The Role of Intraligand Charge Transfer Processes in Iridium(III) Complexes with Morpholine-Decorated 4′-Phenyl-2,2′:6′,2″-terpyridine
Abstract
:1. Introduction
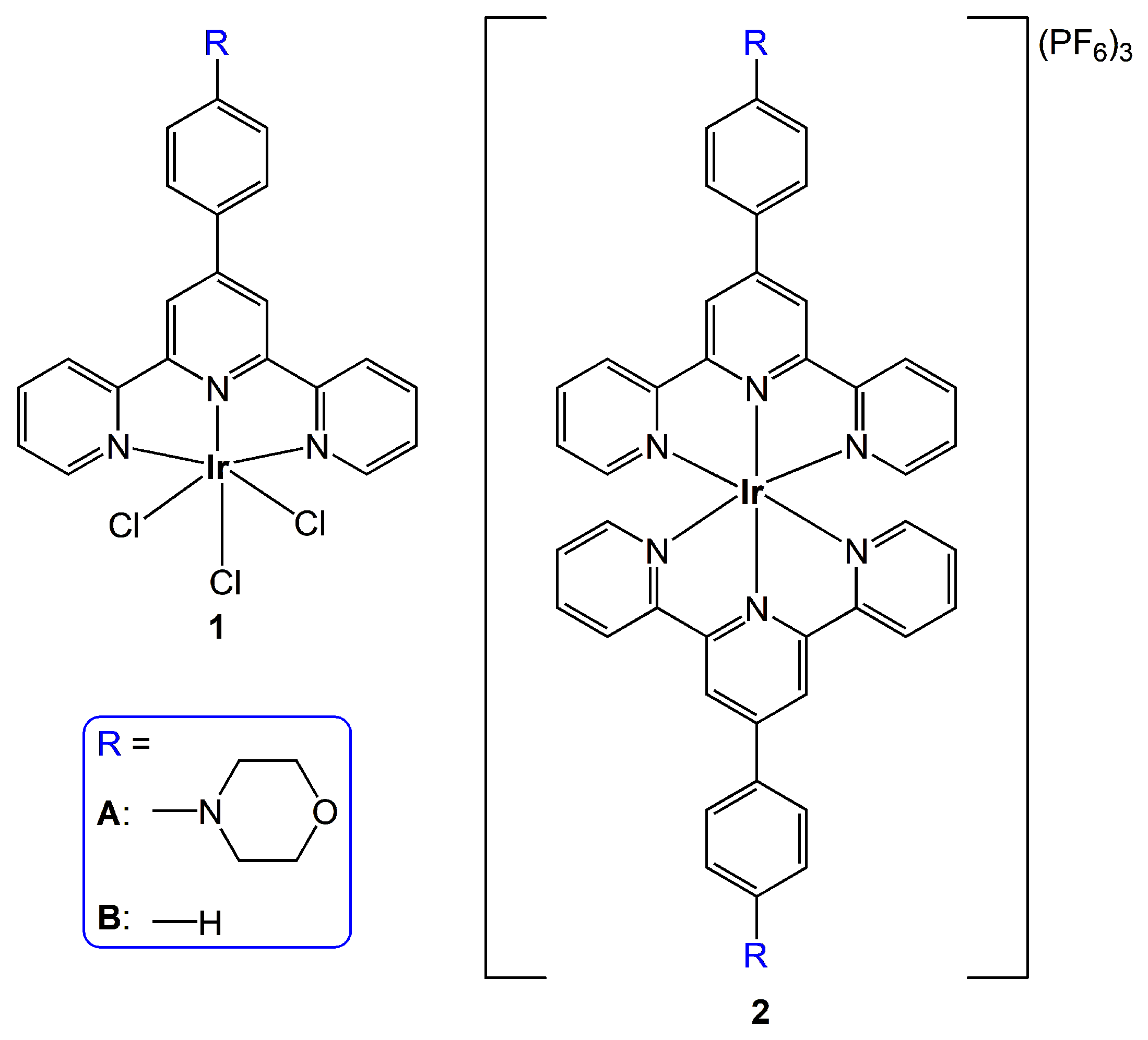
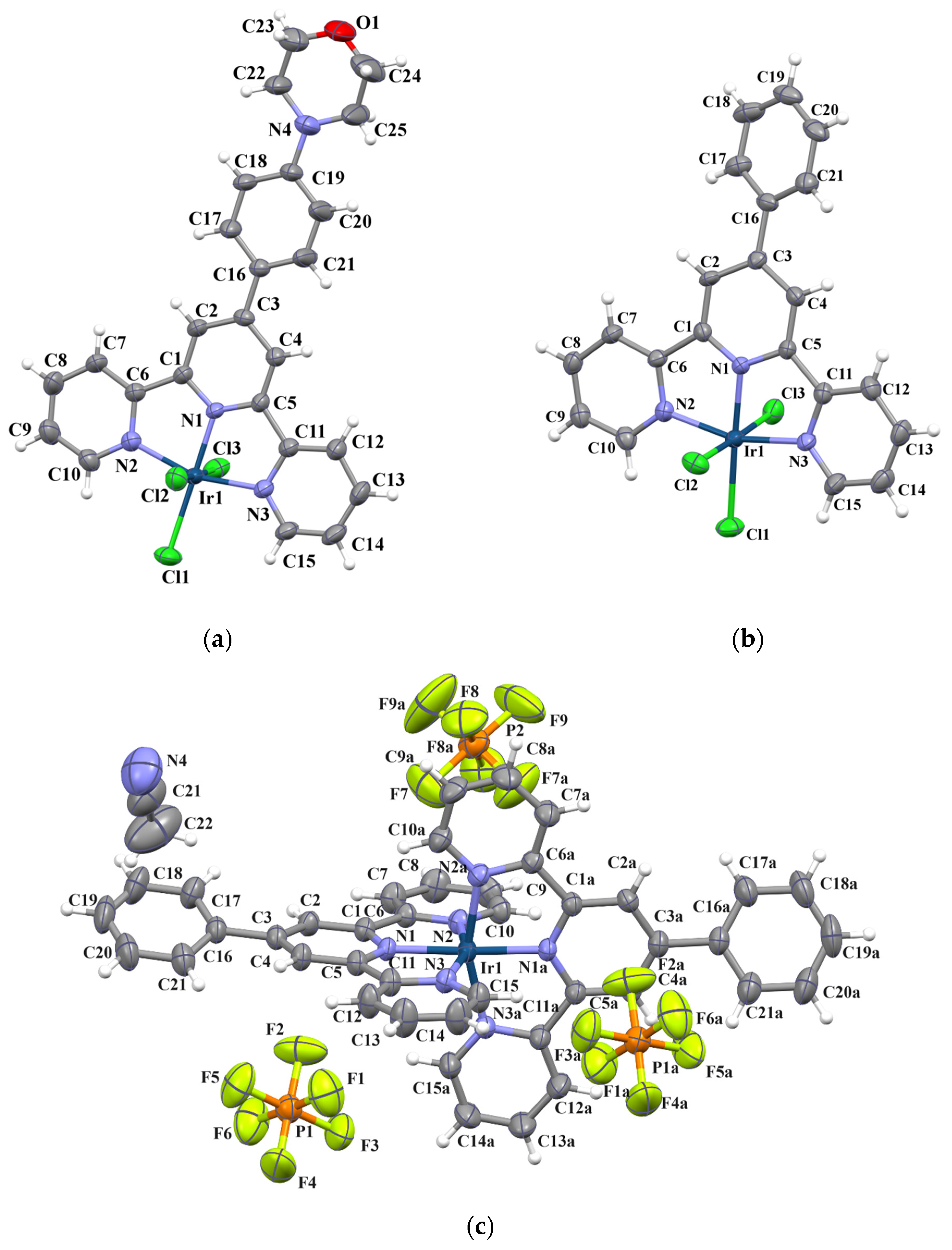
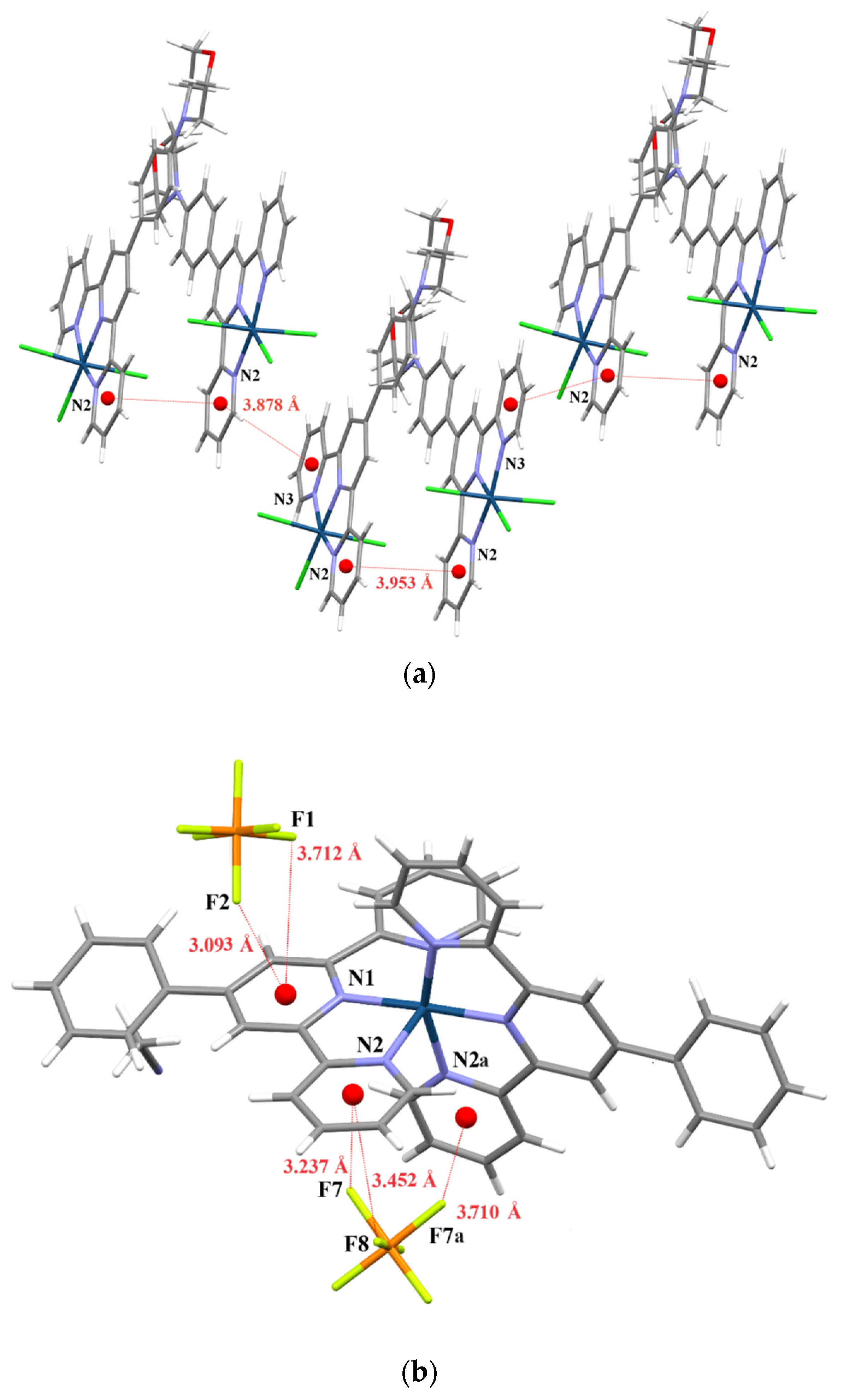
2. Results and Discussion
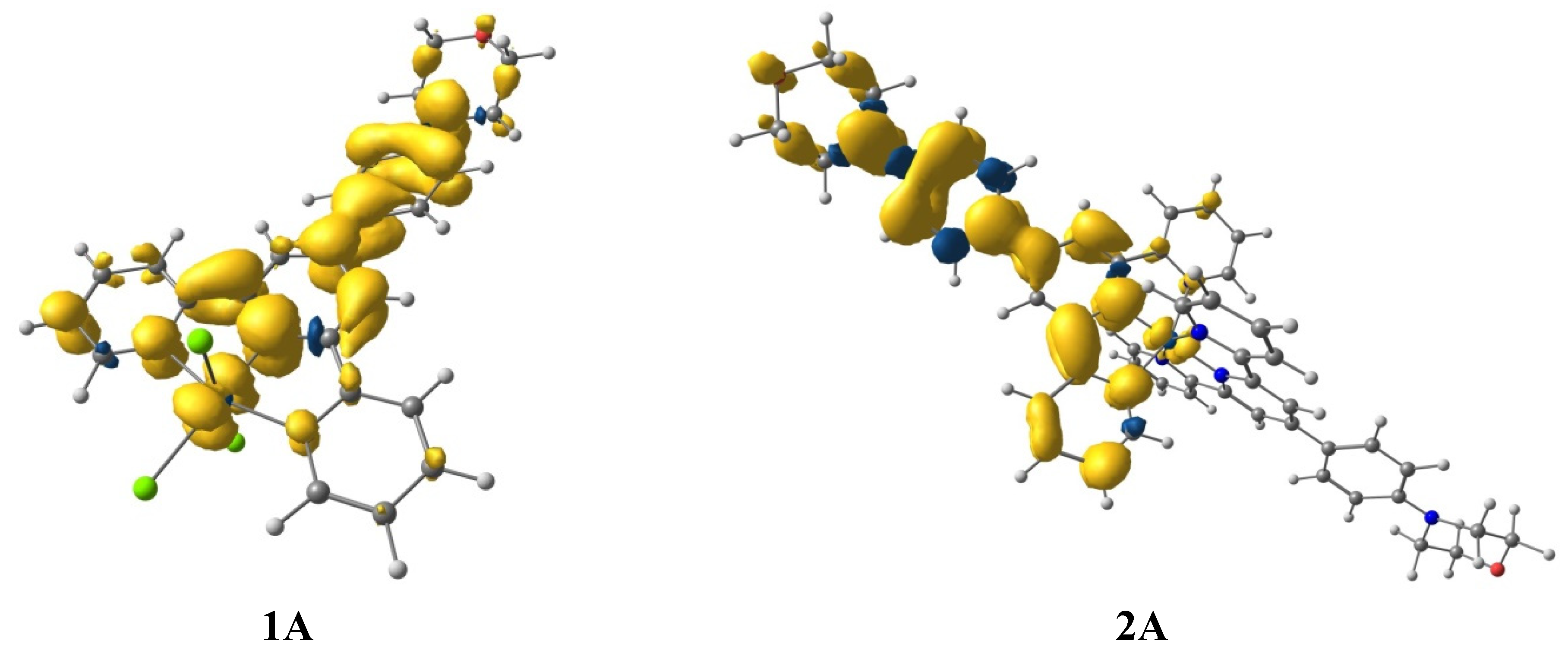
3. Ground-State Molecular Orbitals
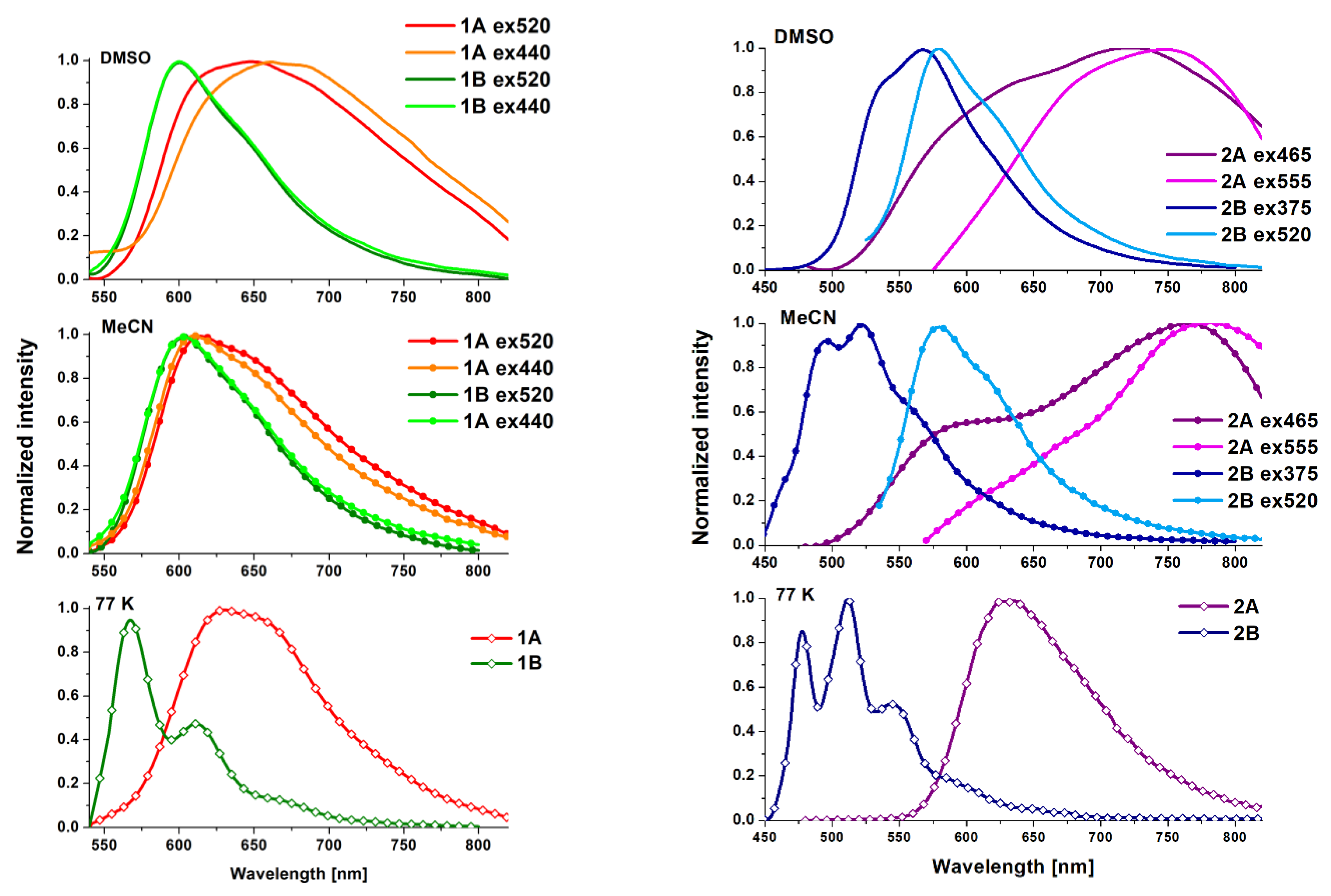
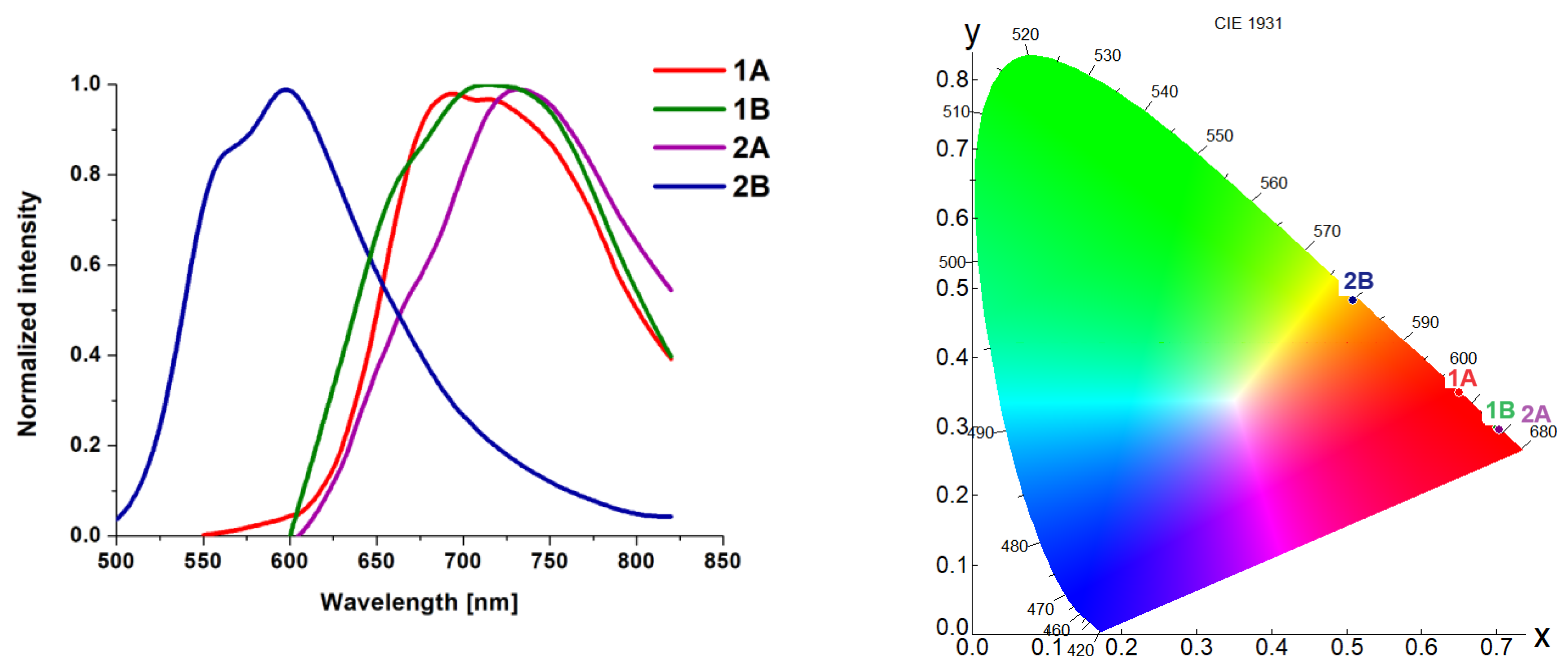
4. Photophysical Properties–Experimental and Theoretical Insights
5. Femtosecond Transient Absorption Spectra
6. Conclusions
7. Experimental
7.1. Materials and Methods
7.2. Preparation of Ir(III) Complexes
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, C.; He, Y.; Shi, X.; Song, Z. Terpyridine-Metal Complexes: Applications in Catalysis and Supramolecular Chemistry. Coord. Chem. Rev. 2019, 385, 1–19. [Google Scholar] [CrossRef]
- Rupp, M.T.; Shevchenko, N.; Hanan, G.S.; Kurth, D.G. Enhancing the Photophysical Properties of Ru(II) Complexes by Specific Design of Tridentate Ligands. Coord. Chem. Rev. 2021, 446, 214127. [Google Scholar] [CrossRef]
- Panicker, R.R.; Sivaramakrishna, A. Remarkably Flexible 2,2′:6′,2″-Terpyridines and Their Group 8–10 Transition Metal Complexes—Chemistry and Applications. Coord. Chem. Rev. 2022, 459, 214426. [Google Scholar] [CrossRef]
- Momeni, B.Z.; Abd-El-Aziz, A.S. Recent Advances in the Design and Applications of Platinum-Based Supramolecular Architectures and Macromolecules. Coord. Chem. Rev. 2023, 486, 215113. [Google Scholar] [CrossRef]
- Palion-Gazda, J.; Choroba, K.; Maroń, A.M.; Malicka, E.; Machura, B. Structural and Photophysical Trends in Rhenium(I) Carbonyl Complexes with 2,2′:6′,2″-Terpyridines. Molecules 2024, 29, 1631. [Google Scholar] [CrossRef] [PubMed]
- Momeni, B.Z.; Davarzani, N.; Janczak, J.; Ma, N.; Abd-El-Aziz, A.S. Progress in Design and Applications of Supramolecular Assembly of 2,2′:6′,2″-Terpyridine-Based First Row d-Block Elements. Coord. Chem. Rev. 2024, 506, 215619. [Google Scholar] [CrossRef]
- Breivogel, A.; Kreitner, C.; Heinze, K. Redox and Photochemistry of Bis(Terpyridine)Ruthenium(II) Amino Acids and Their Amide Conjugates—From Understanding to Applications. Eur. J. Inorg. Chem. 2014, 2014, 5468–5490. [Google Scholar] [CrossRef]
