Increasing 1,4-Diaminobutane Production in Escherichia coli by Optimization of Cofactor PLP and NADPH Synthesis
Abstract
:1. Introduction
2. Results
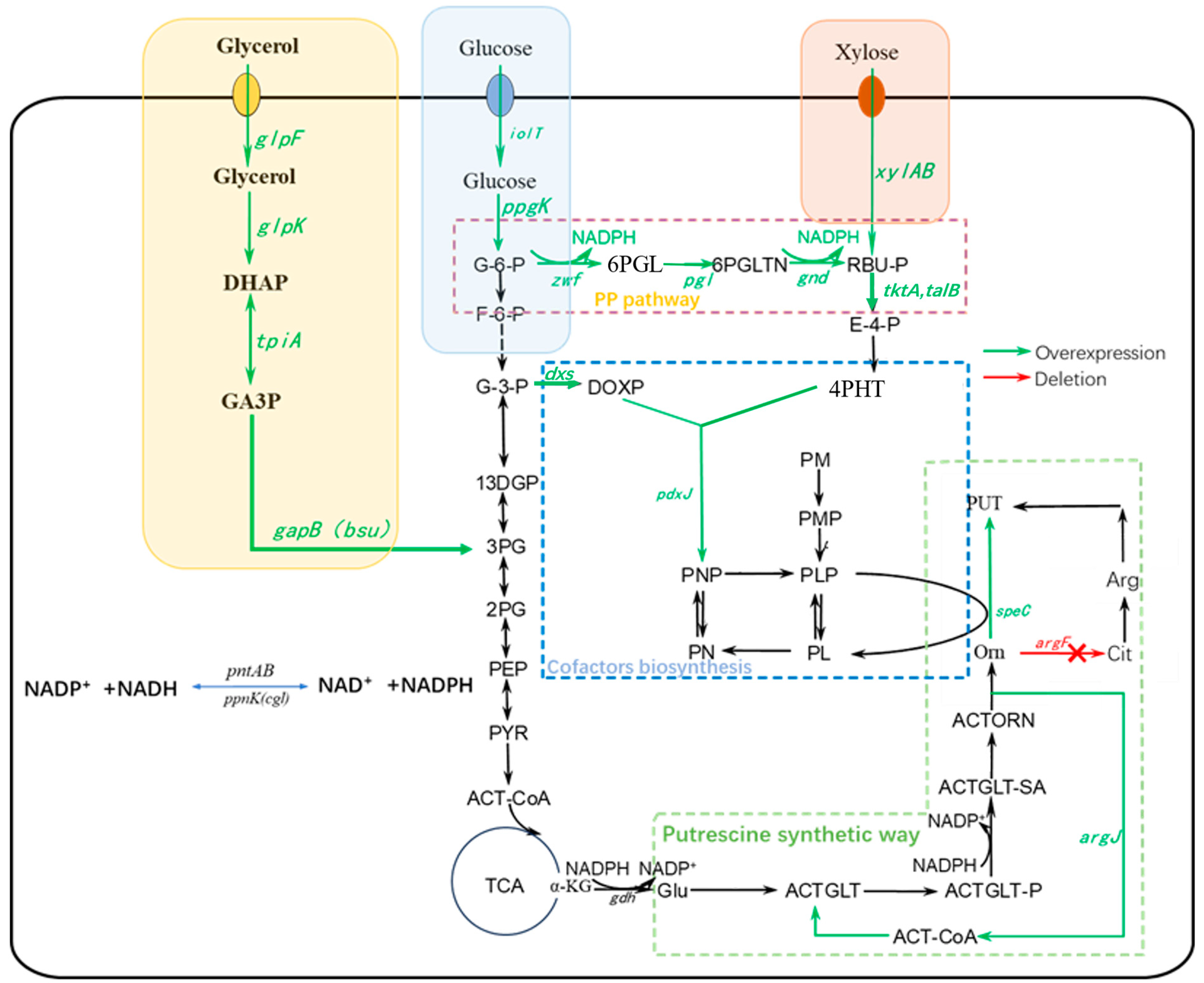
2.1. Optimisation of NADPH Synthesis in the 1,4-Diaminobutane Synthetic Pathway
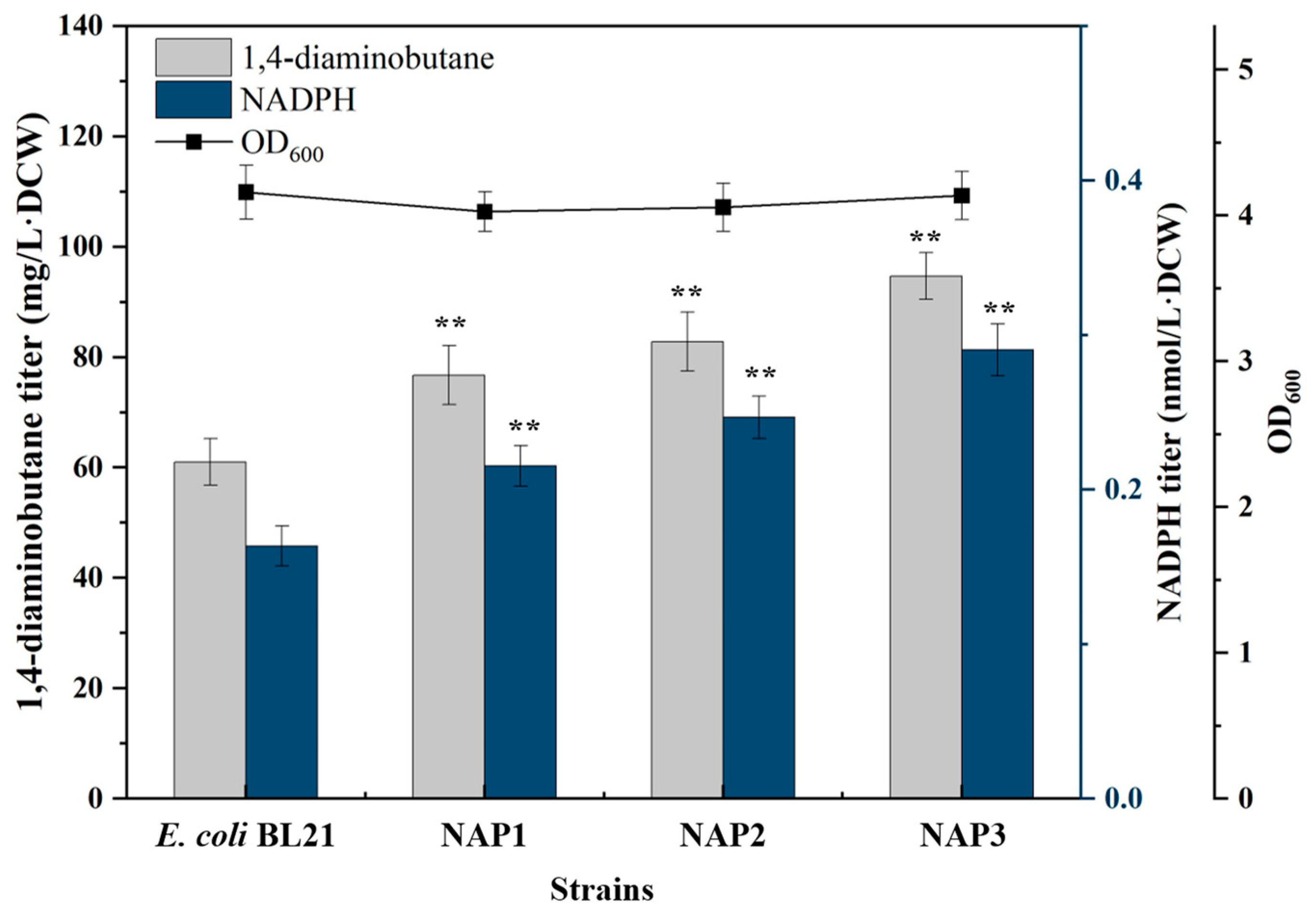
