Lattice Matching and Microstructure of the Aromatic Amide Fatty Acid Salts Nucleating Agent on the Crystallization Behavior of Recycled Polyethylene Terephthalate
Abstract
:1. Introduction
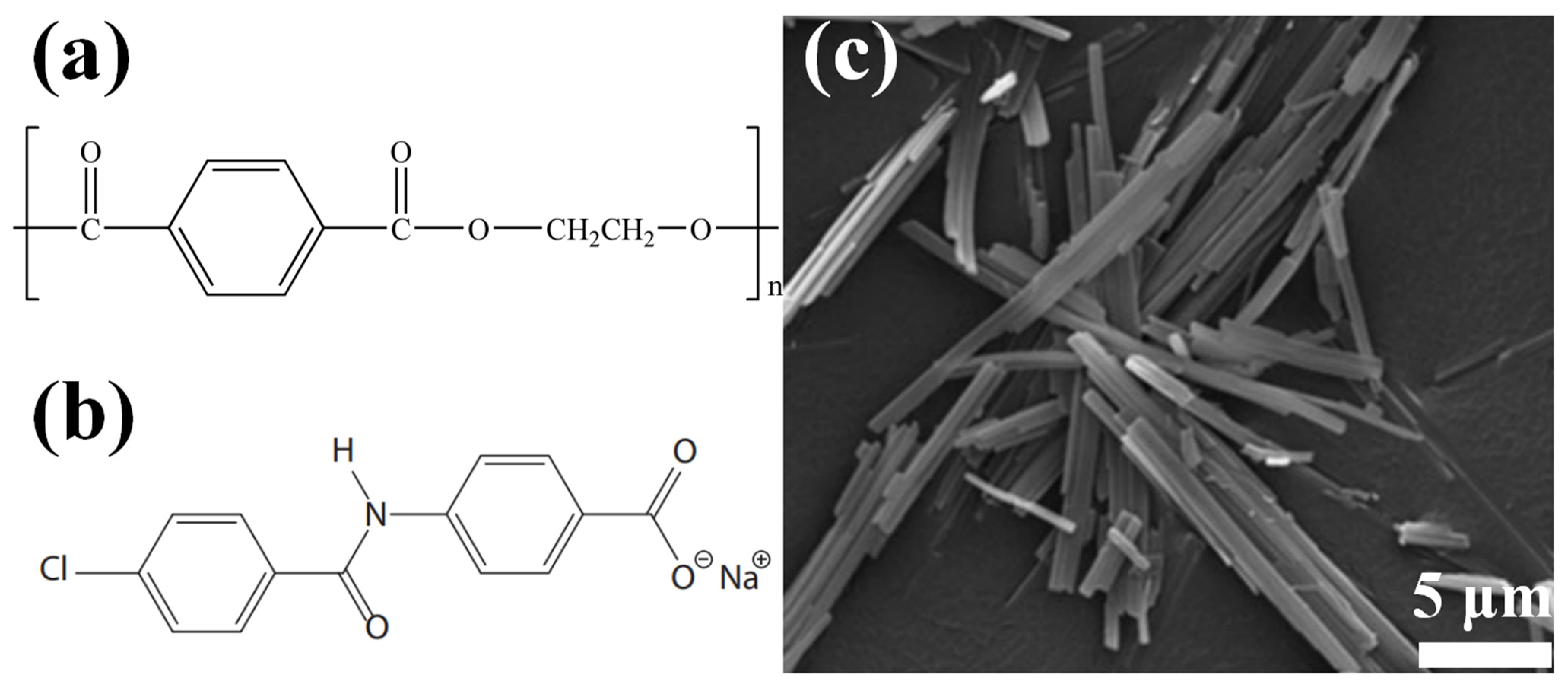
2. Results
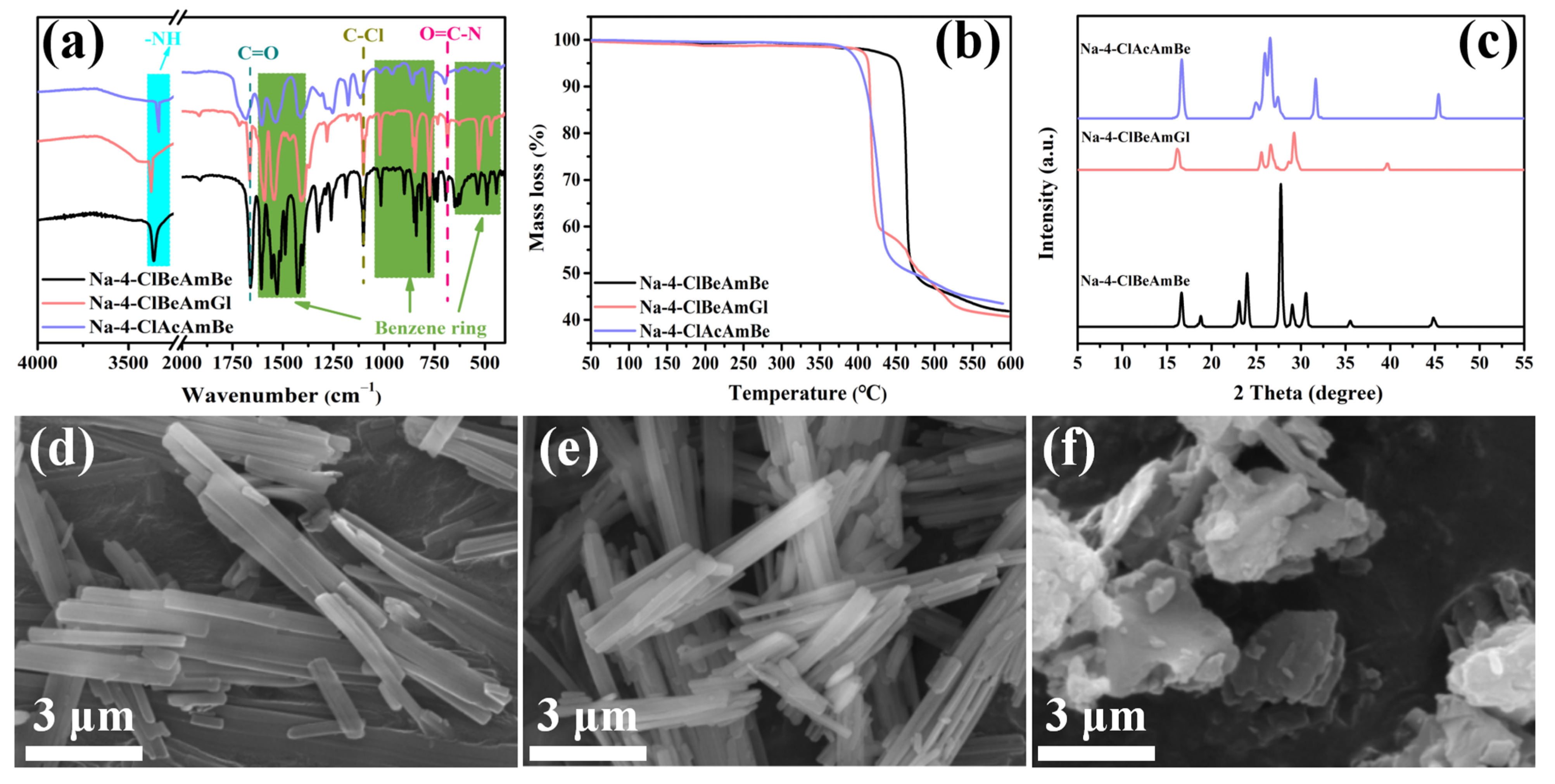
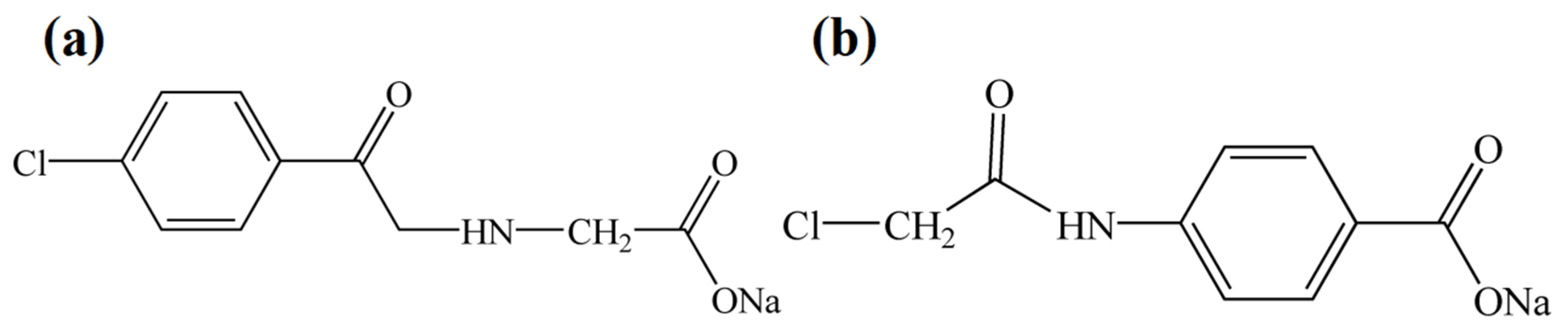
2.1. The Characterization Analysis of the Aromatic Amide Fatty Acid Salt Nucleating Agent
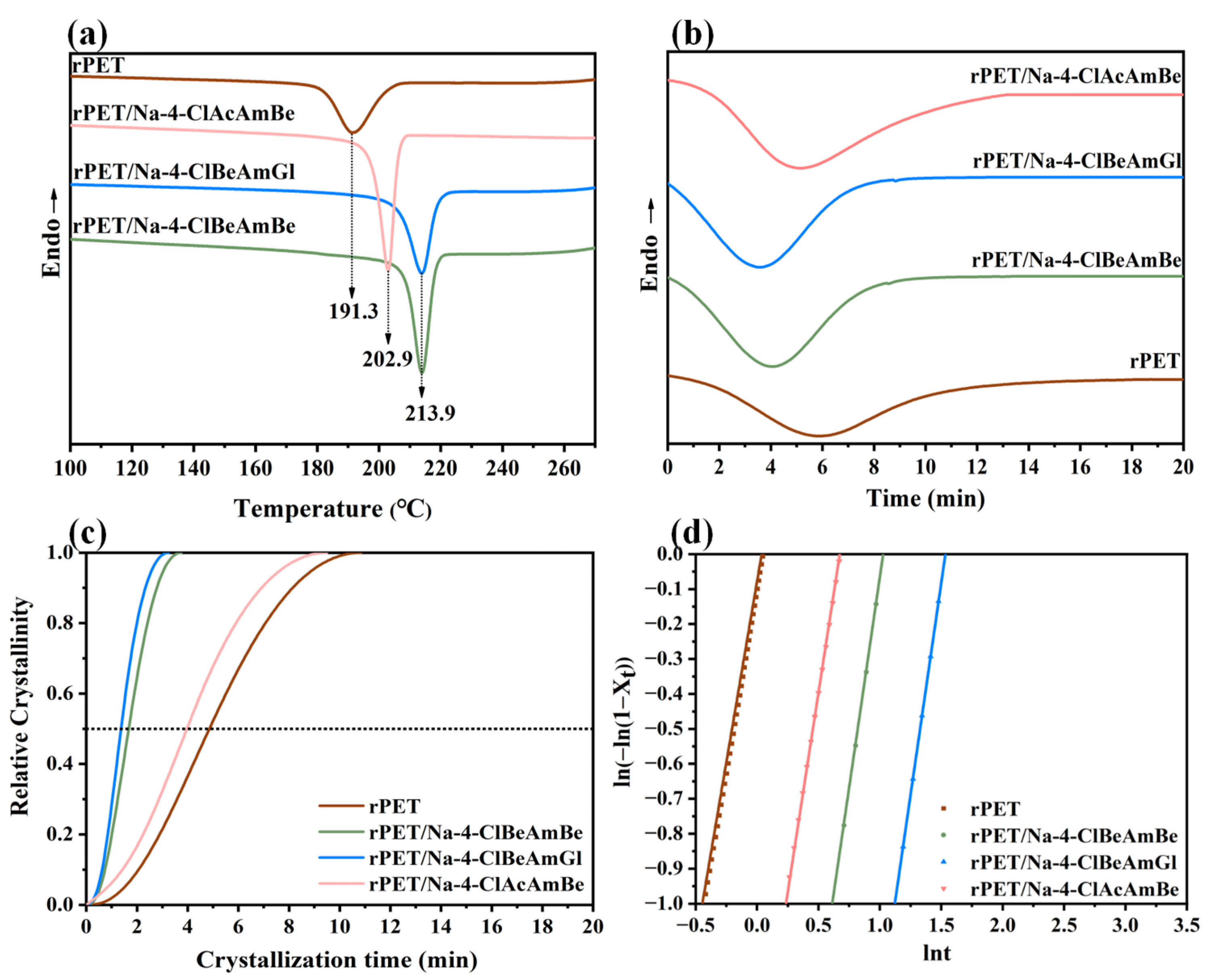
2.2. The Crystallization Behavior of the rPET/Nucleating Agent Blend
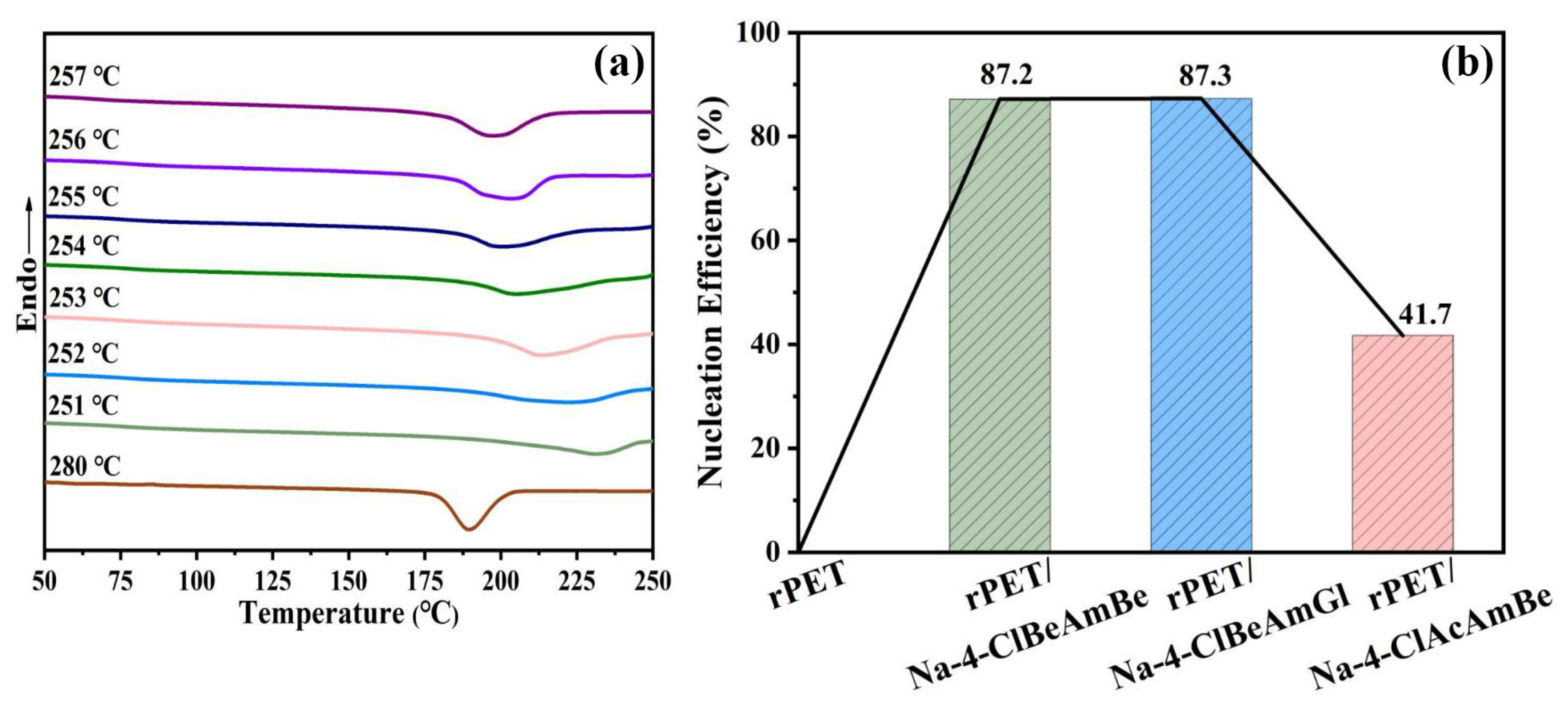
2.3. The Nucleation Process of the rPET/Nucleating Agent Blend
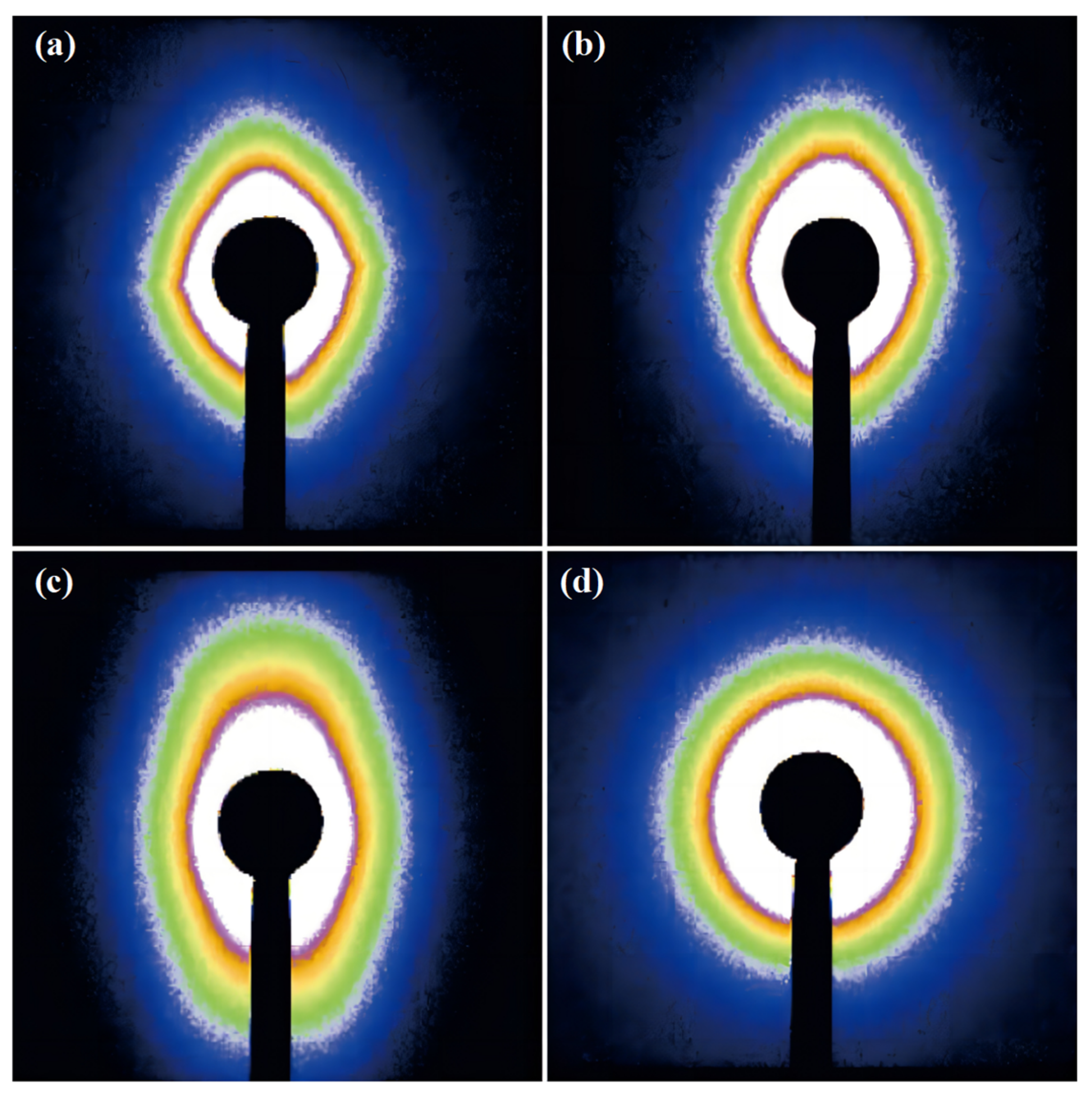
2.4. The Crystal Growth Process of the rPET/Nucleating Agent Blend
2.5. The Mechanical and Thermal Properties of the rPET/Nucleating Agent Blend
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Aromatic Amide Fatty Acid Salts Nucleating Agent
3.3. Characterization of the Aromatic Amide Fatty Acid Salt Nucleating Agent
3.4. Preparation of the rPET/Nucleating Agent Blend
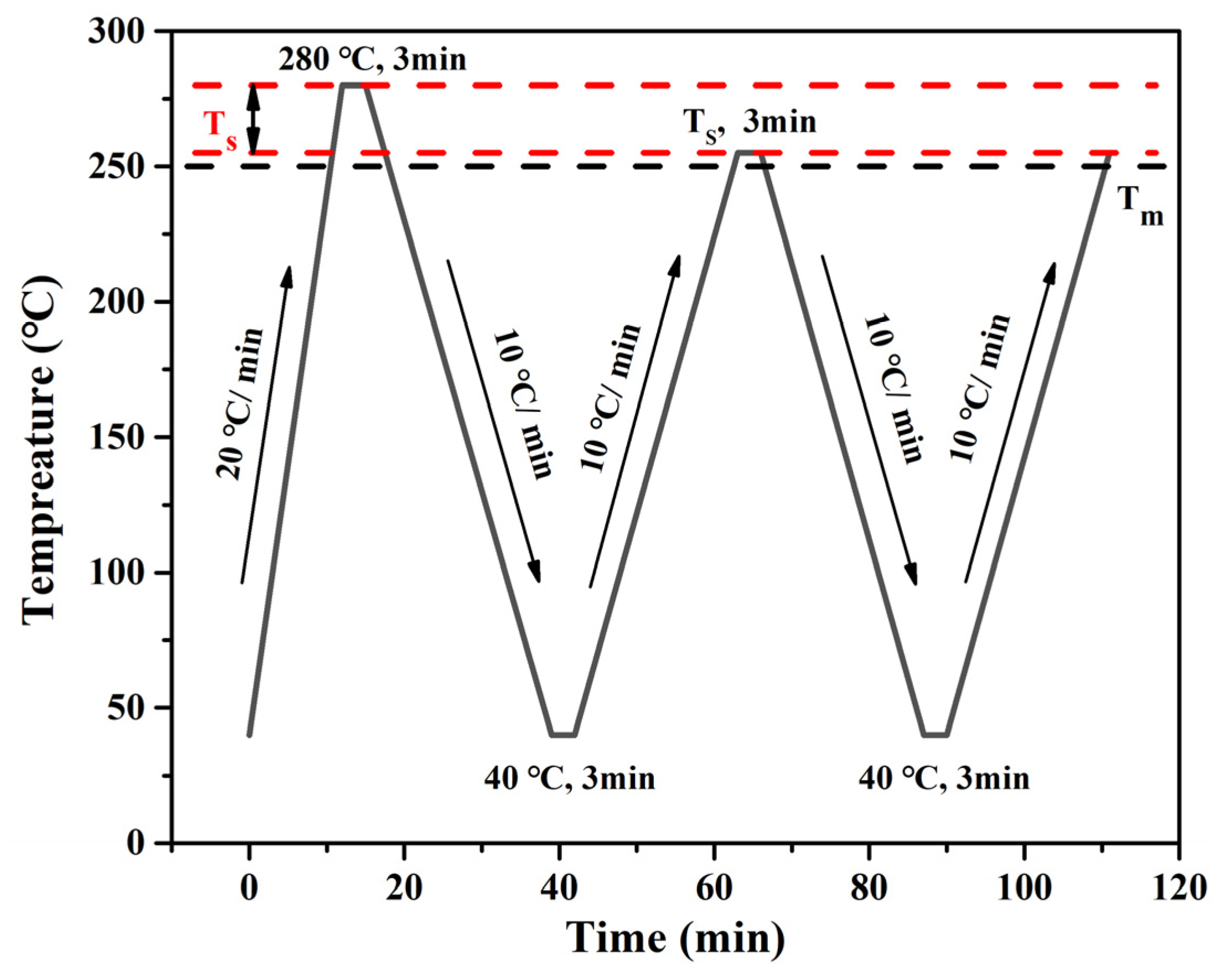
3.5. The Crystal Behavior of the rPET/Nucleating Agent Blend
3.6. The SAXS of the rPET/Nucleating Agent Blend
3.7. The Mechanical Properties of the rPET/Nucleating Agent Blend
3.8. The Thermal Properties of the rPET/Nucleating Agent Blend
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giyahchi, M.; Moghimi, H. Acceleration a yeast-based biodegradation process of polyethylene terephthalate microplastics by Tween 20: Efficiency, by-product analysis, and metabolic pathway Prediction. Environ. Pollut. 2024, 351, 124106. [Google Scholar] [CrossRef]
- Muszynski, M.; Nowicki, J.; Zygadlo, M.; Dudek, G. Comparsion of catalyst effectiveness in different chemical depolymerization methods of poly(ethylene terephthalate). Molecules 2023, 28, 6385. [Google Scholar] [CrossRef]
