An Investigation of N-Hydroxyphthalimide Catalyzed Aerobic Oxidation of Toluene without Metal Ions in Liquid Phase: Effect of Solvents and Phase Transfer Catalysts
Abstract
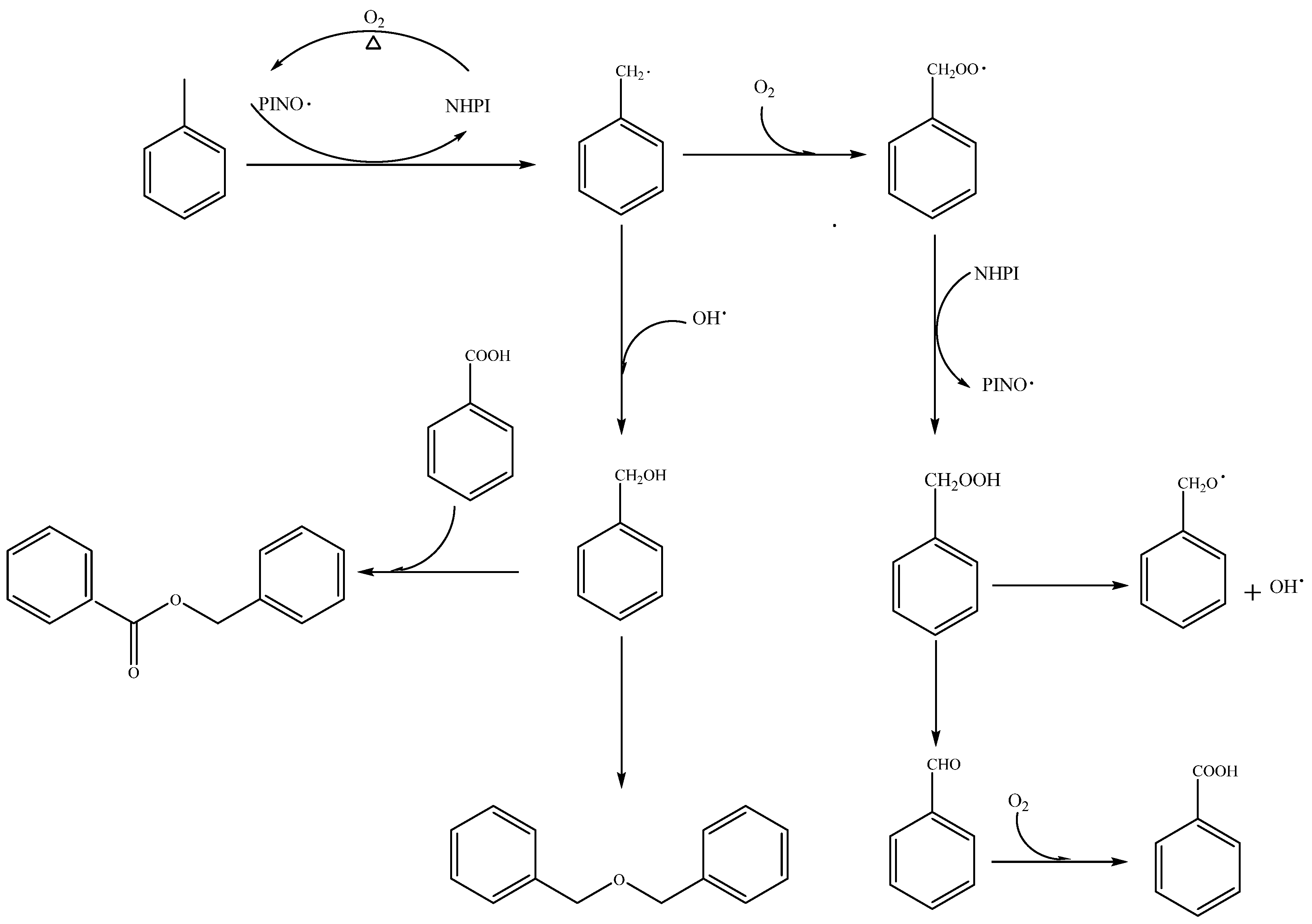
:1. Introduction
2. Results and Discussion
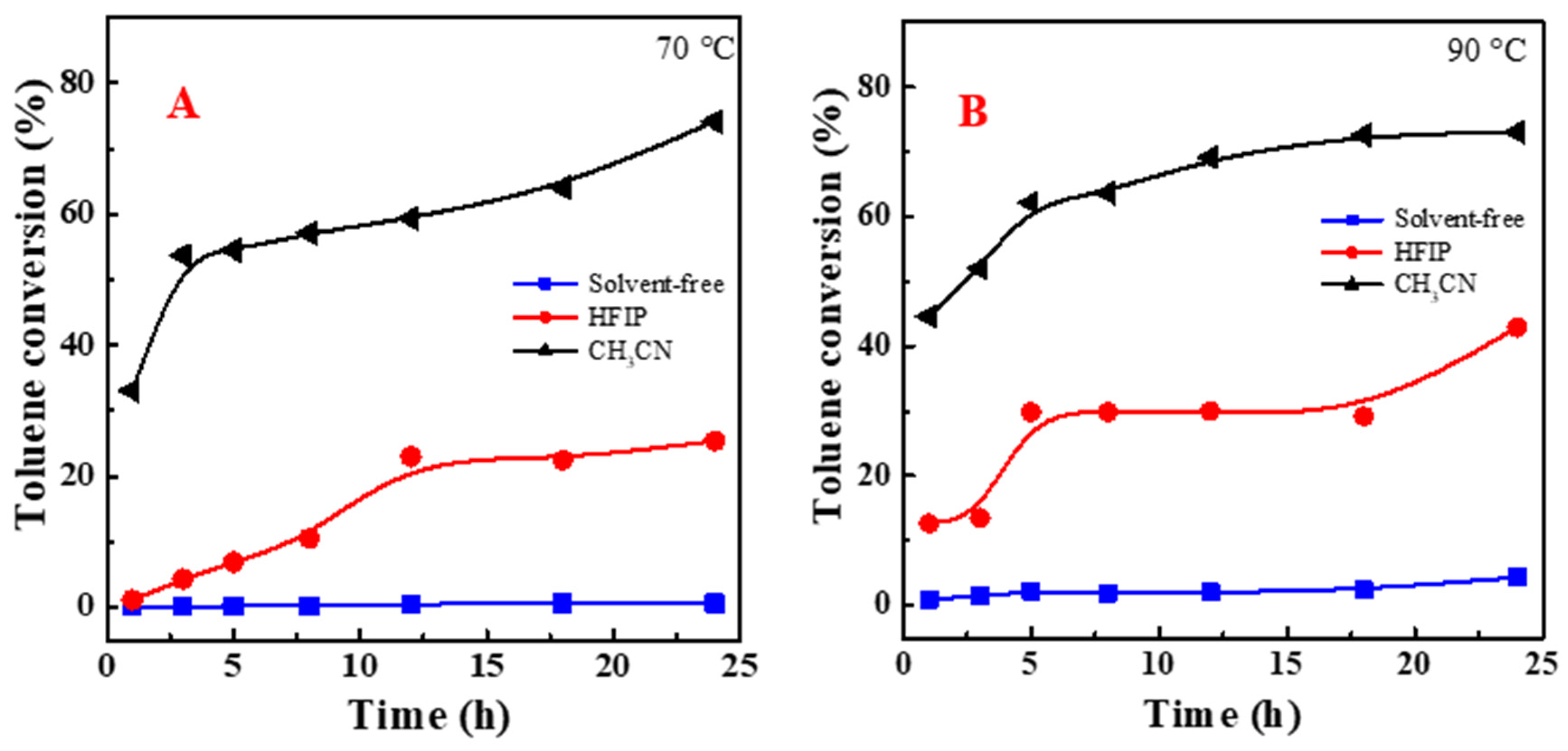
2.1. Effect of Solvents on Metal-Free Oxofunctionalization of Toluene
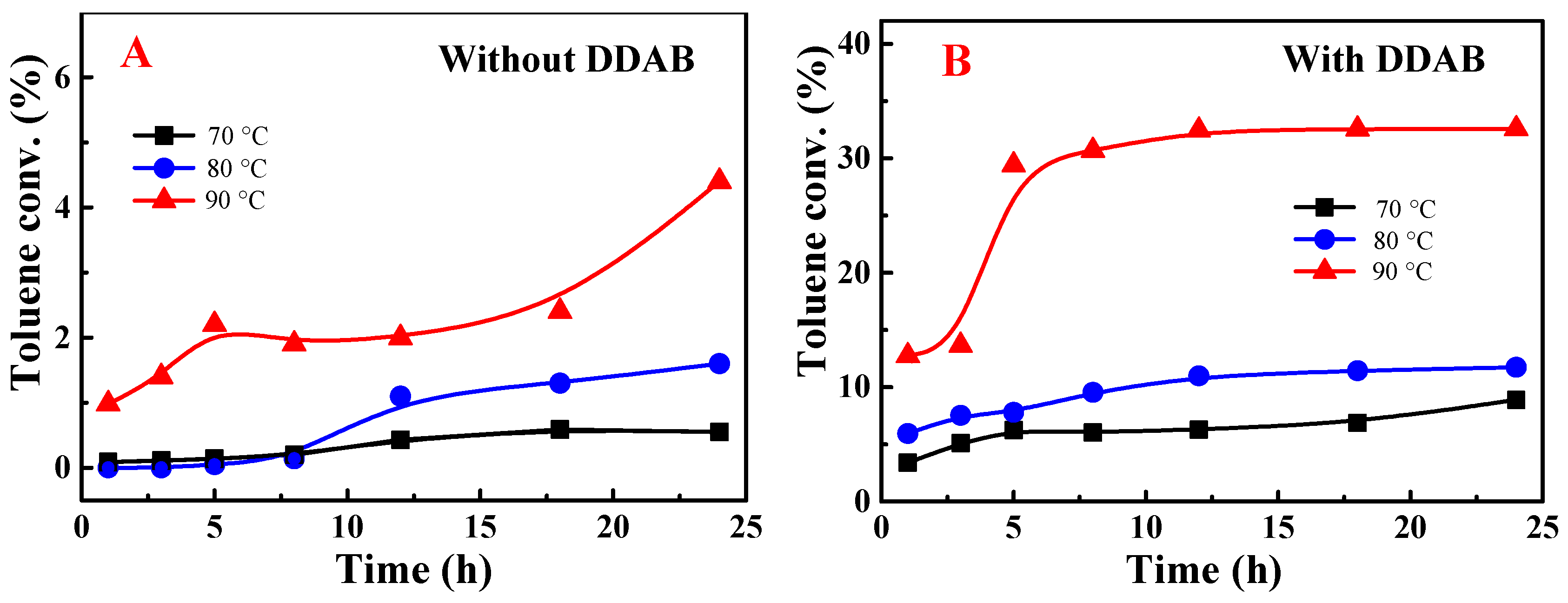
2.2. Role of Phase Transfer Catalysts on Metal-Free Oxofunctionalization of Toluene under Solvent-Free Conditions
3. Experimental Section
3.1. Aerobic Oxidation of Toluene in Different Solvents
3.2. Solvent-Free Aerobic Oxidation of Toluene Mediated by Different Phase Transfer Catalysts
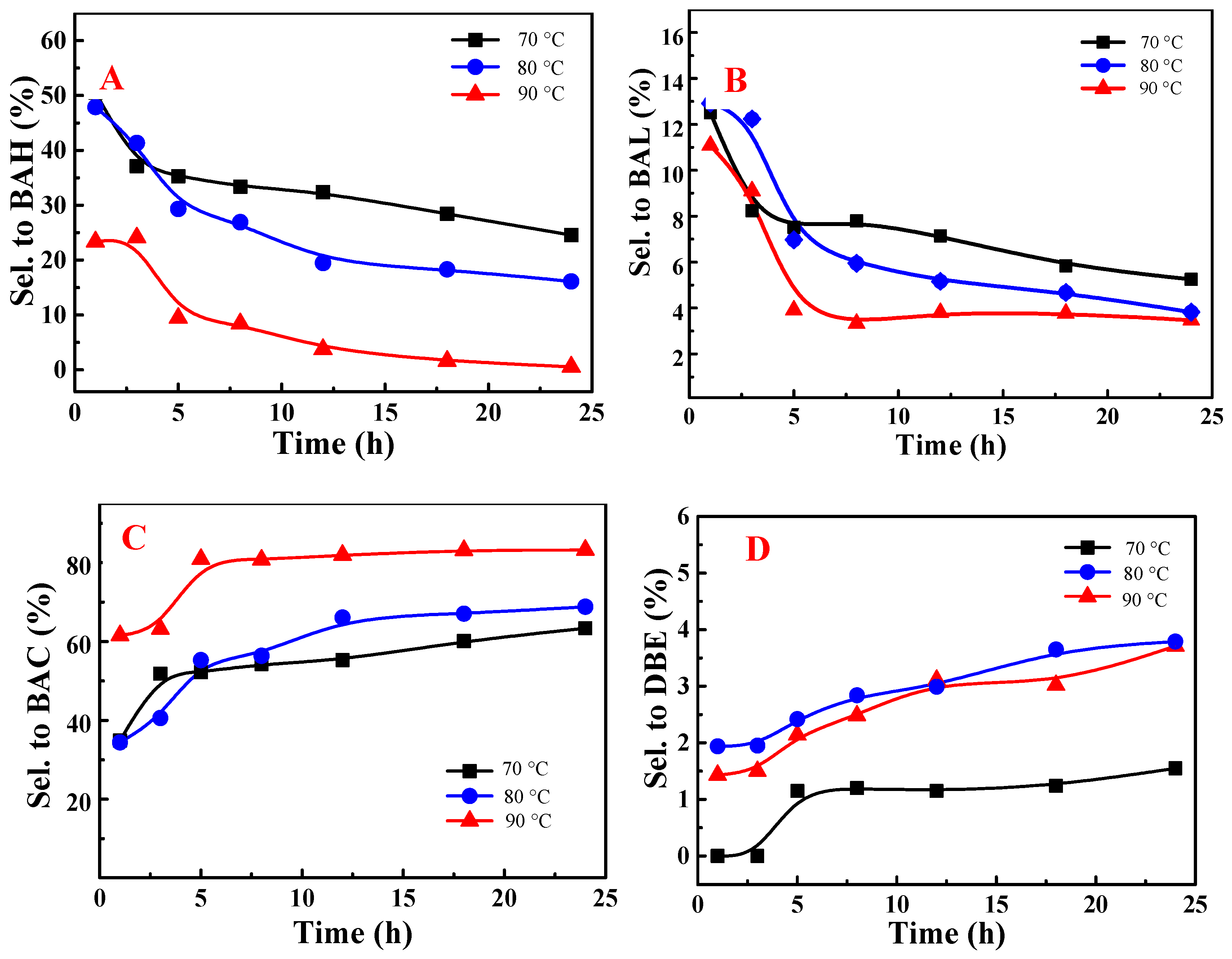
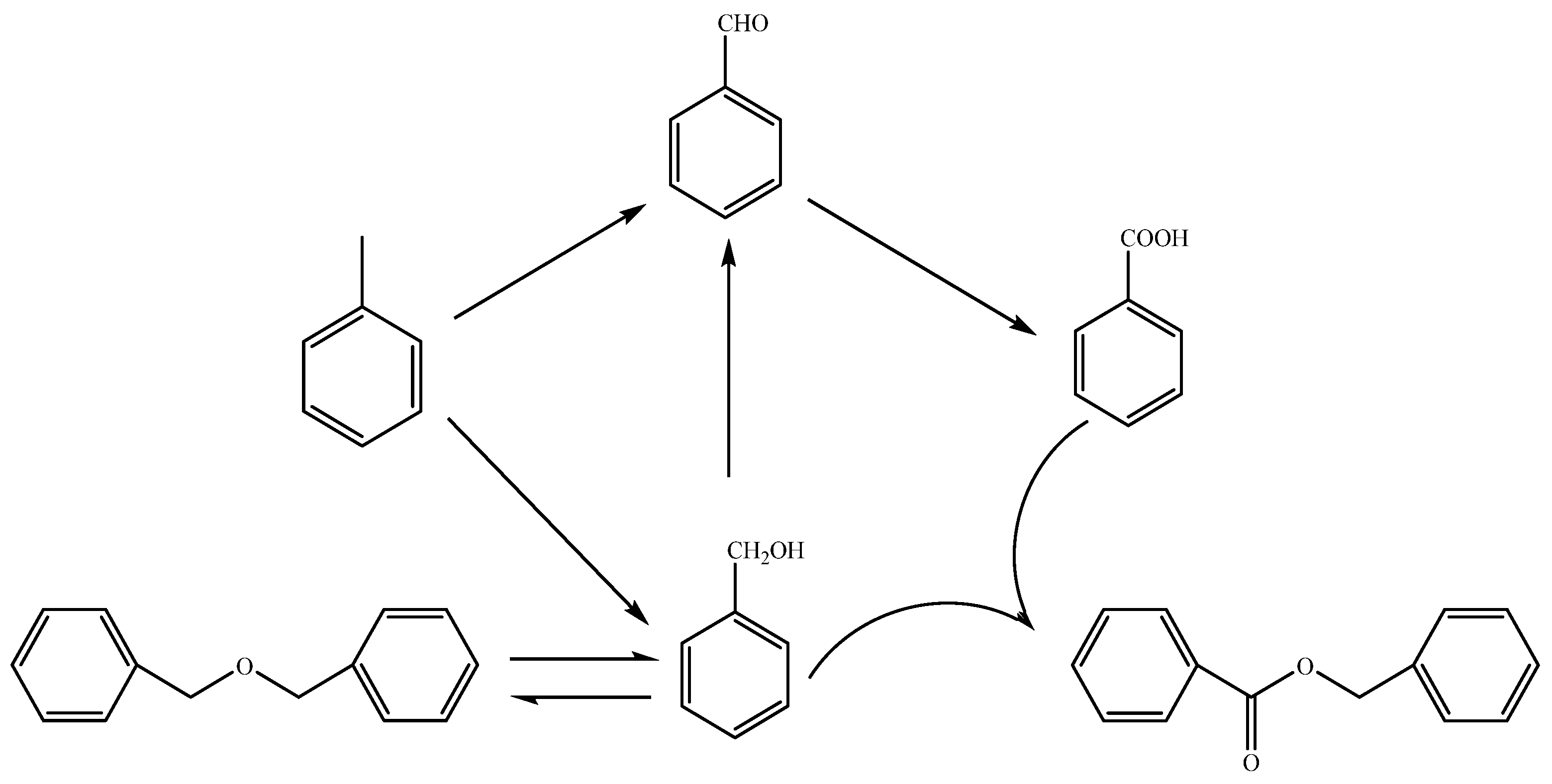
3.3. Identification of Products and Intermediates by GC-MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.L.; Wu, G.D.; Wang, F.; Liu, H.; Jin, T.F. Solvent-free selective oxidation of toluene with O2 catalysed by anion modified mesoporous mixed oxides with high thermal stability. Catal. Commun. 2017, 98, 107–111. [Google Scholar] [CrossRef]
- Xu, J.; Shi, G.; Liang, Y.; Lu, Q.; Ji, L. Selective aerobic oxidation of toluene to benzaldehyde catalyzed by covalently anchored N-hydroxyphthalimide and cobaltous ions. Mol. Catal. 2021, 503, 111440. [Google Scholar] [CrossRef]