2.1.1. Overexpression of ppnK and pntAB Increased NADPH Supply
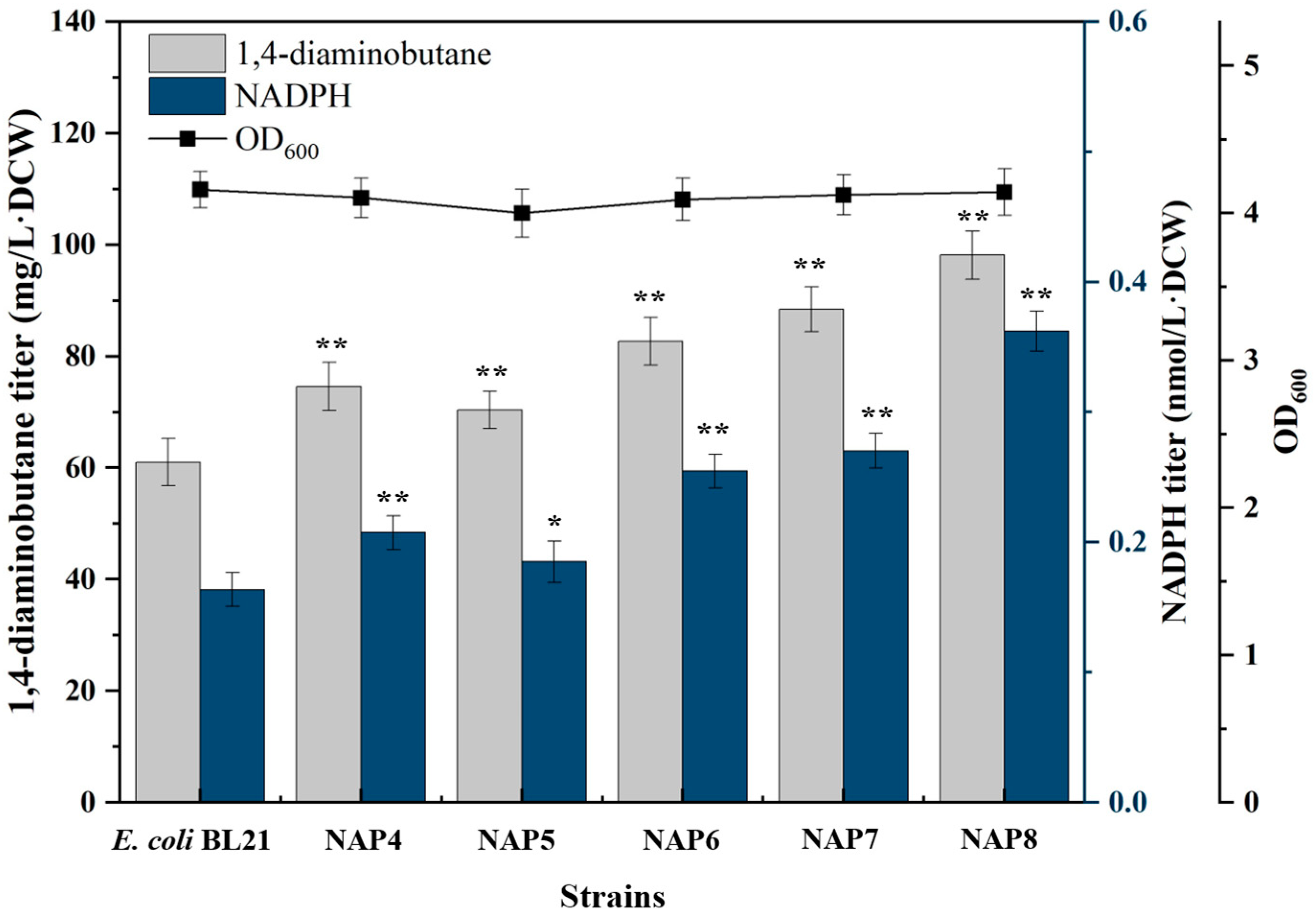
2.1.2. Overexpression of PP Pathway Genes Increased NADPH Supply
2.2. Optimization of PLP Supply in the 1,4-Diaminobutane Synthesis Pathway
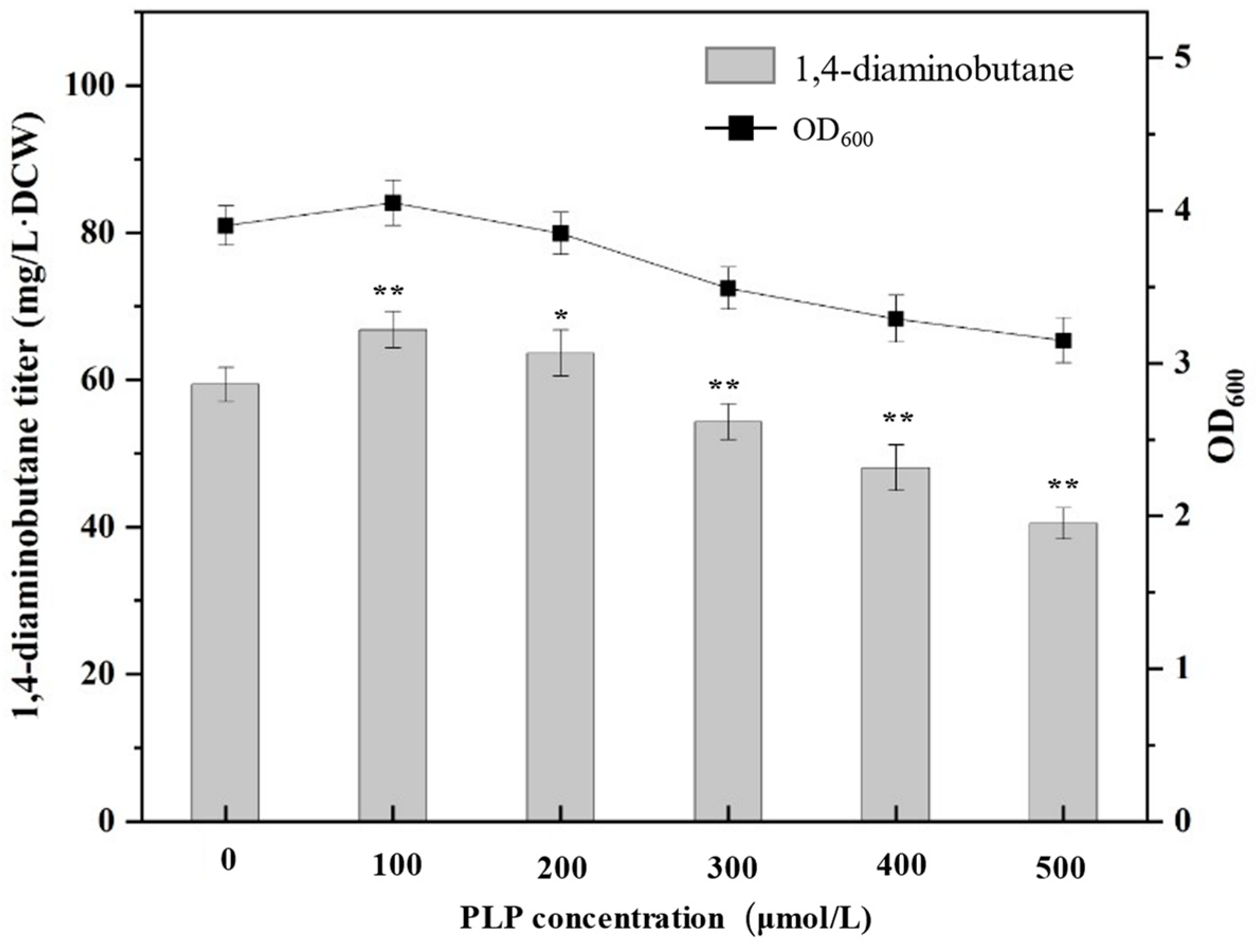
