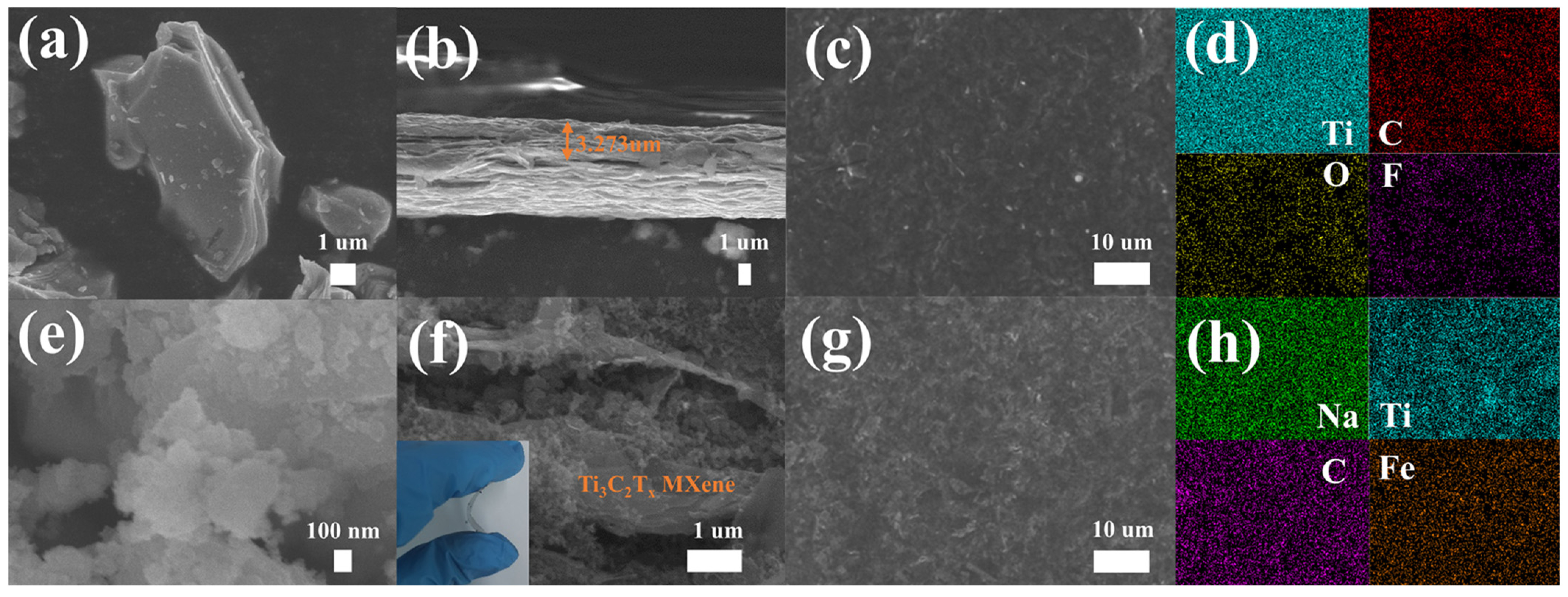
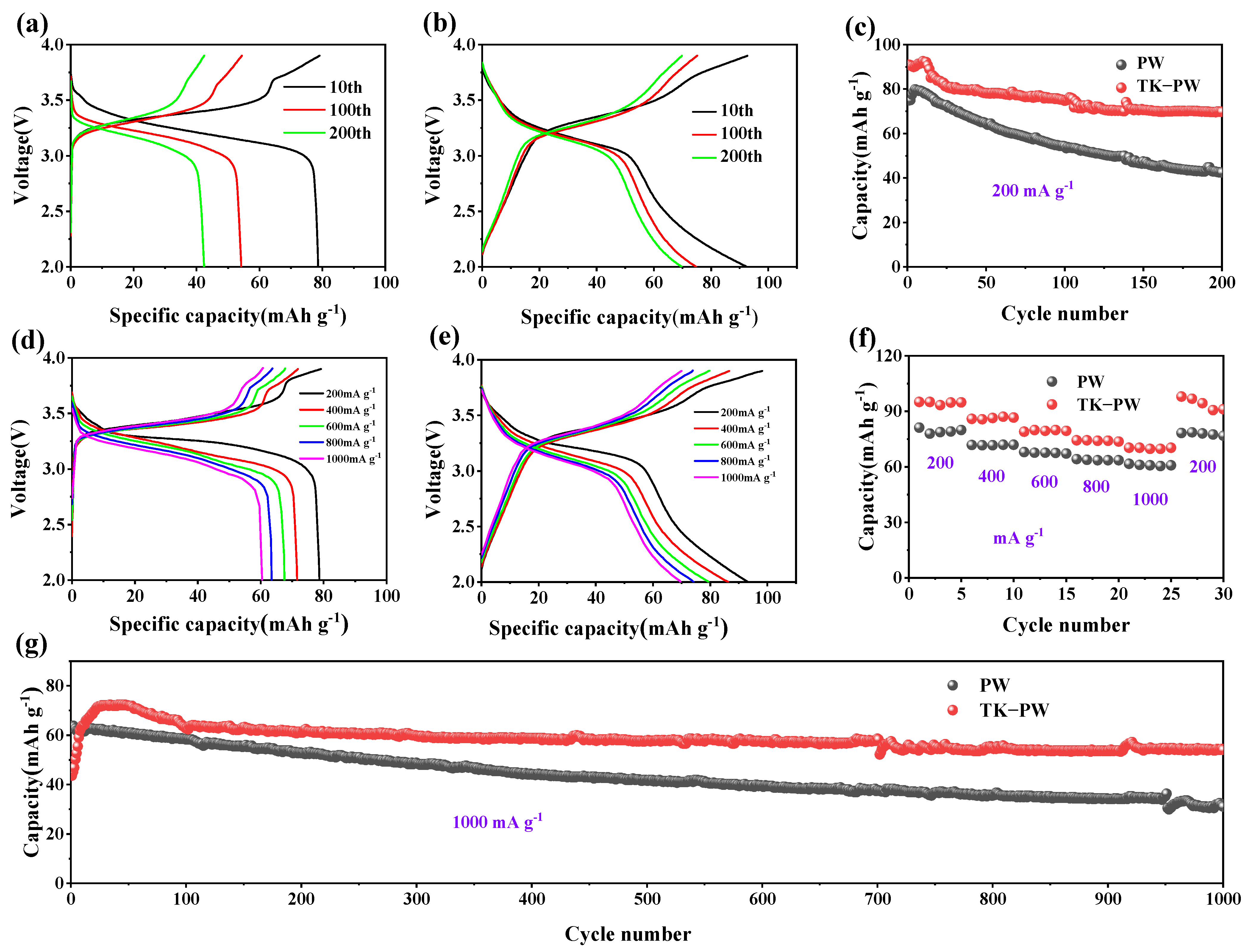
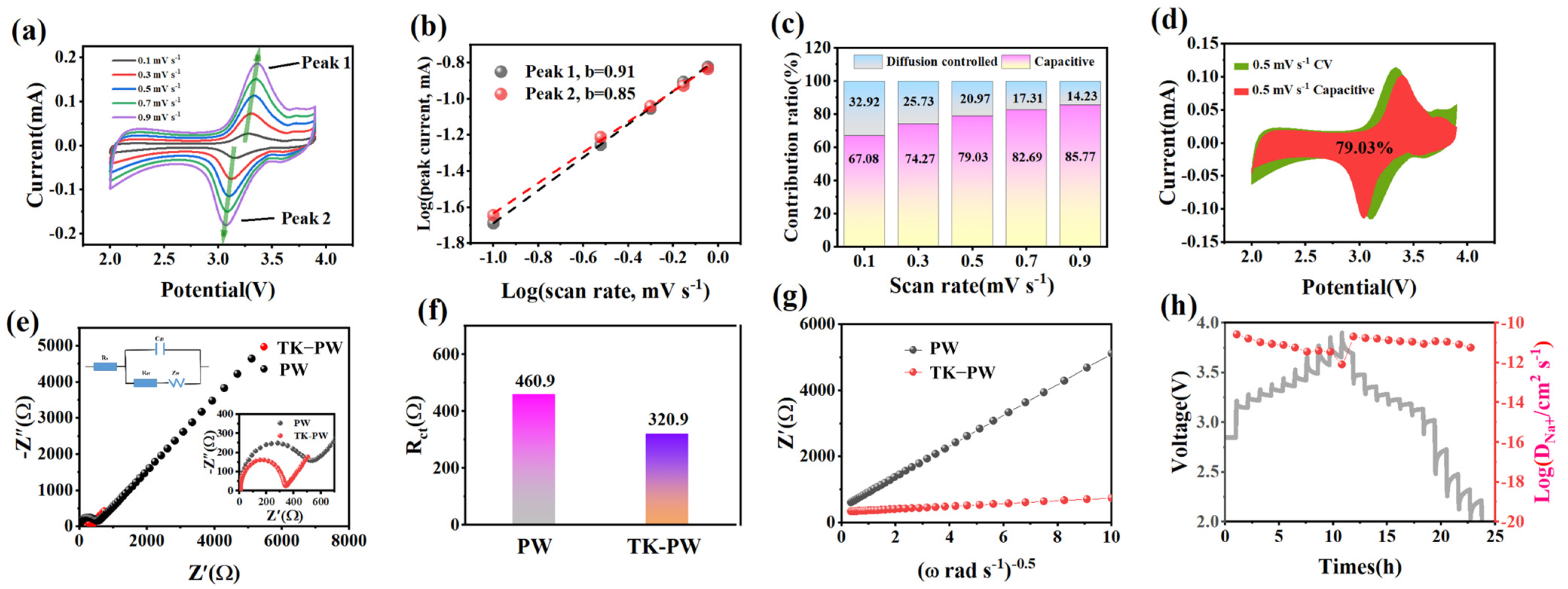
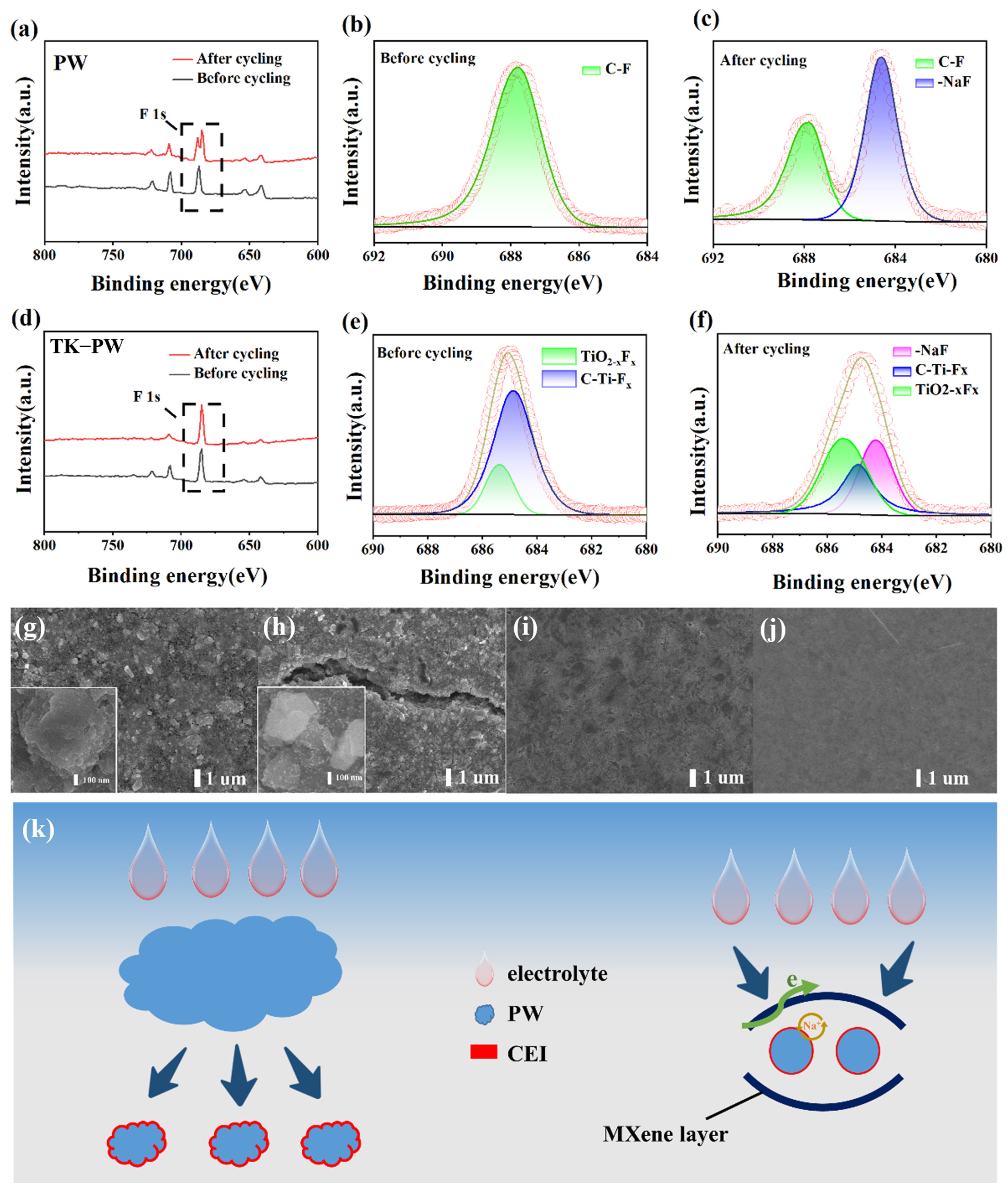
2. Results and Discussion
3. Conclusions
4. Experimental Methods
4.1. Preparation of Ti3C2Tx MXene Film
4.2. Preparation of TK−PW Film
4.3. Materials Characterization
4.4. Electrochemical Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kandari, R.; Neeraj, N.; Micallef, A. Review on recent strategies for integrating energy storage systems in microgrids. Energies 2022, 16, 317. [Google Scholar] [CrossRef]
- Hjalmarsson, J.; Thomas, K.; Boström, C. Service stacking using energy storage systems for grid applications–A review. J. Energy Storage 2023, 60, 106639. [Google Scholar] [CrossRef]
- Huang, B.; Pan, Z.; Su, X.; An, L. Recycling of lithium-ion batteries: Recent advances and perspectives. J. Power Sources 2018, 399, 274–286. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, M.; Tao, P.; Song, C.; Wu, J.; Wang, J.; Deng, T.; Shang, W. Temperature effect and thermal impact in lithium-ion batteries: A review. Prog. Nat. Sci. Mater. Int. 2018, 28, 653–666. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical Processes for Recycling Spent Lithium-Ion Batteries: A Critical Review. ACS Sustain. Chem. Eng. 2018, 6, 13611–13627. [Google Scholar] [CrossRef]
- Xu, M.; Liu, M.; Yang, Z.; Wu, C.; Qian, J. Research Progress on Presodiation Strategies for High Energy Sodium-Ion Batteries. Acta Phys. Chim. Sin. 2022, 39, 2210043. [Google Scholar] [CrossRef]
- Huang, Z.-X.; Gu, Z.-Y.; Heng, Y.-L.; Huixiang Ang, E.; Geng, H.-B.; Wu, X.-L. Advanced layered oxide cathodes for sodium/potassium-ion batteries: Development, challenges and prospects. Chem. Eng. J. 2023, 452, 139438. [Google Scholar] [CrossRef]
