Biological Activities of Novel Oleanolic Acid Derivatives from Bioconversion and Semi-Synthesis
Abstract
:1. Introduction
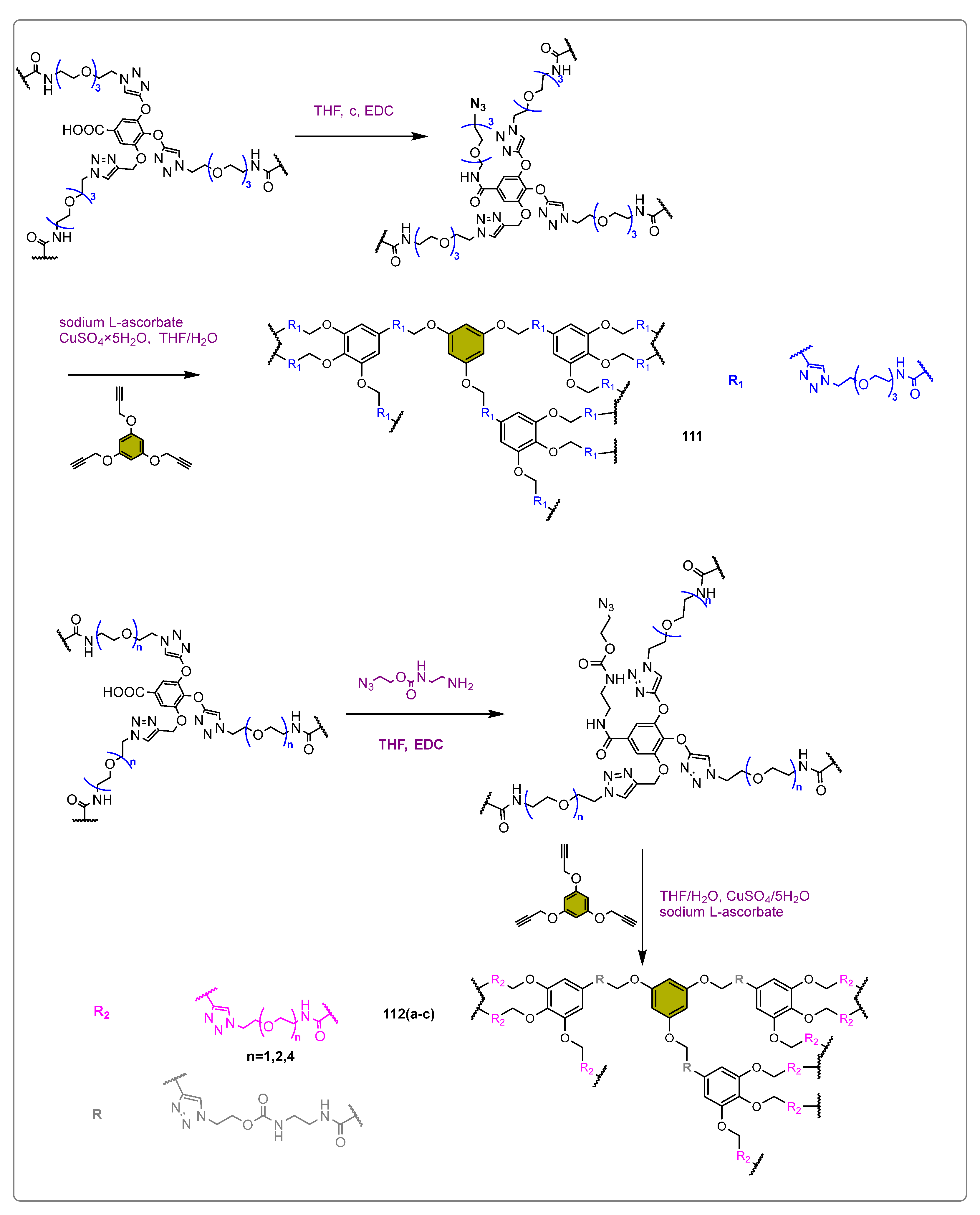
2. Enzymatic Production of Oleanolic Acid Derivatives
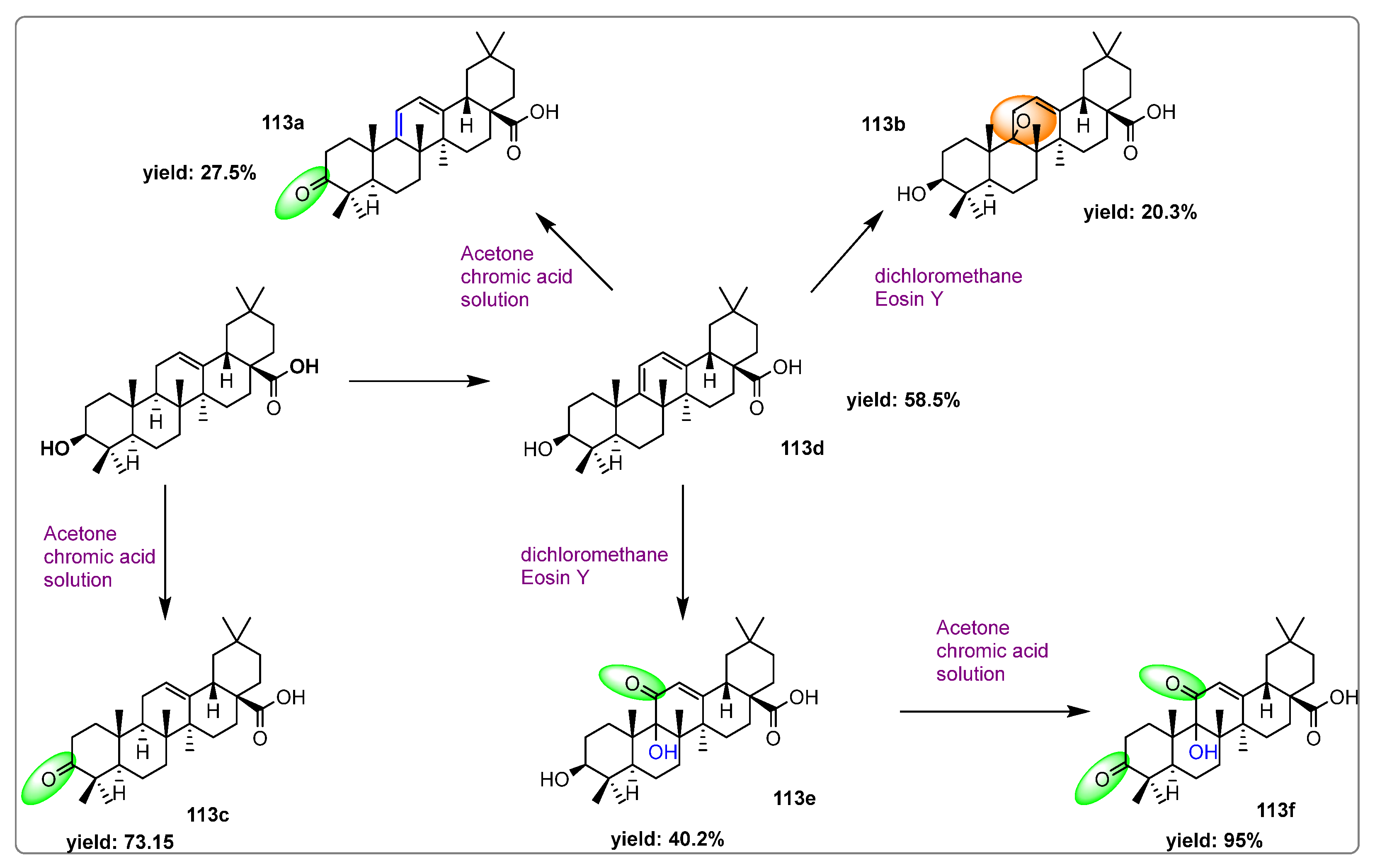
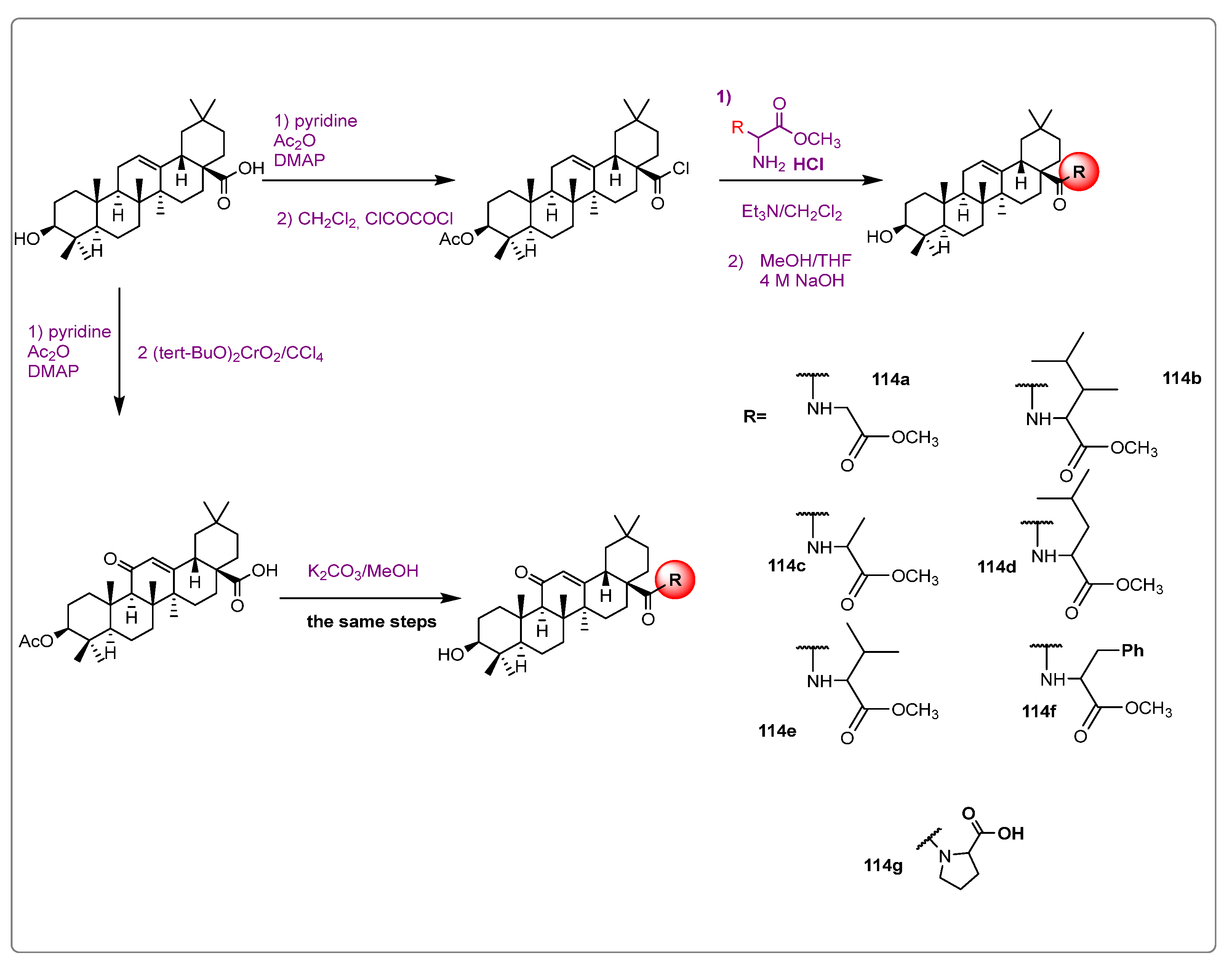
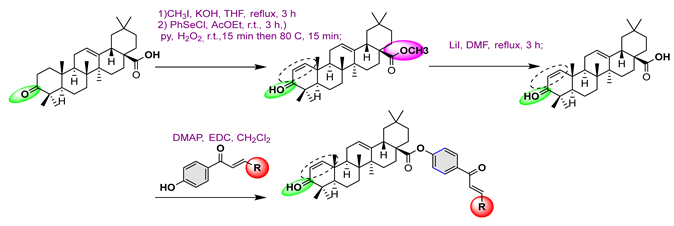
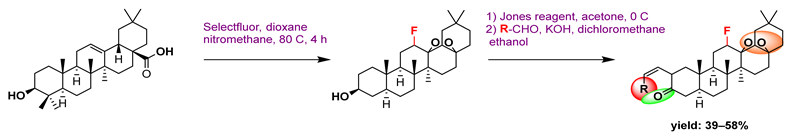
3. Semi-Synthesis of OA and Biological Activities of Its Derivatives
3.1. Anti-Cancer Activity
3.2. Anti-Diabetic Activity
3.3. Anti-Inflammatory Activity
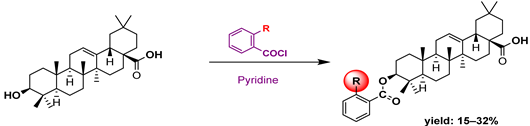
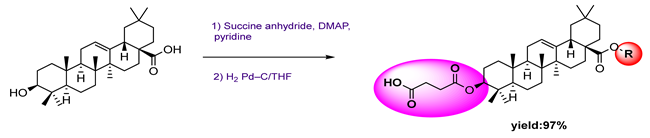
3.4. Antimicrobial Activity
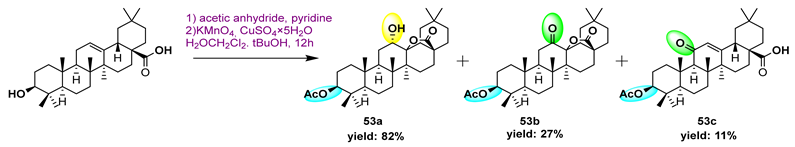 | |||||||
| SA MIC/MBC | EC MIC/MBC | PA MIC/MBC | ST MIC/MBC | SE MIC/MBC | MS MIC/MBC | Reference | |
53a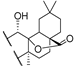 | >188/- | >188/- | >/- | 126/583 | - | - | [101] |
53b 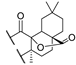 | 175/1756 | 175/1756 | >189/- | 189/585 | - | - | |
53c 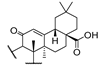 | 175/975 | 175/1756 | 189/1756 | 175/1756 | - | - | |
 | |||||||
53d 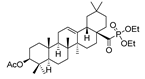 | >165/- | >165/- | >165/- | >165 - | - | - | [101] |
53e 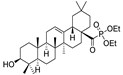 | 156/1285 | 156/1653 | 165/1653 | 165/1653 | - | - | |
 | |||||||
53f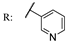 | 156/1566 | 156/1566 | 156/522 | 156/1218 | - | - | [101] |
53g 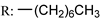 | - | - | - | - | - | - | |
53h  | - | - | - | - | - | - | |
53i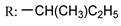 | >175/- | >175/- | >175/- | >175/- | - | - | |
53j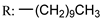 | >152/- | >152/- | >152/- | >152/- | - | - | |
 | |||||||
53k  | - | - | - | - | - | - | |
53l 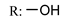 | - | - | - | - | - | - | |
53m 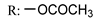 | - | - | - | - | - | - | |
 | [105] | ||||||
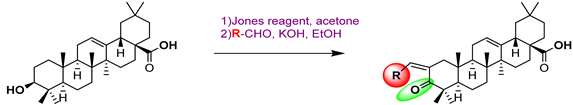
54a 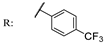 | - | - | - | - | >200/- | >200/- | |
54b 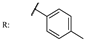 | - | - | - | - | >200/- | >200/- | |
| Gatifloxacin | ND | ND | |||||
 | |||||||||
| Gram-Positive Bacteria | Gram-Negative Bacteria | Reference | |||||||
| SA | EF | EC | PA | [102] | |||||
| R | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
55a 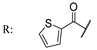 | 26 | 52 | 8 | 44 | 8 | 26 | 8 | 26 | |
55b 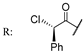 | 32 | 164 | 24 | 41 | 8 | 41 | 8 | 49 | |
55c 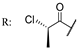 | 27 | 45 | 9 | 45 | 9 | 45 | 27 | 45 | |
55d | 30 | 52 | 30 | 52 | 8 | 1.76 | 8 | 88 | |
55e  | - | - | - | - | - | - | - | - | |
 | [104] | ||||||||
56a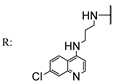 | 3.71 | - | 1.85 | - | 1.85 | - | 3.71 | - | |
56b 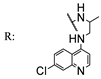 | 3.71 | - | 3.71 | - | 3.71 | - | 3.71 | - | |
56c 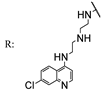 | 1.77 | - | 1.77 | - | 1.77 | - | 3.55 | - | |
56d 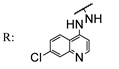 | 3.95 | - | 1.97 | - | 1.97 | - | 3.95 | - | |
 | |||||||||
56e | 1.89 | - | 1.89 | - | 3.78 | - | 3.78 | - | |
56f | 3.54 | - | 1.77 | - | 1.77 | - | 3.54 | - | |
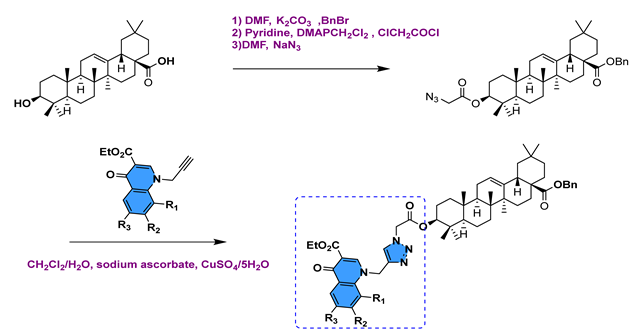 | [107] | ||||||||
57a  | 217 | - | 54 | - | 217 | - | 108 | - | |
57b 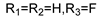 | 138 | - | 33.51 | - | 69.25 | - | 69.25 | - | |
57c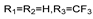 | 65.61 | - | 32.80 | - | 65.61 | - | 65.61 | - | |
57d | 258 | - | 32.26 | - | 129 | - | 32.26 | - | |
57e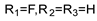 | 34.62 | - | 69.25 | - | 34.62 | - | 69.25 | - | |
57f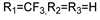 | 131.23 | - | 65.61 | - | 131.23 | - | 65.61 | - | |
57g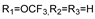 | 64.53 | - | 32.26 | - | 64.53 | - | 64.53 | - | |
57h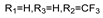 | 65.61 | - | 65.61 | - | 65.61 | -- | 65.61 | - | |
57i | 254 | - | 31.87 | - | 127 | - | 31.87 | - | |
57j | 243 | - | 30.38 | - | 121 | - | 30.38 | - | |
57k 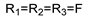 | 133 | - | 33.29 | - | 66.59 | -- | 66.59 | - | |
3.5. Anti-Influenza Activity
3.6. Hepatitis Activity
3.7. Osteoporosis Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OA | Oleanolic acid |
| TCM | Traditional Chinese Medicine |
| WHO | World Health Organization |
| GPa | Rabbit muscle glycogen phosphorylase a |
| PTP1B | Protein tyrosine phosphatase 1B |
| LPS | Lipopolysaccharide |
| NOS | Nitric oxide synthase |
| NO | Nitric oxide |
| PBMCs | Human peripheral blood mononuclear cells |
| ASP | Aspirin |
| NF-κB | Nuclear factor-κB |
| COX-2 | Cyclooxygenase-2 |
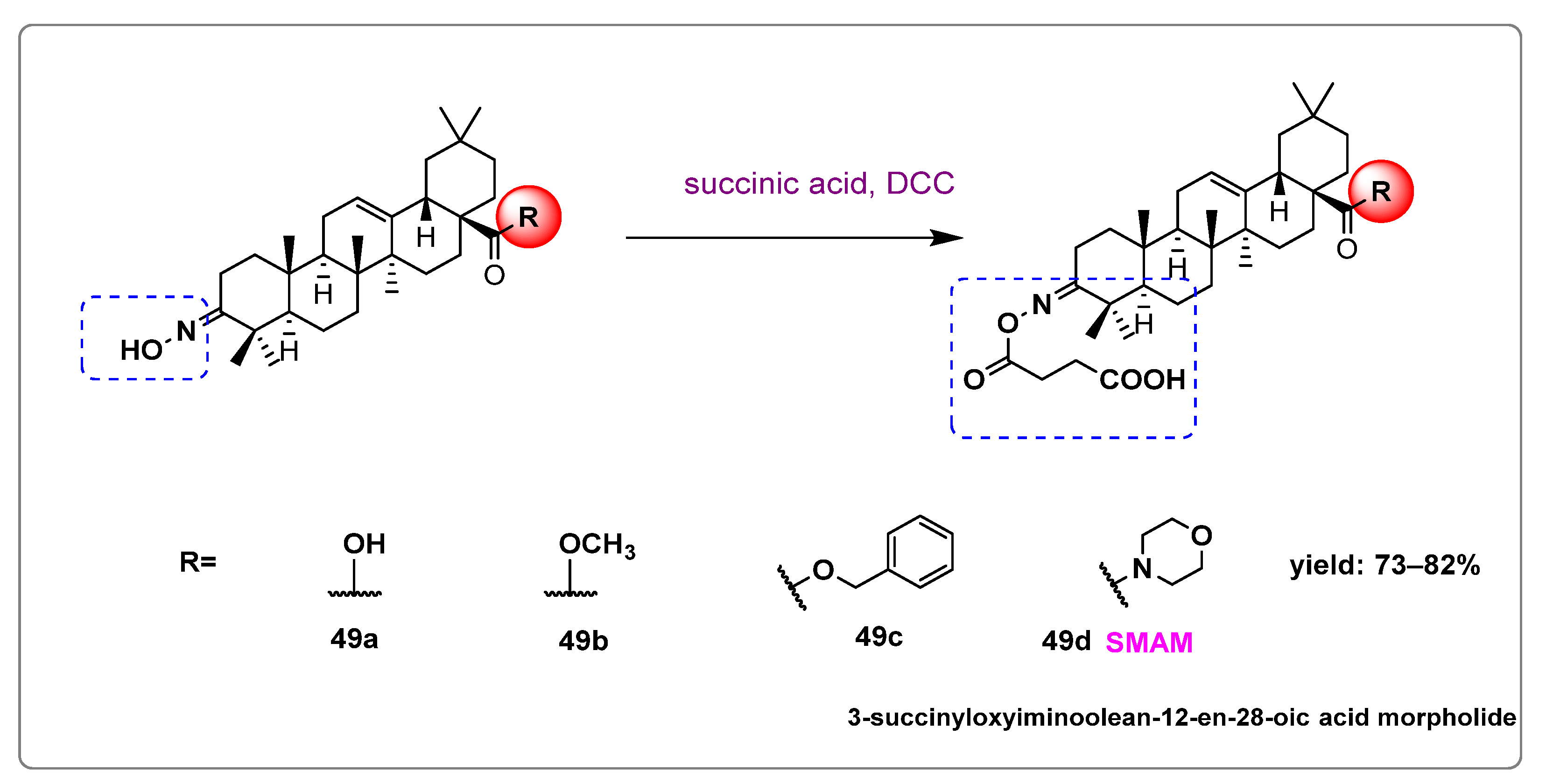
| SMAM | 3-succinyloxyiminoolean-12-en-28-oic acid morpholide |
| TNF-α | Tumor necrosis factor α |
| IL-6 | Interleukin-6 |
| iNOS | Inducible nitric oxide synthase |
| HO-1 | Heme oxygenase-1 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
References
- Che, C.T.; Zhang, H. Plant Natural Products for Human Health. Int. J. Mol. Sci. 2019, 20, 830. [Google Scholar] [CrossRef] [PubMed]
- Namdeo, P.; Gidwani, B.; Tiwari, S.; Jain, V.; Joshi, V.; Shukla, S.S.; Pandey, R.K.; Vyas, A. Therapeutic Potential and Novel Formulations of Ursolic Acid and Its Derivatives: An Updated Review. J. Sci. Food Agric. 2023, 103, 4275–4292. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, K.; Wang, G.; Liu, H.; Shao, L.; Zhou, X.; Liu, L.; Yang, S. Discovery of Novel Pentacyclic Triterpene Acid Amide Derivatives as Excellent Antimicrobial Agents Dependent on Generation of Reactive Oxygen Species. Int. J. Mol. Sci. 2023, 24, 10566. [Google Scholar] [CrossRef] [PubMed]
- Sinda, P.V.K.; Tchuenguem, R.T.; Ponou, B.K.; Kühlborn, J.; Kianfé, B.Y.; Dzoyem, J.P.; Teponno, R.B.; Opatz, T.; Barboni, L.; Tapondjou, L.A. Antimicrobial Activities of Extract, Fractions and Compounds from the Medicinal Plant Helichrysum odoratissimun (L.) Sweet (Asteraceae). S. Afr. J. Bot. 2022, 147, 937–941. [Google Scholar] [CrossRef]
- Martial, D.E.; Dimitry, M.Y.; Selestin, S.D.; Nicolas, N.Y. Hypolipidemic and Antioxidant Activity of Aqueous Extract of Clerodendrum thomsoniae Linn. (Verbenaceae) Leaves in Albino Rats, Rattus norvegicus (Muridae). J. Pharmacogn. Phytochem. 2020, 9, 595–602. [Google Scholar]
