The Effect of Expanded Graphite Content on the Thermal Properties of Fatty Acid Composite Materials for Thermal Energy Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fatty Acid Binary Eutectic Mixtures/EG CPCMs
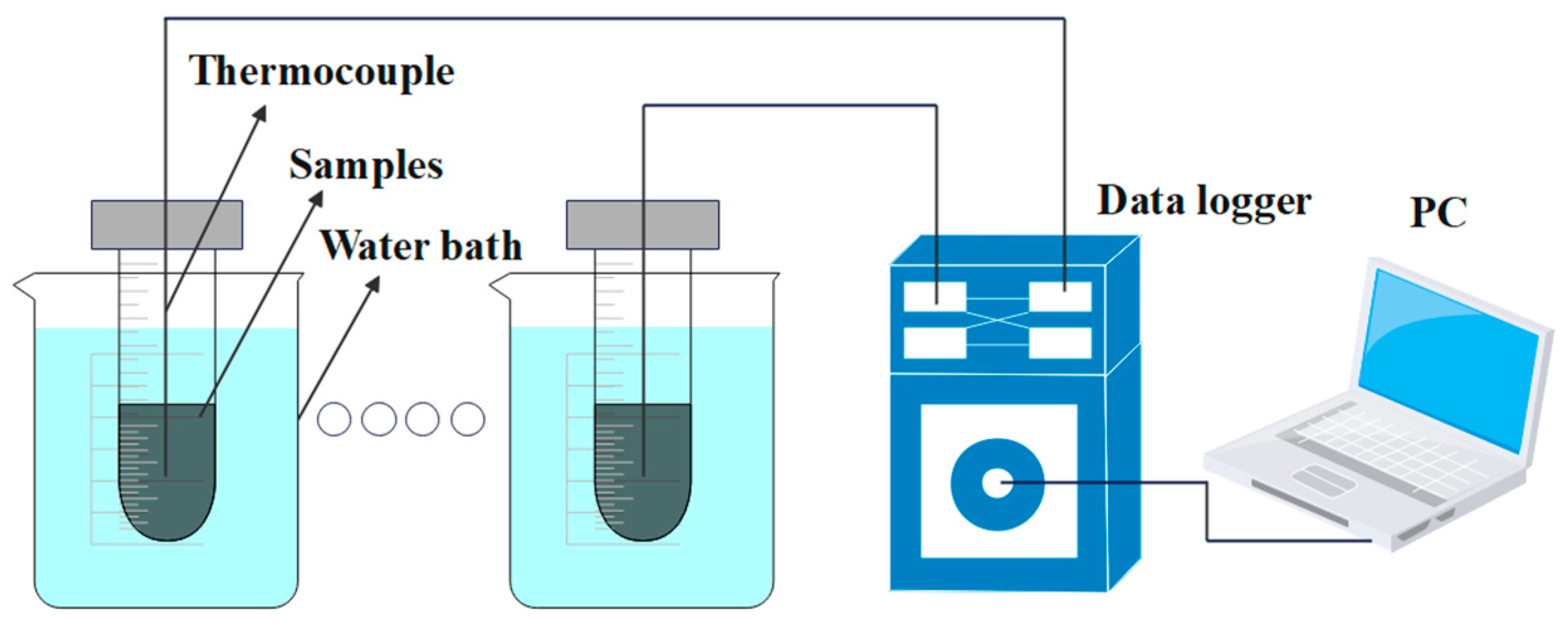
2.3. Methods
2.4. Experimental Uncertainty
3. Results and Discussion
3.1. The Adsorption Properties of EG on PCMs
3.2. Thermal Properties of the CPCMs