- Lin, X.-M.; Yang, X.-T.; Chen, H.-N.; Deng, Y.-L.; Chen, W.-H.; Dong, J.-C.; Wei, Y.-M.; Li, J.-F. In situ characterizations of advanced electrode materials for sodium-ion batteries toward high electrochemical performances. J. Energy Chem. 2023, 76, 146–164. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Yang, M.; Chen, W. High-safety separators for lithium-ion batteries and sodium-ion batteries: Advances and perspective. Energy Storage Mater. 2021, 41, 522–545. [Google Scholar] [CrossRef]
- Lee, H.W.; Wang, R.Y.; Pasta, M.; Woo Lee, S.; Liu, N.; Cui, Y. Manganese hexacyanomanganate open framework as a high-capacity positive electrode material for sodium-ion batteries. Nat. Commun. 2014, 5, 5280. [Google Scholar] [CrossRef]
- Oz, E.; Altin, S.; Avci, S. Investigation of physical and electrochemical properties of Ni-doped Tunnel/P2 hybrid Na0.44MnO2 cathode material for sodium-ion batteries. J. Solid State Chem. 2023, 318, 123741. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, Z.; Li, W.; Zou, C.; Jin, H.; Wang, S.; Chou, S.; Dou, S.-X. Sodium transition metal oxides: The preferred cathode choice for future sodium-ion batteries? Energy Environ. Sci. 2021, 14, 158–179. [Google Scholar] [CrossRef]
- Pahari, D.; Kumar, A.; Das, D.; Puravankara, S. P2-type NaxTmO2 oxides as cathodes for non-aqueous sodium-ion batteries—Structural evolution and commercial prospects. Int. J. Energy Res. 2022, 46, 21894–21927. [Google Scholar] [CrossRef]
- Lv, Z.; Ling, M.; Yue, M.; Li, X.; Song, M.; Zheng, Q.; Zhang, H. Vanadium-based polyanionic compounds as cathode materials for sodium-ion batteries: Toward high-energy and high-power applications. J. Energy Chem. 2021, 55, 361–390. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Y.; Wen, T.; Chen, L.; Pu, X.; Chen, Z. Advances in Vanadium-Redoxed Polyanions for High-Voltage Sodium-Ion Batteries. Batteries 2023, 9, 56. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Cheng, J.; Goodenough, J.B. Prussian blue: A new framework of electrode materials for sodium batteries. Chem. Commun. 