- Saleem, M.; Nazir, M.; Ali, M.S.; Hussain, H.; Lee, Y.S.; Riaz, N.; Jabbar, A. Antimicrobial Natural Products: An Update on Future Antibiotic Drug Candidates. Nat. Prod. Rep. 2010, 27, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Antiviral Activities of Oleanolic Acid and Its Analogues. Molecules 2018, 23, 2300. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Ahmad, N.; Naqvi, A.A.; Shehzad, A.; Al-Ghamdi, M.S. Role of Traditional Islamic and Arabic Plants in Cancer Therapy. J. Tradit. Complement. Med. 2017, 7, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Li, J.; Lai, X.; Zhang, M.; Kuang, Y.; Bao, Y.-O.; Yu, R.; Hong, W.; Muturi, E.; Xue, H. Natural Triterpenoids from Licorice Potently Inhibit SARS-CoV-2 Infection. J. Adv. Res. 2022, 36, 201–210. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Sinou, V.; Babkov, D.A.; Kazakova, O.; Brunel, J.M. Development of New Antimicrobial Oleanonic Acid Polyamine Conjugates. Antibiotics 2022, 11, 94. [Google Scholar] [CrossRef]
- Gudoityte, E.; Arandarcikaite, O.; Mazeikiene, I.; Bendokas, V.; Liobikas, J. Ursolic and Oleanolic Acids: Plant Metabolites with Neuroprotective Potential. Int. J. Mol. Sci. 2021, 22, 4599. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, L.; Szakiel, A.; Glowacka, A.; Rozpara, E.; Marszalek, K.; Skąpska, S. Triterpenoids of Three Apple Cultivars—Biosynthesis, Antioxidative and Anti-Inflammatory Properties, and Fate during Processing. Molecules 2023, 28, 2584. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2013, 30, 1028–1065. [Google Scholar] [CrossRef]
- Baev, D.S.; Blokhin, M.E.; Chirkova, V.Y.; Belenkaya, S.V.; Luzina, O.A.; Yarovaya, O.I.; Salakhutdinov, N.F.; Shcherbakov, D.N. Triterpenic Acid Amides as Potential Inhibitors of the SARS-CoV-2 Main Protease. Molecules 2022, 28, 303. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2017, 34, 90–122. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhou, Q.; Zhou, L.; Sun, Y.; Han, X.; Cheng, X.; Wu, M.; Lv, W.; Wang, J.; Zhao, W. Quinoa (Chenopodium quinoa Willd) Bran Saponins Alleviate Hyperuricemia and Inhibit Renal Injury by Regulating the PI3K/AKT/NFκB Signaling Pathway and Uric Acid Transport. J. Agric. Food Chem. 2023, 71, 6635–6649. [Google Scholar] [CrossRef] [PubMed]
- Mioc, M.; Mioc, A.; Prodea, A.; Milan, A.; Balan-Porcarasu, M.; Racoviceanu, R.; Ghiulai, R.; Iovanescu, G.; Macasoi, I.; Draghici, G. Novel Triterpenic Acid—Benzotriazole Esters Act as Pro-Apoptotic Antimelanoma Agents. Int. J. Mol. Sci. 2022, 23, 9992. [Google Scholar] [CrossRef]
- Ghante, M.H.; Jamkhande, P.G. Role of Pentacyclic Triterpenoids in Chemoprevention and Anticancer Treatment: An Overview on Targets and Underling Mechanisms. J. Pharmacopunct. 2019, 22, 55. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Sun, W.; Peng, W.; Yu, R.; Li, G.; Jiang, T. Pharmacokinetics in Vitro and in Vivo of Two Novel Prodrugs of Oleanolic Acid in Rats and Its Hepatoprotective Effects against Liver Injury Induced by CCl4. Mol. Pharm. 2016, 13, 1699–1710. [Google Scholar] [CrossRef]
- Pollier, J.; Goossens, A. Oleanolic acid. Phytochemistry 2012, 77, 10–15. [Google Scholar] [CrossRef]
- Yu, J.; Cen, X.; Chen, G.; Tang, M.; Mo, L.; Li, J. iTRAQ-Based Quantitative Proteomic Analysis in Liver of Pomacea canaliculata Induced by Oleanolic Acid Stress. Pest Manag. Sci. 2022, 78, 3467–3478. [Google Scholar] [CrossRef]
- Tang, Z.Y.; Li, Y.; Tang, Y.T.; Ma, X.D.; Tang, Z.Y. Anticancer Activity of Oleanolic Acid and Its Derivatives: Recent Advances in Evidence, Target Profiling and Mechanisms of Action. Biomed. Pharmacother. 2022, 145, 112397. [Google Scholar] [CrossRef]
- Dale, M.P.; Moses, T.; Johnston, E.J.; Rosser, S.J. A Systematic Comparison of Triterpenoid Biosynthetic Enzymes for the Production of Oleanolic Acid in Saccharomyces cerevisiae. PLoS ONE 2020, 15, e0231980. [Google Scholar] [CrossRef]
- Sapkota, A.; Choi, J.W. Oleanolic Acid Provides Neuroprotection against Ischemic Stroke through the Inhibition of Microglial Activation and NLRP3 Inflammasome Activation. Biomol. Ther. 2022, 30, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Sen, A. Prophylactic and Therapeutic Roles of Oleanolic Acid and Its Derivatives in Several Diseases. WJCC 2020, 8, 1767–1792. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.; Su, C.; Huang, J.; Liu, J.; Chen, Z.; Cheng, K. Synthesis of Acyl Oleanolic Acid-Uracil Conjugates and Their Anti-Tumor Activity. Chem. Cent. J. 2016, 10, 69. [Google Scholar] [CrossRef]
- Sun, X.; Xue, S.; Cui, Y.; Li, M.; Chen, S.; Yue, J.; Gao, Z. Characterization and Identification of Chemical Constituents in Corni fructus and Effect of Storage Using UHPLC-LTQ-Orbitrap-MS. Food Res. Int. 2023, 164, 112330. [Google Scholar] [CrossRef]
- Reguigui, A.; Ott, P.G.; Darcsi, A.; Bakonyi, J.; Romdhane, M.; Móricz, Á.M. Nine-Dimensional Bioprofiles of Tunisian Sages (Salvia officinalis, S. aegyptiaca and S. verbenaca) by High-Performance Thin-Layer Chromatography–Effect-Directed Analyses. J. Chromatogr. A 2023, 1688, 463704. [Google Scholar] [CrossRef]
- Kabbash, E.M.; Abdel-Shakour, Z.T.; El-Ahmady, S.H.; Wink, M.; Ayoub, I.M. Comparative Metabolic Profiling of Olive Leaf Extracts from Twelve Different Cultivars Collected in Both Fruiting and Flowering Seasons. Sci. Rep. 2023, 13, 612. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Cecere, M.; Argyropoulou, A.; Hellwig, E.; Skaltsounis, A.L.; Wittmer, A.; Tchorz, J.P.; Al-Ahmad, A. Compounds from Olea europaea and Pistacia lentiscus Inhibit Oral Microbial Growth. BMC Complement. Altern. Med. 2019, 19, 51. [Google Scholar] [CrossRef] [PubMed]
- Odun-Ayo, F.; Chetty, K.; Reddy, L. Determination of the Ursolic and Oleanolic Acids Content with the Antioxidant Capacity in Apple Peel Extract of Various Cultivars. Braz. J. Biol. 2022, 82, e258442. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, M.; Dopierała, K.; Prochaska, K. The Biomimetic System of Oleanolic Acid and Oleic Acid at the Air-Water Interface–Interactions in Terms of Nanotechnology-Based Drug Delivery Systems. Membranes 2022, 12, 1215. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Dai, W.; Xia, M.; Guo, W.; Zhao, Y.; Zhang, S.; Gao, W.; You, X. Expression of PnSS Promotes Squalene and Oleanolic Acid (OA) Accumulation in Aralia elata via Methyl Jasmonate (MeJA) Induction. Genes 2023, 14, 1132. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Ata, A. Oleanolic Acid and Related Derivatives as Medicinally Important Compounds. J. Enzym. Inhib. Med. Chem. 2008, 23, 739–756. [Google Scholar] [CrossRef] [PubMed]
