Unpredictable Dynamic Behaviour of Ruthenium Chelate Pyrrole Derivatives
Abstract
1. Introduction
2. Results and Discussion
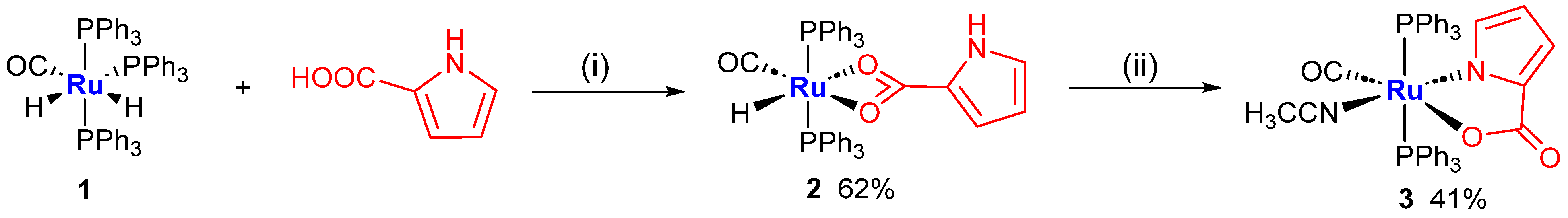
2.1. Syntheses of Complexes 2–3
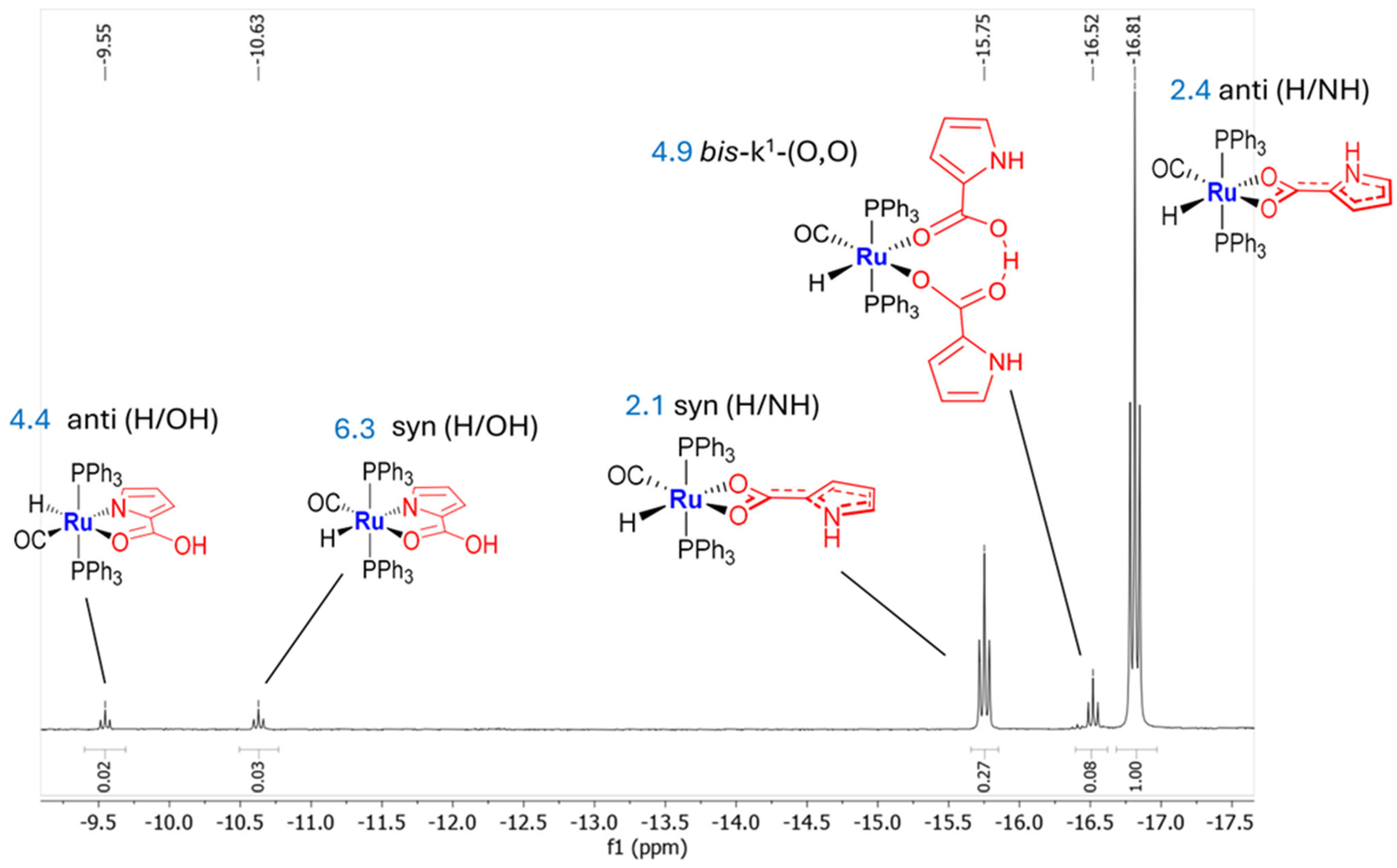
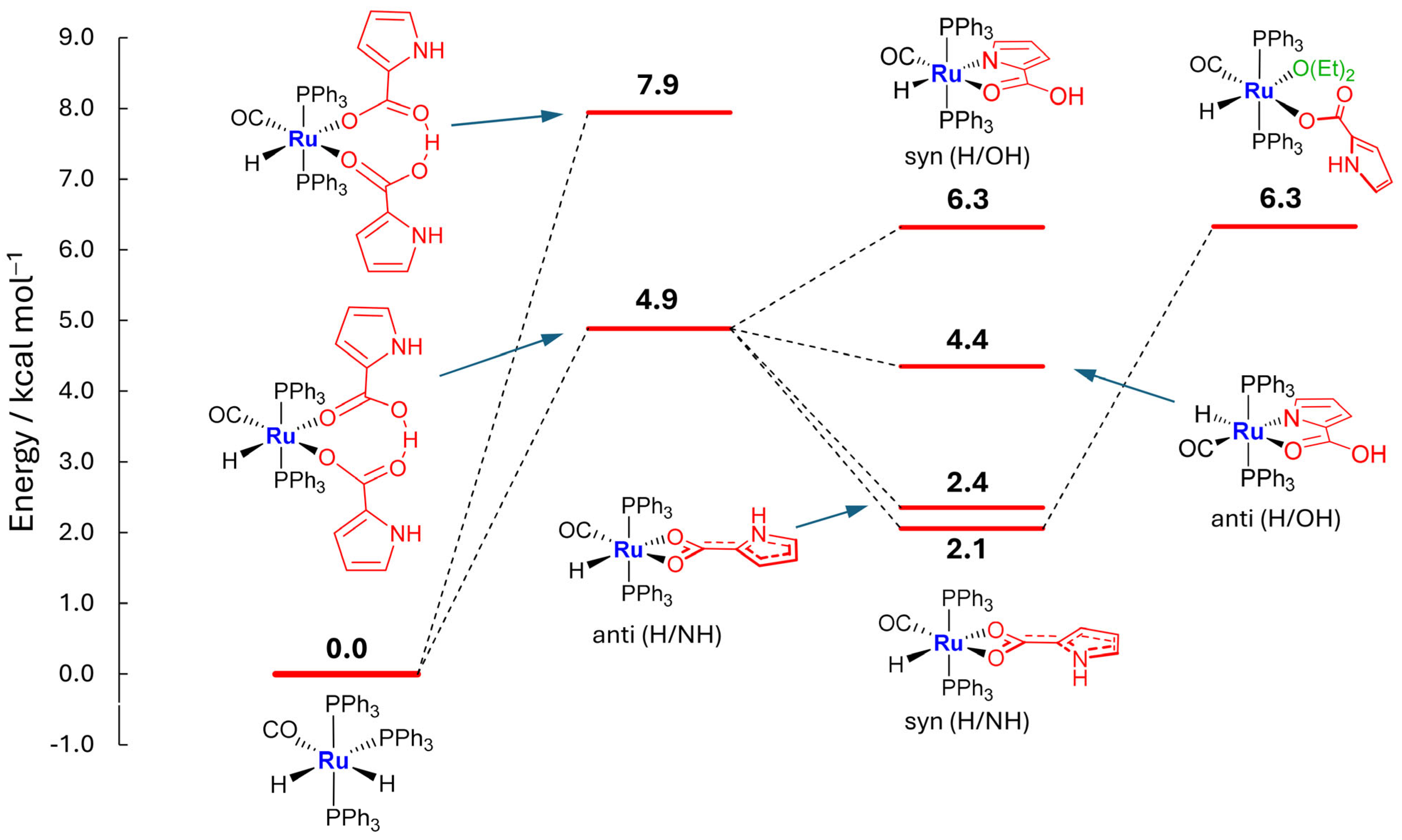
2.2. Kinetic Mixture
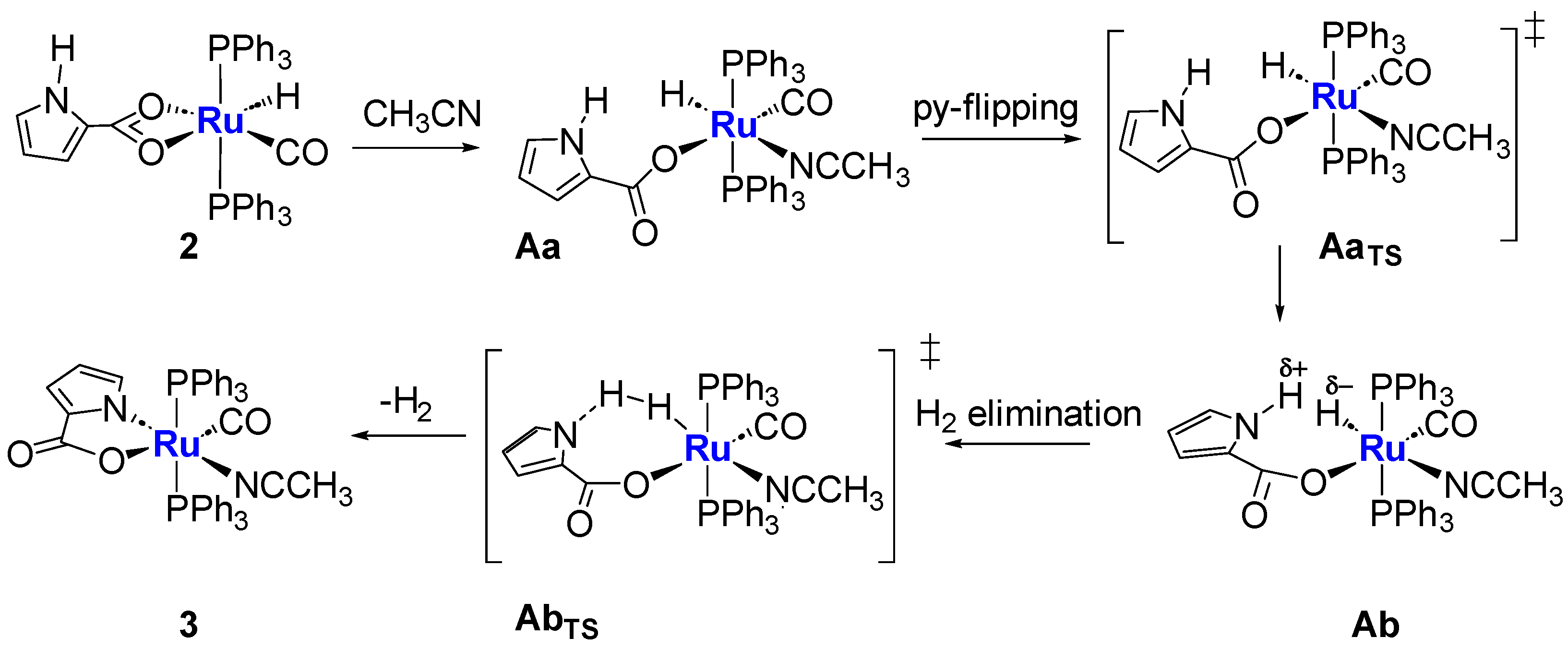
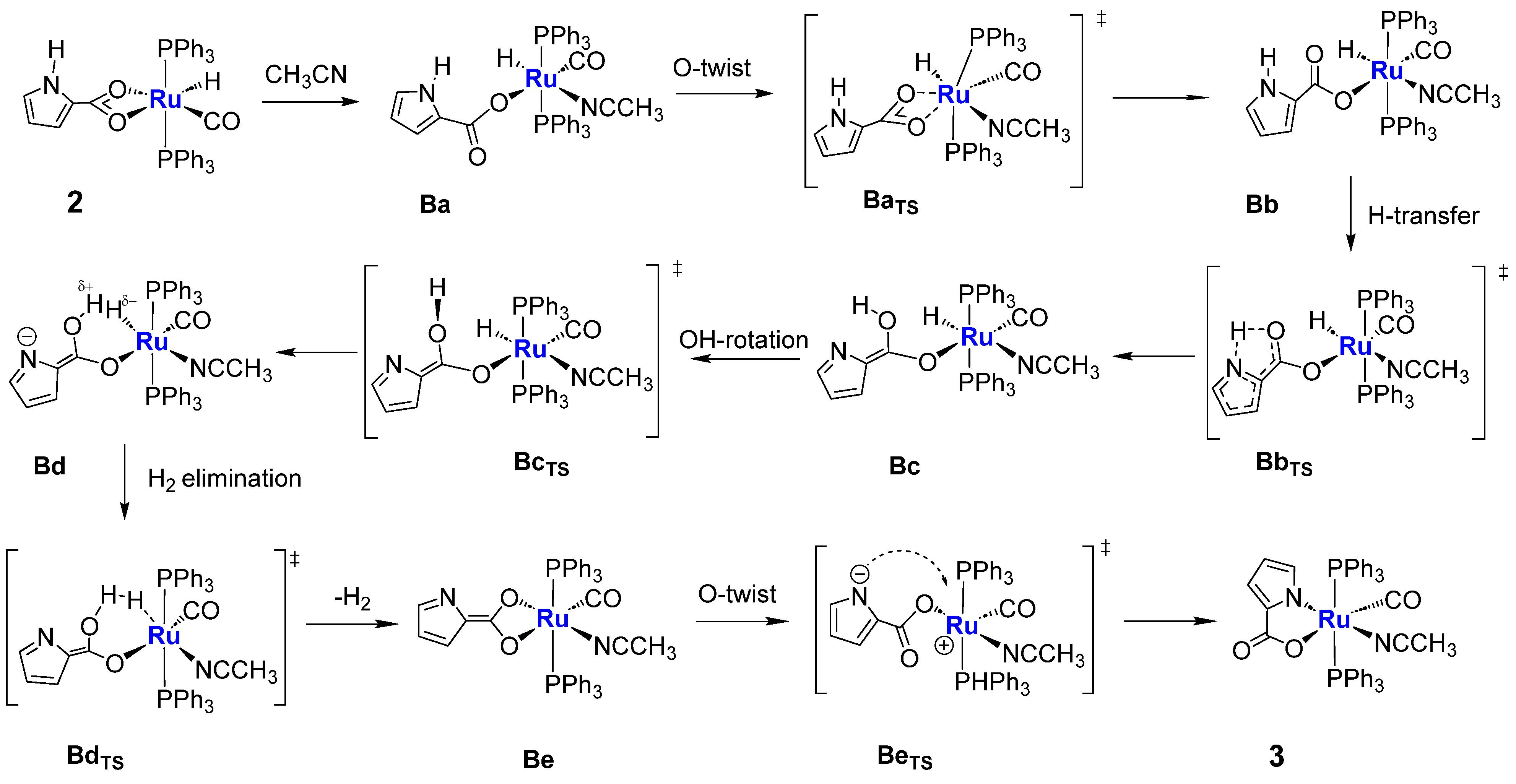
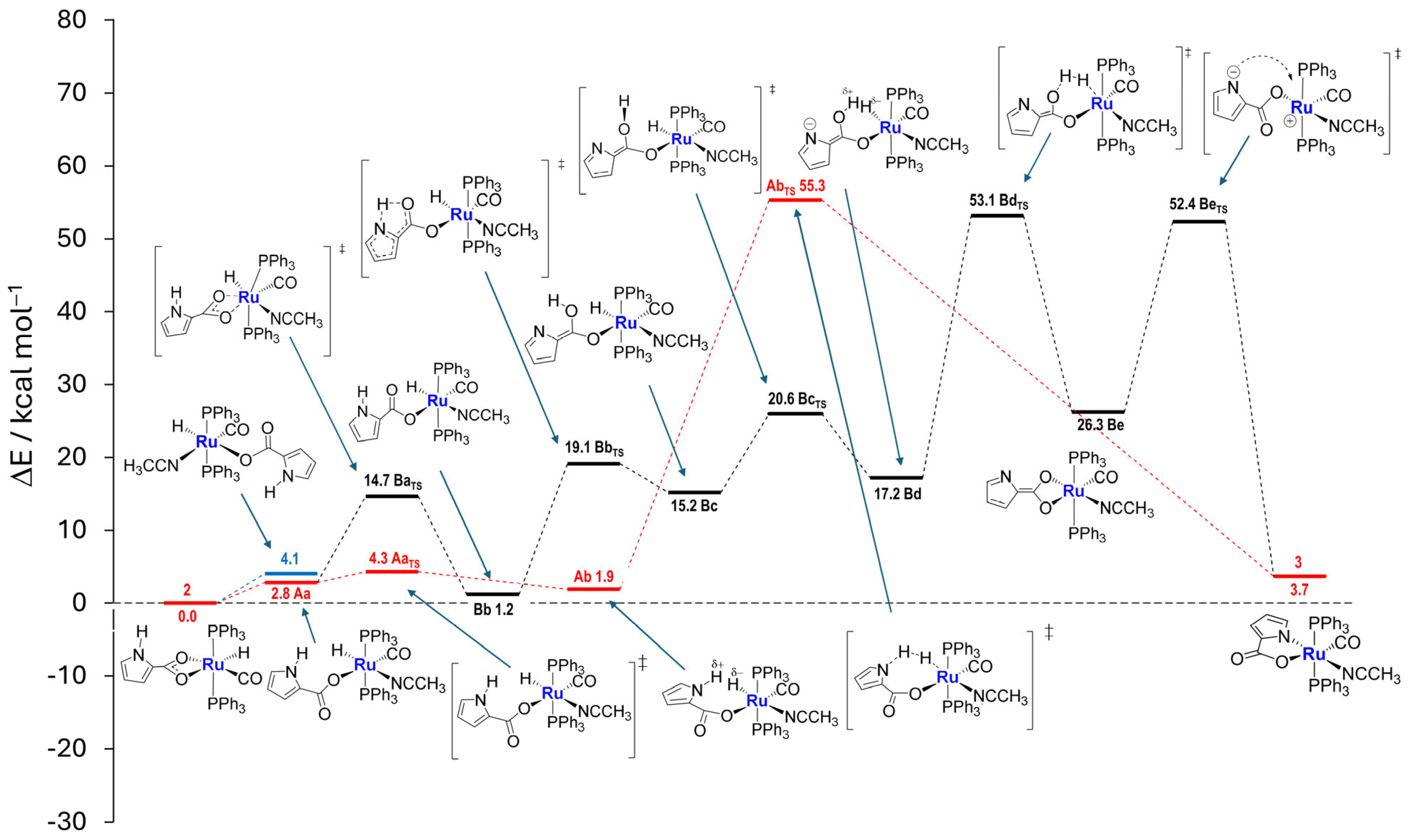
2.3. Coordination of MeCN and Proposed Mechanisms A and B
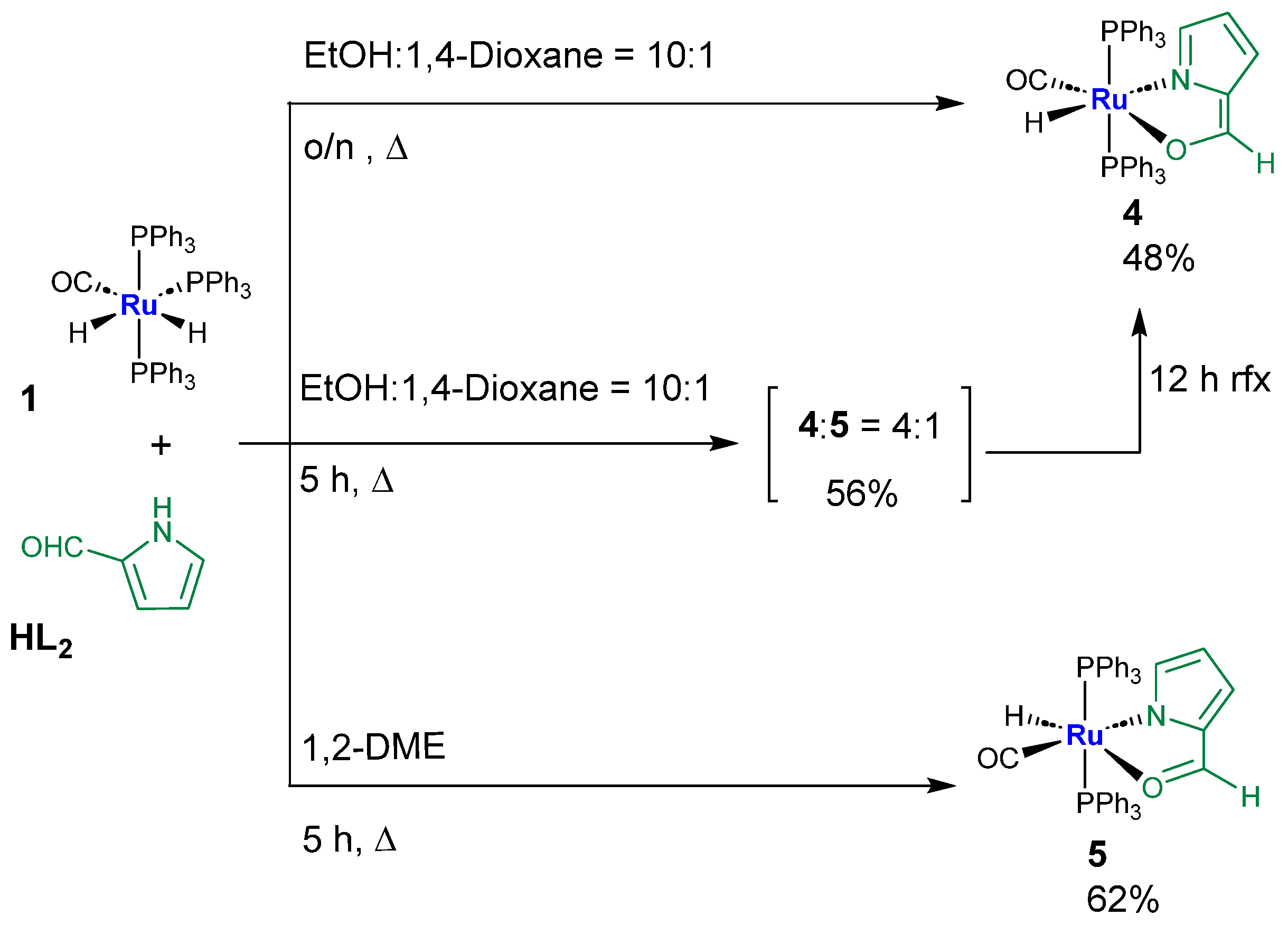
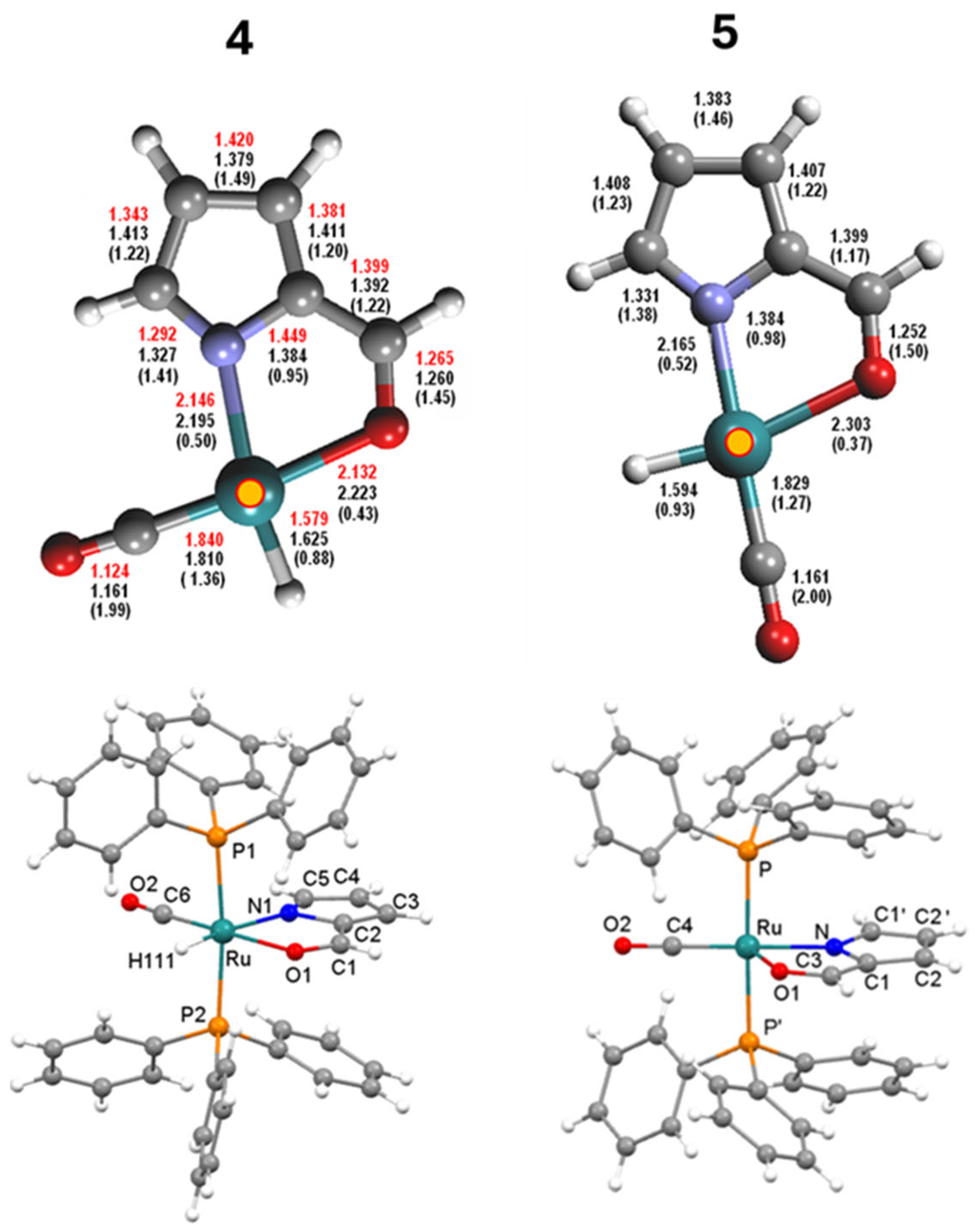
2.4. Syntheses of Complexes 4–5
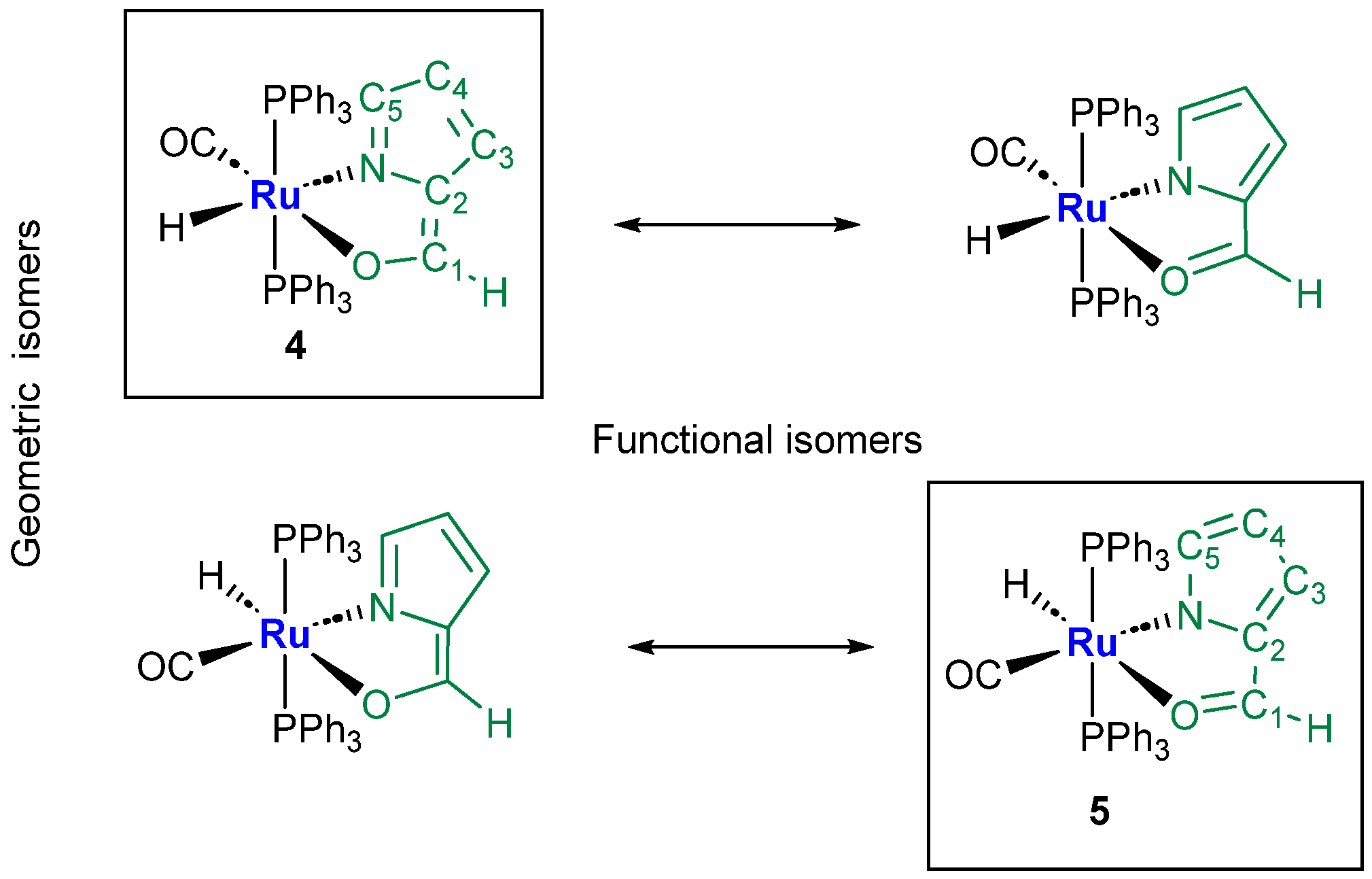
Aldehyde Isomer Description and Extended π−Aromaticity of Five-Membered Fused Rings
- The IR Ru-H stretching appears at ν 1989 for 5, while the related absorption results were overlapped by the strong Ru-CO at 1992 cm−1 in the case of 4;
- As expected, the 1H NMR ethenoyl and the C5H imino-pyrrole signals of 4 fall at 7.57 and 6.84 ppm, respectively (Supplementary Material Figure S21), whereas the analogous carboxaldehyde and the pyrrolide C5H-unit resonances concerning complex 5 appear conversely shifted at δ 8.06 and 6.13 ppm (Supplementary Material Figure S29);
- The adjacent C5=N bond, measured by X-ray (1.29 Å) or evaluated by DFT calculations (MBO = 1.41), is slightly elongated in 4; conversely, in the case of the Ru- carboxaldehyde pyrrole 5 species, the C5-N bond is calculated to be 1.33 Å with a bond order of 1.38 Å, showing a trend in accordance with the proposed isomers.