2012, 48, 6544–6546. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Sun, B.; Gao, T.; Ma, Y.; Yin, G.; Zuo, P. Recent progress of Prussian blue analogues as cathode materials for nonaqueous sodium-ion batteries. Coord. Chem. Rev. 2022, 460, 214478. [Google Scholar] [CrossRef]
- Wang, H.g.; Zhang, X.b. Organic carbonyl compounds for sodium-ion batteries: Recent progress and future perspectives. Chem.–A Eur. J. 2018, 24, 18235–18245. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Whittaker, A.K.; Zhao, X.S. Polymer Electrode Materials for Sodium-ion Batteries. Materials 2018, 11, 2567. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Xia, M.; Qi, R.; Liang, X.; Zhao, M.; Zhang, Z.; Lu, X.; Cao, G. Improved rate performance of Prussian blue cathode materials for sodium ion batteries induced by ion-conductive solid-electrolyte interphase layer. J. Power Sources 2018, 399, 42–48. [Google Scholar] [CrossRef]
- Lim, C.Q.X.; Tan, Z.-K. Prussian White with Near-Maximum Specific Capacity in Sodium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 6214–6220. [Google Scholar] [CrossRef]
- Nielsen, I.; Dzodan, D.; Ojwang, D.O.; Henry, P.F.; Ulander, A.; Ek, G.; Häggström, L.; Ericsson, T.; Boström, H.L.B.; Brant, W.R. Water driven phase transitions in Prussian white cathode materials. J. Phys. Energy 2022, 4, 044012. [Google Scholar] [CrossRef]
- Li, C.; Zang, R.; Li, P.; Man, Z.; Wang, S.; Li, X.; Wu, Y.; Liu, S.; Wang, G. High Crystalline Prussian White Nanocubes as a Promising Cathode for Sodium-ion Batteries. Chem. Asian J. 2018, 13, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Song, W.-L.; Li, X.; Fan, L.-Z. Biomass derivative/graphene aerogels for binder-free supercapacitors. Energy Storage Mater. 2016, 3, 113–122. [Google Scholar] [CrossRef]