2.2.1. Effect of Exogenous Supplementation of PLP on the Synthesis of 1,4-Diaminobutane in E. coli
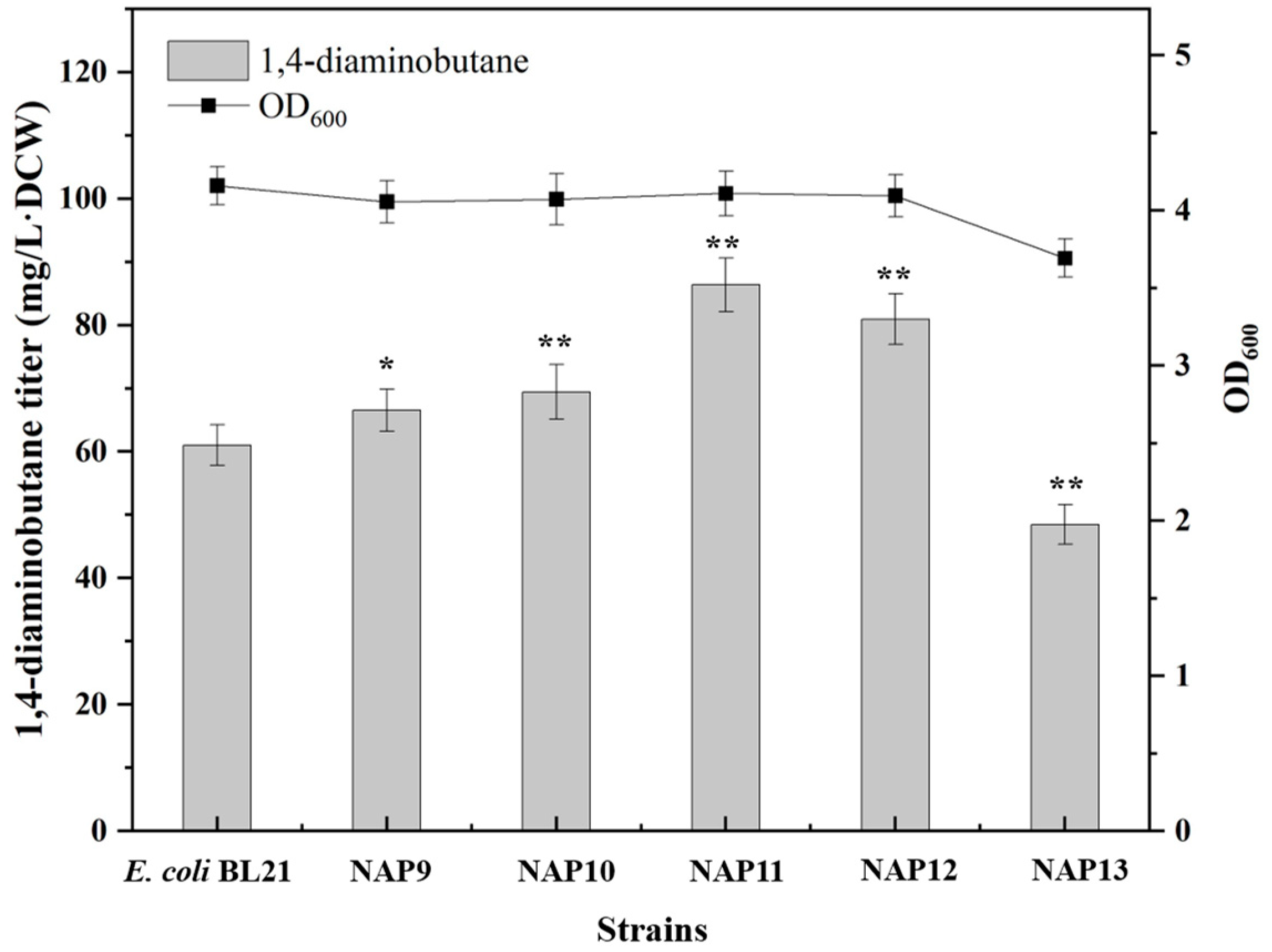
2.2.2. Enhancing the Synthesis of PLP
2.3. Overexpression of 1,4-Diaminobutane Synthesis Module
2.4. Effect of Increasing NADPH and PLP Synthesis on 1,4-Diaminobutane Yield When Utilizing Different Carbon Sources