- Deng, C.; Cui, Y.; Chen, J.; Chen, T.; Guo, X.; Ji, W.; Peng, L.; Ding, W. Enzyme-like mechanism of selective toluene oxidation to benzaldehyde over organophosphoric acid-bonded nano-oxide. Chin. J. Catal. 2021, 42, 1509–1518. [Google Scholar] [CrossRef]
- Che, C.M.; Lo, V.K.Y.; Zhou, C.Y.; Huang, J.S. Selective functionalisation of saturated C–H bonds with metalloporphyrin catalysts. Chem. Soc. Rev. 2011, 40, 1950–1975. [Google Scholar] [CrossRef]
- Gast, S.; Tuttlies, U.S.; Nieken, U. Kinetic study of the toluene oxidation in homogeneous liquid phase. Chem. Eng. Sci. 2020, 217, 115500. [Google Scholar] [CrossRef]
- Mal, D.D.; Khilari, S.; Pradhan, D. Efficient and selective oxidation of toluene to benzaldehyde on manganese tungstate nanobars: A noble metal-free approach. Green Chem. 2018, 20, 2279–2289. [Google Scholar] [CrossRef]
- Martins, N.M.R.; Pombeiro, A.J.L.; Martins, L.M.D.R.S. A green methodology for the selective catalytic oxidation of styrene by magnetic metal-transition ferrite nanoparticles. Catal. Commun. 2018, 116, 10–15. [Google Scholar] [CrossRef]
- Mu, C.; Cao, Y.; Wang, H.; Yu, H.; Peng, F. A kinetics study on cumene oxidation catalyzed by carbon nanotubes: Effect of N-doping. Chem. Eng. Sci. 2018, 177, 391–398. [Google Scholar] [CrossRef]