- Saccone, D.; Magistris, C.; Barbero, N.; Quagliotto, P.; Barolo, C.; Viscardi, G. Terpyridine and Quaterpyridine Complexes as Sensitizers for Photovoltaic Applications. Materials 2016, 9, 137. [Google Scholar] [CrossRef]
- Kreitner, C.; Mengel, A.K.C.; Lee, T.K.; Cho, W.; Char, K.; Kang, Y.S.; Heinze, K. Strongly Coupled Cyclometalated Ruthenium Triarylamine Chromophores as Sensitizers for DSSCs. Chem. Eur. J. 2016, 22, 8915–8928. [Google Scholar] [CrossRef]
- Mills, I.N.; Porras, J.A.; Bernhard, S. Judicious Design of Cationic, Cyclometalated Ir(III) Complexes for Photochemical Energy Conversion and Optoelectronics. Acc. Chem. Res. 2018, 51, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Shi, G.; Chen, X. Terpyridine-Containing π-Conjugated Polymers for Light-Emitting and Photovoltaic Materials. Front. Chem. 2020, 8, 592055. [Google Scholar] [CrossRef] [PubMed]
- Laschuk, N.O.; Ebralidze, I.I.; Easton, E.B.; Zenkina, O.V. Osmium- and Cobalt-Terpyridine-Based Electrochromic Devices for “Smart” Signage Application: The Effect of Lighting on Color Perception. Adv. Electron. Mater. 2021, 7, 2100460. [Google Scholar] [CrossRef]
- Genoni, A.; Chirdon, D.N.; Boniolo, M.; Sartorel, A.; Bernhard, S.; Bonchio, M. Tuning Iridium Photocatalysts and Light Irradiation for Enhanced CO2 Reduction. ACS Catal. 2017, 7, 154–160. [Google Scholar] [CrossRef]
- Fernández-Terán, R.; Sévery, L. Living Long and Prosperous: Productive Intraligand Charge-Transfer States from a Rhenium(I) Terpyridine Photosensitizer with Enhanced Light Absorption. Inorg. Chem. 2021, 60, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Tang, Y.; Li, X.; Zhang, B.; Zhou, A.; Qiao, Z.; Tong, L. A Photofunctional Platform of Bis-Terpyridine Ruthenium Complex-Linked Coordination Polymers with Structural Diversity. J. Mater. Chem. A 2022, 10, 25063–25069. [Google Scholar] [CrossRef]
- Ranjan Jena, S.; Mandal, T.; Choudhury, J. Metal-Terpyridine Assembled Functional Materials for Electrochromic, Catalytic and Environmental Applications. Chem. Rec. 2022, 22, e202200165. [Google Scholar] [CrossRef]
- Saha, S.; Doughty, T.; Banerjee, D.; Patel, S.K.; Mallick, D.; Iyer, E.S.S.; Roy, S.; Mitra, R. Electrocatalytic Reduction of CO2 to CO by a Series of Organometallic Re(I)-Tpy Complexes. Dalton Trans. 2023, 52, 15394–15411. [Google Scholar] [CrossRef]