2.5. ESI-Ms Spectra
2.6. Description of the X-ray Crystal Structure of 3–5
2.7. Antimicrobial Activity
3. Materials and Methods
3.1. General
3.2. Synthesis of k2(O,O)-[RuH(CO)(HL1)(PPh3)2], 2
3.3. Synthesis of k2(N,O)-[Ru(MeCN)(CO)(L1)(PPh3)2], 3
3.4. Synthesis of k2(N,O)-[RuH(CO)(HL2)(PPh3)2], 4
3.5. Synthesis of k2(N,O)-[RuH(CO)(HL2)(PPh3)2], 5
3.6. Synthesis of the Mixture of Isomers 4 and 5
3.7. Computational Details
3.8. Crystallography
3.9. Antimicrobial Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, R.D.; Maccoss, M.; Lawson, A.D.G. Rings in Drugs. J. Med. Chem. 2014, 57, 5845–5859. [Google Scholar] [CrossRef] [PubMed]
- Shearer, J.; Castro, J.L.; Lawson, A.D.G.; MacCoss, M.; Taylor, R.D. Rings in Clinical Trials and Drugs: Present and Future. J. Med. Chem. 2022, 65, 8699–8712. [Google Scholar] [CrossRef] [PubMed]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Young, R.J.; Leeson, P.D. Mapping the Efficiency and Physicochemical Trajectories of Successful Optimizations. J. Med. Chem. 2018, 61, 6421–6467. [Google Scholar] [CrossRef] [PubMed]
- Pennington, L.D.; Moustakas, D.T. The Necessary Nitrogen Atom: A Versatile High-Impact Design Element for Multiparameter Optimization. J. Med. Chem. 2017, 60, 3552–3579. [Google Scholar] [CrossRef] [PubMed]
- Meanwell, N.A. The Influence of Bioisosteres in Drug Design: Tactical Applications to Address Developability Problems. Top Med. Chem. 2014, 9, 283–382. [Google Scholar] [CrossRef]