- Jiang, J.; Luo, R.; Zhou, X.; Wang, F.; Ji, H. Metalloporphyrin-mediated aerobic oxidation of hydrocarbons in cumene: Co-substrate specificity and mechanistic consideration. Mol. Catal. 2017, 440, 36–42. [Google Scholar] [CrossRef]
- Miao, C.; Zhao, H.; Zhao, Q.; Xia, C.; Sun, W. NHPI and ferric nitrate: A mild and selective system for aerobic oxidation of benzylic methylenes. Catal. Sci. Technol. 2016, 6, 1378–1383. [Google Scholar] [CrossRef]
- Huang, H.; Ye, W.; Song, C.; Liu, Y.; Zhang, X.; Shan, Y.; Ge, Y.; Zhang, S.; Lu, R. Confinement of Au3+-rich clusters by using silicalite-1 for selective solvent-free oxidation of toluene. J. Mater. Chem. A 2021, 9, 14710–14721. [Google Scholar] [CrossRef]
- Yoshino, Y.; Hayashi, Y.; Iwahama, T.; Sakaguchi, S.; Ishii, Y. Catalytic oxidation of alkylbenzenes with molecular oxygen under normal pressure and temperature by N-hydroxyphthalimide combined with Co(OAc)2. J. Org. Chem. 1997, 62, 6810–6813. [Google Scholar] [CrossRef]
- Opeida, I.A.; Plekhov, A.L.; Kushch, O.V.; Kompanets, M.A. On the mechanism of oxidation process initiation by the N-hydroxyphthalimide-cobalt (II) acetate system. Russ. J. Phys. Chem. A 2012, 86, 366–368. [Google Scholar] [CrossRef]