- Jacques, A.; Devaux, A.; Rubay, C.; Pennetreau, F.; Desmecht, A.; Robeyns, K.; Hermans, S.; Elias, B. Polypyridine Iridium(III) and Ruthenium(II) Complexes for Homogeneous and Graphene-Supported Photoredox Catalysis. ChemCatChem 2023, 15, e202201672. [Google Scholar] [CrossRef]
- Eryazici, I.; Moorefield, C.N.; Newkome, G.R. Square-Planar Pd(II), Pt(II), and Au(III) Terpyridine Complexes: Their Syntheses, Physical Properties, Supramolecular Constructs, and Biomedical Activities. Chem. Rev. 2008, 108, 1834–1895. [Google Scholar] [CrossRef]
- Grau, J.; Caubet, A.; Roubeau, O.; Montpeyó, D.; Lorenzo, J.; Gamez, P. Time-Dependent Cytotoxic Properties of Terpyridine-Based Copper Complexes. ChemBioChem 2020, 21, 2348–2355. [Google Scholar] [CrossRef]
- Musiol, R.; Malecki, P.; Pacholczyk, M.; Mularski, J. Terpyridines as Promising Antitumor Agents: An Overview of Their Discovery and Development. Expert Opin. Drug Discov. 2022, 17, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Abhijnakrishna, R.; Magesh, K.; Ayushi, A.; Velmathi, S. Advances in the Biological Studies of Metal-Terpyridine Complexes: An Overview from 2012 to 2022. Coord. Chem. Rev. 2023, 496, 215380. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Chen, Z.; Xue, X.; Lin, K.; Chen, H.; Pan, L.; Yuan, Y.; Ma, Z. Silver Complexes with Substituted Terpyridines as Promising Anticancer Metallodrugs and Their Crystal Structure, Photoluminescence, and DNA Interactions. Dalton Trans. 2023, 52, 9607–9621. [Google Scholar] [CrossRef] [PubMed]
- Gil-Moles, M.; Concepción Gimeno, M. The Therapeutic Potential in Cancer of Terpyridine-Based Metal Complexes Featuring Group 11 Elements. ChemMedChem 2024, 19, e202300645. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.A.; Singh, V.; Saha, S.; Peters, S.; Sadhukhan, T.; Kushwaha, R.; Yadav, A.K.; Mandal, A.; Upadhyay, A.; Bera, A.; et al. Green Light-Triggered Photocatalytic Anticancer Activity of Terpyridine-Based Ru(II) Photocatalysts. Inorg. Chem. 2024, 63, 7493–7503. [Google Scholar] [CrossRef] [PubMed]
- Smoleński, P.; Śliwińska-Hill, U.; Kwiecień, A.; Wolińska, J.; Poradowski, D. Design, Synthesis, and Anti-Cancer Evaluation of Novel Water-Soluble Copper(I) Complexes Bearing Terpyridine and PTA Ligands. Molecules 2024, 29, 945. [Google Scholar] [CrossRef] [PubMed]