3.3. Microstructure of the CPCMs
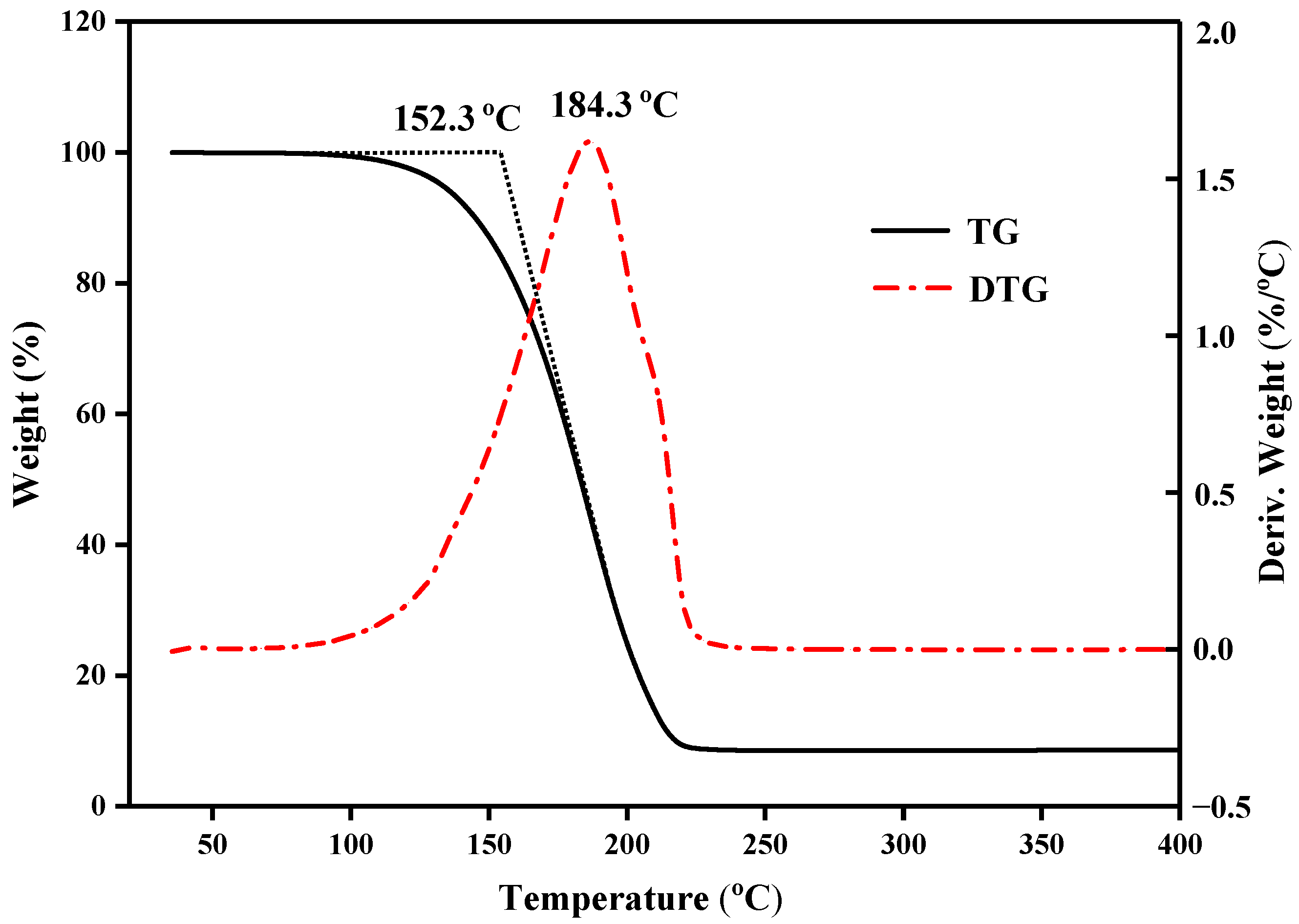
3.4. Thermostability of the CPCMs
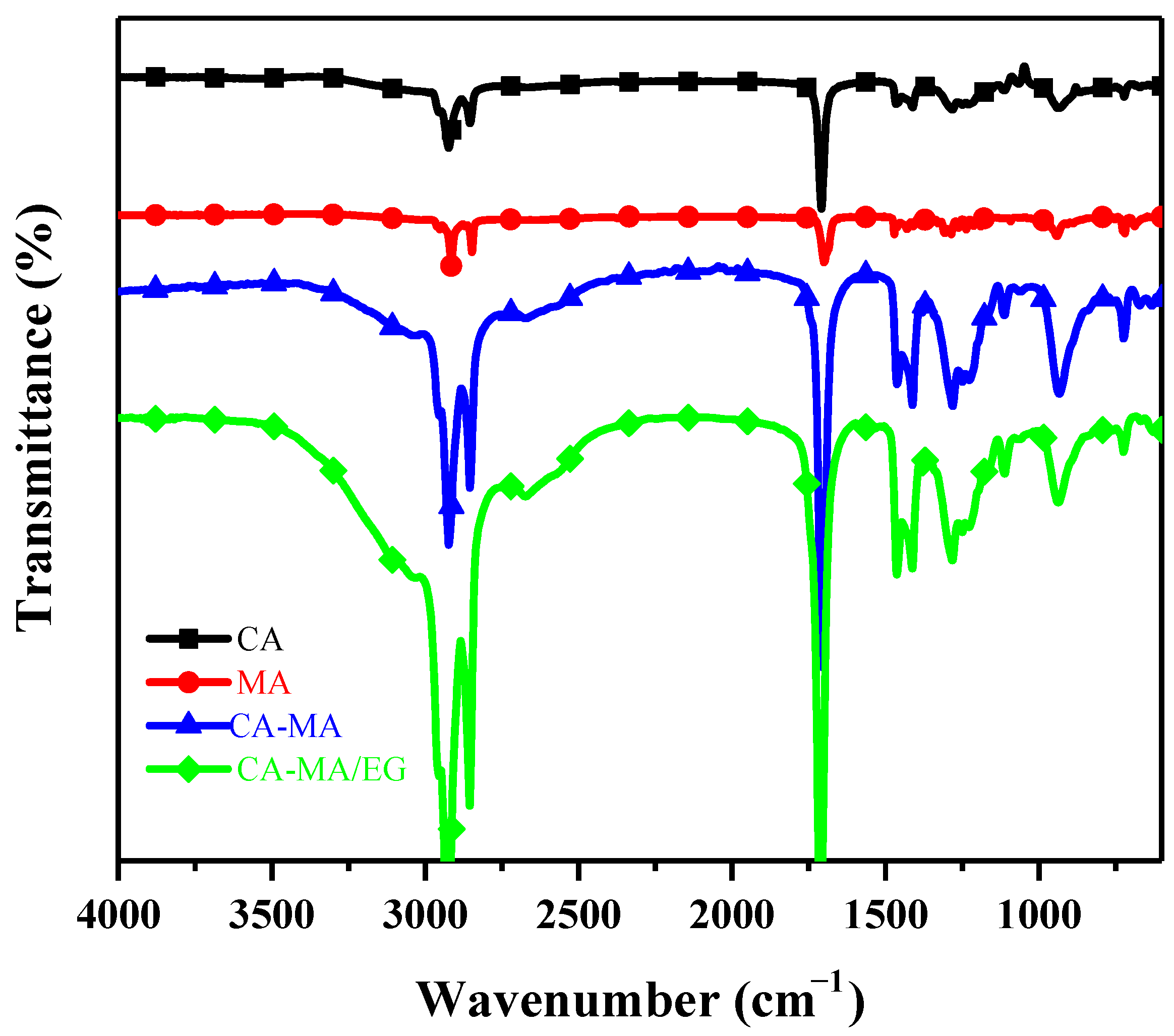
3.5. Infrared Spectral Analysis
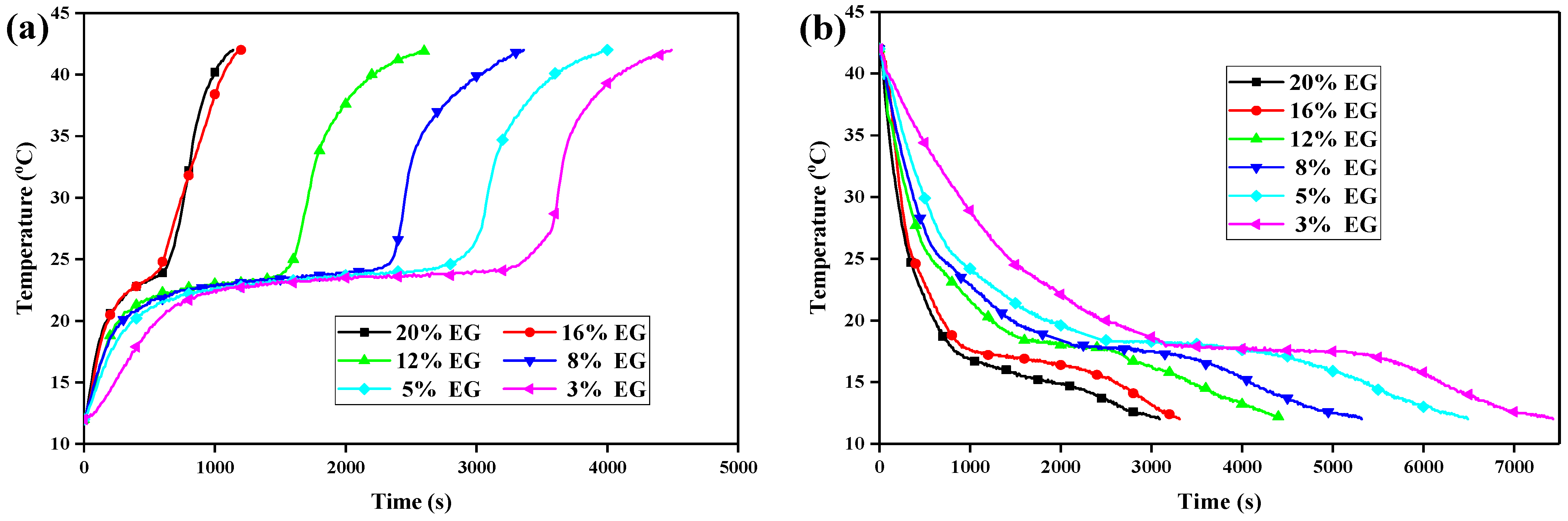
3.6. Heat Transfer Performance Experiments
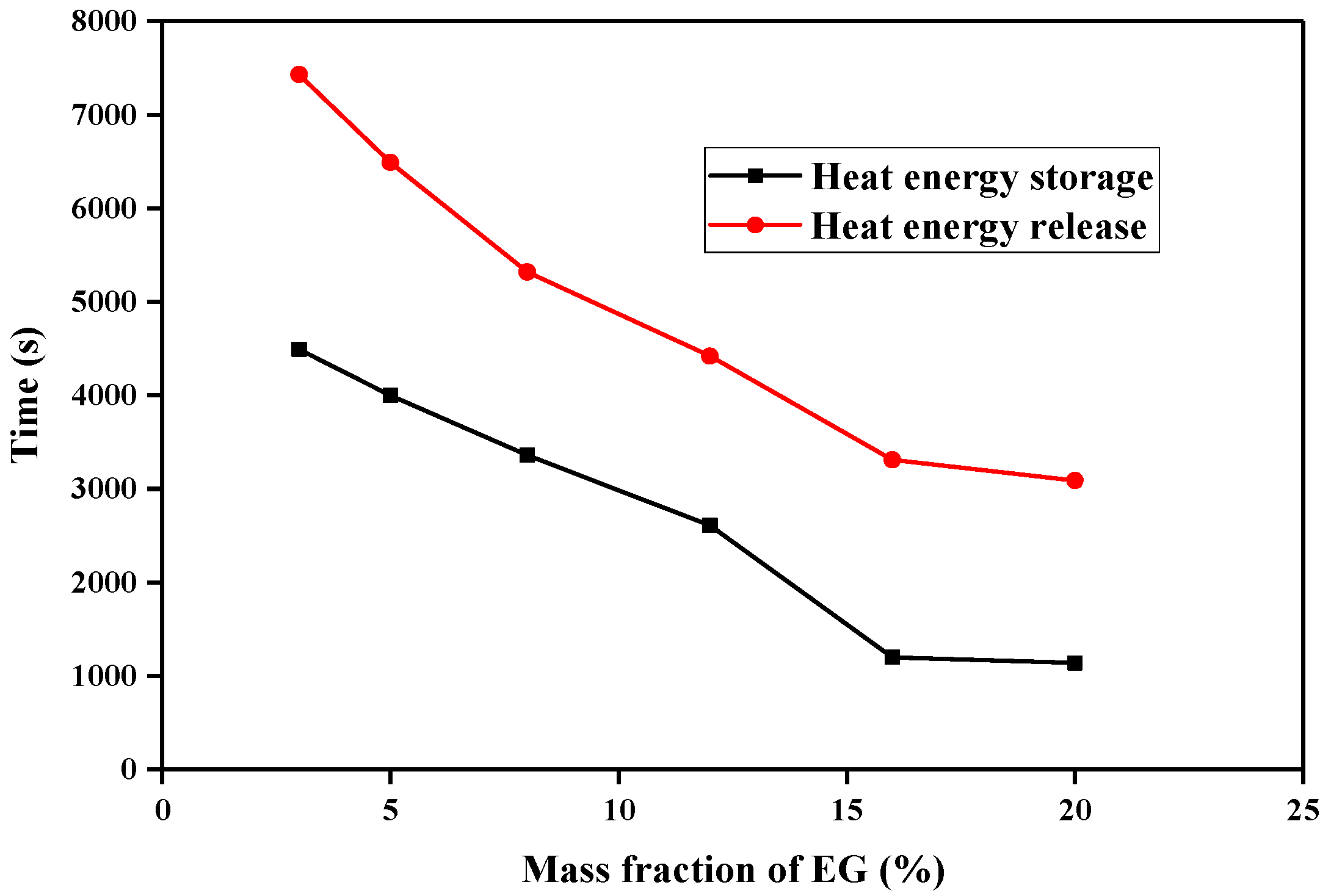
3.7. Heat Energy Storage and Release of the CPCMs
4. Conclusions
- (1)
- The minimum expanded graphite mass content in capric–myristic acid/expanded graphite composite phase-change materials is 7.6%. When the mass content of expanded graphite in composite phase-change materials exceeds the minimum content, the liquid fatty acid phase-change materials are completely filled into the porous structure of expanded graphite. Following the addition of expanded graphite, the structures of phase-change materials remain constant, and no new compounds are produced.
- (2)
- The latent heat of composite phase-change materials decreases almost linearly with an increase in the expanded graphite mass content, and expanded graphite has almost no effect on the phase-change temperature. In low-temperature applications below 100 °C, capric–myristic acid/expanded graphite composite phase-change materials exhibit good thermal stability.