- Peng, Y.T.; Yang, J.; Deng, C.Q.; Deng, J.; Shen, L.; Fu, Y. Acetolysis of waste polyethylene terephthalate for upcycling and life-cycle assessment study. Nat. Commun. 2023, 14, 3249. [Google Scholar] [CrossRef]
- Myren, T.H.T.; Stinson, T.A.; Mast, Z.J.; Huntzinger, C.G.; Luca, O.R. Chemical and electrochemical recycling of end-use poly(ethylene terephthalate) (PET) plastics in batch, microwave and electrochemical reactors. Molecules 2020, 25, 2742. [Google Scholar] [CrossRef]
- Babaei, M.; Jalilian, M.; Shahbaz, K. Chemical recycling of polyethylene terephthalate: A mini-review. J. Environ. 2024, 12, 112507. [Google Scholar] [CrossRef]
- Ren, T.X.; Zhan, H.H.; Xu, H.Z.; Chen, L.F.; Shen, W.; Xu, Y.D.; Zhao, D.F.; Shao, Y.Y.; Wang, Y.T. Recycling and high-value utilization of polyethylene terephthalate wastes: A review. Environ. Res. 2024, 249, 118428. [Google Scholar] [CrossRef]
- Cusano, I.; Campagnolo, L.; Aurilia, M.; Costanzo, S.; Grizzuti, N. Rheology of recycled PET. Materials 2023, 16, 3358. [Google Scholar] [CrossRef]
- Zare, Y. Recent progress on preparation and properties of nanocomposites from recycled polymers: A review. Waste. Manag. 2013, 33, 598–604. [Google Scholar] [CrossRef]
- Lendvai, L.; Singh, T.; Ronkay, F. Thermal, thermomechanical and structural properties of recycled polyethylene terephthalate (rPET)/waste marble dust composites. Heliyon 2024, 10, 25015. [Google Scholar] [CrossRef]
- Wang, D.R.; Luo, F.L.; Shen, Z.Y.; Wu, X.J.; Qi, Y.P. A study on the crystallization behavior and mechanical properties of poly(ethylene terephthalate) induced by chemical degradation nucleation. RSC Adv. 2017, 7, 37139–37147. [Google Scholar] [CrossRef]