- Ma, P.; Fang, D.; Liu, Y.; Shang, Y.; Shi, Y.; Yang, H.Y. MXene-Based Materials for Electrochemical Sodium-Ion Storage. Adv. Sci. 2021, 8, e2003185. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.-J.; Yan, Z.-C.; Lai, W.-H.; Chou, S.-L.; Wang, Y.-X.; Liu, H.-K.; Dou, S.-X. Tailoring MXene-Based Materials for Sodium-Ion Storage: Synthesis, Mechanisms, and Applications. Electrochem. Energy Rev. 2020, 3, 766–792. [Google Scholar] [CrossRef]
- Xu, H.; Fan, J.; Pang, D.; Zheng, Y.; Chen, G.; Du, F.; Gogotsi, Y.; Dall’Agnese, Y.; Gao, Y. Synergy of ferric vanadate and MXene for high performance Li- and Na-ion batteries. Chem. Eng. J. 2022, 436, 135012. [Google Scholar] [CrossRef]
- Yang, J.; Wang, T.; Guo, X.; Sheng, X.; Li, J.; Wang, C.; Wang, G. Flexible sodium-ion capacitors boosted by high electrochemically-reactive and structurally-stable Sb2S3 nanowire/Ti3C2Tx MXene film anodes. Nano Res. 2021, 16, 5592–5600. [Google Scholar] [CrossRef]
- Sun, N.; Zhu, Q.; Anasori, B.; Zhang, P.; Liu, H.; Gogotsi, Y.; Xu, B. MXene-Bonded Flexible Hard Carbon Film as Anode for Stable Na/K-Ion Storage. Adv. Funct. Mater. 2019, 29, 1906282. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, X.; Zhang, J.; Wu, S.; Wang, J.; Chen, J.S.; Li, T. Enhancing the lithium storage capabilities of TiO2 nanoparticles using delaminated MXene supports. Ceram. Int. 2018, 44, 17660–17666. [Google Scholar] [CrossRef]
- Yang, C.; Wu, Q.; Cao, Y.; Gao, Y.; Li, A.; Liu, X.; Zhang, X.; Tian, Z.; Liu, R. α-MnO2/super-P with conductive carbon network for rechargeable aqueous Zinc ion batteries. Mater. Lett. 2021, 302, 130419. [Google Scholar] [CrossRef]
- Murali, G.; Reddy Modigunta, J.K.; Park, Y.H.; Lee, J.H.; Rawal, J.; Lee, S.Y.; In, I.; Park, S.J. A Review on MXene Synthesis, Stability, and Photocatalytic Applications. ACS Nano 2022, 16, 13370–13429. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Wang, X.; Wei, C.; Wang, Z.; Zhang, Y.; Feng, J. Flexible and free-supporting Prussian blue analogs/MXene film for high-performance sodium-ion batteries. J. Power Sources 2023, 576, 233165. [Google Scholar] [CrossRef]