- (3)
- The thermal conductivity of composite phase-change materials gradually increases with an increase in the expanded graphite mass content. With an increase in the expanded graphite mass content in composite phase-change materials, the heat transfer mainly transitions from phase-change heat transfer to thermal conductivity, but when the expanded graphite mass content exceeds 15%, an increase in the expanded graphite mass content has no significant effect on the composite phase-change material heat storage/release time.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, C.; Tian, Y. Review on thermal energy storage with phase change materials (PCMs) in building applications. Appl. Energy 2012, 92, 593–605. [Google Scholar] [CrossRef]
- Gao, J.; Xu, Z.; Wang, R. Experimental study on a double–stage absorption solar thermal storage system with enhanced energy storage density. Appl. Energy 2020, 262, 114476. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al–Sulaiman, F.A.; Ibrahim, N.I.; Zahir, M.H.; Al–Ahmed, A.; Saidur, R.; Yilbas, B.S.; Sahin, A.Z. A review on current status and challenges of inorganic phase change materials for thermal energy storage systems. Renew. Sustain. Energy Rev. 2017, 70, 1072–1089. [Google Scholar] [CrossRef]
- Baskar, I.; Chellapandian, M.; Jaswanth, S. Development of a novel composite phase change material based paints and mortar for energy storage applications in buildings. J. Energy Storage 2022, 55, 105829. [Google Scholar] [CrossRef]
- Horibe, A.; Jang, H.; Haruki, N.; Sano, Y.; Kanbara, H.; Takahashi, K. Melting and solidification heat transfer characteristics of phase change material in a latent heat storage vessel: Effect of perforated partition plate. Int. J. Heat Mass Transf. 2015, 82, 259–266. [Google Scholar] [CrossRef]
- Chung, O.; Jeong, S.G.; Kim, S. Preparation of energy efficient paraffinic PCMs/expanded vermiculite and perlite composites for energy saving in buildings. Sol. Energy Mater. Sol. Cells 2015, 137, 107–112. [Google Scholar] [CrossRef]
- Yang, J.; Jia, Y.; Bing, N.; Wang, L.; Xie, H.; Yu, W. Reduced graphene oxide and zirconium carbide co–modified melamine sponge/paraffin wax composites as new form–stable phase change materials for photothermal energy conversion and storage. Appl. Therm. Eng. 2019, 163, 114412. [Google Scholar] [CrossRef]
- George, M.; Pandey, A.K.; Abd Rahim, N.; Tyagi, V.V.; Shahabuddin, S.; Saidur, R. A novel polyaniline (PANI)/paraffin wax nano composite phase change material: Superior transition heat storage capacity, thermal conductivity and thermal reliability. Sol. Energy 2020, 204, 448–458. [Google Scholar] [CrossRef]
- Gunasekara, S.N.; Pan, R.J.; Chiu, J.N.; Martin, V. Polyols as phase change materials for surplus thermal energy storage. Appl. Energy 2016, 162, 1439–1452. [Google Scholar] [CrossRef]
- Lv, L.; Huang, S.; Cen, K.; Zhou, H. Experimental study of screening polyols and their binary eutectic phase change materials for long–term thermal energy storage. J. Clean. Prod. 2023, 399, 136636. [Google Scholar] [CrossRef]
- Tao, Y.; Lin, C.; He, Y. Preparation and thermal properties characterization of carbonate salt/carbon nanomaterial composite phase change material. Energy Convers. Manag. 2015, 97, 103–110. [Google Scholar] [CrossRef]
- Li, M.; Jin, B.; Ma, Z.; Yuan, F. Experimental and numerical study on the performance of a new high–temperature packed–bed thermal energy storage system with macroencapsulation of molten salt phase change material. Appl. Energy 2018, 221, 1–15. [Google Scholar] [CrossRef]