- Bertolini, G.R.; Pizzio, L.R.; Kubacka, A.; Batista, M.J.M.; Garcia, M.F. Composite H3PW12O40-TiO2 catalysts for toluene selective photo-oxidation. Appl. Catal. B 2018, 225, 100–109. [Google Scholar] [CrossRef]
- Ishii, Y.; Sakaguchi, S.; Iwahama, T. Innovation of hydrocarbon oxidation with molecular oxygen and related reactions. Adv. Synth. Catal. 2010, 32, 393–427. [Google Scholar] [CrossRef]
- Shi, G.; Lu, Q.; Xu, J.; Wang, J.; Ji, J. Co-immobilization of N-hydroxyphthalimide and cobaltous ions as a recyclable catalyst for selective aerobic oxidation of toluene to benzaldehyde. J. Environ. Chem. Eng. 2021, 9, 106234. [Google Scholar] [CrossRef]
- Deng, W.; Wan, Y.P.; Jiang, H.; Luo, W.P.; Tan, Z.; Jiang, Q.; Guo, C.C. Solvent-free aerobic oxidation of toluene over metalloporphyrin/NHPI/CTAB: Synergy and mechanism. Catal. Lett. 2014, 144, 333–339. [Google Scholar] [CrossRef]
- Yang, H.M.; Wu, H.S. Interfacial mechanism and kinetics of phase-transfer catalysis. Catal. Rev. 2003, 45, 463–540. [Google Scholar] [CrossRef]
- Kurganova, E.A.; Sapunov, V.N.; Koshel, G.N.; Frolov, A.S. Selective aerobic oxidation of cyclohexyl- and sec-alkylarenes to hydroperoxides in the presence of N-hydroxyphthalimide. Russ. Chem. Bull. 2016, 65, 2115–2128. [Google Scholar] [CrossRef]