- Marquina, S.; Maldonado, N.; Garduño-Ramírez, M.L.; Aranda, E.; Villarreal, M.L.; Navarro, V.; Bye, R.; Delgado, G.; Alvarez, L. Bioactive oleanolic acid saponins and other constituents from the roots of Viguiera decurrens. Phytochemistry 2001, 56, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Muthoni, D.K.; Samarakoon, S.R.; Piyathilaka, P.C.; Rajagopalan, U.; Tennekoon, K.H.; Ediriweera, M.K. Identification of 3-O-α-l-arabinosyl oleanolic acid, a triterpenoid saponin, as a new breast cancer stem cell growth inhibitor. Nat. Prod. Res. 2022, 36, 2923–2926. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, M.; Duan, L.; Wang, W.; Zhang, J.; Wang, D.; Liang, X. Efficient Synthesis and Anti-Fungal Activity of Oleanolic Acid Oxime Esters. Molecules 2013, 18, 3615–3629. [Google Scholar] [CrossRef] [PubMed]
- Vite, M.H.; Sonawane, M.I.; Vayal, P.M. Oleanolic Acid as a Potential Drug Molecule: A Review. World J. Adv. Res. Rev. 2023, 17, 249–254. [Google Scholar] [CrossRef]
- Rahman, S.; Islam, R.; Kamruzzaman, M.; Alam, K.; Jamal, A.H.M. Ocimum sanctum L.: A review of phytochemical and pharmacological profile. Am. J. Drug Discov. Dev. 2011, 1, 1–15. [Google Scholar] [CrossRef]
- Eid, A.; Jaradat, N.; Elmarzugi, N. A Review of chemical constituents and traditional usage of Neem plant (Azadirachta indica). Palest. Med. Pharm. J. 2017, 2, 3. [Google Scholar] [CrossRef]
- Akram, M.; Asif, M.; Naveed, A.; Shah, P.A.; Uzair, M.; Shaheen, G.; Shamim, T.; Shah, S.M.A.; Ahmad, K. Tribulus terrestris Linn.: A review article. J. Med. Plants Res. 2011, 5, 3601–3605. [Google Scholar]
- Long, C.; Yang, J.; Yang, H.; Li, X.; Wang, G. Attenuation of Renal Ischemia/Reperfusion Injury by Oleanolic Acid Preconditioning via Its Antioxidant, Anti-inflammatory, and Anti-apoptotic Activities. Mol. Med. Rep. 2016, 13, 4697–4704. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, X.; Liu, R.; Chen, H.; Bai, H.; Liang, X.; Zhang, X.; Wang, Z.; Li, W.; Hai, C. Antioxidant Activities of Oleanolic Acid in Vitro: Possible Role of Nrf2 and MAP Kinases. Chem. Biol. Interact. 2010, 184, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, B.; Maurya, R.; Siddiqui, J.A.; Bid, H.K.; Rajendran, S.M.; Yadav, P.P.; Konwar, R. In Vitro Anti-Breast Cancer Activity of Ethanolic Extract of Wrightia tomentosa: Role of pro-Apoptotic Effects of Oleanolic Acid and Urosolic Acid. J. Ethnopharmacol. 2012, 142, 72–79. [Google Scholar] [CrossRef]
- Fernández-Aparicio, Á.; Correa-Rodríguez, M.; Castellano, J.M.; Schmidt-RioValle, J.; Perona, J.S.; González-Jiménez, E. Potential Molecular Targets of Oleanolic Acid in Insulin Resistance and Underlying Oxidative Stress: A Systematic Review. Antioxidants 2022, 11, 1517. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudieh, M.; Naghavi, M.R.; Sobri, Z.M.; Azzeme, A.M.; Abd-Aziz, N.; Rahman, N.M.A.N.A.; Alitheen, N.B.; Hussin, Y.; Bahmanrokh, G.; Baharum, N.A. Biotechnological approaches in the production of plant secondary metabolites for treating human viral diseases: Prospects and challenges. Biocatal. Agric. Biotechnol. 2024, 103249. [Google Scholar] [CrossRef]
- Banarase, N.B.; Kaur, C.D. Whole Whey Stabilized Oleanolic Acid Nanosuspension: Formulation and Evaluation Study. J. Drug Deliv. Sci. Technol. 2022, 67, 103001. [Google Scholar] [CrossRef]
- Lisiak, N.; Dzikowska, P.; Wisniewska, U.; Kaczmarek, M.; Bednarczyk-Cwynar, B.; Zaprutko, L.; Rubis, B. Biological Activity of Oleanolic Acid Derivatives HIMOXOL and Br-HIMOLID in Breast Cancer Cells Is Mediated by ER and EGFR. Int. J. Mol. Sci. 2023, 24, 5099. [Google Scholar] [CrossRef] [PubMed]
- Huali, Y.; Minghui, D.; Hongwei, J.I.A.; Zhang, K.; Yang, L.I.U.; Cheng, M.; Wei, X. A Review of Structural Modification and Biological Activities of Oleanolic Acid. Chin. J. Nat. Med. 2024, 22, 15–30. [Google Scholar] [CrossRef]
- Iqbal Choudhary, M.; Batool, I.; Nahar Khan, S.; Sultana, N.; Adnan Ali Shah, S.; Ur-Rahman, A. Microbial Transformation of Oleanolic Acid by Fusarium lini and α-Glucosidase Inhibitory Activity of Its Transformed Products. Nat. Prod. Res. 2008, 22, 489–494. [Google Scholar] [CrossRef]
- Gong, T.; Zheng, L.; Zhen, X.; He, H.-X.; Zhu, H.-X.; Zhu, P. Microbial Transformation of Oleanolic Acid by Trichothecium roseum. J. Asian Nat. Prod. Res. 2014, 16, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-L.; Liu, Y.; Qiu, F.; Gao, Y.; Zhang, J.-Z. Biotransformation of Oleanolic Acid by Alternaria longipes and Penicillium adametzi. J. Asian Nat. Prod. Res. 2011, 13, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cheng, Z.-H.; Yu, B.-Y.; Cordell, G.A.; Qiu, S.X. Novel Biotransformation of Pentacyclic Triterpenoid Acids by Nocardia sp. NRRL 5646. Tetrahedron Lett. 2005, 46, 2337–2340. [Google Scholar] [CrossRef]
- Martinez, A.; Rivas, F.; Perojil, A.; Parra, A.; Garcia-Granados, A.; Fernandez-Vivas, A. Biotransformation of Oleanolic and Maslinic Acids by Rhizomucor miehei. Phytochemistry 2013, 94, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, B.; Geib, D.; Haas, C.; Steingroewer, J.; Bley, T.; Muffler, K.; Ulber, R. Whole-cell Biotransformation of Oleanolic Acid by Free and Immobilized Cells of Nocardia Iowensis: Characterization of New Metabolites. Eng. Life Sci. 2015, 15, 108–115. [Google Scholar] [CrossRef]
- Yan, S.; Lin, H.; Huang, H.; Yang, M.; Xu, B.; Chen, G. Microbial Hydroxylation and Glycosidation of Oleanolic Acid by Circinella muscae and Their Anti-Inflammatory Activities. Nat. Prod. Res. 2019, 33, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Luchnikova, N.A.; Grishko, V.V.; Kostrikina, N.A.; Sorokin, V.V.; Mulyukin, A.L.; Ivshina, I.B. Biotransformation of Oleanolic Acid Using Rhodococcus rhodochrous IEGM 757. Catalysts 2022, 12, 1352. [Google Scholar] [CrossRef]
- Gupta, N. A Review on Recent Developments in the Anticancer Potential of Oleanolic Acid and Its Analogs (2017–2020). Mini Rev. Med. Chem. 2022, 22, 600–616. [Google Scholar] [CrossRef]
- Yan, M.C.; Liu, Y.; Chen, H.; Ke, Y.; Xu, Q.C.; Cheng, M. Synthesis and Antitumor Activity of Two Natural N-Acetylglucosamine-Bearing Triterpenoid Saponins: Lotoidoside D and E. Bioorg. Med. Chem. Lett. 2006, 16, 4200–4204. [Google Scholar] [CrossRef]