- Yang, T.; King, W.; Miljkovic, N. Phase change material–based thermal energy storage. Cell Rep. Phys. Sci. 2021, 2, 100540. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, N.; Tao, W.; Cao, X.; He, Y. Fatty acids as phase change materials: A review. Renew. Sustain. Energy Rev. 2014, 29, 482–498. [Google Scholar] [CrossRef]
- Akeiber, H.; Nejat, P.; Abd Majid, M.Z.; Wahid, M.A.; Jomehzadeh, F.; Famileh, I.Z.; Calautit, J.K.; Hughes, B.; Zaki, S.A. A review on phase change material (PCM) for sustainable passive cooling in building envelopes. Renew. Sustain. Energy Rev. 2016, 60, 1470–1497. [Google Scholar] [CrossRef]
- Sun, N.; Xiao, Z. Synthesis and performances of phase change materials microcapsules with a polymer/BN/TiO2 hybrid shell for thermal energy storage. Energy Fuels 2017, 31, 10186–10195. [Google Scholar] [CrossRef]
- He, H.; Yue, Q.; Gao, B.; Zhang, X.; Li, Q.; Wang, Y. The effects of compounding conditions on the properties of fatty acids eutectic mixtures as phase change materials. Energy Convers. Manag. 2013, 69, 116–121. [Google Scholar] [CrossRef]
- Tang, F.; Su, D.; Tang, Y.; Fang, G. Synthesis and thermal properties of fatty acid eutectics and diatomite composites as shape–stabilized phase change materials with enhanced thermal conductivity. Sol. Energy Mater. Sol. Cells 2015, 141, 218–224. [Google Scholar] [CrossRef]
- Qiu, L.; Ning, Z.; Feng, Y.; Michaelides, E.E.; Żyła, G.; Jing, D.; Zhang, X.; Norris, P.M.; Markides, C.N.; Mahian, O. A review of recent advances in thermophysical properties at the nanoscale: From solid state to colloids. Phys. Rep. 2020, 843, 1–81. [Google Scholar] [CrossRef]
- Gorbacheva, S.N.; Makarova, V.V.; Ilyin, S.O. Hydrophobic nanosilica–stabilized graphite particles for improving thermal conductivity of paraffin wax–based phase–change materials. J. Energy Storage 2021, 36, 102417. [Google Scholar] [CrossRef]
- Bulk, A.; Odukomaiya, A.; Simmons, E.; Woods, J. Processing Compressed Expanded Natural Graphite for Phase Change Material Composites. J. Therm. Sci. 2022, 32, 1213–1226. [Google Scholar] [CrossRef]
- Guo, Y.L.; Yang, W.B.; Jiang, Z.N.; He, F.F.; Zhang, K.; He, R.; Wu, J.Y.; Fan, J.H. Silicone rubber/paraffin@silicon dioxide form–stable phase change materials with thermal energy storage and enhanced mechanical property. Sol. Energy Mater. Sol. Cells 2019, 196, 16–24. [Google Scholar] [CrossRef]
- Ben Khedher, N.; Bantan, R.A.; Kolsi, L.; Omri, M. Performance investigation of a vertically configured LHTES via the combination of nano–enhanced PCM and fins: Experimental and numerical approaches. Int. Commun. Heat Mass Transf. 2022, 137, 106246. [Google Scholar] [CrossRef]
- Hua, J.; Yuan, C.; Zhao, X.; Zhang, J.; Du, J. Structure and thermal properties of expanded graphite/paraffin composite phase change material. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 86–93. [Google Scholar] [CrossRef]
- Jeon, J.; Park, J.H.; Wi, S.; Kim, K.H.; Kim, S. Thermal performance enhancement of a phase change material with expanded graphite via ultrasonication. J. Ind. Eng. Chem. 2019, 79, 437–442. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, P.; Meng, Z.; Li, M. Thermal Characterization of Lauric–Stearic Acid/Expanded Graphite Eutectic Mixture as Phase Change Materials. J. Nanosci. Nanotechnol. 2015, 15, 3288–3294. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, G.; Jia, Z.; Gao, Q.; Li, Y.; Gu, Z. Eutectic Fatty Acids Phase Change Materials Improved with Expanded Graphite. Materials 2022, 15, 6856. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, H.; Zhao, Z.; Sun, L.; Xu, F.; Zhang, J.; Jiao, Q.; Bao, Y.; Ma, J. Preparation, Encapsulation and Thermal Properties of Fatty Acid/Expanded Graphite Composites as Shape–stabilized Phase Change Materials. Chem. J. Chin. Univ. 2012, 33, 526–530. [Google Scholar] [CrossRef]