- Ravindra, R.C.; Katrin, N.V.; Florian, R. Recycled poly(ethylene terephthalate)/clay nanocomposites: Rheology, thermal and mechanical properties. J. Polym. Environ. 2018, 10, 37–49. [Google Scholar] [CrossRef]
- Hu, W.B. The physics of polymer chain-folding. Phys. Rep. 2018, 747, 1–50. [Google Scholar] [CrossRef]
- Lauritzen, J.I.J.; Hoffman, J.D. Theory of Formation of Polymer Crystals with folded chains in dilute solution. J. Res. Natl. Bur. Stand. A Phys. Chem. 1960, 64A, 73–102. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Liang, C.S.; Bo, Y.; Xu, S.A. Non-isothermal crystallization behavior of compatibilized polypropylene/recycled polyethylene terephthalate blends. J. Therm. Anal. Calorim. 2015, 119, 2005–2013. [Google Scholar] [CrossRef]
- Da Costa, V.; Le Moigne, J.; Oswald, L.; Pham, T.A.; Thierry, A. Thin film orientation by epitaxy of carbazolyl polydiacetylenes: guest−Host interaction on a crystal surface. Macromolecules 1998, 31, 1635–1643. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X.X.; Jia, D.Z.; Yin, J.; Lei, J.; Xu, L.; Lin, H.; Zhong, G.J.; Li, Z.M. In situ nanofibrillation of polypropylene/polyethylene/poly(ethylene terephthalate) ternary system: A strategy of upgrade recycling. Polymer 2023, 269, 125729. [Google Scholar] [CrossRef]
- Sun, Y.J.; Li, H.H.; Huang, Y.; Chen, E.Q.; Gan, Z.H.; Yan, S.K. Epitaxial crystallization of poly(butylene adipate) on highly oriented isotactic polypropylene thin film. Polymer 2006, 47, 2455–2459. [Google Scholar] [CrossRef]
- Yan, S.; Petermann, J.; Yang, D. Epitaxial crystallization in the sPP/nylon-12 semicrystalline polymer system. Polymer 1996, 37, 2681–2685. [Google Scholar] [CrossRef]