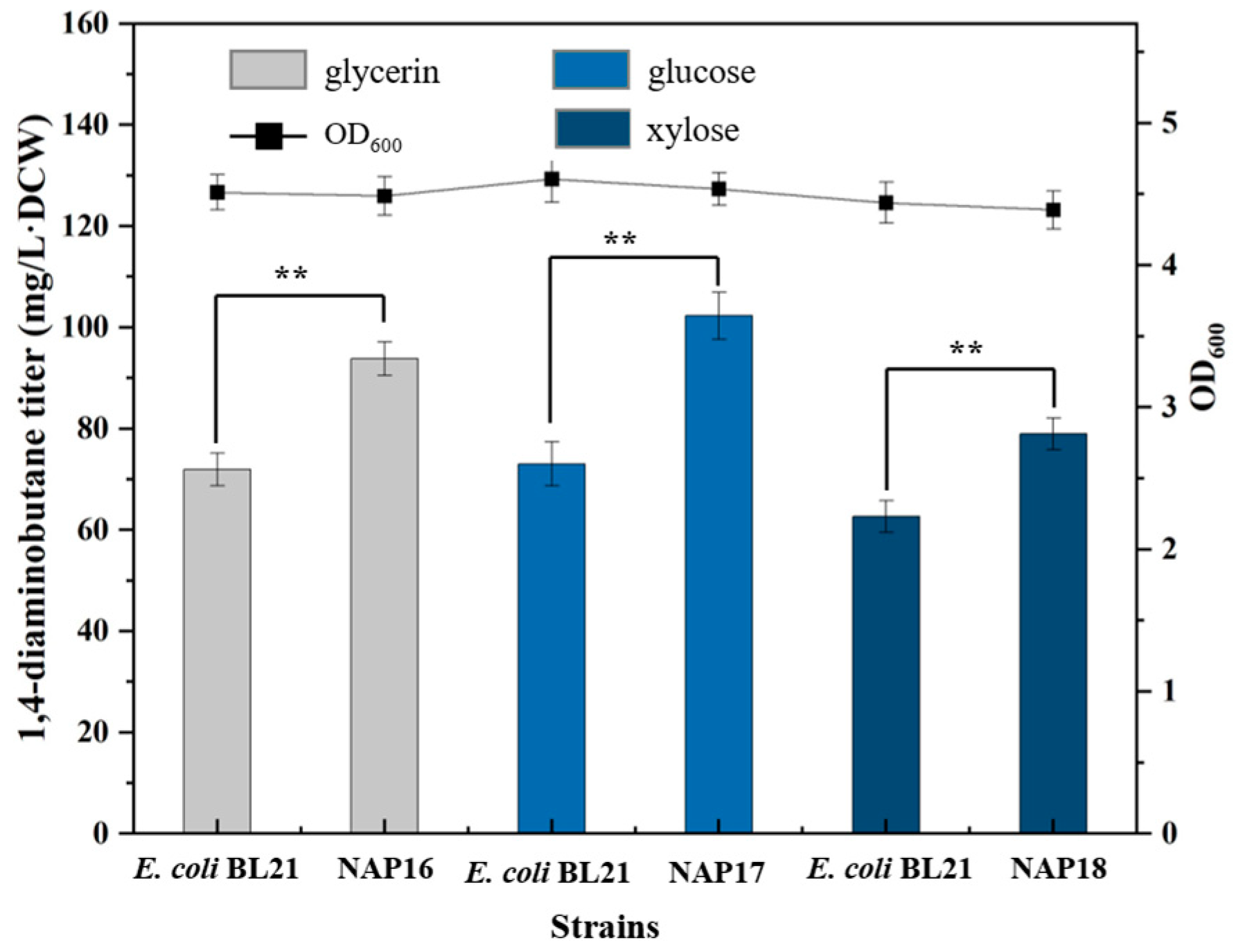
Overexpression of Carbon Source Utilization Pathway Genes
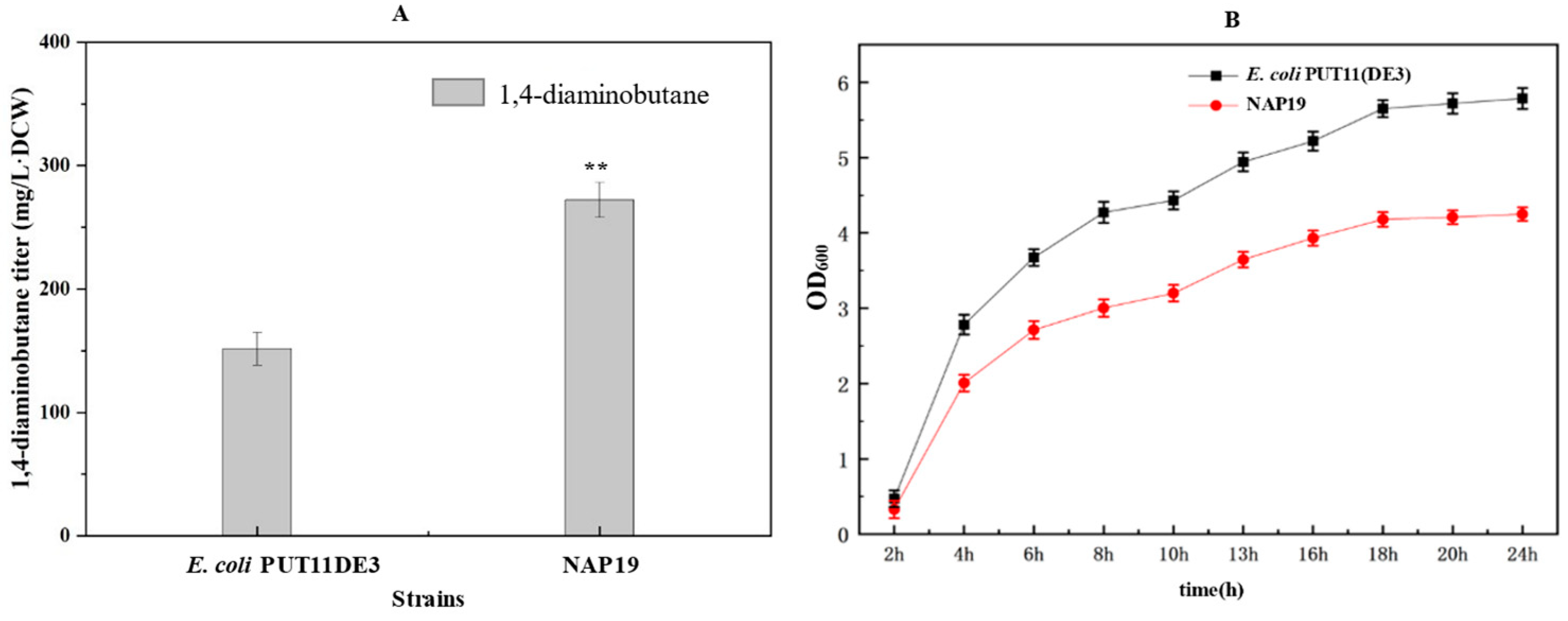
2.5. Fermentation Results of Recombinant Strains
3. Discussion
4. Materials and Methods
4.1. Strain, Plasmid and Culture Conditions
4.2. Plasmids Construction
4.3. Shake Flask Culture and Fermentation
- (1)
- SOB medium (Super Optimal Broth): 20 g/L tryptone, 5 g/L yeast extract, 0.5 g/L NaCl, add 10 mL of 250 mmol/L KCl solution, and adjust pH to 7.0. Supplemented with 1 g/L carbon source. The solution was autoclaved at 115 °C for 20 min. 5 mL of sterilised 2 mol/L MgCl2 was added before use.