- Zhang, W.X.; Zhang, J.H.; Zhang, Y.K.; He, C.; Zhao, P. NiS2 nanoparticles anchored on MXene conductive frameworks with enhanced lithium and sodium storage properties. Ionics 2022, 28, 4621–4629. [Google Scholar] [CrossRef]
- Wei, C.; Xi, B.; Wang, P.; Wang, Z.; An, X.; Li, Y.; Feng, J.; Xiong, S. Rapid Growth of Bi2Se3 Nanodots on MXene Nanosheets at Room Temperature for Promoting Sulfur Redox Kinetics. Inorg. Chem. 2024, 63, 8853–8862. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Jiang, H.; Wei, C.; An, Y.; Tan, L.; Xiong, S.; Feng, J. Free-standing Na2C6O6/MXene composite paper for high-performance organic sodium-ion batteries. Nano Res. 2023, 16, 458–465. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, P.; Li, S.; Zhou, C.; Guo, S.; Liu, J.; Zhou, J.; Jian, X.; Yang, Y.; Lei, Y.; et al. Nitrogen-doped carbon-wrapped porous FeMnO3 nanocages derived from etched prussian blue analogues as high-performance anode for lithium ion batteries. J. Power Sources 2020, 475, 228683. [Google Scholar] [CrossRef]
- Chen, Y.; Woo, H.J.; Syed Mohd Fadzil, S.A.F.; Tan, W.; Wang, F.; Mohd Arof, A.K. Cage-Like Porous Prussian Blue as High-Capacity Cathode for Sodium-Ion Batteries. ACS Appl. Nano Mater. 2022, 5, 4833–4840. [Google Scholar] [CrossRef]
- Cheng, F.; Liang, J.; Tao, Z.; Chen, J. Functional materials for rechargeable batteries. Adv. Mater. 2011, 23, 1695–1715. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zheng, H.; Wang, S.; Chen, X.; Yang, S.; Feng, C. Construction of Na3V2(PO4)3/C nanoplate as cathode for stable sodium ion storage. Ionics 2022, 28, 981–988. [Google Scholar] [CrossRef]
- Xie, B.; Wang, L.; Li, H.; Huo, H.; Cui, C.; Sun, B.; Ma, Y.; Wang, J.; Yin, G.; Zuo, P. An interface-reinforced rhombohedral Prussian blue analogue in semi-solid state electrolyte for sodium-ion battery. Energy Storage Mater. 2021, 36, 99–107. [Google Scholar] [CrossRef]