- Amin, A.; Qadir, T.; Sharma, P.K.; Jeelani, I.; Abe, H. A Review on The Medicinal And Industrial Applications of N-Containing Heterocycles. Open J. Med. Chem. 2022, 16. [Google Scholar] [CrossRef]
- Bai, H.-F.; Zhang, S.-Y.; Yan, Y.-M.; Cheng, Y.-X. N-Containing Phenolic Compounds from Periplaneta Americana with Triple Negative Breast Cancer Inhibitory Activity. Phytochemistry 2024, 218, 113936. [Google Scholar] [CrossRef] [PubMed]
- Pennington, L.D.; Collier, P.N.; Comer, E. Harnessing the Necessary Nitrogen Atom in Chemical Biology and Drug Discovery. Med. Chem. Res. 2023, 32, 1278–1293. [Google Scholar] [CrossRef]
- Kuznietsova, H.; Dziubenko, N.; Byelinska, I.; Hurmach, V.; Bychko, A.; Lynchak, O.; Milokhov, D.; Khilya, O.; Rybalchenko, V. Pyrrole Derivatives as Potential Anti-Cancer Therapeutics: Synthesis, Mechanisms of Action, Safety. J. Drug Target 2020, 28, 547–563. [Google Scholar] [CrossRef]
- Wilkerson, W.W.; Copeland, R.A.; Covington, M.; Trzaskos, J.M. Anti Inflammatory 4,5-Diarylpyrroles. 2. Activity as a Function of Cyclooxygenase-2 Inhibition. J. Med. Chem. 1995, 38, 3895–3901. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.M.; Johnson, R.E. Pyrrole Antibacterial Agents. 4,5-Dihalopyrrole-2-Carboxylic Acid Derivatives. J. Med. Chem. 1973, 16, 1300–1302. [Google Scholar] [CrossRef]
- Di Santo, R.; Costi, R.; Artico, M.; Massa, S.; Lampis, G.; Deiddat, D.; Pompei~, R. Pyrrolnitrin and Related Pyrroles Endowed with Antibacterial Activities against Mycobacterium Tuberculosis. Bioorg. Med. Chem. Lett. 1998, 8, 2931–2936. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, K.M.; Corrêa, R.S.; Barbosa, M.I.F.; Ellena, J.; Cominetti, M.R.; Batista, A.A. Ruthenium(II)/Triphenylphosphine Complexes: An Effective Way to Improve the Cytotoxicity of Lapachol. Polyhedron 2017, 130, 108–114. [Google Scholar] [CrossRef]
- Nayeem, N.; Contel, M. Exploring the Potential of Metallodrugs as Chemotherapeutics for Triple Negative Breast Cancer. Chem. Eur. J. 2021, 27, 8891–8917. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.; Shamsi, F.; Azam, A. Ruthenium Complexes: An Emerging Ground to the Development of Metallopharmaceuticals for Cancer Therapy. Mini-Rev. Med. Chem. 2016, 16, 772–786. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Zhao, Z.-Z.; Bo, H.-B.; Hao, X.-J.; Wang, J.-Q. Applications of Ruthenium Complex in Tumor Diagnosis and Therapy. Front. Pharmacol. 2018, 9, 1323. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, V.; Gumber, D.; Dhiman, S.; Sharma, P. Pyrrole: A Resourceful Small Molecule in Key Medicinal Hetero-Aromatics. RSC Adv. 2015, 5, 15233–15266. [Google Scholar] [CrossRef]
- Buil, M.L.; Esteruelas, M.A.; Oñate, E.; Picazo, N.R. Osmathiazole Ring: Extrapolation of an Aromatic Purely Organic System to Organometallic Chemistry. Organometallics 2023, 42, 327–338. [Google Scholar] [CrossRef]
- Chen, D.; Hua, Y.; Xia, H. Metallaaromatic Chemistry: History and Development. Chem. Rev. 2020, 120, 12994–13086. [Google Scholar] [CrossRef]