- Choroba, K.; Machura, B.; Erfurt, K.; Casimiro, A.R.; Cordeiro, S.; Baptista, P.V.; Fernandes, A.R. Copper(II) Complexes with 2,2′:6′,2″-Terpyridine Derivatives Displaying Dimeric Dichloro−μ–Bridged Crystal Structure: Biological Activities from 2D and 3D Tumor Spheroids to In Vivo Models. J. Med. Chem. 2024, 67, 5813–5836. [Google Scholar] [CrossRef] [PubMed]
- Kröhnke, F. The Specific Synthesis of Pyridines and Oligopyridines. Synthesis 1976, 1976, 1–24. [Google Scholar] [CrossRef]
- Fernández-Terán, R.J.; Sucre-Rosales, E.; Echevarria, L.; Hernández, F.E. Dissecting Conjugation and Electronic Effects on the Linear and Non-Linear Optical Properties of Rhenium(I) Carbonyl Complexes. Phys. Chem. Chem. Phys. 2022, 24, 28069–28079. [Google Scholar] [CrossRef]
- Klemens, T.; Świtlicka-Olszewska, A.; Machura, B.; Grucela, M.; Janeczek, H.; Schab-Balcerzak, E.; Szlapa, A.; Kula, S.; Krompiec, S.; Smolarek, K.; et al. Synthesis, Photophysical Properties and Application in Organic Light Emitting Devices of Rhenium(I) Carbonyls Incorporating Functionalized 2,2′:6′,2″-Terpyridines. RSC Adv. 2016, 6, 56335–56352. [Google Scholar] [CrossRef]
- Klemens, T.; Świtlicka, A.; Szlapa-Kula, A.; Krompiec, S.; Lodowski, P.; Chrobok, A.; Godlewska, M.; Kotowicz, S.; Siwy, M.; Bednarczyk, K.; et al. Experimental and Computational Exploration of Photophysical and Electroluminescent Properties of Modified 2,2′:6′,2″-Terpyridine, 2,6-Di(Thiazol-2-yl)Pyridine and 2,6-Di(Pyrazin-2-yl)Pyridine Ligands and Their Re(I) Complexes. Appl. Organomet. Chem. 2018, 32, e4611. [Google Scholar] [CrossRef]
- Klemens, T.; Świtlicka, A.; Machura, B.; Kula, S.; Krompiec, S.; Łaba, K.; Korzec, M.; Siwy, M.; Janeczek, H.; Schab-Balcerzak, E.; et al. A Family of Solution Processable Ligands and Their Re(I) Complexes towards Light Emitting Applications. Dyes Pigment. 2019, 163, 86–101. [Google Scholar] [CrossRef]
- Klemens, T.; Świtlicka, A.; Szlapa-Kula, A.; Łapok, Ł.; Obłoza, M.; Siwy, M.; Szalkowski, M.; Maćkowski, S.; Libera, M.; Schab-Balcerzak, E.; et al. Tuning Optical Properties of Re(I) Carbonyl Complexes by Modifying Push–Pull Ligands Structure. Organometallics 2019, 38, 4206–4223. [Google Scholar] [CrossRef]
- Maroń, A.M.; Szlapa-Kula, A.; Matussek, M.; Kruszynski, R.; Siwy, M.; Janeczek, H.; Grzelak, J.; Maćkowski, S.; Schab-Balcerzak, E.; Machura, B. Photoluminescence Enhancement of Re(I) Carbonyl Complexes Bearing D–A and D–π–A Ligands. Dalton Trans. 2020, 49, 4441–4453. [Google Scholar] [CrossRef] [PubMed]