- Shi, G.; Feng, Y.; Xu, S.; Lu, Q.; Liang, Y.; Yuan, E.; Ji, L. Covalent anchoring of N-hydroxyphthalimide on silica via robust imide bonds as a reusable catalyst for the selective aerobic oxidation of ethylbenzene to acetophenone. New J. Chem. 2021, 45, 13441–13450. [Google Scholar] [CrossRef]
- Lu, Q.; Shi, G.; Zhou, H.; Yuan, E.; Chen, C.; Ji, L. A highly efficient transformation from cumene to cumyl hydroperoxide via catalytic aerobic oxidation at room temperature and investigations into solvent effects, reaction networks and mechanisms. Appl. Catal. A 2022, 630, 118441. [Google Scholar] [CrossRef]
- Gunchenko, P.A.; Li, J.; Liu, B.F.; Chen, H.Y.; Pashenko, A.E.; Bakhonsky, V.V.; Zhuk, T.S.; Fokin, A.A. Aerobic oxidations with N-hydroxyphthalimide in trifluoroacetic acid. Mol. Catal. 2018, 447, 72–79. [Google Scholar] [CrossRef]
- Gaster, E.; Vainer, Y.; Regev, A.; Narute, S.; Sudheendran, K.; Werbeloff, A.; Shalit, H.; Pappo, D. Significant enhancement in the efficiency and selectivity of iron-catalyzed oxidative cross-coupling of phenols by fluoroalcohols. Angew. Chem. Int. Ed. 2015, 54, 4198–4202. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Xu, S.; Bao, Y.; Xu, J.; Liang, Y. Selective aerobic oxidation of toluene to benzaldehyde on immobilized CoOx on SiO2 catalyst in the presence of N-hydroxyphthalimide and hexafluoropropan-2-ol. Catal. Commun. 2019, 123, 73–78. [Google Scholar] [CrossRef]