- Wang, B.; Lin, F.H.; Li, X.Y.; Ji, X.R.; Liu, S.X.; Han, X.J.; Yuan, Z.Q.; Luo, J. Transcrystallization of isotactic polypropylene/bacterial cellulose hamburger composite. Polymer 2019, 11, 508. [Google Scholar] [CrossRef]
- Wang, B.J.; Zhang, Y.J.; Zhang, J.Q.; Li, H.Y.; Chen, P.; Wang, Z.B.; Gu, Q. Noncovalent method for improving the interaction between reduced graphene oxide and poly(ε-caprolactone). Ind. Eng. Chem. Res. 2013, 52, 15824–15828. [Google Scholar] [CrossRef]
- Zhang, X.X.; Li, Y.K.; Sun, Z.Y. Acceleration of crystal transformation from crystal form II to form I in polybutene-1 induced by nanoparticles. Polymer 2018, 150, 119–129. [Google Scholar] [CrossRef]
- Ouchiar, S.; Stoclet, G.; Cabaret, C.; Gloaguen, V. Influence of the filler nature on the crystalline structure of polylactide-based nanocomposites: New insights into the nucleating effect. Macromolecules 2016, 49, 2782–2790. [Google Scholar] [CrossRef]
- Fujii, L.C.; Shiino, M.Y. Development of polyurethane/polyethylene terephthalate/fiber glass polymeric composite from internal auto parts waste. Prog. Rubber Plast. Recycl. Technol. 2023, 39, 64–80. [Google Scholar] [CrossRef]
- Ravindra, R.C.; Katrin, N.V.; Florian, R. Recycled polyethylene terephthalate/carbon nanotube composites with improved processability and performance. J. Mater. Sci-Mater. M. 2018, 53, 7017–7029. [Google Scholar] [CrossRef]
- Liang, R.; Chen, Y.C.; Zhang, C.Q.; Yin, J.; Liu, X.L.; Wang, L.K.; Kong, R.; Feng, X.; Yang, J.J. Crystallization behavior of biodegradable poly(ethylene adipate) modulated by a benign nucleating agent: Zinc phenylphosphonate. Chin. J. Polym. Sci. 2017, 35, 558–568. [Google Scholar] [CrossRef]
- Horrocks, M.; Kerscher, C.; Dotson, D. A Novel Nucleating Agent for Polyethylene; The Plastics and Rubber Institute of Singapore: Singapore, 2008. [Google Scholar]