- Wilton-Ely, J.D.E.T.; Pogorzelec, P.J.; Honarkha, S.J.; Reid, D.H.; Tocher, D.A. Mixed-Donor Ligands: Pyrrolecarbaldehyde and Pyrrolecarbothioaldehyde σ-Organyl Complexes of Ruthenium(II) and Osmium(II). Organometallics 2005, 24, 2862–2874. [Google Scholar] [CrossRef]
- Jazzar, R.F.R.; Varrone, M.; Burrows, A.D.; MacGregor, S.A.; Mahon, M.F.; Whittlesey, M.K. Synthesis and Isomerisation of Two Metallated N,O-Complexes of Ruthenium: Models for the Murai Reaction. Inorg. Chim. Acta 2006, 359, 815–820. [Google Scholar] [CrossRef]
- Lundrigan, T.; Jackson, C.L.M.; Uddin, M.I.; Tucker, L.A.; Ali, A.A.S.; Linden, A.; Cameron, T.S.; Thompson, A. Synthesis of Heteroleptic Pyrrolide/Bipyridyl Complexes of Ruthenium(II). Can. J. Chem. 2012, 90, 693–700. [Google Scholar] [CrossRef][Green Version]
- Tsai, Y.W.; Chen, Y.F.; Li, Y.J.; Chen, K.H.; Lin, C.H.; Huang, J.H. Structural Determination of Ruthenium Complexes Containing Bi-Dentate Pyrrole-Ketone Ligands. Molecules 2018, 23, 159. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Lai, H.T.; Hu, C.H.; Chen, J.H.; Lin, C.H.; Huang, J.H. Geometric Isomerization and Geometry Controlled Catalytic Alcohol Aminations of Ruthenium Hydride Compounds Containing Bidentate Pyrrolyl-Imines. J. Organomet. Chem. 2019, 902, 120957. [Google Scholar] [CrossRef]
- Ovcharenko, V.; Kuznetsova, O.; Fursova, E.; Romanenko, G.; Polushkin, A.; Sagdeev, R. Redox-Induced Change in the Ligand Coordination Mode. Inorg. Chem. 2014, 53, 10033–10035. [Google Scholar] [CrossRef] [PubMed]