- Mostafa, M.S.; Naga, A.O.A.E.; Galhoum, A.A.; Guibal, E.; Morshedy, A.S. A new route for the synthesis of self-acidified and granulated mesoporous alumina catalyst with superior Lewis acidity and its application in cumene conversion. J. Mater. Sci. 2019, 54, 5424–5444. [Google Scholar] [CrossRef]
- Bao, L.; Li, X.X.; Wu, Z.W.; Yuan, X.; Luo, H.A. N-hydroxyphthalimide incorporated onto Cu-BTC metal organic frameworks: A novel catalyst for aerobic oxidation of toluene. Res. Chem. Intermed. 2016, 42, 5527–5539. [Google Scholar] [CrossRef]
- Chidambaram, M.; Sonavane, S.U.; de la Zerda, J.; Sasson, Y. Didecyldimethylammonium bromide (DDAB): A universal, robust, and highly potent phase-transfer catalyst for diverse organic transformations. Tetrahedron 2007, 63, 7696–7701. [Google Scholar] [CrossRef]
- Patil, R.D.; Fuchs, B.; Taha, N.; Sasson, Y. Solvent-free and selective autooxidation of alkylbenzenes catalyzed by Co/NHPI under phase transfer conditions. ChemistrySelect 2016, 1, 3791–3796. [Google Scholar] [CrossRef]
- Dobras, G.; Orlińska, B. Aerobic oxidation of alkylaromatic hydrocarbons to hydroperoxides catalysed by N-hydroxyimides in ionic liquids as solvents. Appl. Catal. A 2018, 561, 59–67. [Google Scholar] [CrossRef]