- (2)
- R/2 medium: 5 g/L yeast extract, 1 g/L peptone, 2 g/L (NH4)2HPO4, 6.75 g/L KH2PO4, 0.85 g/L citric acid, 0.7 g/L MgSO4·7H2O, 10 g/L glucose, 3 g/L (NH4)2SO4 and 5 mL/L trace metal solution. The pH was adjusted to 6.8 and autoclaved at 115 °C for 20 min. The trace metal solution contained 5 mol/L HCl, 10 g/L FeSO4·7H2O. 2.25 g/L ZnSO4·7H20, 1 g/L CuSO4·5H2O, 0.5 g/L MnSO4·5H2O, 1.23 g/L Na2B4O7·10H2O, 2 g/LCaCl2·2H2O and 0.1 g/L (NH4)6Mo7O24.
4.4. Analytical Method
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Zhao, P.; Kuai, J.; Chang, C.; Yuan, Q. Spermidine alleviates lipopolysaccharide-induced myocardial injury in mice by suppressing apoptosis, ROS production and ferroptosis. J. South. Med. Univ. 2024, 44, 166–172. [Google Scholar]
- Holbert, C.E.; Cullen, M.T.; Casero, R.A., Jr.; Stewart, T.M. Polyamines in cancer: Integrating organismal metabolism and antitumour immunity. Nat. Rev. Cancer 2022, 22, 467–480. [Google Scholar] [CrossRef]
- Pérez-Pertejo, Y.; García-Estrada, C.; Martínez-Valladares, M.; Murugesan, S.; Reguera, R.M.; Balaña-Fouce, R. Polyamine Metabolism for Drug Intervention in Trypanosomatids. Pathogens 2024, 13, 79. [Google Scholar] [CrossRef]
- Lee, P.C.; Kim, S.Y.; Ko, Y.K.; Ha, J.U.; Jeoung, S.K.; Shin, D.; Kim, J.H.; Kim, M.G. Tribological Properties of Polyamide 46/Graphene Nanocomposites. Polymers 2022, 14, 1139. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.Z. Transcriptomic Changes in Response to Putrescine Production in Metabolically Engineered Corynebacterium glutamicum. Front. Microbiol. 2017, 8, 1987. [Google Scholar] [CrossRef]
- Sanders, J.; Scott, E.; Weusthuis, R.; Mooibroek, H. Biorefinery as the bioinspired process to bulk chemicals. Macromol. Biosci. 2007, 7, 105–117. [Google Scholar] [CrossRef]
- Son, J.; Sohn, Y.J.; Baritugo, K.A. Recent advances in microbial production of diamines, aminocarboxylic acids, and diacids as potential platform chemicals and bio-based polyamides monomer. Biotechnol. Adv. 2023, 62, 108070. [Google Scholar] [CrossRef]
- Yang, S.-C.; Ting, W.-W.; Ng, I.-S. Effective whole cell biotransformation of arginine to a four-carbon diamine putrescine using engineered Escherichia coli. Biochem. Eng. J. 2022, 185, 108502. [Google Scholar]
- Qian, Z.G.; Xia, X.X.; Lee, S.Y. Metabolic engineering of Escherichia coli for the production of putrescine: A four carbon diamine. Biotechnol. Bioeng. 2009, 104, 651–662. [Google Scholar] [CrossRef]
- Li, Z.; Shen, Y.P.; Jiang, X.L.; Feng, L.S.; Liu, J.Z. Metabolic evolution and a comparative omics analysis of Corynebacterium glutamicum for putrescine production. J. Ind. Microbiol. Biotechnol. 2018, 45, 123–139. [Google Scholar] [CrossRef]
- Nguyen, A.Q.; Schneider, J.; Wendisch, V.F. Elimination of polyamine N-acetylation and regulatory engineering improved putrescine production by Corynebacterium glutamicum. J. Biotechnol. 2015, 201, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.Y.; Kim, J.Y.; Upadhyay, R.; Park, B.J.; Lee, J.M.; Kim, B.G. Rational engineering of ornithine decarboxylase with greater selectivity for ornithine over lysine through protein network analysis. J. Biotechnol. 2018, 281, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Gou, L.; Fan, T.P.; Cai, Y. Enzymatic properties of ornithine decarboxylase from Clostridium aceticum DSM1496. Biotechnol. Appl. Biochem. 2024, 71, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, F.; Sun, X. Chassis Strain of Escherichia coli Producing 1,4-Diaminobutane and Its Application. CN202311595033.0, 27 March 2024. [Google Scholar]