- Li, Y.P.; Guo, Z.X.; Xue, M.L.; Yan, S.K. Epitaxial recrystallization of IPBu in Form II on an oriented IPS film initially induced by oriented form I IPBu. Macromolecules 2019, 52, 4232–4239. [Google Scholar] [CrossRef]
- Krivoshein, P.K.; Volkov, D.S.; Rogova, O.B.; Proskurnin, M.A. FTIR photoacoustic spectroscopy for identification and assessment of soil components: Chernozems and their size fractions. Photoacoustics 2020, 18, 100162. [Google Scholar] [CrossRef]
- Tkachenko, Y.; Niedzielski, P. FTIR as a method for qualitative assessment of solid samples in geochemical research: A review. Molecules 2022, 27, 8846. [Google Scholar] [CrossRef]
- Shen, Z.Y.; Luo, F.L.; Xing, Q.; Si, P.F.; Lei, X.M.; Ji, L.J.; Ding, S.F.; Wang, K.Z. Effect of an aryl amide derivative on the crystallization behaviour and impact toughness of poly(ethylene terephthalate). CrystEngComm 2016, 18, 2135–2143. [Google Scholar] [CrossRef]
- Daubeny, R.D.P.; Bunn, C.W.; Brown, C.J. The crystal structure of polyethylene terephthalate. Proc. R. Soc. Lond. 1954, 226, 531–542. [Google Scholar] [CrossRef]
- Candal, M.V.; Safari, M.; Fernández, M.; Otaegi, I.; Múgica, A.; Zubitur, M.; Gerrica-Echevarria, G.; Sebastián, V.; Irusta, S.; Loaeza, D.; et al. Structure and properties of reactively extruded opaque post-consumer recycled PET. Polymers 2021, 13, 3531. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. I general theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Xie, H.; Liang, Z.L.; Su, J.Y.; Liu, G.Q.; Li, B. Comparative analysis of thermal behavior, Isothermal crystallization kinetics and polymorphism of palm oil fractions. Molecules 2013, 18, 1036–1052. [Google Scholar] [CrossRef]