- Melone, L.; Prosperini, S.; Ercole, G.; Pastori, N.; Punta, C. Is it possible to implement N-hydroxyphthalimide homogeneous catalysis for industrial applications? A case study of cumene aerobic oxidation. J. Chem. Technol. Biotechnol. 2014, 89, 1370–1378. [Google Scholar] [CrossRef]

| Starting Material | Conversion (%) | Selectivity (%) | ||||
|---|---|---|---|---|---|---|
| BAH | BAL | BAC | DBE | Others | ||
| BAH | 63.3 | - | 1.7 | 94.6 | 3.5 | 0.2 |
| BAL | 6.0 | 100 | - | 0.0 | 0.0 | 0.0 |
| BAC | 0.0 | - | - | - | - | - |
| DBE | 33.2 | 71.8 | 23.6 | 4.0 | - | 0.6 |
| Toluene | 29.8 | 73.3 | 16.9 | 4.6 | 5.0 | 0.2 |
| Entry | Phase Transfer Catalyst | Toluene Conversion (%) | Selectivity (%) | ||||
|---|---|---|---|---|---|---|---|
| BAH | BAL | BAC | DBE | Others | |||
| 1 | - | 2.2 | 79.7 | 20.3 | 0.0 | 0.0 | 0.0 |
| 2 | OP-10 | 2.3 | 80.1 | 19.9 | 0.0 | 0.0 | 0.0 |
| 3 | SDBS | 2.4 | 79.3 | 19.9 | 0.0 | 0.0 | 0.8 |
| 4 | BS-12 | 11.2 | 26.9 | 6.1 | 59.6 | 5.1 | 2.4 |
| 5 | DDAB | 29.4 | 9.4 | 3.9 | 80.9 | 2.1 | 3.6 |
| Starting Material | Conversion (%) | Selectivity (%) | |||||
|---|---|---|---|---|---|---|---|
| BAH | BAL | BAC | DBE | BBE | Others | ||
| Toluene | 29.4 | 9.4 | 3.9 | 80.9 | 2.1 | 0.0 | 3.6 |
| BAL | 100 | 11.3 | - | 71.4 | 0.9 | 7.4 | 9.0 |