- Schneider, J.; Wendisch, V.F. Biotechnological production of polyamines by bacteria: Recent achievements and future perspectives. Appl. Microbiol. Biotechnol. 2011, 91, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.; Yoo, S.M.; Kim, W.J.; Lee, S.Y. Gene Expression Knockdown by Modulating Synthetic Small RNA Expression in Escherichia coli. Cell Syst. 2017, 5, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.V.; Eberhardt, D.; Wendisch, V.F. Modular pathway engineering of Corynebacterium glutamicum for production of the glutamate-derived compounds ornithine, proline, putrescine, citrulline, and arginine. J. Biotechnol. 2015, 214, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lee, J.; Lee, S.Y. Metabolic engineering of Corynebacterium glutamicum for the production of L-ornithine. Biotechnol. Bioeng. 2015, 112, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Sauer, U.; Canonaco, F.; Heri, S.; Perrenoud, A.; Fischer, E. The soluble and membrane-bound transhydrogenases UdhA and PntAB have divergent functions in NADPH metabolism of Escherichia coli. J. Biol. Chem. 2004, 279, 6613–6619. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Mori, S.; Mukai, T.; Suzuki, S.; Yamada, T.; Hashimoto, W.; Murata, K. Inorganic Polyphosphate/ATP-NAD kinase of Micrococcus flavus and Mycobacterium tuberculosis H37Rv. Biochem. Biophys. Res. Commun. 2000, 276, 57–63. [Google Scholar] [CrossRef]
- Shi, F.; Li, Y.; Li, Y.; Wang, X. Molecular properties, functions, and potential applications of NAD kinases. Acta Biochim. Biophys. Sin. 2009, 41, 352–361. [Google Scholar] [CrossRef]
- Okazaki, S.; Suzuki, A.; Mizushima, T.; Kawano, T.; Komeda, H.; Asano, Y.; Yamane, T. The novel structure of a pyridoxal 5′-phosphate-dependent fold-type I racemase, alpha-amino-epsilon-caprolactam racemase from Achromobacter obae. Biochemistry 2009, 48, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.B.; Amrhein, N.; Kappes, B.; Macheroux, P.; Tews, I.; Raschle, T. Two independent routes of de novo vitamin B6 biosynthesis: Not that different after all. Biochem. J. 2007, 407, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Schiroli, D.; Peracchi, A. A subfamily of PLP-dependent enzymes specialized in handling terminal amines. Biochim. Biophys. Acta 2015, 1854, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Bisercić, M.; Feutrier, J.Y.; Reeves, P.R. Nucleotide sequences of the gnd genes from nine natural isolates of Escherichia coli: Evidence of intragenic recombination as a contributing factor in the evolution of the polymorphic gnd locus. J. Bacteriol. 1991, 173, 3894–3900. [Google Scholar] [CrossRef]
- Moccand, C.; Kaufmann, M.; Fitzpatrick, T.B. It takes two to tango: Defining an essential second active site in pyridoxal 5′-phosphate synthase. PLoS ONE 2011, 6, e16042. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, J.; Kim, H.J.; Sathiyanarayanan, G.; Bhatia, S.K.; Song, H.S.; Choi, Y.K.; Kim, Y.G.; Park, K.; Yang, Y.H. Biotransformation of pyridoxal 5′-phosphate from pyridoxal by pyridoxal kinase (pdxY) to support cadaverine production in Escherichia coli. Enzym. Microb. Technol. 2017, 104, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Xu, B.; Zeng, W.; Zhou, J. Regulating the biosynthesis of pyridoxal 5′-phosphate with riboswitch to enhance L-DOPA production by Escherichia coli whole-cell biotransformation. J. Biotechnol. 2020, 321, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Cao, W.; Zhang, B.; Chen, K.; Liu, Q.; Li, Y.; Ouyang, P. Engineering a pyridoxal 5′-phosphate supply for cadaverine production by using Escherichia coli whole-cell biocatalysis. Sci. Rep. 2015, 5, 15630. [Google Scholar] [CrossRef] [PubMed]
- Momany, C.; Ernst, S.; Ghosh, R.; Chang, N.L.; Hackert, M.L. Crystallographic structure of a PLP-dependent ornithine decarboxylase from Lactobacillus 30a to 3.0 A resolution. J. Mol. Biol. 1995, 252, 643–655. [Google Scholar] [CrossRef]
- Oliveira, E.F.; Cerqueira, N.M.; Fernandes, P.A.; Ramos, M.J. Mechanism of formation of the internal aldimine in pyridoxal 5′-phosphate-dependent enzymes. J. Am. Chem. Soc. 2011, 133, 15496–15505. [Google Scholar] [CrossRef]
- Seol, E.; Sekar, B.S.; Raj, S.M.; Park, S. Co-production of hydrogen and ethanol from glucose by modification of glycolytic pathways in Escherichia coli—from Embden-Meyerhof-Parnas pathway to pentose phosphate pathway. Biotechnol. J. 2016, 11, 249–256. [Google Scholar] [CrossRef]
- Moritz, B.; Striegel, K.; De Graaf, A.A.; Sahm, H. Kinetic properties of the glucose-6-phosphate and 6-phosphogluconate dehydrogenases from Corynebacterium glutamicum and their application for predicting pentose phosphate pathway flux in vivo. Eur. J. Biochem. 2000, 267, 3442–3452. [Google Scholar] [CrossRef]