- Ye, M.; You, S.; Xiong, J.; Yang, Y.; Zhang, Y.; Li, C.C. In-situ construction of a NaF-rich cathode–electrolyte interface on Prussian blue toward a 3000-cycle-life sodium-ion battery. Mater. Today Energy 2022, 23, 100898. [Google Scholar] [CrossRef]
- Chun, J.; Wang, X.; Zhang, Y.; Wei, C.; Wang, Z.; Feng, J. Ti3C2Tx film current collectors for high-performance sodium-ion batteries. Vacuum 2023, 207, 111476. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, B.; Jin, F.; Chen, Y.; Jiang, Y.; Bao, C.; Tian, J.; Wang, J.; Xu, R.; Li, Y.; et al. Modified cathode-electrolyte interphase toward high-performance batteries. Cell Rep. Phys. Sci. 2022, 3, 101197. [Google Scholar] [CrossRef]
- You, Y.; He, Z. Phenol degradation in iron-based advanced oxidation processes through ferric reduction assisted by molybdenum disulfide. Chemosphere 2023, 312, 137278. [Google Scholar] [CrossRef] [PubMed]
- ur Rehman, W.; Jiang, Z.; Qu, Z.; Ahmed, S.; Ghani, A.; Xu, Y. Highly crystalline Prussian blue cubes filled with tin oxide as anode materials for lithium-ion batteries. Appl. Surf. Sci. 2022, 604, 154533. [Google Scholar] [CrossRef]
- Fu, B.; Man, H.; Zhao, J.; Wang, F.; Fang, F.; Sun, D.; Song, Y. Less Is More: High-Performance All-Solid-State Electrode Enabled by Multifunctional MXene. ACS Appl. Energy Mater. 2022, 5, 7210–7219. [Google Scholar] [CrossRef]
- Ye, W.; Yu, L.; Sun, M.; Cheng, G.; Fu, S.; Peng, S.; Han, S.; Yang, X. Yolk−shell Prussian blue analogues hierarchical microboxes: Controllably exposing active sites toward enhanced cathode performance for lithium ion batteries. Electrochim. Acta 2019, 319, 237–244. [Google Scholar] [CrossRef]
- Shen, Z.; Guo, S.; Liu, C.; Sun, Y.; Chen, Z.; Tu, J.; Liu, S.; Cheng, J.; Xie, J.; Cao, G. Na-rich Prussian white cathodes for long-life sodium-ion batteries. ACS Sustain. Chem. Eng. 2018, 6, 16121–16129. [Google Scholar] [CrossRef]