- Yang, H.W.; Du, J.H. Crystallinity, rheology, and mechanical properties of low-/high-molecular-weight PLA blended systems. Molecules 2024, 29, 169. [Google Scholar] [CrossRef]
- Fillon, B.; Lotz, B.; Thierry, A.; Wittmann, J.C. Self-nucleation and enhanced nucleation of polymers. Definition of a convenient calorimetric “efficiency scale” and evaluation of nucleating additives in isotactic polypropylene (α phase). J. Polym. Sci. Part B Polym. Phys. 1993, 31, 1395–1405. [Google Scholar] [CrossRef]
- Saeed, H.A.M.; Eltahir, Y.A.; Xia, Y.; Wang, Y. Non-isothermal crystallization kinetics and nucleation activity of hyperbranched polyester (HBPET) in recycled PET. Polym. Bull 2014, 71, 595–612. [Google Scholar] [CrossRef]
- Zhang, R.C.; Lu, A.; Xu, Y.; Min, M.; Xia, J.Q.; Zhou, J.H.; Huang, Y.G.; Li, Z.M. Equilibrium melting temperature and spherulitic growth rate of poly(phenylene sulfide). Eur. Polym. J. 2009, 45, 2867–2872. [Google Scholar] [CrossRef]
- Strobl, G. From the melt via mesomorphic and granular crystalline layers to lamellar crystallites: A major route followed in polymer crystallization? Eur. Phys. J. E 2000, 3, 165–183. [Google Scholar] [CrossRef]
- Li, X.Y.; Ding, J.J.; Chen, P.J.; Zheng, K.; Zhang, X.; Tian, X.Y. Detection and characterization of folded-chain clusters in the structured melt of isotactic polypropylene. IUCrJ 2021, 8, 595–607. [Google Scholar] [CrossRef]
- Wang, B.; Nie, K.; Xue, X.R.; Lin, F.H.; Li, X.Y.; Xue, Y.B.; Luo, J. Preparation of maleic anhydride grafted polybutene and its application in isotactic polybutene-1/microcrystalline cellulose composites. Polymers 2018, 10, 393. [Google Scholar] [CrossRef]
- Baldrian, J.; Steinhart, M.; Vlček, P.; Horký, M.; Laggner, P.; Amenitsch, H.; Bernstorff, S. Time-resolved SAXS/WAXS study of phase behavior and crystallization in polymer blends. J. Macromol. Sci. B. 2002, 41, 1023–1032. [Google Scholar] [CrossRef]