- Wen, R.; Zhang, X.; Huang, Y.; Yin, Z.; Huang, Z.; Fang, M.; Liu, Y.; Wu, X. Preparation and properties of fatty acid eutectics/expanded perlite and expanded vermiculite shape–stabilized materials for thermal energy storage in buildings. Energy Build. 2017, 139, 197–204. [Google Scholar] [CrossRef]
- Luo, K.; Wu, D.; Wang, Y.; Fei, H.; Jiang, H.; Ye, Z. Preparation and characterization of lauric acid–stearic acid/fumed silica/expanded graphite thermally conductive enhanced composites. J. Energy Storage 2023, 73, 109151. [Google Scholar] [CrossRef]
- Fei, H.; He, Q.; Du, W.; Li, P.; Zhou, J.; Pan, Y.; Liang, X. Structural characteristics and thermal performances of capric acid–stearic acid–octadecanol adsorbed into porous expanded graphite under vacuum condition. J. Energy Storage 2023, 72, 108326. [Google Scholar] [CrossRef]
- Fei, H.; Zhou, J.; He, Q.; Wang, L.; Liang, X.; Pan, Y. Characteristic and Properties of Ternary Shape–Stabilized Composite Phase Change Materials Based on Expanded Graphite. ACS Omega 2021, 6, 29215–29222. [Google Scholar] [CrossRef] [PubMed]
- Badenhorst, H. A review of the application of carbon materials in solar thermal energy storage. Sol. Energy 2019, 192, 35–68. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Zhu, X.; Xin, S. A Low–Temperature Phase Change Material Based on Capric–Stearic Acid/Expanded Graphite for Thermal Energy Storage. ACS Omega 2021, 6, 17988–17998. [Google Scholar] [CrossRef] [PubMed]
- Ao, C.; Yan, S.; Zhao, S.; Hu, W.; Zhao, L.; Wu, Y. Stearic acid/expanded graphite composite phase change material with high thermal conductivity for thermal energy storage. Energy Rep. 2022, 8, 4834–4843. [Google Scholar] [CrossRef]
- Sari, A.; Karaipekli, A. Preparation, thermal properties and thermal reliability of palmitic acid/expanded graphite composite as form–stable PCM for thermal energy storage. Sol. Energy Mater. Sol. Cells 2009, 93, 571–576. [Google Scholar] [CrossRef]
- Huang, D.; Ma, G.; Yu, Z.; Lv, P.; Zhou, Q.; Liu, Q.; Peng, C.; Xiong, F.; Huang, Y. Highly thermal conductive shape–stabilized composite phase change materials based on boron nitride and expanded graphite for solar thermal applications. RSC Adv. 2023, 13, 13252–13262. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, C.; Alva, G.; Fang, G. Palmitic acid/polyvinyl butyral/expanded graphite composites as form–stable phase change materials for solar thermal energy storage. Appl. Energy 2019, 228, 1801–1809. [Google Scholar] [CrossRef]
- Said, A.; Salah, A.; Fattah, G. Thermo–optic switching properties of paraffin–wax hosting carbon fillers. J. Energy Storage 2018, 19, 260–271. [Google Scholar] [CrossRef]
- Said, A.; Salah, A.; Fattah, G. Enhanced Thermo–Optical Switching of Paraffin–Wax Composite Spots under Laser Heating. Materials 2017, 10, 525. [Google Scholar] [CrossRef] [PubMed]
- Atinafu, D.G.; Dong, W.; Huang, X.; Gao, H.; Wangc, G. Introduction of organic–organic eutectic PCM in mesoporous N–doped carbons for enhanced thermal conductivity and energy storage capacity. Appl. Energy 2018, 211, 1203–1215. [Google Scholar] [CrossRef]
- Zhou, D.; Xiao, S.; Xiao, X.; Liu, Y. Preparation, Phase Diagrams and Characterization of Fatty Acids Binary Eutectic Mixtures for Latent Heat Thermal Energy Storage. Separations 2023, 10, 49. [Google Scholar] [CrossRef]
- Zhou, D.; Xiao, S.; Xiao, X. Preparation and thermal performance of fatty acids binary eutectic mixtures/expanded graphite as form–stable phase change materials for thermal energy storage. ACS Omega 2023, 8, 8596–8604. [Google Scholar] [CrossRef]