- Cunha, B.N.; Colina-Vegas, L.; Plutín, A.M.; Silveira, R.G.; Honorato, J.; Oliveira, K.M.; Cominetti, M.R.; Ferreira, A.G.; Castellano, E.E.; Batista, A.A. Hydrolysis Reaction Promotes Changes in Coordination Mode of Ru(II)/Acylthiourea Organometallic Complexes with Cytotoxicity against Human Lung Tumor Cell Lines. J. Inorg. Biochem. 2018, 186, 147–156. [Google Scholar] [CrossRef]
- Gonzalez, P.; Vileno, B.; Bossak, K.; El Khoury, Y.; Hellwig, P.; Bal, W.; Hureau, C.; Faller, P. Cu(II) Binding to the Peptide Ala-His-His, a Chimera of the Canonical Cu(II)-Binding Motifs Xxx-His and Xxx-Zzz-His. Inorg. Chem. 2017, 56, 14870–14879. [Google Scholar] [CrossRef]
- Miyashita, A.; Sugai, R.-J.; Yamamoto, J.-I. Synthesis and Reactivities of Novel η2-(C,O) Alkylphenylketene Complexes of Nickel. Coordination-Mode Switching Reaction of the Ketene Ligand. J. Organomet. Chem. 1992, 428, 239–247. [Google Scholar] [CrossRef]
- Morris, R.; Habtemariam, A.; Guo, Z.; Parsons, S.; Sadler, P.J. Chelate Ring-Opening Aminophosphine Complexes of Ruthenium(II). Inorg. Chim. Acta 2002, 339, 551–559. [Google Scholar] [CrossRef]
- Dubis, A.T.; Wojtulewski, S.; Filipkowski, K. Spectroscopic and Theoretical Studies on the Aromaticity of Pyrrol-2-Yl-Carbonyl Conformers. J. Mol. Struct. 2013, 1041, 92–99. [Google Scholar] [CrossRef]
- Dubis, A.T.; Grabowski, S.J. Spectroscopic and Theoretical Studies on the Monomeric and Dimeric Forms of Methyl Pyrrole-2-Carboxylate. New J. Chem. 2002, 26, 165–169. [Google Scholar] [CrossRef]