- Gupta, S.; Kalani, K.; Saxena, M.; Srivastava, S.K.; Agrawal, S.K.; Suri, N.; Saxena, A.K. Cytotoxic Evaluation of Semisynthetic Ester and Amide Derivatives of Oleanolic Acid. Nat. Prod. Commun. 2010, 5, 1934578X1000501010. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Z.-F.; Meng, F.; Xu, C.-S.; Zhang, Y.-H. Synthesis and Cytotoxicity of Oleanolic Acid/N-Aryl-N’-Hydroxyguanidine Hybrids. Chin. J. Nat. Med. 2010, 8, 436–440. [Google Scholar] [CrossRef]
- Hao, J.; Liu, J.; Wen, X.; Sun, H. Synthesis and Cytotoxicity Evaluation of Oleanolic Acid Derivatives. Bioorg. Med. Chem. Lett. 2013, 23, 2074–2077. [Google Scholar] [CrossRef] [PubMed]
- Mallavadhani, U.V.; Mahapatra, A.; Pattnaik, B.; Vanga, N.; Suri, N.; Saxena, A.K. Synthesis and Anti-Cancer Activity of Some Novel C-17 Analogs of Ursolic and Oleanolic Acids. Med. Chem. Res. 2013, 22, 1263–1269. [Google Scholar] [CrossRef]
- Cheng, K.; Su, C.; Huang, J.; Liu, J.; Zheng, Y.; Chen, Z. Conjugation of Uridine with Oleanolic Acid Derivatives as Potential Antitumor Agents. Chem. Biol. Drug Des. 2016, 88, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Chouaïb, K.; Romdhane, A.; Delemasure, S.; Dutartre, P.; Elie, N.; Touboul, D. Regiospecific Synthesis, Anti-Inflammatory and Anticancer Evaluation of Novel 3,5-Disubstituted Isoxazoles from the Natural Maslinic and Oleanolic Acids. Ind. Crops Prod. 2016, 85, 287–299. [Google Scholar] [CrossRef]
- Chouaïb, K.; Romdhane, A.; Delemasure, S.; Dutartre, P.; Elie, N.; Touboul, D.; Jannet, H.B. Regiospecific Synthesis by Copper-and Ruthenium-Catalyzed Azide–Alkyne 1,3-Dipolar Cycloaddition, Anticancer and Anti-Inflammatory Activities of Oleanolic Acid Triazole Derivatives. Arab. J. Chem. 2019, 12, 3732–3742. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Luan, T.; Liu, C. Design and Synthesis of Novel Oleanolic Acid-Linked Disulfide, Thioether, or Selenium Ether Moieties as Potent Cytotoxic Agents. Chem. Biodivers. 2022, 19, e202100831. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zeng, Q.; Wang, R.; Wang, B.; Chen, R.; Wang, N.; Lu, Y.; Shi, F.; Dehaen, W.; Huai, Q. Synthesis and Discovery of Mitochondria-Targeting Oleanolic Acid Derivatives for Potential PI3K Inhibition. Fitoterapia 2022, 162, 105291. [Google Scholar] [CrossRef] [PubMed]
- Şenol, H.; Çokuludağ, K.; Aktaş, A.S.; Atasoy, S.; Dağ, A.; Topçu, G. Synthesis of New Fatty Acid Derivatives of Oleanane and Ursane Triterpenoids and Investigation of Their in Vitro Cytotoxic Effects on 3T3 Fibroblast and PC3 Prostate Cancer Cell linesLines. Org. Commun. 2020, 13, 126. [Google Scholar] [CrossRef]
- Halil, Ş.; Berre, M.; Büşra, Ş.R.; Burak, K.H.; Ebru, H. Synthesis of Oleanolic Acid Hydrazide-Hydrazone Hybrid Derivatives and Investigation of Their Cytotoxic Effects on A549 Human Lung Cancer Cells. Results Chem. 2022, 4, 100317. [Google Scholar] [CrossRef]
- Şenol, H.; Ghaffari-Moghaddam, M.; Bulut, Ş.; Akbaş, F.; Köse, A.; Topçu, G. Synthesis and Anticancer Activity of Novel Derivatives of α, β-Unsaturated Ketones Based on Oleanolic Acid: In Vitro and in Silico Studies against Prostate Cancer Cells. Chem. Biodivers. 2023, 20, e202301089. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Huang, J.; Liu, C.; Zhang, J.; Cheng, K. Synthesis of Oleanolic Acid/Ursolic Acid/Glycyrrhetinic Acid-Hydrogen Sulfide Donor Hybrids and Their Antitumor Activity. Med. Chem. Res. 2019, 28, 1212–1222. [Google Scholar] [CrossRef]
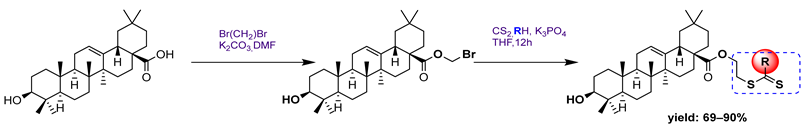
- Tang, L.; Zhang, Y.; Xu, J.; Yang, Q.; Du, F.; Wu, X.; Li, M.; Shen, J.; Deng, S.; Zhao, Y. Synthesis of Oleanolic Acid-Dithiocarbamate Conjugates and Evaluation of Their Broad-Spectrum Antitumor Activities. Molecules 2023, 28, 1414. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yuan, W.; Yuan, J.; Wei, W.; He, Q.; Zhang, X.; He, S.; Yang, C. Synthesis and Biological Evaluation of Pyrazole-Fused Oleanolic Acid Derivatives as Novel Inhibitors of Inflammatory and Osteoclast Differentiation. Bioorg. Med. Chem. 2023, 80, 117177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jia, X.; Dong, J.; Chen, D.; Liu, J.; Zhang, L.; Wen, X. Synthesis and Evaluation of Novel Oleanolic Acid Derivatives as Potential Antidiabetic Agents. Chem. Biol. Drug Des. 2014, 83, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.S.G.; Oliveira, P.J.; Duarte, M.F. Oleanolic, Ursolic, and Betulinic Acids as Food Supplements or Pharmaceutical Agents for Type 2 Diabetes: Promise or Illusion? J. Agric. Food Chem. 2016, 64, 2991–3008. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Jahangir, M.; ul Hussan, S.S.; Choudhary, M.I. Inhibition of α-Glucosidase by Oleanolic Acid and Its Synthetic Derivatives. Phytochemistry 2002, 60, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, J.; Zhang, L.; Wu, G.; Hua, W.; Wu, X.; Sun, H. Pentacyclic Triterpenes. Part 3: Synthesis and Biological Evaluation of Oleanolic Acid Derivatives as Novel Inhibitors of Glycogen Phosphorylase. Bioorg. Med. Chem. Lett. 2006, 16, 2915–2919. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Zhang, W.; Hong, D.; Shi, L.; Shen, Q.; Li, J.-Y.; Li, J.; Hu, L.-H. Oleanolic Acid and Its Derivatives: New Inhibitor of Protein Tyrosine Phosphatase 1B with Cellular Activities. Bioorg. Med. Chem. 2008, 16, 8697–8705. [Google Scholar] [CrossRef]
- Cheng, K.; Liu, J.; Sun, H.; Xie, J. Synthesis of Nucleoside Conjugates as Potential Inhibitors of Glycogen Phosphorylase. Synthesis 2010, 2010, 1046–1052. [Google Scholar] [CrossRef]
- Cheng, K.; Liu, J.; Sun, H.; Xie, J. Synthesis of Oleanolic Acid Dimers as Inhibitors of Glycogen Phosphorylase. Chem. Biodivers. 2010, 7, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Li, H.; Chen, Y.; Zhang, W.; Yang, S.; Wu, Y. Synthesis and Biological Evaluation of Oleanolic Acid Derivatives As Inhibitors of Protein Tyrosine Phosphatase 1B. J. Nat. Prod. 2010, 73, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Luo, J.-G.; Wang, X.-B.; Yin, H.; Sun, H.-B.; Yao, H.-Q.; Kong, L.-Y. Synthesis of New α-Glucosidase Inhibitors Based on Oleanolic Acid Incorporating Cinnamic Amides. Chem. Pharm. Bull. 2011, 59, 1051–1056. [Google Scholar] [CrossRef]
- Liu, Q.-C.; Guo, T.-T.; Zhang, L.; Yu, Y.; Wang, P.; Yang, J.-F.; Li, Y.-X. Synthesis and Biological Evaluation of Oleanolic Acid Derivatives as PTP1B Inhibitors. Eur. J. Med. Chem. 2013, 63, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zhu, L.; Chen, Y.; Qin, R.; Mei, Z.; Xu, J.; Yang, G. Synthesis and Biological Evaluation of Oleanolic Acid Derivative–Chalcone Conjugates as α-Glucosidase Inhibitors. RSC Adv. 2014, 4, 10862–10874. [Google Scholar] [CrossRef]