- Choroba, K.; Maroń, A.; Świtlicka, A.; Szłapa-Kula, A.; Siwy, M.; Grzelak, J.; Maćkowski, S.; Pedzinski, T.; Schab-Balcerzak, E.; Machura, B. Carbazole Effect on Ground- and Excited-State Properties of Rhenium(I) Carbonyl Complexes with Extended Terpy-like Ligands. Dalton Trans. 2021, 50, 3943–3958. [Google Scholar] [CrossRef] [PubMed]
- Palion-Gazda, J.; Szłapa-Kula, A.; Penkala, M.; Erfurt, K.; Machura, B. Photoinduced Processes in Rhenium(I) Terpyridine Complexes Bearing Remote Amine Groups: New Insights from Transient Absorption Spectroscopy. Molecules 2022, 27, 7147. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Monro, S.; Li, Z.; Jabed, M.A.; Ramirez, D.; Cameron, C.G.; Colón, K.; Roque, J.I.; Kilina, S.; Tian, J.; et al. New Class of Homoleptic and Heteroleptic Bis(Terpyridine) Iridium(III) Complexes with Strong Photodynamic Therapy Effects. ACS Appl. Bio Mater. 2019, 2, 2964–2977. [Google Scholar] [CrossRef] [PubMed]
- Moura, N.M.M.; Castro, K.A.D.F.; Biazzotto, J.C.; Prandini, J.A.; Lodeiro, C.; Faustino, M.A.F.; Simões, M.M.Q.; da Silva, R.S.; Neves, M.G.P.M.S. Ruthenium and Iridium Complexes Bearing Porphyrin Moieties: PDT Efficacy against Resistant Melanoma Cells. Dyes Pigment. 2022, 205, 110501. [Google Scholar] [CrossRef]
- Williams, J.A.G.; Wilkinson, A.J.; Whittle, V.L. Light-Emitting Iridium Complexes with Tridentate Ligands. Dalton Trans. 2008, 2008, 2081–2099. [Google Scholar] [CrossRef]
- Qin, Q.-P.; Meng, T.; Tan, M.-X.; Liu, Y.-C.; Luo, X.-J.; Zou, B.-Q.; Liang, H. Synthesis and in Vitro Biological Evaluation of Three 4′-(4-Methoxyphenyl)-2,2′:6′,2″-Terpyridine Iridium(III) Complexes as New Telomerase Inhibitors. Eur. J. Med. Chem. 2018, 143, 1387–1395. [Google Scholar] [CrossRef]
- Ma, Y.; Shen, L.; She, P.; Hou, Y.; Yu, Y.; Zhao, J.; Liu, S.; Zhao, Q. Constructing Multi-Stimuli-Responsive Luminescent Materials through Outer Sphere Electron Transfer in Ion Pairs. Adv. Opt. Mater. 2019, 7, 1801657. [Google Scholar] [CrossRef]
- Heyns, A.M.; van Schalkwyk, G.J. A Study of the Infrared and Raman Spectra of Ammonium Hexafluorophosphate NH4PF6 over a Wide Range of Temperatures. Spectrochim. Acta Part Mol. Spectrosc. 1973, 29, 1163–1175. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01 2016. Available online: https://gaussian.com/gaussian16/ (accessed on 1 June 2024).
- Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuß, H. Energy-Adjustedab Initio Pseudopotentials for the Second and Third Row Transition Elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Tang, K.-C.; Liu, K.L.; Chen, I.-C. Rapid Intersystem Crossing in Highly Phosphorescent Iridium Complexes. Chem. Phys. Lett. 2004, 386, 437–441. [Google Scholar] [CrossRef]
- Yoshikawa, N.; Yamabe, S.; Kanehisa, N.; Inoue, T.; Takashima, H.; Tsukahara, K. Detailed Description of the Metal-to-Ligand Charge-Transfer State in Monoterpyridine IrIII Complexes. Eur. J. Inorg. Chem. 2009, 2009, 2067–2073. [Google Scholar] [CrossRef]
- Kuang, Z.; Wang, X.; Wang, Z.; He, G.; Guo, Q.; He, L.; Xia, A. Phosphorescent Cationic Iridium(III) Complexes with 1,3,4-Oxadiazole Cyclometalating Ligands: Solvent-Dependent Excited-State Dynamics. Chin. J. Chem. Phys. 2017, 30, 259–267. [Google Scholar] [CrossRef]
- Lo, K.K.-W.; Chung, C.-K.; Ng, D.C.-M.; Zhu, N. Syntheses, Characterisation and Photophysical Studies of Novel Biological Labelling Reagents Derived from Luminescent Iridium(III) Terpyridine Complexes. New J. Chem. 2002, 26, 81–88. [Google Scholar] [CrossRef]
- Ito, W.; Hattori, S.; Kondo, M.; Sakagami, H.; Kobayashi, O.; Ishimoto, T.; Shinozaki, K. Dual Emission from an Iridium(III) Complex/Counter Anion Ion Pair. Dalton Trans. 2021, 50, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Collin, J.-P.; Dixon, I.M.; Sauvage, J.-P.; Williams, J.A.G.; Barigelletti, F.; Flamigni, L. Synthesis and Photophysical Properties of Iridium(III) Bisterpyridine and Its Homologues: A Family of Complexes with a Long-Lived Excited State. J. Am. Chem. Soc. 1999, 121, 5009–5016. [Google Scholar] [CrossRef]
- Arm, K.J.; Leslie, W.; Williams, J.A.G. Synthesis and pH-Sensitive Luminescence of Bis-Terpyridyl Iridium(III) Complexes Incorporating Pendent Pyridyl Groups. Inorganica Chim. Acta 2006, 359, 1222–1232. [Google Scholar] [CrossRef]
- Otaif, H.Y.; Adams, S.J.; Horton, P.N.; Coles, S.J.; Beames, J.M.; Pope, S.J.A. Bis-Cyclometalated Iridium(III) Complexes with Terpyridine Analogues: Syntheses, Structures, Spectroscopy and Computational Studies. RSC Adv. 2021, 11, 39718–39727. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, L.; Mastropietro, T.F.; Ghedini, M.; La Deda, M.; Szerb, E.I. Ionic-Pair Effect on the Phosphorescence of Ionic Iridium(III) Complexes. J. Organomet. Chem. 2014, 772–773, 307–313. [Google Scholar] [CrossRef]
- Guo, S.; Huang, T.; Liu, S.; Zhang, K.Y.; Yang, H.; Han, J.; Zhao, Q.; Huang, W. Luminescent Ion Pairs with Tunable Emission Colors for Light-Emitting Devices and Electrochromic Switches. Chem. Sci. 2016, 8, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Ilic, S.; Cairnie, D.R.; Bridgewater, C.M.; Morris, A.J. Investigation into Dual Emission of a Cyclometalated Iridium Complex: The Role of Ion-Pairing. J. Photochem. Photobiol. 2021, 8, 100084. [Google Scholar] [CrossRef]
- Earley, J.; Zieleniewska, A.; Ripberger, H.; Lazorski, M.; Mast, Z.; Sayre, H.; Knowles, R.; McCusker, J.; Scholes, G.; Reid, O.; et al. Dipole Moment and Charge Reorganization in Photoredox Catalysts. ChemRxiv 2021. [CrossRef]
- Leslie, W.; Batsanov, A.S.; Howard, J.A.K.; Williams, J.A.G. Cross-Couplings in the Elaboration of Luminescent Bis-Terpyridyl Iridium Complexes: The Effect of Extended or Inhibited Conjugation on Emission. Dalton Trans. 2004, 2004, 623–631. [Google Scholar] [CrossRef]
- Leslie, W.; Poole, R.A.; Murray, P.R.; Yellowlees, L.J.; Beeby, A.; Williams, J.A.G. Near Infra-Red Luminescence from Bis-Terpyridyl Iridium(III) Complexes Incorporating Electron-Rich Pendants. Polyhedron 2004, 23, 2769–2777. [Google Scholar] [CrossRef]
- Flamigni, L.; Ventura, B.; Barigelletti, F.; Baranoff, E.; Collin, J.-P.; Sauvage, J.-P. Luminescent Iridium(III)-Terpyridine Complexes—Interplay of Ligand Centred and Charge Transfer States. Eur. J. Inorg. Chem. 2005, 2005, 1312–1318. [Google Scholar] [CrossRef]