- Ren, W.; Zhu, Z.; Qin, M.; Chen, S.; Yao, X.; Li, Q.; Xu, X.; Wei, Q.; Mai, L.; Zhao, C. Prussian white hierarchical nanotubes with surface-controlled charge storage for sodium-ion batteries. Adv. Funct. Mater. 2019, 29, 1806405. [Google Scholar] [CrossRef]
- Tang, X.; Liu, H.; Su, D.; Notten, P.H.; Wang, G. Hierarchical sodium-rich Prussian blue hollow nanospheres as high-performance cathode for sodium-ion batteries. Nano Res. 2018, 11, 3979–3990. [Google Scholar] [CrossRef]
- Wang, L.; Lu, Y.; Liu, J.; Xu, M.; Cheng, J.; Zhang, D.; Goodenough, J.B. A superior low-cost cathode for a Na-ion battery. Angew. Chem. 2013, 125, 2018. [Google Scholar] [CrossRef]
- Li, W.-J.; Chou, S.-L.; Wang, J.-Z.; Kang, Y.-M.; Wang, J.-L.; Liu, Y.; Gu, Q.-F.; Liu, H.-K.; Dou, S.-X. Facile method to synthesize Na-enriched Na1+xFeFe(CN)6 frameworks as cathode with superior electrochemical performance for sodium-ion batteries. Chem. Mater. 2015, 27, 1997–2003. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, S.; Wang, B.; Li, Y.; Sun, W.; Lu, Y.; Yan, M.; Song, B.; Dou, S. Prussian blue@ C composite as an ultrahigh-rate and long-life sodium-ion battery cathode. Adv. Funct. Mater. 2016, 26, 5315–5321. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, L.; Tang, H.; Du, F.; Guo, Y.; Qiu, T.; Yang, J. Synthesis of two-dimensional Ti3C2Tx MXene using HCl + LiF etchant: Enhanced exfoliation and delamination. J. Alloys Compd. 2017, 695, 818–826. [Google Scholar] [CrossRef]


| Electrode Material | Preparation Method | Rate Capability | Cycle Performance | Ref. | ||
|---|---|---|---|---|---|---|
| Current Density (mA·g−1) | Capacity (mAh·g−1) | Cycle Number | Capacity Retention (mAh·g−1) | |||
| TK−PW | Vacuum Filtration Method | 200 | 93 | 1000 | 54.1 at 1000 mA·g−1 | This work |
| PW | Coating Method | 200 | 78.7 | 1000 | 31.3 at 1000 mA·g−1 | This work |