| DBE | 96.9 | 6.5 | 3.0 | 28.2 | - | 28.8 | 33.5 |
| Catalyst | Conversion (%) | Selectivity (%) | ||||
|---|---|---|---|---|---|---|
| BAH | BAL | BAC | DBE | Others | ||
| None | 0.3 | 86.8 | 13.2 | 0.0 | 0.0 | 0.0 |
| NHPI | 2.2 | 79.7 | 20.3 | 0.0 | 0.0 | 0.0 |
| DDAB | 0.2 | 100 | 0.0 | 0.0 | 0.0 | 0.0 |
| NHPI, DDAB | 29.4 | 9.4 | 3.9 | 80.9 | 2.1 | 3.6 |
| NHPI, DDAB, TEMPO b | 0.0 | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, G.; Dong, L.; Feng, Y. An Investigation of N-Hydroxyphthalimide Catalyzed Aerobic Oxidation of Toluene without Metal Ions in Liquid Phase: Effect of Solvents and Phase Transfer Catalysts. Molecules 2024, 29, 3066. https://doi.org/10.3390/molecules29133066
Shi G, Dong L, Feng Y. An Investigation of N-Hydroxyphthalimide Catalyzed Aerobic Oxidation of Toluene without Metal Ions in Liquid Phase: Effect of Solvents and Phase Transfer Catalysts. Molecules. 2024; 29(13):3066. https://doi.org/10.3390/molecules29133066
Chicago/Turabian StyleShi, Guojun, Longsheng Dong, and Ya Feng. 2024. "An Investigation of N-Hydroxyphthalimide Catalyzed Aerobic Oxidation of Toluene without Metal Ions in Liquid Phase: Effect of Solvents and Phase Transfer Catalysts" Molecules 29, no. 13: 3066. https://doi.org/10.3390/molecules29133066