- Nonato, R.C.; Bonse, B.C. A study of PP/PET composites: Factorial design, mechanical and thermal properties. Polym. Test. 2016, 56, 167–173. [Google Scholar] [CrossRef]

| Sample | Crystal System | Density (g/cm3) | z | Unit Cell Volume (nm3) | Lattice Parameters | ||
|---|---|---|---|---|---|---|---|
| a (nm) | b (nm) | c (nm) | |||||
| rPET [31] | triclinic | 1.455 | 1 | 0.291 | 0.456 | 0.594 | 1.075 |
| Na-4-ClBeAmBe | monoclinic | 0.403 | 1 | 1.228 | 2.215 | 0.518 | 1.161 |
| Na-4-ClBeAmGl | monoclinic | 1.158 | 1 | 0.338 | 0.348 | 0.589 | 1.717 |
| Na-4-ClAcAmBe | cubic | 1.413 | 1 | 0.277 | 0.652 | 0.652 | 0.652 |
| Sample | Non-Isothermal | Isothermal | |||
|---|---|---|---|---|---|
| ΔHm (J/g) | X (%) | n | K (min−n) | t1/2 (min) | |
| rPET | 29.47 | 21.0 | 2.06 | 2.6 10−2 | 4.85 |
| rPET/Na-4-ClBeAmBe | 36.91 | 26.3 | 2.41 | 2.1 10−1 | 1.65 |
| rPET/Na-4-ClBeAmGl | 38.78 | 27.6 | 2.43 | 3.3 10−1 | 1.36 |
| rPET/Na-4-ClAcAmBe | 35.57 | 25.4 | 2.28 | 3.0 10−2 | 3.94 |
| Sample | Tc (°C) | Tm (°C) | (°C) |
|---|---|---|---|
| rPET | 225 | 250.8 | 264.5 |
| 227 | 251.4 | ||
| 230 | 252.4 | ||
| rPET/Na-4-ClBeAmBe | 225 | 248.7 | 276.2 |
| 227 | 250.9 | ||
| 230 | 252.0 | ||
| rPET/Na-4-ClBeAmGl | 225 | 248.5 | 283.1 |
| 227 | 250.6 | ||
| 230 | 252.1 | ||
| rPET/Na-4-ClAcAmBe | 225 | 250.7 | 266.4 |
| 227 | 251.5 | ||
| 230 | 252.6 |
| Sample | Flexural Strength (MPa) | Impact Strength (KJ/m2) | Tensile Strength (MPa) | HDT (°C) |
|---|---|---|---|---|
| rPET | 66.7 | 3.06 | 54.2 | 69.1 |
| rPET/Na-4-ClBeAmBe | 78.2 | 2.93 | 57.8 | 98.9 |
| rPET/Na-4-ClBeAmGl | 80.3 | 2.85 | 60.8 | 101.4 |
| rPET/Na-4-ClAcAmBe | 69.7 | 3.03 | 56.7 | 88.1 |