| PW-1 | Coating Method | 300 (2 C) | 108 | 700 | 87.1 at 0.5 C | [49] |
| PW-2 | Coating Method | 300 (2 C) | 110 | 580 | 65.3 at 0.5 C | [49] |
| PW-HN | Coating Method | 240 (2 C) | 115 | 5000 | 80% at 10 C | [50] |
| Na1.58Fe[Fe(CN)6]0.92 | Coating Method | 340 (2 C) | 113 | 800 | 90% at 2 C | [51] |
| NMHFCs | Coating Method | 102 (0.85 C) | 92 | 30 | 121 at 1/20 C | [52] |
| PB-1 | Coating Method | 20 | 89.5 | 400 | 81.45 at 20 mA·g−1 | [53] |
| PB@C | Coating Method | 2000 (20 C) | 100 | 1000 | 100 at 2000 mA·g−1 | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, X.; Chun, J.; Wang, X.; Xv, T.; Wang, Z.; Wei, C.; Feng, J. Rational Design of Flexible, Self-Supporting, and Binder-Free Prussian White/KetjenBlack/MXene Composite Electrode for Sodium-Ion Batteries with Boosted Electrochemical Performance. Molecules 2024, 29, 3048. https://doi.org/10.3390/molecules29133048
Dai X, Chun J, Wang X, Xv T, Wang Z, Wei C, Feng J. Rational Design of Flexible, Self-Supporting, and Binder-Free Prussian White/KetjenBlack/MXene Composite Electrode for Sodium-Ion Batteries with Boosted Electrochemical Performance. Molecules. 2024; 29(13):3048. https://doi.org/10.3390/molecules29133048
Chicago/Turabian StyleDai, Xiaowen, Jingyun Chun, Xiaolong Wang, Tianao Xv, Zhengran Wang, Chuanliang Wei, and Jinkui Feng. 2024. "Rational Design of Flexible, Self-Supporting, and Binder-Free Prussian White/KetjenBlack/MXene Composite Electrode for Sodium-Ion Batteries with Boosted Electrochemical Performance" Molecules 29, no. 13: 3048. https://doi.org/10.3390/molecules29133048
APA StyleDai, X., Chun, J., Wang, X., Xv, T., Wang, Z., Wei, C., & Feng, J. (2024). Rational Design of Flexible, Self-Supporting, and Binder-Free Prussian White/KetjenBlack/MXene Composite Electrode for Sodium-Ion Batteries with Boosted Electrochemical Performance. Molecules, 29(13), 3048. https://doi.org/10.3390/molecules29133048