- Chernyshov, I.Y.; Ananyev, I.V.; Pidko, E.A. Revisiting van Der Waals Radii: From Comprehensive Structural Analysis to Knowledge-Based Classification of Interatomic Contacts. Chemphyschem 2020, 21, 370–376. [Google Scholar] [CrossRef]
- Ackermann, L. Carboxylate-Assisted Ruthenium-Catalyzed Alkyne Annulations by C-H/Het-H Bond Functionalizations. Acc. Chem. Res. 2014, 47, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, L. Carboxylate-Assisted Transition-Metal-Catalyzed C-H Bond Functionalizations: Mechanism and Scope. Chem. Rev. 2011, 111, 1315–1345. [Google Scholar] [CrossRef]
- Li, F.; Collins, J.G.; Keene, F.R. Ruthenium Complexes as Antimicrobial Agents. Chem. Soc. Rev. 2015, 44, 2529–2542. [Google Scholar] [CrossRef]
- Samouei, H.; Grushin, V.V. New, Highly Efficient, Simple, Safe, and Scalable Synthesis of [(Ph3P)3Ru(CO)(H)2]. Organometallics 2013, 32, 4440–4443. [Google Scholar] [CrossRef]
- Neese, F. The ORCA Program System. Wiley Interdiscip Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Kesharwani, M.K.; Brauer, B.; Martin, J.M.L. Frequency and Zero-Point Vibrational Energy Scale Factors for Double-Hybrid Density Functionals (and Other Selected Methods): Can Anharmonic Force Fields Be Avoided? J.Phys. Chem A 2015, 119, 1701–1714. [Google Scholar] [CrossRef]
- Martin, R.L.; Hay, P.J.; Pratt, L.R. Hydrolysis of Ferric Ion in Water and Conformational Equilibrium. J. Phys. Chem. A 1998, 102, 3565–3573. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. A New Local Density Functional for Main-Group Thermochemistry, Transition Metal Bonding, Thermochemical Kinetics, and Noncovalent Interactions. J. Chem. Phys. 2006, 125, 194101. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- APEX3 Software Package V2019; Bruker AXS Inc.: Madison, WI, USA, 2019.