- Zhong, Y.-Y.; Chen, H.-S.; Wu, P.-P.; Zhang, B.-J.; Yang, Y.; Zhu, Q.-Y.; Zhang, C.-G.; Zhao, S.-Q. Synthesis and Biological Evaluation of Novel Oleanolic Acid Analogues as Potential α-Glucosidase Inhibitors. Eur. J. Med. Chem. 2019, 164, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.-Y.; Ke, J.-J.; Zheng, Y.-Y.; Li, D.-L.; Zhang, K.; Zheng, X.; Wu, J.-Y.; Xiong, Z.; Wu, P.-P.; Xu, X.-T. Synthesis and Bioactivities Evaluation of Oleanolic Acid Oxime Ester Derivatives as α-Glucosidase and α-Amylase Inhibitors. J. Enzym. Inhib. Med. Chem. 2022, 37, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Ma, H.; Liu, X.; Zhang, Y.; Tang, L.; Zheng, Z.; Zhang, X.; Jiang, C.; Lin, L.; Sun, H. Synthesis and Biological Evaluation of Substituted Pyrazole-Fused Oleanolic Acid Derivatives as Novel Selective α-Glucosidase Inhibitors. Chem. Biodivers. 2023, 20, e202201178. [Google Scholar] [CrossRef]
- Petrova, A.V.; Babkov, D.A.; Khusnutdinova, E.F.; Baikova, I.P.; Kazakova, O.B.; Sokolova, E.V.; Spasov, A.A. α-Glucosidase Inhibitors Based on Oleanolic Acid for the Treatment of Immunometabolic Disorders. Appl. Sci. 2023, 13, 9269. [Google Scholar] [CrossRef]
- Nkeh-Chungag, B.N.; Oyedeji, O.O.; Oyedeji, A.O.; Ndebia, E.J. Anti-Inflammatory and Membrane-Stabilizing Properties of Two Semisynthetic Derivatives of Oleanolic Acid. Inflammation 2015, 38, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk-Cwynar, B.; Zaprutko, L.; Marciniak, J.; Lewandowski, G.; Szulc, M.; Kaminska, E.; Wachowiak, N.; Mikolajczak, P.L. The Analgesic and Anti-Inflammatory Effect of New Oleanolic Acid Acyloxyimino Derivative. Eur. J. Pharm. Sci. 2012, 47, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, P.; Patel, N.K.; Gangwal, R.P.; Sangamwar, A.T.; Bhutani, K.K. Oleanolic Acid Analogs as NO, TNF-α and IL-1β Inhibitors: Synthesis, Biological Evaluation and Docking Studies. Bioorg. Med. Chem. Lett. 2014, 24, 4114–4119. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.T.; Camelio, A.M.; Claussen, K.R.; Cho, J.; Tremmel, L.; DiGiovanni, J.; Siegel, D. Synthesis of Oxygenated Oleanolic and Ursolic Acid Derivatives with Anti-Inflammatory Properties. Bioorg. Med. Chem. Lett. 2015, 25, 4342–4346. [Google Scholar] [CrossRef] [PubMed]
- Rali, S.; Oyedeji, O.O.; Aremu, O.O.; Oyedeji, A.O.; Nkeh-Chungag, B.N. Semisynthesis of Derivatives of Oleanolic Acid from Syzygium Aromaticum and Their Antinociceptive and Anti-Inflammatory Properties. Mediat. Inflamm. 2016, 2016, 8401843. [Google Scholar] [CrossRef] [PubMed]
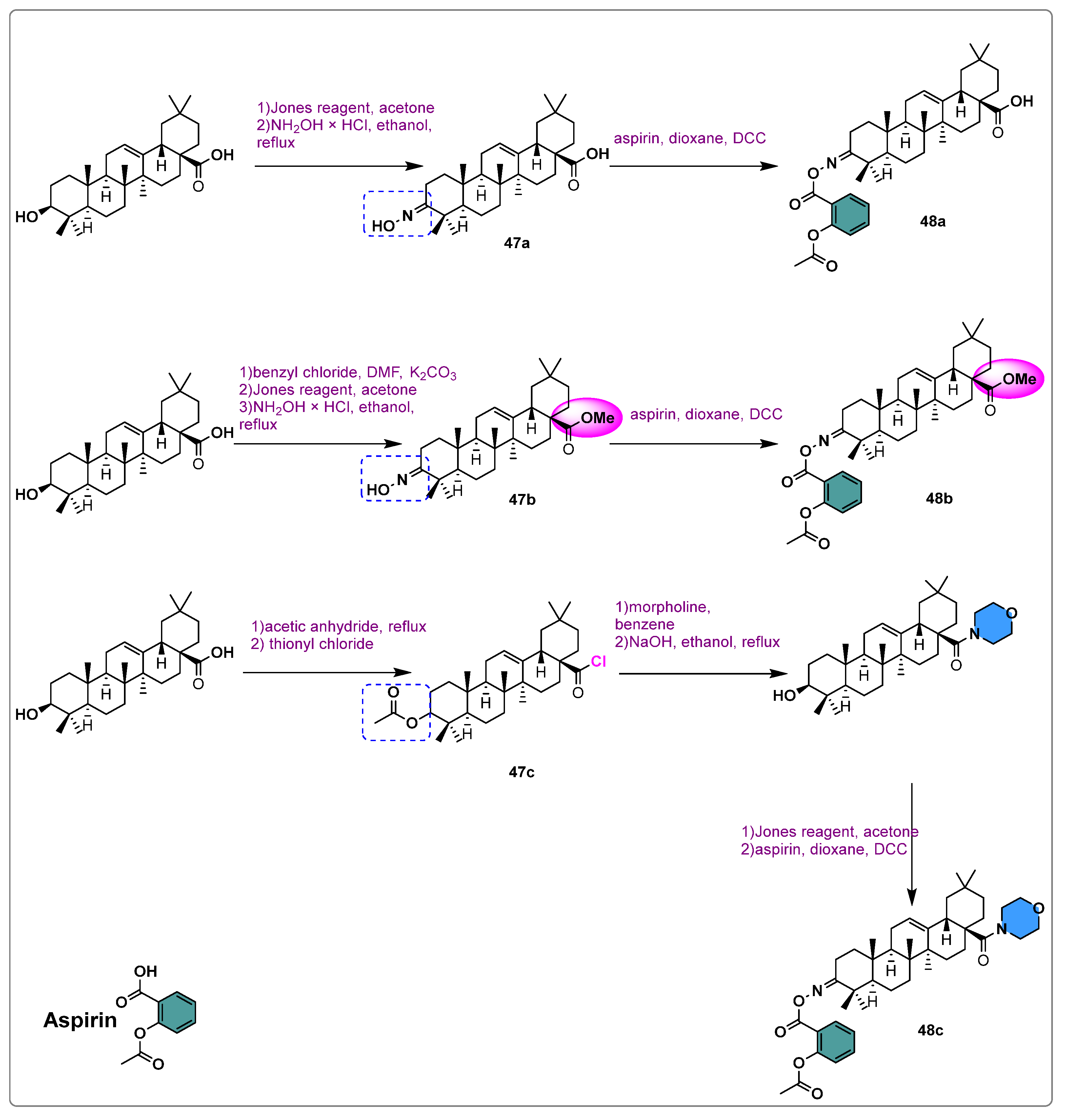
- Krajka-Kuźniak, V.; Bednarczyk-Cwynar, B.; Paluszczak, J.; Szaefer, H.; Narożna, M.; Zaprutko, L.; Baer-Dubowska, W. Oleanolic Acid Oxime Derivatives and Their Conjugates with Aspirin Modulate the NF-κB-Mediated Transcription in HepG2 Hepatoma Cells. Bioorg. Chem. 2019, 93, 103326. [Google Scholar] [CrossRef] [PubMed]
- Krajka-Kuźniak, V.; Bednarczyk-Cwynar, B.; Narożna, M.; Szaefer, H.; Baer-Dubowska, W. Morpholide Derivative of the Novel Oleanolic Oxime and Succinic Acid Conjugate Diminish the Expression and Activity of NF-κB and STATs in Human Hepatocellular Carcinoma Cells. Chem. Biol. Interact. 2019, 311, 108786. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, H.; Hu, K.; Xu, Q.; Wen, X.; Cheng, K.; Chen, C.; Yuan, H.; Dai, L.; Sun, H. Synthesis and Anti-Inflammatory Activity of Saponin Derivatives of δ-Oleanolic Acid. Eur. J. Med. Chem. 2021, 209, 112932. [Google Scholar] [CrossRef] [PubMed]
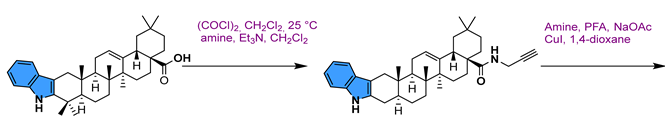
- Jin, J.; He, H.; Zhang, X.; Wu, R.; Gan, L.; Li, D.; Lu, Y.; Wu, P.; Wong, W.-L.; Zhang, K. The in Vitro and in Vivo Study of Oleanolic Acid Indole Derivatives as Novel Anti-Inflammatory Agents: Synthesis, Biological Evaluation, and Mechanistic Analysis. Bioorg. Chem. 2021, 113, 104981. [Google Scholar] [CrossRef]
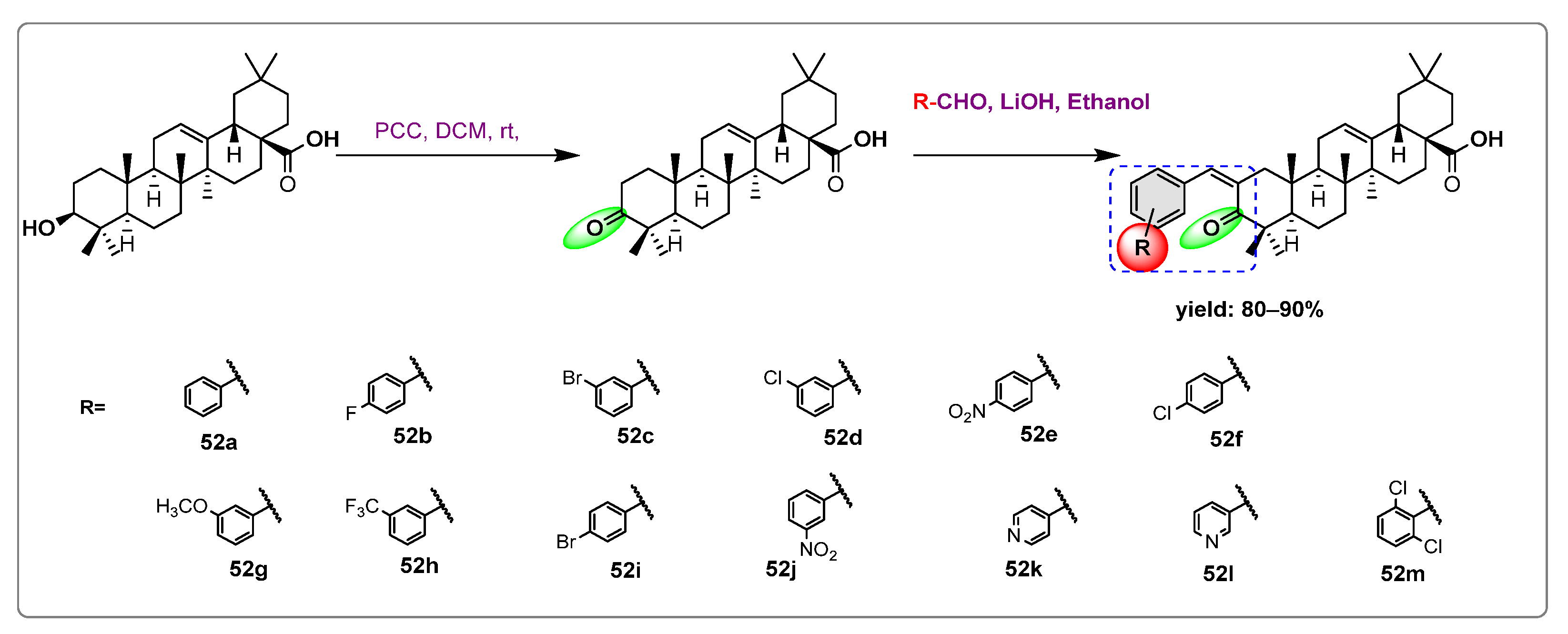
- Hassan Mir, R.; Godavari, G.; Siddiqui, N.A.; Ahmad, B.; Mothana, R.; Ullah, R.; Almarfadi, O.; Jachak, S.; Masoodi, M. Design, Synthesis, Molecular Modelling, and Biological Evaluation of Oleanolic Acid-Arylidene Derivatives as Potential Anti-Inflammatory Agents. Drug Des. Devel. Ther. 2021, 15, 385–397. [Google Scholar] [CrossRef]
- Hichri, F.; Jannet, H.B.; Cheriaa, J.; Jegham, S.; Mighri, Z. Antibacterial Activities of a Few Prepared Derivatives of Oleanolic Acid and of Other Natural Triterpenic Compounds. C. R. Chim. 2003, 6, 473–483. [Google Scholar] [CrossRef]
- Chouaïb, K.; Hichri, F.; Nguir, A.; Daami-Remadi, M.; Elie, N.; Touboul, D.; Jannet, H.B. Semi-Synthesis of New Antimicrobial Esters from the Natural Oleanolic and Maslinic Acids. Food Chem. 2015, 183, 8–17. [Google Scholar] [CrossRef]
- Blanco-Cabra, N.; Vega-Granados, K.; Moya-Andérico, L.; Vukomanovic, M.; Parra, A.; Álvarez De Cienfuegos, L.; Torrents, E. Novel Oleanolic and Maslinic Acid Derivatives as a Promising Treatment against Bacterial Biofilm in Nosocomial Infections: An in Vitro and in Vivo Study. ACS Infect. Dis. 2019, 5, 1581–1589. [Google Scholar] [CrossRef]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A.; Morifi, E.; Fonkui, T.Y.; Ndinteh, D.T.; Steenkamp, V. Synthesis, Antibacterial, and Cytotoxicity Evaluation of Oleanolic Acid-4-Aminoquinoline Based Hybrid Compounds. Recent Adv. Anti-Infect. Drug Discov. 2021, 16, 122–136. [Google Scholar] [CrossRef]
- Wu, P.; Tu, B.; Liang, J.; Guo, S.; Cao, N.; Chen, S.; Luo, Z.; Li, J.; Zheng, W.; Tang, X. Synthesis and Biological Evaluation of Pentacyclic Triterpenoid Derivatives as Potential Novel Antibacterial Agents. Bioorg. Chem. 2021, 109, 104692. [Google Scholar] [CrossRef] [PubMed]
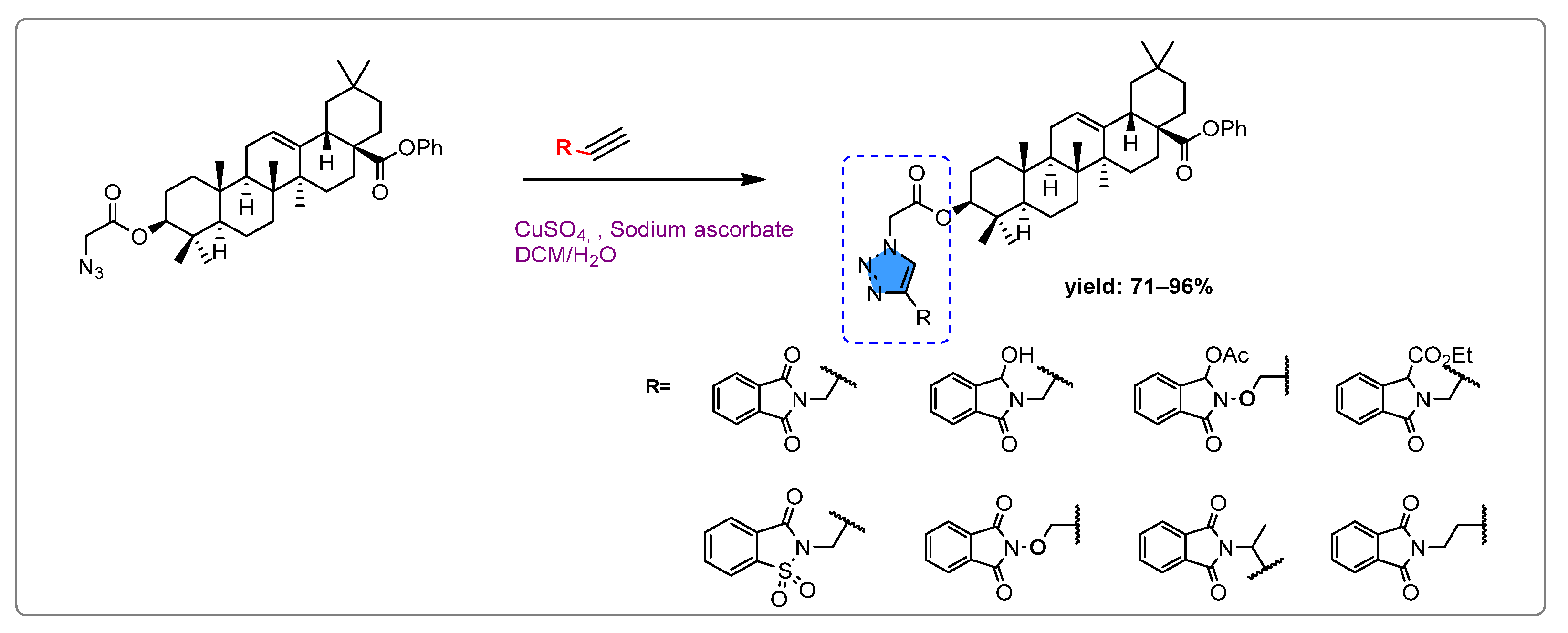
- Lahmadi, G.; Horchani, M.; Dbeibia, A.; Mahdhi, A.; Romdhane, A.; Lawson, A.M.; Daïch, A.; Harrath, A.H.; Ben Jannet, H.; Othman, M. Novel Oleanolic Acid-Phtalimidines Tethered 1,2,3 Triazole Hybrids as Promising Antibacterial Agents: Design, Synthesis, In Vitro Experiments and In Silico Docking Studies. Molecules 2023, 28, 4655. [Google Scholar] [CrossRef] [PubMed]
- Boulila, B.; Horchani, M.; Duval, R.; Othman, M.; Daïch, A.; Jannet, H.B.; Romdhane, A.; Lawson, A.M. Design, Semi-Synthesis and Molecular Docking of New Antibacterial and Antibiofilm Triazole Conjugates from Hydroxy-Triterpene Acids and Fluoroquinolones. New J. Chem. 2023, 47, 15973–15986. [Google Scholar] [CrossRef]