| Sample | rPET (wt%) | Na-4-ClBeAmBe (wt%) | Na-4-ClBeAmGl (wt%) | Na-4-ClAcAmBe (wt%) |
|---|---|---|---|---|
| rPET | 100 | 0 | 0 | 0 |
| rPET/Na-4-ClBeAmBe | 100 | 0.3 | - | - |
| rPET/Na-4-ClBeAmGl | 100 | - | 0.3 | - |
| rPET/Na-4-ClAcAmBe | 100 | - | - | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, T.; Lin, F.; Dong, Y.; Wang, M.; Ning, D.; Hao, X.; Hao, J.; Zhang, Y.; Zhou, D.; Zhao, Y.; et al. Lattice Matching and Microstructure of the Aromatic Amide Fatty Acid Salts Nucleating Agent on the Crystallization Behavior of Recycled Polyethylene Terephthalate. Molecules 2024, 29, 3100. https://doi.org/10.3390/molecules29133100
Zhao T, Lin F, Dong Y, Wang M, Ning D, Hao X, Hao J, Zhang Y, Zhou D, Zhao Y, et al. Lattice Matching and Microstructure of the Aromatic Amide Fatty Acid Salts Nucleating Agent on the Crystallization Behavior of Recycled Polyethylene Terephthalate. Molecules. 2024; 29(13):3100. https://doi.org/10.3390/molecules29133100
Chicago/Turabian StyleZhao, Tianjiao, Fuhua Lin, Yapeng Dong, Meizhen Wang, Dingyi Ning, Xinyu Hao, Jialiang Hao, Yanli Zhang, Dan Zhou, Yuying Zhao, and et al. 2024. "Lattice Matching and Microstructure of the Aromatic Amide Fatty Acid Salts Nucleating Agent on the Crystallization Behavior of Recycled Polyethylene Terephthalate" Molecules 29, no. 13: 3100. https://doi.org/10.3390/molecules29133100
APA StyleZhao, T., Lin, F., Dong, Y., Wang, M., Ning, D., Hao, X., Hao, J., Zhang, Y., Zhou, D., Zhao, Y., Luo, J., Lu, J., & Wang, B. (2024). Lattice Matching and Microstructure of the Aromatic Amide Fatty Acid Salts Nucleating Agent on the Crystallization Behavior of Recycled Polyethylene Terephthalate. Molecules, 29(13), 3100. https://doi.org/10.3390/molecules29133100