- Goldstein, D.C.; Cheng, Y.Y.; Schmidt, T.W.; Bhadbhade, M.; Thordarson, P. Photophysical Properties of a New Series of Water Soluble Iridium Bisterpyridine Complexes Functionalised at the 4′ Position. Dalton Trans. 2011, 40, 2053–2061. [Google Scholar] [CrossRef]
- Xiang, H.; Cheng, J.; Ma, X.; Zhou, X.; Chruma, J.J. Near-Infrared Phosphorescence: Materials and Applications. Chem. Soc. Rev. 2013, 42, 6128–6185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qiao, J. Near-Infrared Emitting Iridium Complexes: Molecular Design, Photophysical Properties, and Related Applications. iScience 2021, 24, 102858. [Google Scholar] [CrossRef] [PubMed]
- Chelushkin, P.S.; Shakirova, J.R.; Kritchenkov, I.S.; Baigildin, V.A.; Tunik, S.P. Phosphorescent NIR Emitters for Biomedicine: Applications, Advances and Challenges. Dalton Trans. 2022, 51, 1257–1280. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lystrom, L.; Brown, S.L.; Hobbie, E.K.; Kilina, S.; Sun, W. Impact of Benzannulation Site at the Diimine (N^N) Ligand on the Excited-State Properties and Reverse Saturable Absorption of Biscyclometalated Iridium(III) Complexes. Inorg. Chem. 2019, 58, 5483–5493. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Lu, C.; Cui, P.; Kilina, S.; Sun, W. Impacts of Extending the π-Conjugation of the 2,2′-Biquinoline Ligand on the Photophysics and Reverse Saturable Absorption of Heteroleptic Cationic Iridium(III) Complexes. J. Mater. Chem. C 2021, 9, 15932–15941. [Google Scholar] [CrossRef]
- Lu, J.; Pan, Q.; Zhu, S.; Liu, R.; Zhu, H. Ligand-Mediated Photophysics Adjustability in Bis-Tridentate Ir(III) Complexes and Their Application in Efficient Optical Limiting Materials. Inorg. Chem. 2021, 60, 12835–12846. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Jiang, Z.; Tang, M.; Jiang, X.; Tu, H.; Zhu, S.; Liu, R.; Zhu, H. Synthesis, Photophysics and Tunable Reverse Saturable Absorption of Bis-Tridentate Iridium(III) Complexes via Modification on Diimine Ligand. Molecules 2023, 28, 566. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wu, L.-Z.; Si, G.; Pan, J.; Yang, Q.-Z.; Zhang, L.-P.; Tung, C.-H. Switching between Ligand-to-Ligand Charge-Transfer, Intraligand Charge-Transfer, and Metal-to-Ligand Charge-Transfer Excited States in Platinum(II) Terpyridyl Acetylide Complexes Induced by pH Change and Metal Ions. Chem. Eur. J. 2007, 13, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Choroba, K.; Kotowicz, S.; Maroń, A.; Świtlicka, A.; Szłapa-Kula, A.; Siwy, M.; Grzelak, J.; Sulowska, K.; Maćkowski, S.; Schab-Balcerzak, E.; et al. Ground- and Excited-State Properties of Re(I) Carbonyl Complexes—Effect of Triimine Ligand Core and Appended Heteroaromatic Groups. Dyes Pigment. 2021, 192, 109472. [Google Scholar] [CrossRef]
- Palion-Gazda, J.; Machura, B.; Klemens, T.; Szlapa-Kula, A.; Krompiec, S.; Siwy, M.; Janeczek, H.; Schab-Balcerzak, E.; Grzelak, J.; Maćkowski, S. Structure-Dependent and Environment-Responsive Optical Properties of the Trisheterocyclic Systems with Electron Donating Amino Groups. Dyes Pigment. 2019, 166, 283–300. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Ernzerhof, M.; Scuseria, G.E. Assessment of the Perdew–Burke–Ernzerhof Exchange-Correlation Functional. J. Chem. Phys. 1999, 110, 5029–5036. [Google Scholar] [CrossRef]
- Martin, J.M.L.; Sundermann, A. Correlation Consistent Valence Basis Sets for Use with the Stuttgart–Dresden–Bonn Relativistic Effective Core Potentials: The Atoms Ga–Kr and In–Xe. J. Chem. Phys. 2001, 114, 3408–3420. [Google Scholar] [CrossRef]
- Pritchard, B.P.; Altarawy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. New Basis Set Exchange: An Open, Up-to-Date Resource for the Molecular Sciences Community. J. Chem. Inf. Model. 2019, 59, 4814–4820. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- CrysAlis PRO 2011. Available online: https://rigaku.com/ja/products/crystallography/x-ray-diffraction/crysalispro (accessed on 1 June 2024).