- Sander, W.J.; O’Neill, H.G.; Pohl, C.H. Prostaglandin E2 as a Modulator of Viral Infections. Front. Physiol. 2017, 8, 89. [Google Scholar] [CrossRef]
- Wimmerová, M.; Bildziukevich, U.; Wimmer, Z. Selected Plant Triterpenoids and Their Derivatives as Antiviral Agents. Molecules 2023, 28, 7718. [Google Scholar] [CrossRef]
- Schulz, L.; Hornung, F.; Häder, A.; Radosa, L.; Brakhage, A.A.; Löffler, B.; Deinhardt-Emmer, S. Influenza Virus-Induced Paracrine Cellular Senescence of the Lung Contributes to Enhanced Viral Load. Aging Dis. 2023, 14, 1331. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Wang, H.; Xiong, Y. Recent Advances in Antiviral Activities of Triterpenoids. Pharmaceuticals 2022, 15, 1169. [Google Scholar] [CrossRef] [PubMed]
- Shirahata, T.; Nagai, T.; Hirata, N.; Yokoyama, M.; Katsumi, T.; Konishi, N.; Nishino, T.; Makino, K.; Yamada, H.; Kaji, E. Syntheses and Mucosal Adjuvant Activity of Simplified Oleanolic Acid Saponins Possessing Cinnamoyl Ester. Bioorg. Med. Chem. 2017, 25, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Meng, L.; Sun, J.; Li, W.; Shao, L.; Chen, K.; Zhou, D.; Yang, F.; Yu, F. Design, Synthesis of Oleanolic Acid-Saccharide Conjugates Using Click Chemistry Methodology and Study of Their Anti-Influenza Activity. Eur. J. Med. Chem. 2019, 182, 111622. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Su, Y.; Yang, F.; Xiao, S.; Yin, Z.; Liu, J.; Zhong, J.; Zhou, D.; Yu, F. Design, Synthesis and Biological Evaluation of Amino Acids-Oleanolic Acid Conjugates as Influenza Virus Inhibitors. Bioorg. Med. Chem. 2019, 27, 115147. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, F.; Meng, L.; Sun, J.; Su, Y.; Shao, L.; Zhou, D.; Yu, F. Synthesis, Structure Activity Relationship and Anti-Influenza A Virus Evaluation of Oleanolic Acid-Linear Amino Derivatives. Chem. Pharm. Bull. 2019, 67, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Su, Y.; Zhang, Y.; Yang, F.; Zhang, J.; Tang, T.; Yu, F. Nine-Valent Oleanolic Acid Conjugates as Potent Inhibitors Blocking the Entry of Influenza A Virus. Eur. J. Med. Chem. 2023, 258, 115562. [Google Scholar] [CrossRef] [PubMed]
- Purcell, R.H. Hepatitis Viruses: Changing Patterns of Human Disease. Proc. Natl. Acad. Sci. USA 1994, 91, 2401–2406. [Google Scholar] [CrossRef]
- He, W.; Gao, Y.; Wen, Y.; Ke, X.; Ou, Z.; Li, Y.; He, H.; Chen, Q. Detection of Virus-Related Sequences Associated with Potential Etiologies of Hepatitis in Liver Tissue Samples from Rats, Mice, Shrews, and Bats. Front. Microbiol. 2021, 12, 653873. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhang, C.; Li, B.; Xu, X.; Liang, M.; Gu, S.; Chu, F.; Xu, B.; Ren, J.; Wang, P. A Series of Oleanolic Acid Derivatives as Anti-Hepatitis B Virus Agents: Design, Synthesis, and in Vitro and in Vivo Biological Evaluation. Molecules 2016, 21, 402. [Google Scholar] [CrossRef]
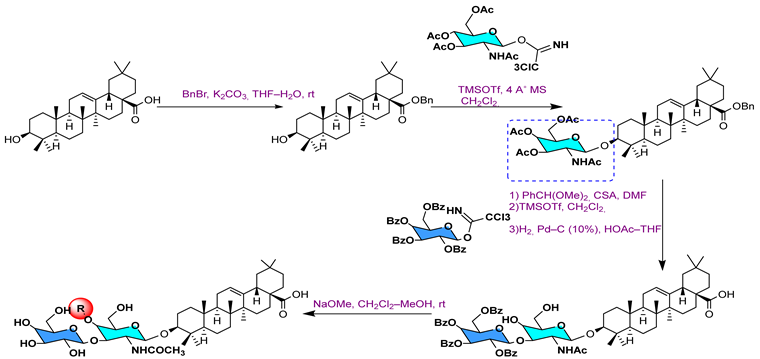
- Zhang, Y.; Li, J.; Zhao, J.; Wang, S.; Pan, Y.; Tanaka, K.; Kadota, S. Synthesis and Activity of Oleanolic Acid Derivatives, a Novel Class of Inhibitors of Osteoclast Formation. Bioorg. Med. Chem. Lett. 2005, 15, 1629–1632. [Google Scholar] [CrossRef]
- Li, J.-F.; Zhao, Y.; Cai, M.-M.; Li, X.-F.; Li, J.-X. Synthesis and Evaluation of a Novel Series of Heterocyclic Oleanolic Acid Derivatives with Anti-Osteoclast Formation Activity. Eur. J. Med. Chem. 2009, 44, 2796–2806. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Bao, B.-H.; Shen, Q.; Zhang, Y.-C.; Jiang, Q.; Li, J.-X. Novel Heterocyclic Ring-Fused Oleanolic Acid Derivatives as Osteoclast Inhibitors for Osteoporosis. MedChemComm 2016, 7, 371–377. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Q.; Zhu, M.; Wang, J.; Du, Y.; Wu, J.; Li, J. Modified Quinoxaline-Fused Oleanolic Acid Derivatives as Inhibitors of Osteoclastogenesis and Potential Agent in Anti-Osteoporosis. ChemistrySelect 2020, 5, 1526–1533. [Google Scholar] [CrossRef]
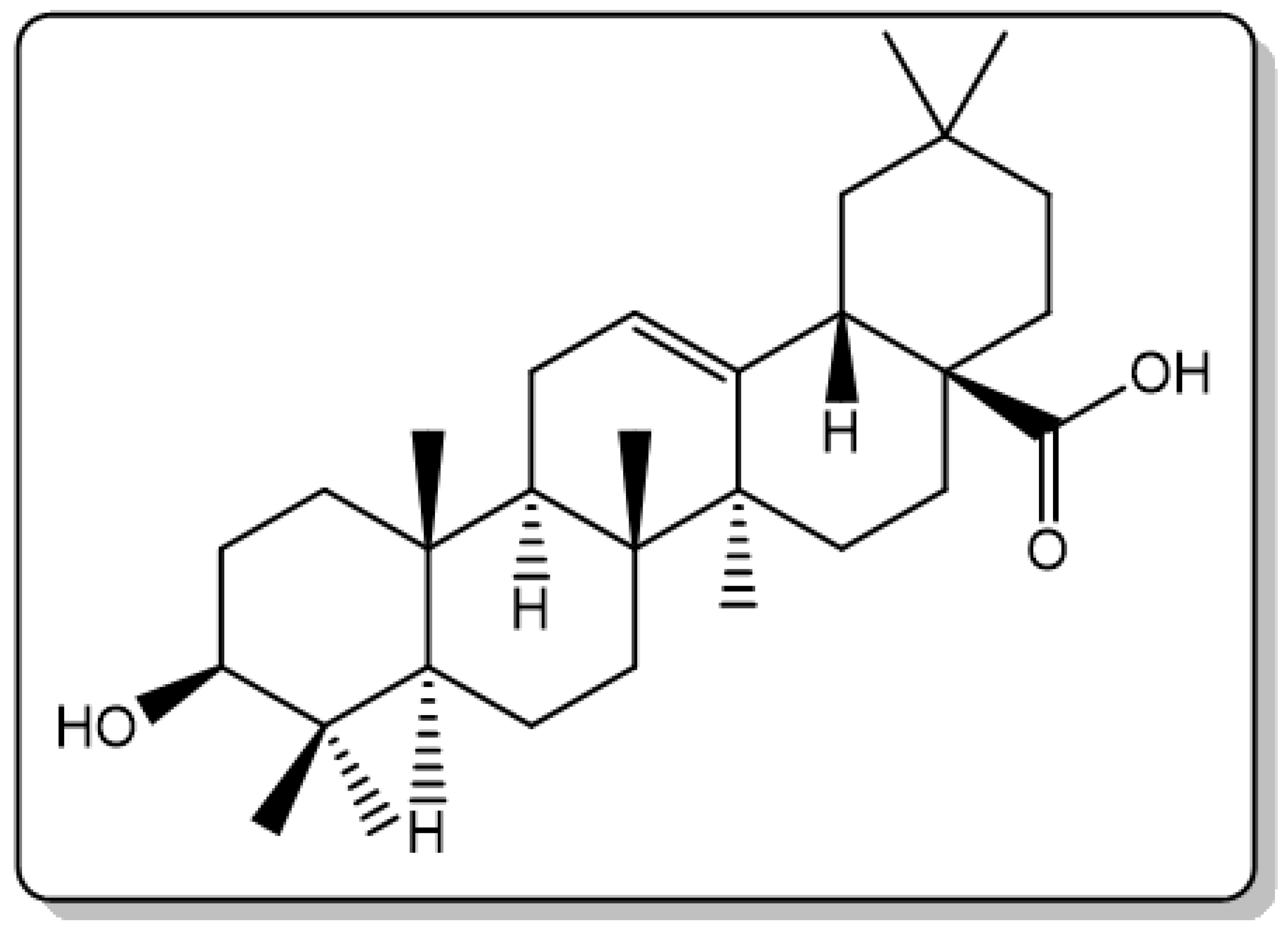
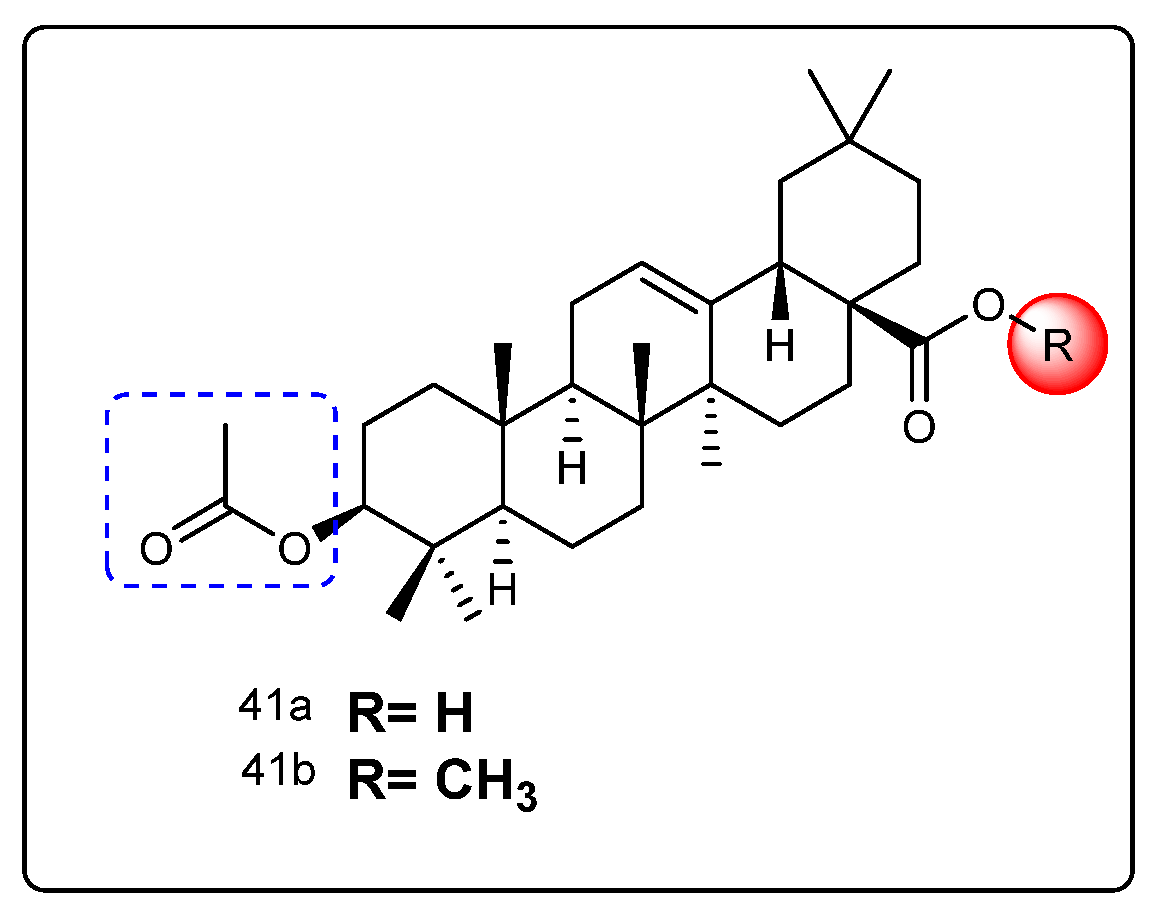
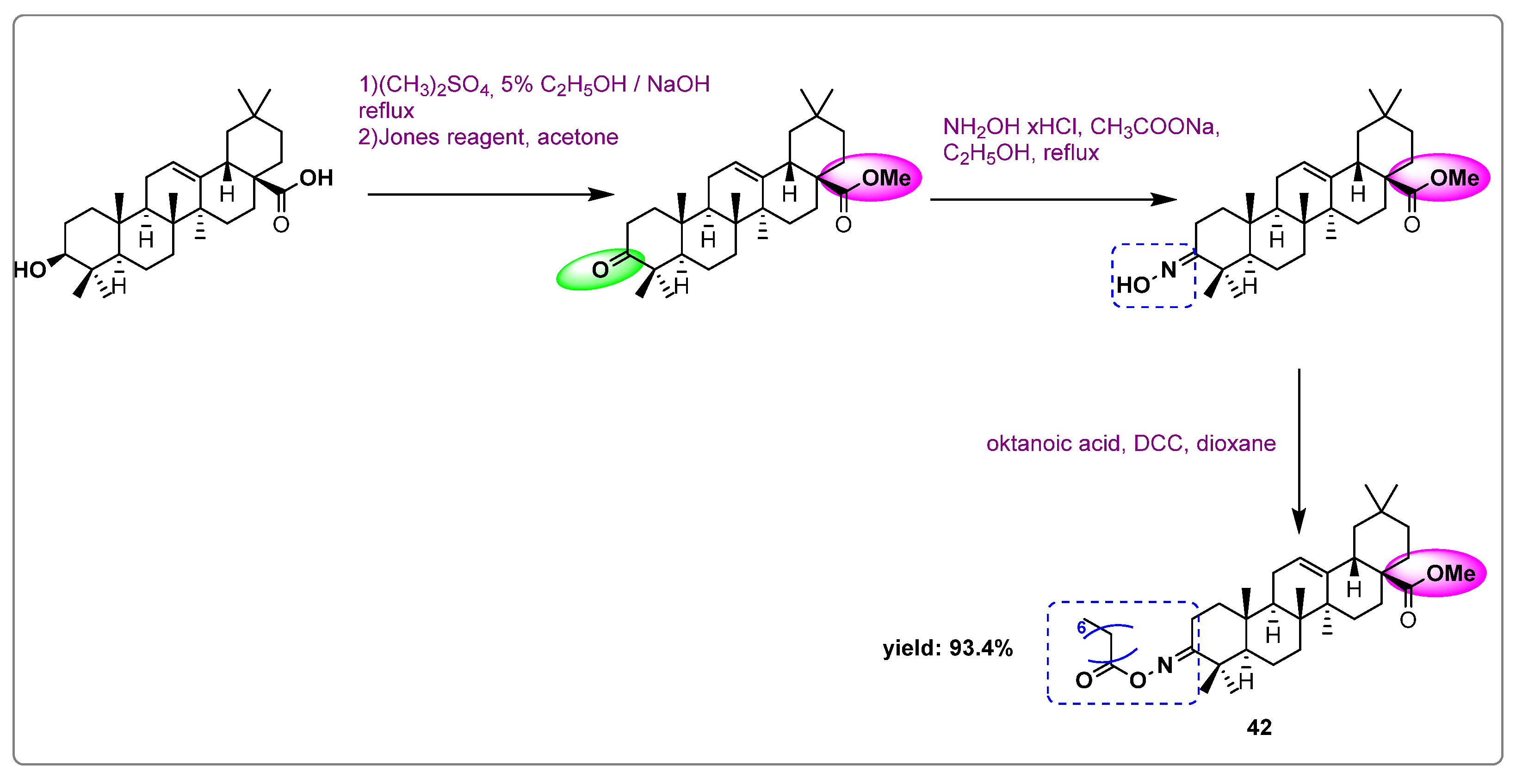
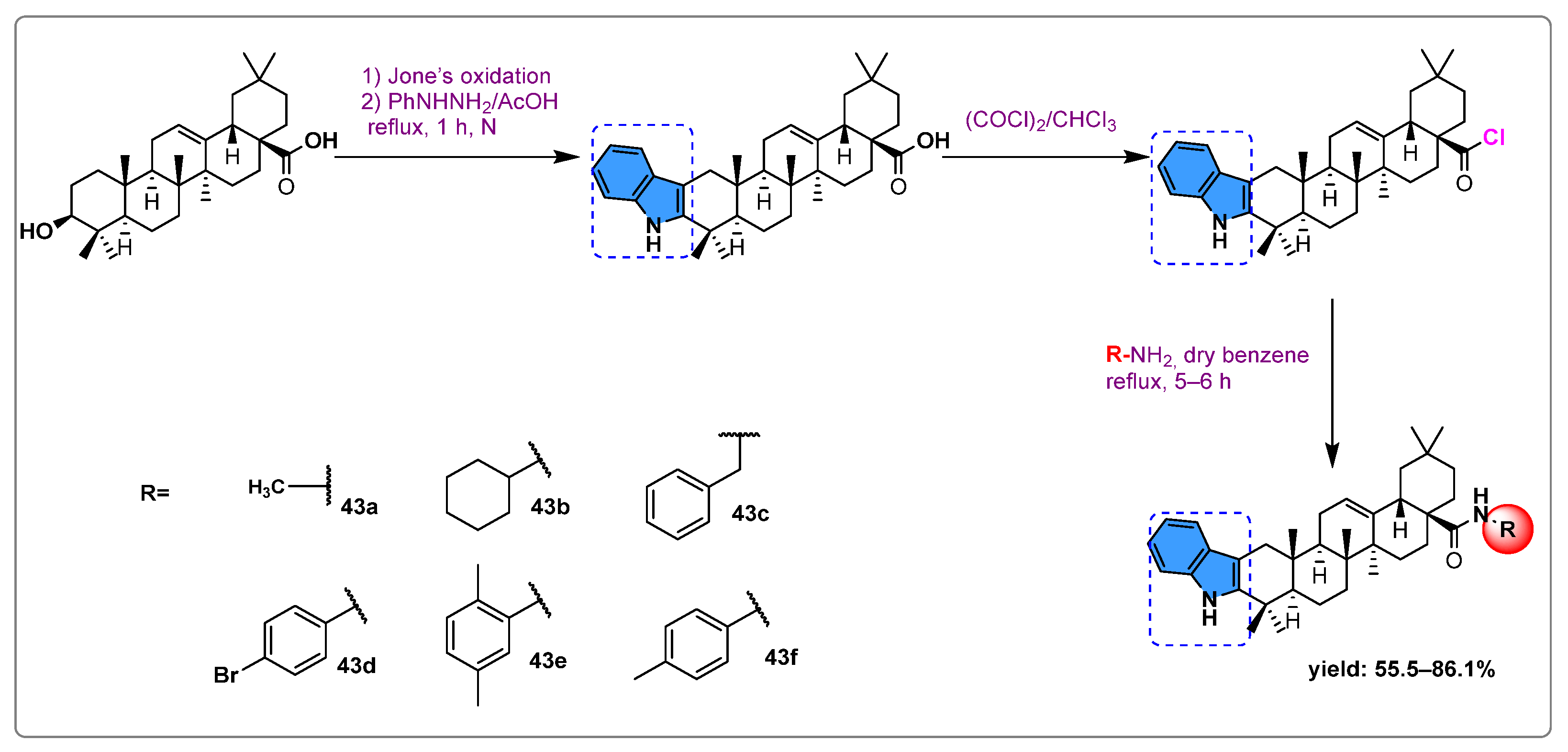

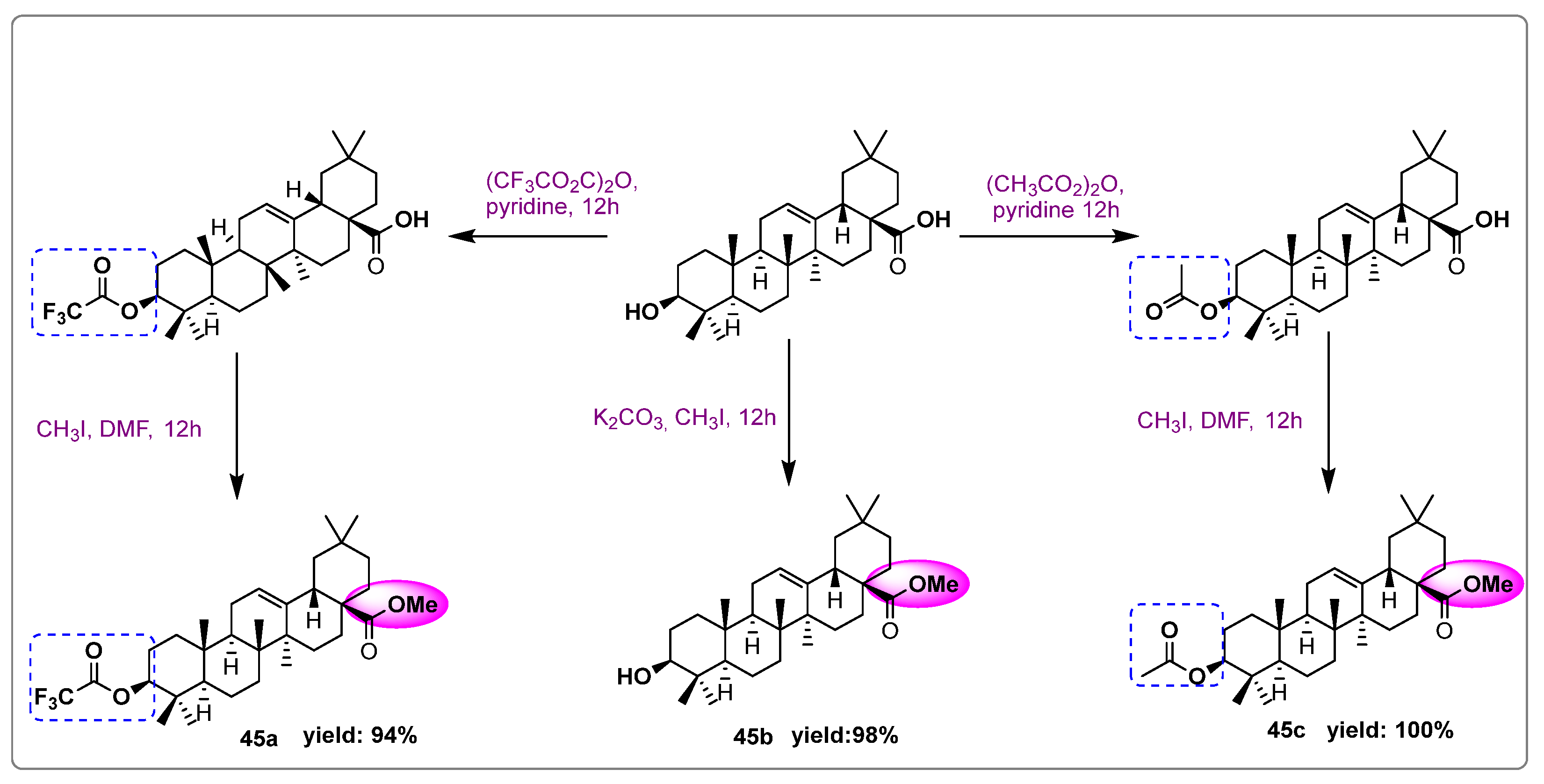



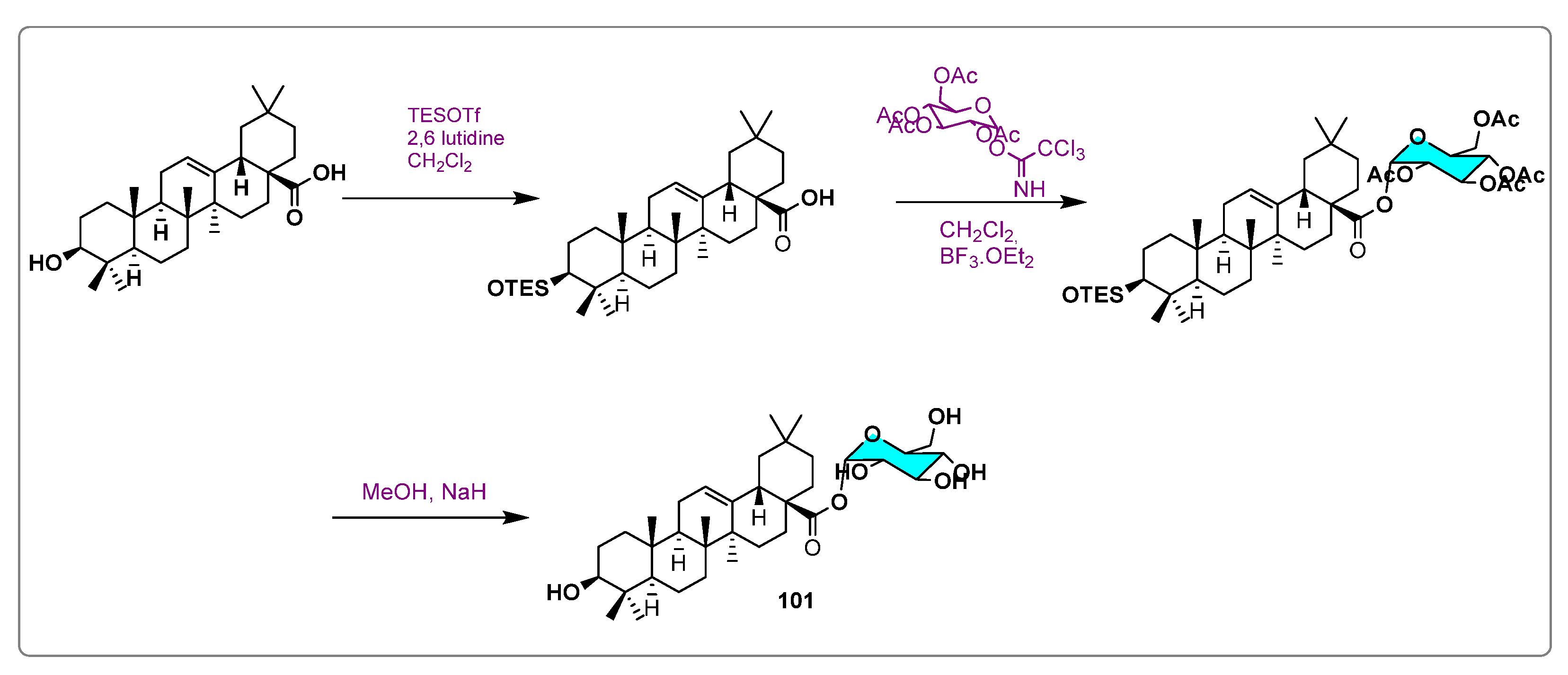
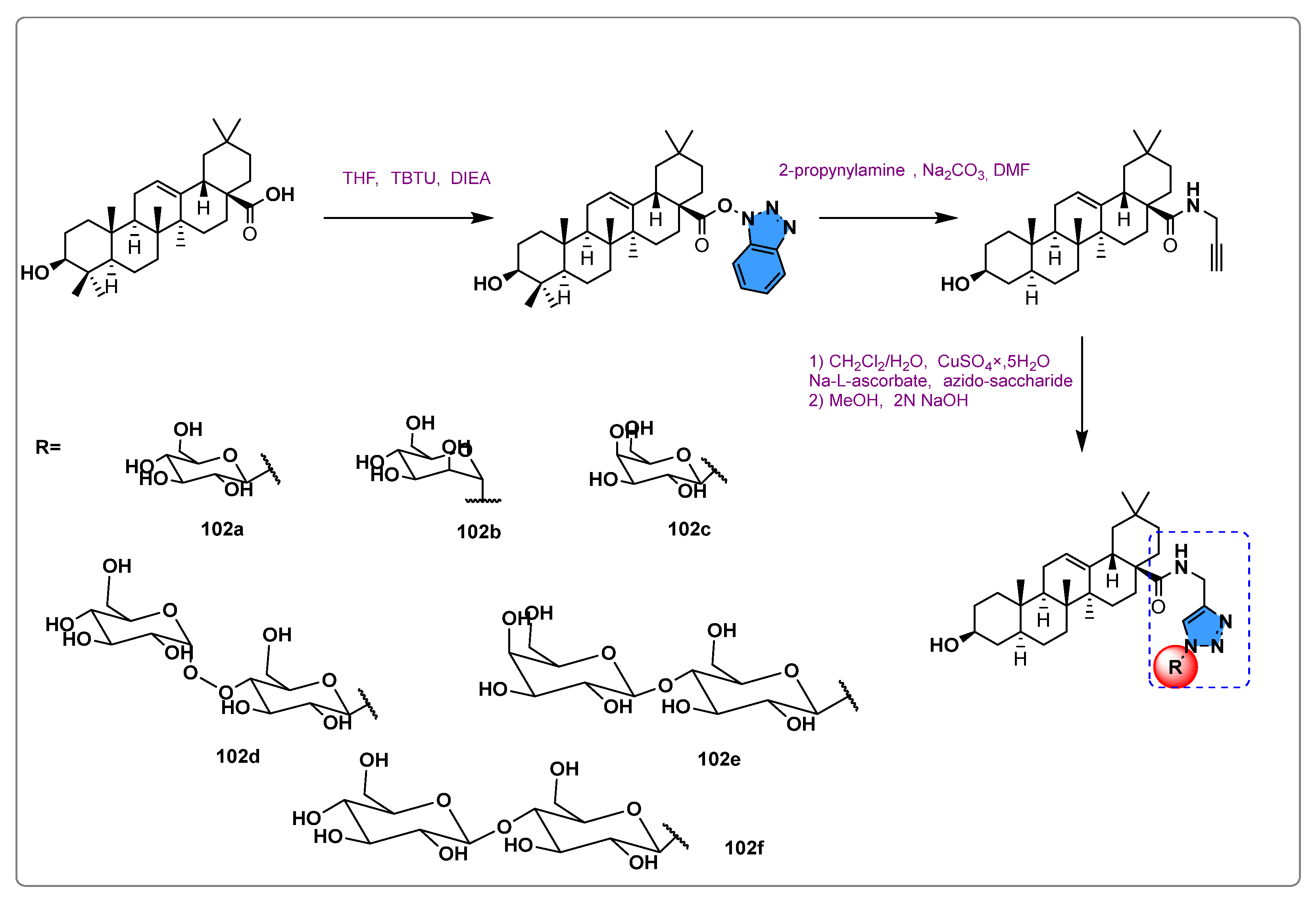
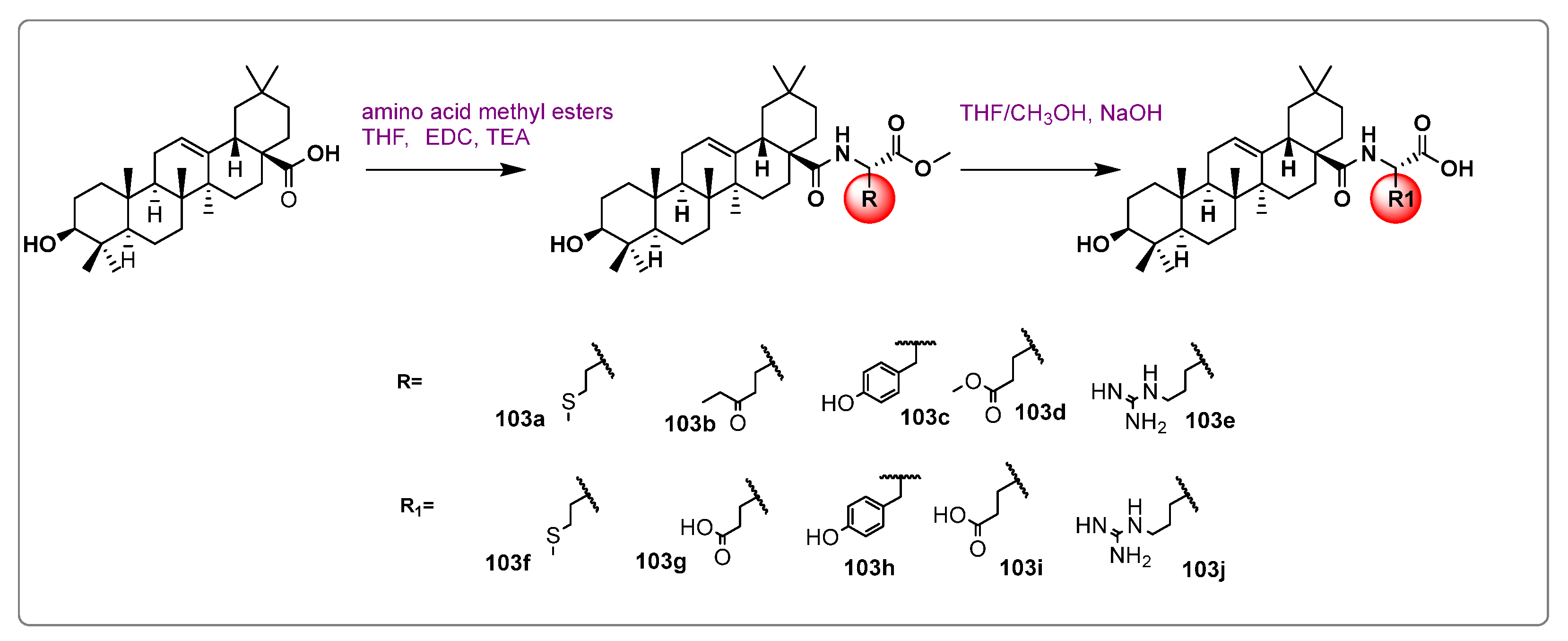
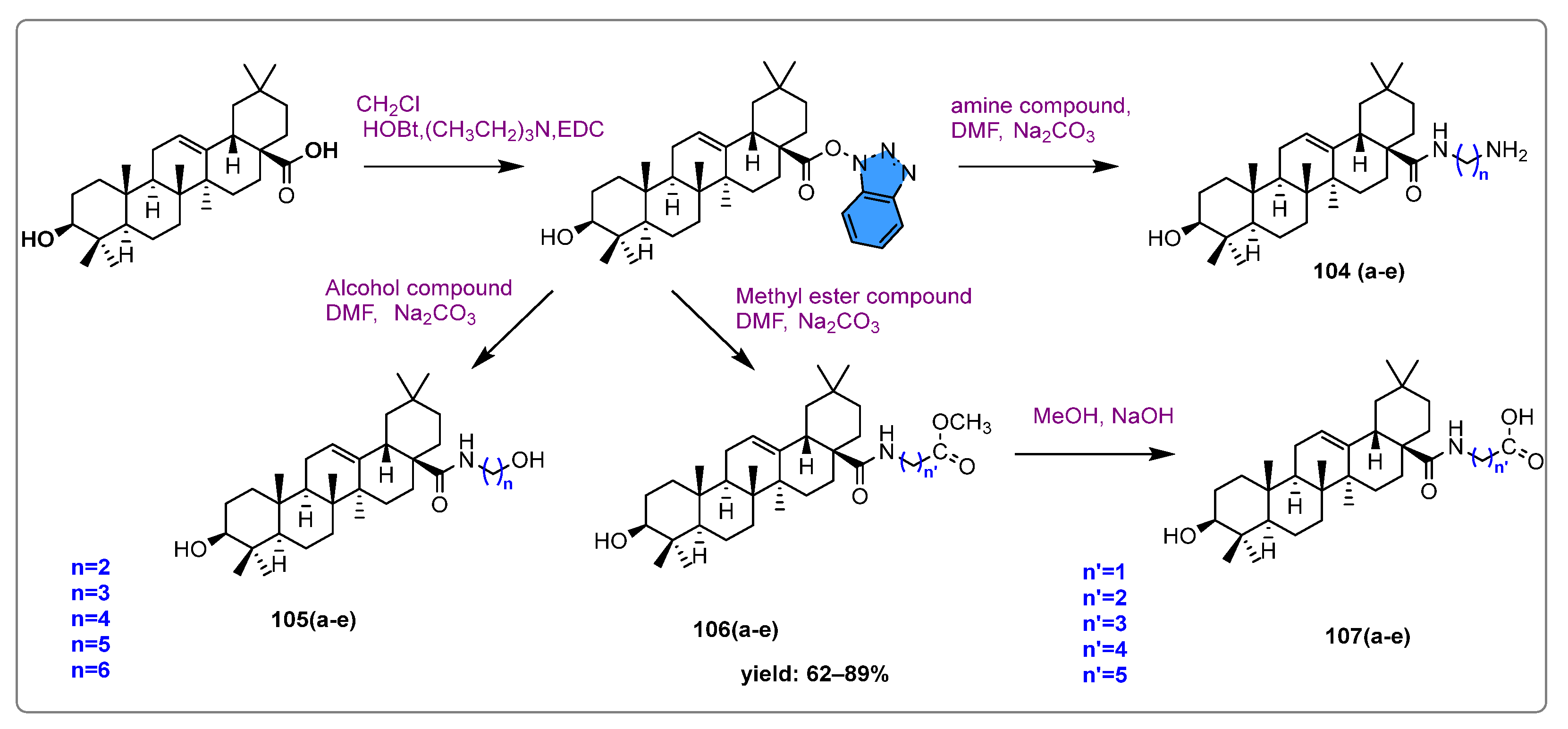





| Names of the Micro-Organisms | Derivatives | Biological Activities | References |
|---|---|---|---|
| Nocardia sp. NRRL 5646 |  | - | [53] |
| Furarium lini |  | Anti-α-glycosidase activity IC50: 2: 444 µM 3: 666 µM | [50] |
| Alternaria longipes | 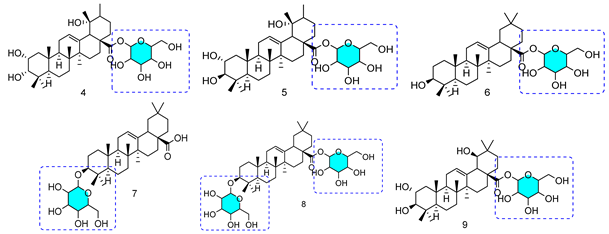 | Anticancer activity HeLa Cell Line IC50: 4: 7.40 μM | [52] |
| Penicillium adametzi | 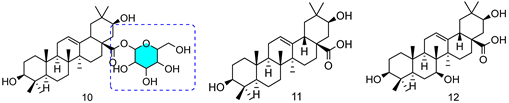 | 10: 25.00 μM/11: 7.60 μM/12: 0.78 μM | |
| Rhizomucor miehei |  | - | [54] |
| Trichothecium roseum |  | - | [51] |
| Nocardia iowensis |  | - | [55] |
| Circinella muscae |  | Anti-inflammatory activities Cell: RAW 264.7 IC50: 20: 9.24 μM/21: 56.13 μM/22: 68.39 μM 23: 10.06 μM/24: 34.63 μM/25: 39.83 μM 26: 11.28 μM/27: 40.74 μM/28: 8.28 μM | [56] |
| Rhodococcus rhodochrous | 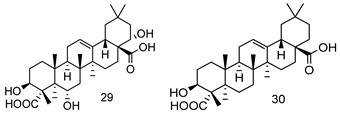 | - | [57] |
 | |
| 1 | Cell Line Hela IC50 (μM) |
1a  | 2.74 |
1b  | >10 |
 | ||
| 2 | Cell Line: Conc. (50 μM) | |
| IGR-OV-1 | HOP-62 | |
2a  | 25.00 | - |
2b 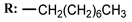 | 38.00 | - |
2c  | 45.00 | - |
2d 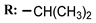 | 44.00 | - |
2e 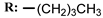 | 36.00 | - |
2f 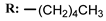 | 31.00 | - |
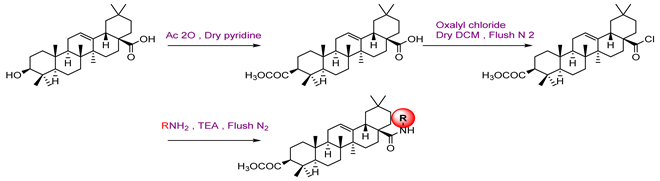 3 | ||
3a 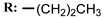 | - | 22.00 |
3b  | - | 13.00 |
3c 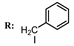 | - | 27.00 |
3d  | - | 53.00 |
3e 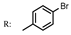 | - | 32.00 |
3f  | - | 22.00 |
3g  | - | 16.00 |
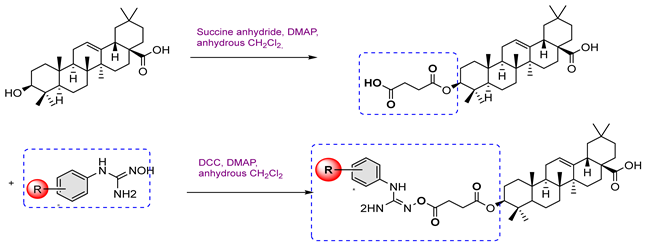 | ||||
| 4 | Cell Line: Conc. (10 μM) | |||
| A549 | HT-29 | BEL-7402 | SMMC7721 | |
4a  | 42.30 | 48.53 | 47.17 | 46.63 |
4b  | 88.43 | 77.90 | 73.24 | 85.86 |
4c 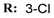 | 46.15 | 40.59 | 40.21 | 36.53 |
4d 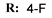 | 38.99 | 45.22 | 35.41 | 44.80 |
4e  | 92.30 | 58.53 | 77.17 | 95.26 |