- Hao, N.; Mu, J.; Hu, N.; Xu, S.; Shen, P.; Yan, M.; Li, Y.; Xu, L. Implication of ornithine acetyltransferase activity on l-ornithine production in Corynebacterium glutamicum. Biotechnol. Appl. Biochem. 2016, 63, 15–21. [Google Scholar] [CrossRef]
- Hao, N.; Mu, J.; Hu, N.; Xu, S.; Yan, M.; Li, Y.; Guo, K.; Xu, L. Improvement of L-citrulline production in Corynebacterium glutamicum by ornithine acetyltransferase. J. Ind. Microbiol. Biotechnol. 2015, 42, 307–313. [Google Scholar] [CrossRef]
- Caldovic, L.; Tuchman, M. N-acetylglutamate and its changing role through evolution. Biochem. J. 2003, 372 Pt 2, 279–290. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, G.; Chu, X.H.; Ye, B.C. Metabolic engineering of Corynebacterium glutamicum S9114 to enhance the production of l-ornithine driven by glucose and xylose. Bioresour. Technol. 2019, 284, 204–213. [Google Scholar] [CrossRef]
- Kim, S.M.; Choi, B.Y.; Ryu, Y.S.; Jung, S.H.; Park, J.M.; Kim, G.H.; Lee, S.K. Simultaneous utilization of glucose and xylose via novel mechanisms in engineered Escherichia coli. Metab. Eng. 2015, 30, 141–148. [Google Scholar] [CrossRef]
- Shams Yazdani, S.; Gonzalez, R. Engineering Escherichia coli for the efficient conversion of glycerol to ethanol and co-products. Metab. Eng. 2008, 10, 340–351. [Google Scholar] [CrossRef]
- Hawi, A.A.; Yip, H.; Sullivan, T.S.; Digenis, G.A. Development of an HPLC assay for the analysis of tetrafluoroputrescine—A putrescine analog. Anal. Biochem. 1988, 172, 235–240. [Google Scholar] [CrossRef]

| Strains | Genotypes | Source |
|---|---|---|
| E. coli str. K12 substr. MG1655 | Template for gene from E.coli cloning | Kept in our lab |
| C. glutamicum 13032 | Wild type, donor of ppnk, argJ gene | Kept in our lab |
| B. subtilis subsp. subtilis str. 168 | Wild type, donor of gapB gene | Kept in our lab |
| E. coli PUT11(DE3) | K12 MG1655△argR△patA△puuA△speED△speG△puuP△argF△ydcSTUV△potFGHI△plaP::DE3 | Kept in our lab |
| NAP1 | K12 harbouring pETM6-ppnk, Amp | This study |
| NAP2 | K12 harbouring pETM6-pntAB, Amp | This study |
| NAP3 | K12 harbouring pETM6-ppnk -pntAB, Amp | This study |
| NAP4 | BL21(DE3) harbouring pETM6-zwf, Amp | This study |
| NAP5 | BL21(DE3) harbouring pETM6-pgl, Amp | This study |
| NAP6 | BL21(DE3) harbouring pETM6-gnd, Amp | This study |
| NAP7 | BL21(DE3) harbouring pETM6-zwf-pgl, Amp | This study |
| NAP8 | BL21(DE3) harbouring pETM6-zwf-pgl-gnd, Amp | This study |
| NAP9 | BL21(DE3) harbouring pETM6-pdxJ, Amp | This study |
| NAP10 | BL21(DE3) harbouring pETM6-dxs, Amp | This study |
| NAP11 | BL21(DE3) harbouring pETM6-tktA, Amp | This study |
| NAP12 | BL21(DE3) harbouring pETM6-talB, Amp | This study |
| NAP13 | BL21(DE3) harbouring pETM6-pdxJ-dxs-tktA-talB, Amp | This study |
| NAP14 | NAP13 harbouring pRSM3-speC-argJ, Kan | This study |
| NAP15 | NAP14 harbouring pCDM4-pntAB-ppnK and pACM4-zwf-gnd-pgl, Cl | This study |
| NAP16 | BL21(DE3) harbouring pETM6-glpFK-tpiA-gapB, Amp | This study |
| NAP17 | BL21(DE3) harbouring pETM6-iolT-ppgK, Amp | This study |
| NAP18 | BL21(DE3) harbouring pETM6-xylAB, Amp | This study |
| NAP19 | PUT11(DE3) harbouring the pRSM3-glpFK-tpiA-gapB, pTrc99A-speC-argJ, pACM4-zwf-gnd-pgl, pCDM4-pdxJ-dxs-tktA-talB-pntAB-ppnk. Kan, Amp, Cl, Str | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, T.; Zhao, Y.; Wang, J.; Kang, W.; Sun, X.; Sun, Y.; Chu, M.; Liu, Z.; Lu, F.; Li, M. Increasing 1,4-Diaminobutane Production in Escherichia coli by Optimization of Cofactor PLP and NADPH Synthesis. Molecules 2024, 29, 3094. https://doi.org/10.3390/molecules29133094
Sun T, Zhao Y, Wang J, Kang W, Sun X, Sun Y, Chu M, Liu Z, Lu F, Li M. Increasing 1,4-Diaminobutane Production in Escherichia coli by Optimization of Cofactor PLP and NADPH Synthesis. Molecules. 2024; 29(13):3094. https://doi.org/10.3390/molecules29133094
Chicago/Turabian StyleSun, Tong, Yongcan Zhao, Jinjin Wang, Wenke Kang, Xiangxiang Sun, Yanling Sun, Meixue Chu, Zhengyu Liu, Fuping Lu, and Ming Li. 2024. "Increasing 1,4-Diaminobutane Production in Escherichia coli by Optimization of Cofactor PLP and NADPH Synthesis" Molecules 29, no. 13: 3094. https://doi.org/10.3390/molecules29133094