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Szlapa-Kula, A.; Małecka, M.; Maroń, A.M.; Janeczek, H.; Siwy, M.; Schab-Balcerzak, E.; Szalkowski, M.; Maćkowski, S.; Pedzinski, T.; Erfurt, K.; et al. In-Depth Studies of Ground- and Excited-State Properties of Re(I) Carbonyl Complexes Bearing 2,2′:6′,2″-Terpyridine and 2,6-Bis(Pyrazin-2-Yl)Pyridine Coupled with π-Conjugated Aryl Chromophores. Inorg. Chem. 2021, 60, 18726–18738. [Google Scholar] [CrossRef]
- Małecka, M.; Szlapa-Kula, A.; Maroń, A.M.; Ledwon, P.; Siwy, M.; Schab-Balcerzak, E.; Sulowska, K.; Maćkowski, S.; Erfurt, K.; Machura, B. Impact of the Anthryl Linking Mode on the Photophysics and Excited-State Dynamics of Re(I) Complexes [ReCl(CO)3(4′-An-Terpy-κ2N)]. Inorg. Chem. 2022, 61, 15070–15084. [Google Scholar] [CrossRef]
- van Wilderen, L.J.G.W.; Lincoln, C.N.; van Thor, J.J. Modelling Multi-Pulse Population Dynamics from Ultrafast Spectroscopy. PLoS ONE 2011, 6, e17373. [Google Scholar] [CrossRef]

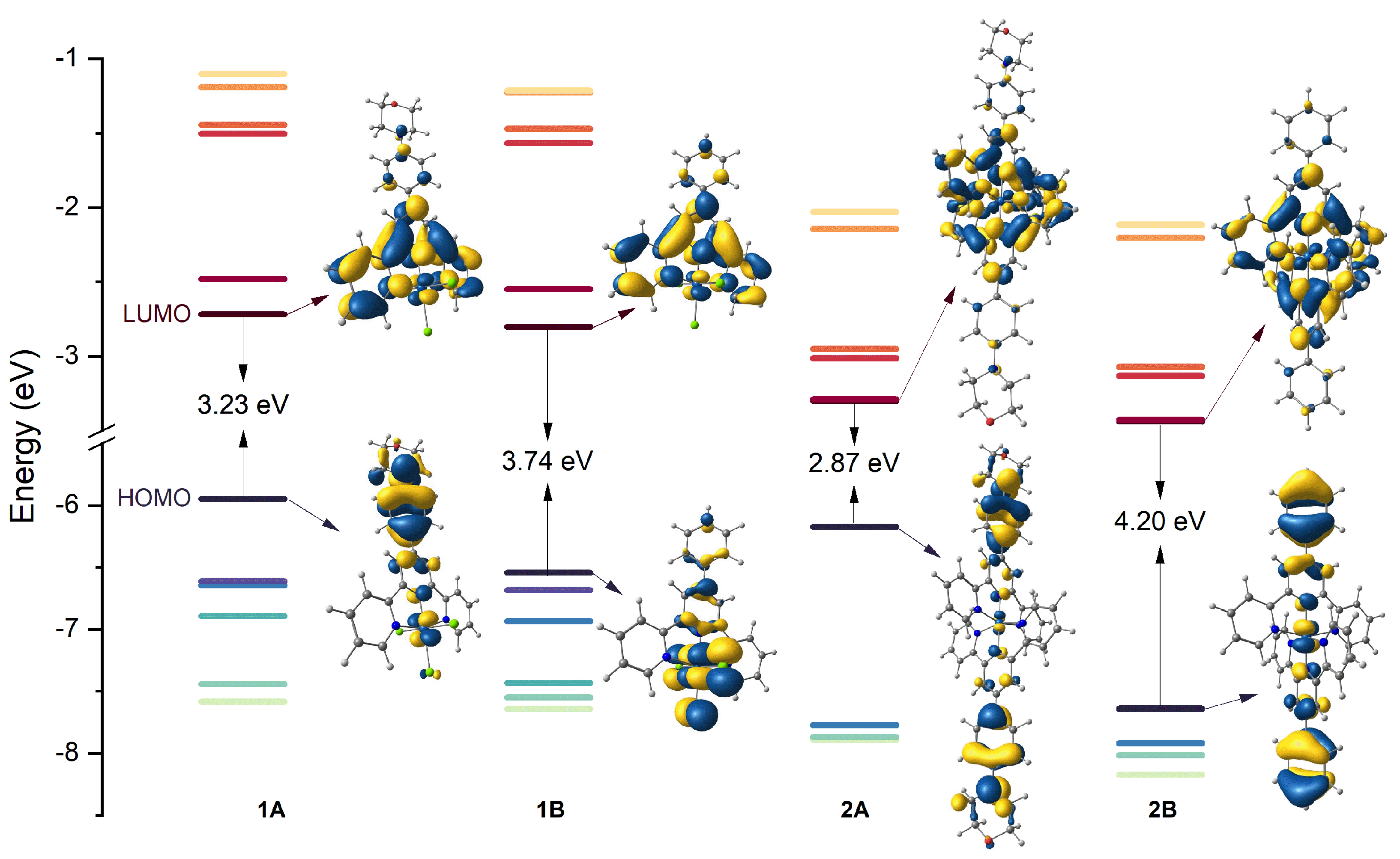



| 1A | 1B | 2B | |
|---|---|---|---|
| Bond length [Å] | |||
| Ir(1)–Cl(1) | 2.372(1) | 2.375(2) | |
| Ir(1)–Cl(2) | 2.361(2) | 2.347(2) | |
| Ir(1)–Cl(3) | 2.363(1) | 2.362(2) | |
| Ir(1)–N(1) | 1.935(4) | 1.938(5) | 1.974(3) |
| Ir(1)–N(2) | 2.046(4) | 2.040(5) | 2.051(3) |
| Ir(1)–N(3) | 2.028(4) | 2.045(5) | 2.056(3) |
| Bond angle [°] | |||
| Cl(1)–Ir(1)–Cl(2) | 92.55(60) | 90.03(60) | |
| Cl(1)–Ir(1)–Cl(3) | 91.40(60) | 91.29 (60) | |
| Cl(2)–Ir(1)–Cl(3) | 175.67(50) | 178.43(60) | |
| Cl(1)–Ir(1)–N(1) | 179.39(13) | 177.15(14) | |
| Cl(1)–Ir(1)–N(2) | 98.69(12) | 99.80(15) | |
| Cl(1)–Ir(1)–N(3) | 99.39(12) | 99.00(14) | |
| Cl(2)–Ir(1)–N(1) | 86.93(12) | 92.80(14) | |
| Cl(2)–Ir(1)–N(2) | 91.46(12) | 90.56(14) | |
| Cl(2)–Ir(1)–N(3) | 87.92(12) | 88.66(15) | |
| Cl(3)–Ir(1)–N(1) | 89.13(12) | 85.88(14) | |
| Cl(3)–Ir(1)–N(2) | 89.69(12) | 88.37(14) | |
| Cl(3)–Ir(1)–N(3) | 89.70(13) | 91.98(15) | |
| N(1)–Ir(1)–N(1a) | 177.47(16) | ||
| N(1)–Ir(1)–N(2) | 80.99(16) | 80.53(19) | 79.82 (11) |
| N(1)–Ir(1)–N(2a) | 98.43(11) | ||
| N(1)–Ir(1)–N(3) | 80.93(16) | 80.73(19) | 79.90(11) |
| N(1)–Ir(1)–N(3a) | 101.88(11) | ||
| N(2)–Ir(1)–N(2a) | 93.62(17) | ||
| N(2)–Ir(1)–N(3) | 161.92(16) | 161.18(19) | 159.70(11) |
| N(2)–Ir(1)–N(3a) | 90.27(12) | ||
| N(3)–Ir(1)–N(3a) | 92.97(17) |
| Medium | Compound | λex a [nm] | λem b [nm] | τav c [μs] | Φ d [%] | Compound | λex [nm] | λem [nm] | τav [μs] | Φ [%] |
|---|---|---|---|---|---|---|---|---|---|---|
| 77 K | 1A | 520 | 639 | 10.90 | – | 1B | 520 | 565 | 12.43 | – |
| DMSO | 440/520 | 670/650 | 1.22 | 3.74 | 440/520 | 600 | 1.75 | 37.80 | ||
| MeCN | 440/520 | 611/614 | 1.45 | 1.63 | 440/520 | 604 | 1.96 | 5.86 | ||
| solid | 530 | 710 | 0.21 | 4.38 | 525 | 745 | 0.21 | 2.48 | ||
| 77 K | 2A | 465 | 632 | 90.45 | – | 2B | 370 | 512 | 40.08 | – |
| DMSO | 465/555 | 720/750 | 0.10 | 7.40 | 375/520 | 567/578 | 4.46 | 4.22 | ||
| MeCN | 465/555 | 765/780 | 0.07 | 0.16 | 375/520 | 522/580 | 6.50 | 10.18 | ||
| solid | 500 | 735 | 0.58 | <0.05 | 500 | 600 | 10.30 | 4.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palion-Gazda, J.; Kwiecień, A.; Choroba, K.; Penkala, M.; Kryczka, A.; Machura, B. The Role of Intraligand Charge Transfer Processes in Iridium(III) Complexes with Morpholine-Decorated 4′-Phenyl-2,2′:6′,2″-terpyridine. Molecules 2024, 29, 3074. https://doi.org/10.3390/molecules29133074
Palion-Gazda J, Kwiecień A, Choroba K, Penkala M, Kryczka A, Machura B. The Role of Intraligand Charge Transfer Processes in Iridium(III) Complexes with Morpholine-Decorated 4′-Phenyl-2,2′:6′,2″-terpyridine. Molecules. 2024; 29(13):3074. https://doi.org/10.3390/molecules29133074
Chicago/Turabian StylePalion-Gazda, Joanna, Aleksandra Kwiecień, Katarzyna Choroba, Mateusz Penkala, Anna Kryczka, and Barbara Machura. 2024. "The Role of Intraligand Charge Transfer Processes in Iridium(III) Complexes with Morpholine-Decorated 4′-Phenyl-2,2′:6′,2″-terpyridine" Molecules 29, no. 13: 3074. https://doi.org/10.3390/molecules29133074