4f 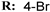 | 48.90 | 51.00 | 45.28 | 43.75 |
4g 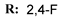 | 88.43 | 77.90 | 73.24 | 8.86 |
4h  | 78.64 | 85.02 | 65.40 | 84.80 |
 | ||||
| 5 | Cell Line IC50 (μM) | |||
| MCF7 | PC-3 | A549 | BGC-823 | |
5a  | 60.70 | 7.11 | 6.57 | 5.59 |
5b  | 805.00 | 7.72 | 11.90 | 13.40 |
 6 | ||||
6a  | 5.19 | 0.83 | 1.31 | 7.32 |
6b  | 51.70 | 10.40 | 268.10 | 84.50 |
 7 | ||||
7a  | 16.20 | 0.39 | 0.71 | 4.18 |
7b  | 35.10 | 8.07 | 19.30 | 9.36 |
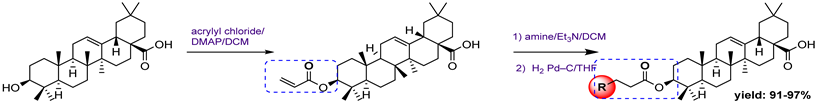 8 | ||||
8a 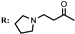 | 1.98 | 5.45 | 6.12 | 27.20 |
8b 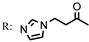 | - | 50.50 | 0.22 | 76.30 |
8c 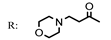 | 7.18 | 6.38 | 727.50 | 81.90 |
 | ||||||
| 9 | Cell Line: Conc. (50 μM) | |||||
| Colo-205 | SW-620 | SiHa | HeLa | A-549 | IMR-32 | |
9a  | 0 | 1.00 | 15 | 0 | 31.00 | 16.00 |
9b  | 0 | 13.00 | 36.00 | 5.00 | 25.00 | 16.00 |
9c 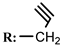 | 15.00 | 2.00 | 30.00 | 27.00 | 20.00 | 16.00 |
 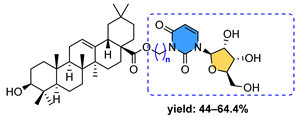 | |||||
| 10 | Cell line IC50 (μM) | ||||
| MCF-7 | A-549 | Hep-G2 | BGC-823 | PC-3 | |
| 10a n = 2 | 1.76 | 2.68 | 3.92 | 5.09 | 1.02 |
| 10b n = 6 | 2.77 | 6.04 | 3.89 | 3.49 | 167.46 |
| 10c n = 8 | 4.45 | 1.67 | 3.54 | 2.24 | 15.09 |
 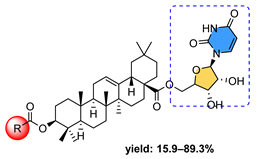 11 | |||||
11a  | 0.07 | 2.27 | 4.24 | <0.1 | 2.37 |
11b 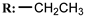 | 1.06 | 1.38 | 4.81 | 0.14 | 1.34 |
11c 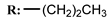 | 0.83 | 1.83 | 5.43 | 0.29 | 51.52 |
11d 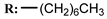 | 0.85 | 5.89 | 4.79 | <0.1 | 2.29 |
11e 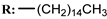 | 1.85 | 0.70 | 4.46 | - | 1.21 |
11f 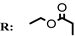 | 1.48 | 1.21 | 3.96 | 5.91 | 7.57 |
 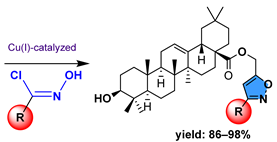 | |||
| 12 | Cell Line Viability (% Control) Conc. (100 μM) | Reference | |
| EMT-6 | SW480 | ||
12a 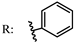 | 108.00 | 82.00 | [65] |
12b  | 40.00 | 71.00 | |
12c  | 44.00 | 61.00 | |
12d 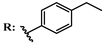 | 116.00 | 106.00 | |
12e 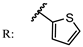 | 32.00 | 57.00 | |
12f 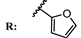 | 55.00 | 90.00 | |
13  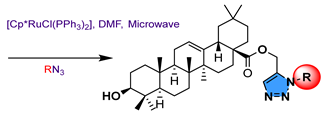 14 | |||
13–14a 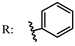 | - | - | [66] |
13b 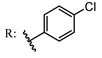 14b | 42.00 94.00 | 15.00 102.00 | |
13c 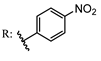 14c | - | - | |
13d 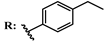 14d | 78.00 74.00 | 108.00 105.00 | |
13e  | 102.00 60.00 | 112.00 80.00 | |
| 14e | |||
13f  | 106.00 | 104.00 | |
 | |||||
| 15 | Cell Line IC50 (μM) | Reference | |||
| 16 BEL-7 | HepG2 | LO2 | A549 | ||
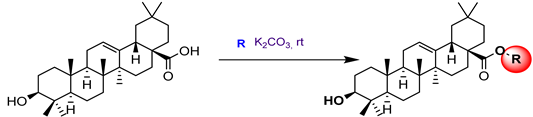
15a 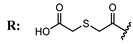 | 9.81 | 40.91 | 26.73 | - | [67] |
15b 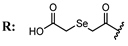 | 7.08 | 46.67 | 30.25 | - | |
15c 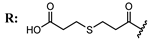 | 5.70 | 47.22 | 25.47 | - | |
15d 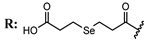 | 10.12 | 30.06 | 13.28 | - | |
 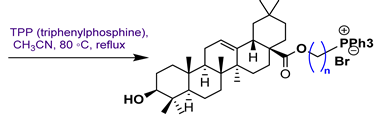 16 | |||||
| 16a n = 3 | - | 1.06 | - | 0.93 | [68] |
| 16b n = 5 | - | 1.32 | - | 0.81 | |
 | ||||
| 17 | Cell Line: IC50 (μM) | Reference | ||
| BEAS-2B | A549 | PC3 | ||
17a  | - | - | 3.71 | [69] |
17b 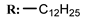 | - | - | 14.63 | |
17c 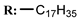 | - | - | 8.26 | |
17d 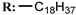 | - | - | 21.93 | |
17e 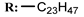 | - | - | 15.90 | |
17f  | - | - | 6.08 | |
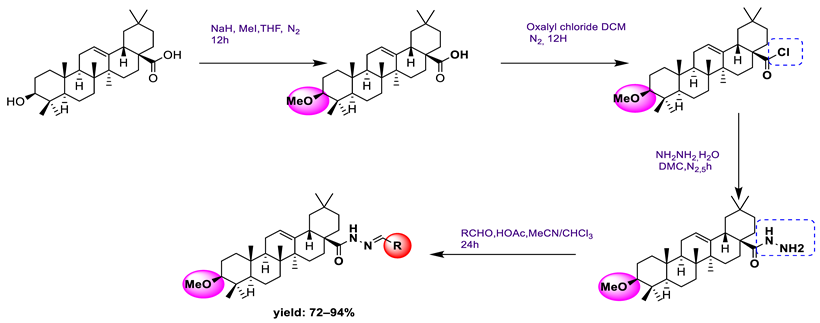 18 | [70] | |||
18a  | 1.15 | 0.25 | - | |
18b 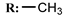 | 2.96 | 0.08 | - | |
18c 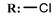 | 2.76 | 0.35 | - | |
18d  | 2.31 | 0.31 | - | |
18e 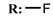 | 5.26 | 1.72 | - | |
 19 | [71] | |||
19a  | - | - | 17.08 | |
19b  | - | - | 7.87 | |
19c  | - | - | 8.86 | |
19d  | - | - | 9.90 | |
19e 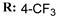 | - | - | 8.76 | |
19f 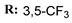 | - | - | 17.08 | |
19g 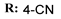 | - | - | 13.92 | |
19h  | - | - | 10.90 | |
19i  | - | - | 17.62 | |
 20 | |||
| Cell Line IC50 (µM) | Reference | ||
| K562 | K562/ADR | ||
| [72] | |||
| 20a n = 2 | 89.63 | >200 | |
| 20b n = 4 | 92.39 | >200 | |
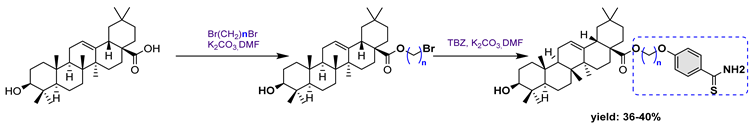 21 | |||
| 21a n = 6 | 55.20 | 74.60 | |
| 21b n = 8 | 95.00 | 181.00 | |
 22 | ||||||||
| Cell Line IC50 (µM) | Reference | |||||||
| A549 | Hep3B | Huh-7 | HT-29 | Hela | LO2 | RAW264.7 | ||
| [73] | ||||||||
22a 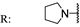 | 92.10 | >200 | 144.90 | - | 89.40 | 113.40 | - | |
22b  | 135.80 | >200 | >200 | 100.30 | 77.10 | 136.00 | - | |
22c  | 64.30 | - | >200 | >200 | 133.70 | - | - | |
22d 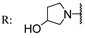 | 42.50 | 26.30 | 64.60 | 18.30 | 11.90 | 34.10 | - | |
22e 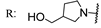 | 28.80 | 15.20 | 29.90 | 17.60 | 7.00 | 62.80 | - | |
22f 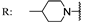 | - | 176.50 | 96.90 | 106.7 | 106.20 | >200 | - | |
22g 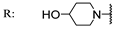 | 24.40 | 18.70 | 70.60 | 18.4 | 7.80 | 30.30 | - | |
22h  | 33.60 | 16.90 | 49.40 | 7.60 | 10.90 | 25.20 | - | |
22i 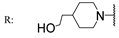 | >200 | >200 | 106.70 | >200 | 49.80 | >200 | - | |
 23 | [74] | |||||||
23a 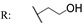 | - | - | - | - | - | - | - | |
23b  | - | - | - | - | - | - | - | |
23c 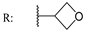 | - | - | - | - | - | - | 27.30 | |
23d 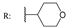 | - | - | - | - | - | - | 40.45 | |
23e 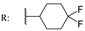 | - | - | - | - | - | - | 39.28 | |
23f 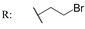 | - | - | - | - | - | - | 2.67 | |
23g 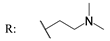 | - | - | - | - | - | - | 9.17 | |
23h 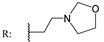 | - | - | - | - | - | - | 9.31 | |
23i 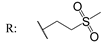 | - | - | - | - | - | - | 28.30 | |
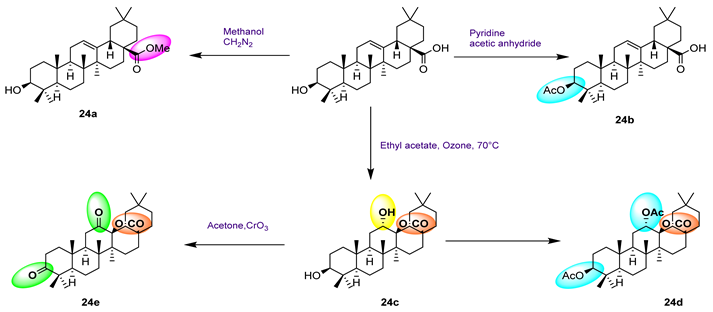 | ||||||
| 24 | IC50 (μM) | Reference | ||||
| α-Glucosidase | α-Amylase | GPa | PTP1B | Positive Control | ||