- Bruker SAINT, v8.40A: Part of the APEX3 Software Package V2019; Bruker AXS Inc.: Madison, WI, USA, 2019.
- Bruker SADABS V2016/2: Part of the APEX3 Software Package V2019; Bruker AXS Inc.: Madison, WI, USA, 2019.
- Rigaku, O.D. CrysAlis PRO; Rigaku Oxford Diffraction: Yarnton, UK, 2019. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drius, G.; Tarroni, R.; Birchmeier, M.; Parolin, C.; Boga, C.; Monari, M.; Bordoni, S. Unpredictable Dynamic Behaviour of Ruthenium Chelate Pyrrole Derivatives. Molecules 2024, 29, 3068. https://doi.org/10.3390/molecules29133068
Drius G, Tarroni R, Birchmeier M, Parolin C, Boga C, Monari M, Bordoni S. Unpredictable Dynamic Behaviour of Ruthenium Chelate Pyrrole Derivatives. Molecules. 2024; 29(13):3068. https://doi.org/10.3390/molecules29133068
Chicago/Turabian StyleDrius, Giacomo, Riccardo Tarroni, Matteo Birchmeier, Carola Parolin, Carla Boga, Magda Monari, and Silvia Bordoni. 2024. "Unpredictable Dynamic Behaviour of Ruthenium Chelate Pyrrole Derivatives" Molecules 29, no. 13: 3068. https://doi.org/10.3390/molecules29133068
APA StyleDrius, G., Tarroni, R., Birchmeier, M., Parolin, C., Boga, C., Monari, M., & Bordoni, S. (2024). Unpredictable Dynamic Behaviour of Ruthenium Chelate Pyrrole Derivatives. Molecules, 29(13), 3068. https://doi.org/10.3390/molecules29133068