24a  | 55.09 | Deoxynojirimycin: 330.00 | [78] | |||
24b  | 19.01 | |||||
24c 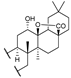 | 7.97 | |||||
24d 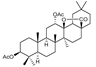 | 89.71 | |||||
24e 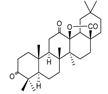 | 21.63 | |||||
 25 | ||||||
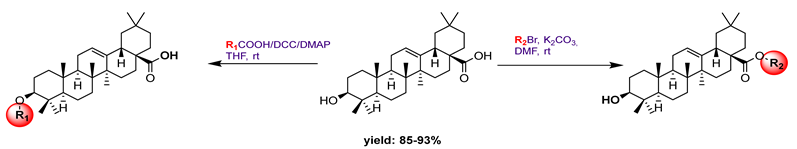
25a R1:  R2: R2:  | 34.30 | Caffeine: 114 | [79] | |||
25b R1: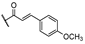 R2: R2: 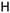 | 3.30 | |||||
25c R1: 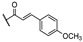 R2: R2: 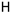 | 16.90 | |||||
25d R1:  R2: R2: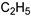 | - | |||||
25e R1: 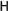 R2: R2: 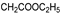 | - | |||||
25f R1: 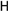 R2: R2:  | 62.60 | |||||
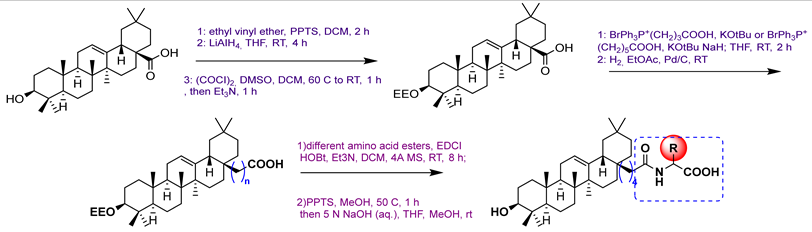 26 | ||||||
26a 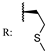 | 0.55 | - | [80] | |||
26b  | 0.51 | |||||
26c 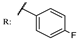 | 0.57 | |||||
26d 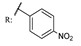 | 0.45 | |||||
26e 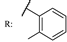 | 0.55 | |||||
26f 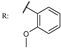 | 0.53 | |||||
26g 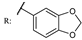 | 0.44 | |||||
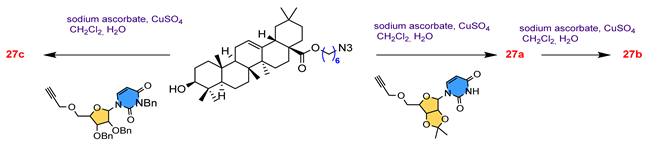 27 | ||||||
27a 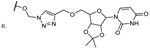 | 35.00 | Caffeine: 98.50 | [81] | |||
27b 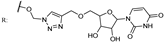 | 15.50 | |||||
27c 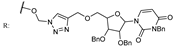 | 85.50 | |||||
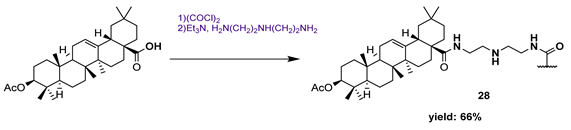 | Caffeine: 102.30 | [82] | ||||
| 28 | 3.25 | |||||
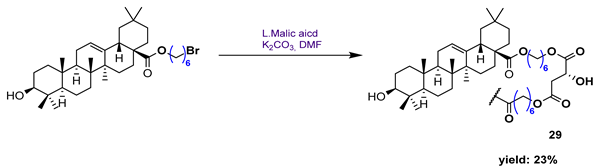 | ||||||
| 29 | 12.30 | |||||
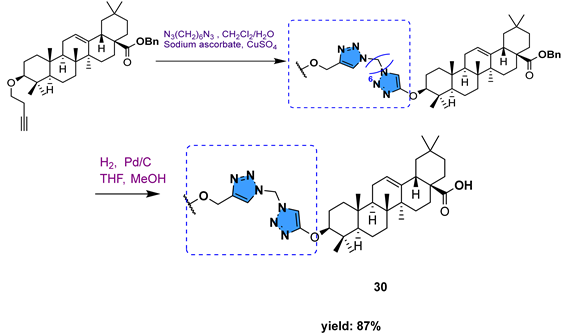 | ||||||
| 30 | 2.59 | |||||
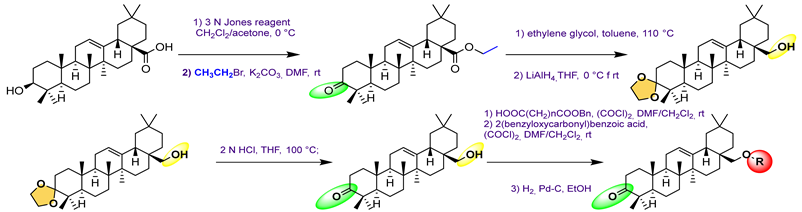 31 | [83] | |||||
31a 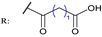 | 4.11 | Sodium Orthovanadate: - | ||||
31b  | 4.61 | |||||
31c  | 6.39 | |||||
31d 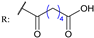 | - | |||||
31e 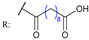 | - | |||||
31f 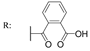 | 3.12 | |||||
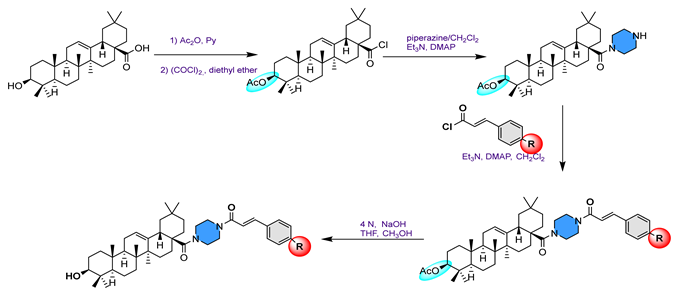 32 | [84] | |||||
32a  | 92.60 | Acarbose: 388.100 | ||||
32b 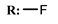 | 149.70 | |||||
32c 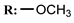 | 130.00 | |||||
32d 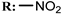 | 35.50 | |||||
 33 | ||||||
33a 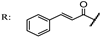 | 5.90 | Acarbose: 388.100 | ||||
33b  | 19.70 | |||||
33c 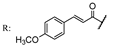 | 7.90 | |||||
33d 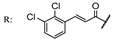 | 1.90 | |||||
33e 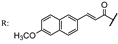 | 3.90 | |||||
33f 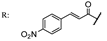 | 5.90 | |||||
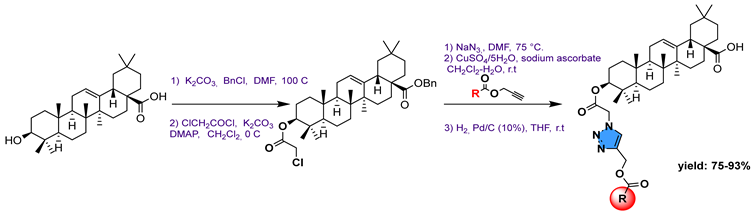 34 | ||||||
34a  | 77.10 | Caffeine: 144.00 | [75] | |||
34b 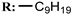 | 25.60 | |||||
34c 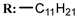 | 8.40 | |||||
34e 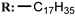 | 11.20 | |||||
34f 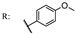 | 34.60 | |||||
34g 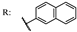 | 5.40 | |||||
 | [85] | |||||
| 35a | 1.91 | Sodium Orthovanadate: 8.54 | ||||
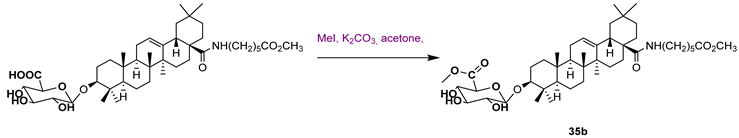 | ||||||
| 35b | 12.20 | |||||
 | ||||||
| 35c | 0.56 | |||||
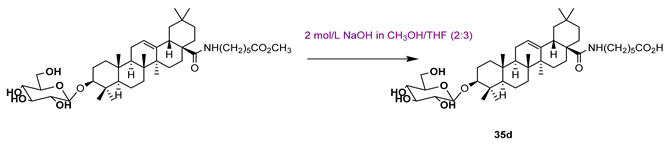 | ||||||
| 35d | 9.21 | |||||
 | [86] | |||||
| 36a | 3.20 | Acarbose: >300.00 | ||||
 | ||||||
36b  | 76.90 | |||||
36c 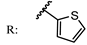 | 13.50 | |||||
 | ||||||
36d  | 4.10 | |||||
36e  | 11.50 | |||||
 37 | [87] | |||||
37a 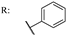 | 3.43 | Acarbose: 579.15 | ||||
37b  | 0.98 | |||||
37c 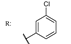 | 0.72 | |||||
37d 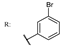 | 1.29 | |||||
37e 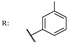 | 2.74 | |||||
37f 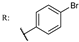 | 3.29 | |||||
37g 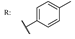 | 0.33 | |||||
37h 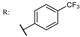 | 1.74 | |||||
37i  | 0.69 | |||||
37j 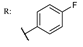 | 1.92 | |||||
37k 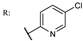 | 3.67 | |||||
37l  | 1.17 | |||||
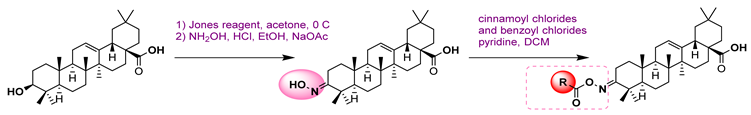 38 | [88] | |||||
38a  | 0.35 | 7.86 | Acarbose: 665.56 | |||
38b  | 0.68 | 15.26 | ||||
38c  | 0.89 | 25.37 | ||||
38d 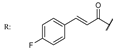 | 1.45 | 17.47 | ||||
38e 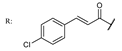 | 0.95 | 5.64 | ||||
38f 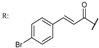 | 1.28 | 3.80 | ||||
38g  | 3.13 | 12.57 | ||||
38h 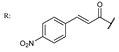 | 1.36 | 18.29 | ||||
38i  | 0.67 | 25.57 | ||||
38j  | 0.99 | 55.30 | ||||
38k 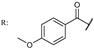 | 1.10 | 35.47 | ||||
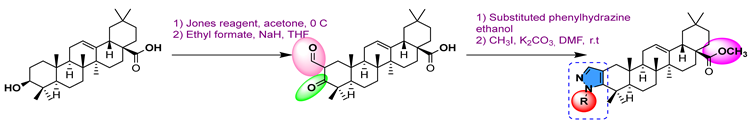 39 | [89] | |||||
39a  | 2.44 | 40.36 | Acarbose: 70.82 | |||
39b 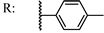 | 2.60 | 55.12 | ||||
39c 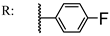 | 4.51 | 62.18 | ||||
39d 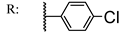 | 2.64 | 87.23 | ||||
39e 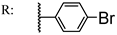 | 2.79 | 20.46 | ||||
39f  | 4.86 | 36.18 | ||||
 40 | [90] | |||||
40a  | 2.40 | Acarbose: 436.00 | ||||
40b 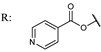 | 4.56 | |||||
40c  | 752.60 | |||||
  | ||||||
| 40d | 3.01 | |||||
 | ||||||
| 40e | 12.37 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triaa, N.; Znati, M.; Ben Jannet, H.; Bouajila, J. Biological Activities of Novel Oleanolic Acid Derivatives from Bioconversion and Semi-Synthesis. Molecules 2024, 29, 3091. https://doi.org/10.3390/molecules29133091
Triaa N, Znati M, Ben Jannet H, Bouajila J. Biological Activities of Novel Oleanolic Acid Derivatives from Bioconversion and Semi-Synthesis. Molecules. 2024; 29(13):3091. https://doi.org/10.3390/molecules29133091
Chicago/Turabian StyleTriaa, Nahla, Mansour Znati, Hichem Ben Jannet, and Jalloul Bouajila. 2024. "Biological Activities of Novel Oleanolic Acid Derivatives from Bioconversion and Semi-Synthesis" Molecules 29, no. 13: 3091. https://doi.org/10.3390/molecules29133091
APA StyleTriaa, N., Znati, M., Ben Jannet, H., & Bouajila, J. (2024). Biological Activities of Novel Oleanolic Acid Derivatives from Bioconversion and Semi-Synthesis. Molecules, 29(13), 3091. https://doi.org/10.3390/molecules29133091