- Venkitaraj, K.; Suresh, S. Experimental study on thermal and chemical stability of pentaerythritol blended with low melting alloy as possible PCM for latent heat storage. Exp. Therm. Fluid Sci. 2017, 88, 73–87. [Google Scholar] [CrossRef]
- Li, J.; Wang, W.; Deng, Y.; Gao, L.; Bai, J.; Xu, L.; Chen, J.; Yuan, Z. Thermal Performance Analysis of Composite Phase Change Material of Myristic Acid–Expanded Graphite in Spherical Thermal Energy Storage Unit. Energies 2023, 16, 4527. [Google Scholar] [CrossRef]
- Zhou, D.; Yuan, J.; Zhou, Y.; Liu, Y. Preparation and characterization of myristic acid/expanded graphite composite phase change materials for thermal energy storage. Sci. Rep. 2020, 10, 10889. [Google Scholar] [CrossRef]
- Zhou, D.; Yuan, J.; Zhou, Y.; Liu, Y. Preparation and Properties of Capric–Myristic Acid/Expanded Graphite Composite Phase Change Materials for Latent Heat Thermal Energy Storage. Energies 2020, 13, 2462. [Google Scholar] [CrossRef]




| PCM | Supporting Material | Mass Content | (°C) | (J/g) | Application | Literature |
|---|---|---|---|---|---|---|
| Stearic acid (SA) + benzamide (BN) | Boron nitride + expanded graphite (BG) | 15 wt.% BN + 20 wt.% BG | 65.21 | 132.35 | Solar hot-water system | [38] |
| PA | Polyvinyl butyral + expanded graphite | 3 wt.% EG | 59.1 | 125.88 | Low-temperature solar energy systems | [39] |
| 5 wt.% EG | 58.5 | 124.99 | ||||
| 7 wt.% EG | 56.1 | 122.05 | ||||
| Paraffin wax | Graphite | 0–7 wt.% | / | / | TOS device applications | [40] |
| Graphene | 0.001–0.07 wt.% | / | / | |||
| Paraffin wax | Graphite | 0.007–7 wt.% | Around 52 | 143.03–131.35 | Future networks and electric noise-free remote aerial laser switching applications | [41] |
| Graphene | 0.001–0.7 wt.% | 59.7–52.1 | 134.4–108.8 | |||
| Capric acid + myristic acid | Expanded graphite | 0 wt.% | 19.4 | 150.9 | Low-temperature LHTES systems and backfill materials in ground source heat pump systems | This study |
| 1 wt.% | 18.4 | 148.4 | ||||
| 3 wt.% | 18.8 | 142.5 | ||||
| 5 wt.% | 18.5 | 142.8 | ||||
| 8 wt.% | 18.5 | 138.0 | ||||
| 12 wt.% | 18.6 | 129.9 | ||||
| 16 wt.% | 19.2 | 128.8 | ||||
| 20 wt.% | 19.2 | 111.8 |
| Fatty Acid | Melting | Freezing | ||
|---|---|---|---|---|
| (°C) | (J/g) | (°C) | (J/g) | |
| Capric acid | 31.40 | 169.4 | 31.69 | 170.3 |
| Lauric acid | 43.10 | 183.6 | 44.06 | 183.2 |
| Myristic acid | 52.68 | 188.6 | 51.63 | 193.1 |
| Palmitic acid | 60.60 | 198.1 | 61.10 | 199.3 |
| Stearic acid | 68.90 | 209.8 | 67.60 | 202.2 |
| Mass Content of EG (%) | Exudation Circle Average Diameter (mm) | Leakage Percentage (%) | Assessment Standard | Assessment Result |
|---|---|---|---|---|
| 1% | / | / | / | / |
| 3% | 88.0 | 193.0 | Φ > 50 | Extremely unstable |
| 5% | 46.5 | 55.0 | Φ > 50 | Extremely unstable |
| 8% | 0 | 0 | Φ ≤ 0 | Very stable |
| 12% | 0 | 0 | Φ ≤ 0 | Very stable |
| 16% | 0 | 0 | Φ ≤ 0 | Very stable |
| 20% | 0 | 0 | Φ ≤ 0 | Very stable |
| W (EG)% | Melting | Freezing | ||||||
|---|---|---|---|---|---|---|---|---|
| (°C) | (°C) | |||||||
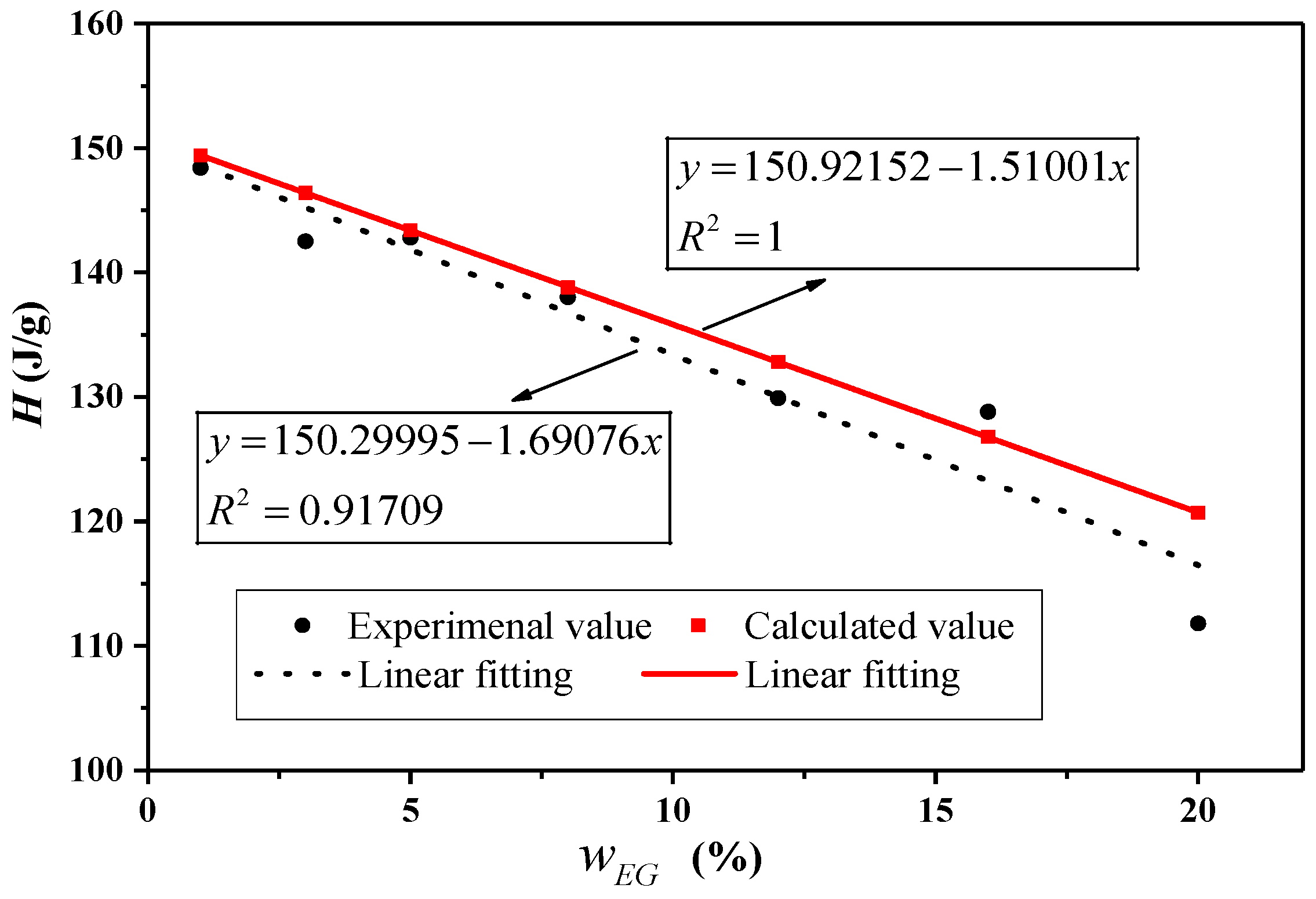
| Experimental Value (J·g−1) | Calculated Value (J·g−1) | Difference (%) | Experimental Value (J·g−1) | Calculated Value (J·g−1) | Difference (%) | |||
| 0 | 19.4 | 150.9 | / | / | 18.4 | 149.2 | / | / |
| 1 | 18.4 | 148.4 | 149.4 | 0.66 | 19.6 | 146.8 | 147.7 | 0.61 |
| 3 | 18.8 | 142.5 | 146.4 | 2.65 | 19.4 | 143.0 | 144.7 | 1.19 |
| 5 | 18.5 | 142.8 | 143.4 | 0.39 | 17.6 | 141.2 | 141.7 | 0.38 |
| 8 | 18.5 | 138.0 | 138.8 | 0.60 | 18.3 | 135.4 | 137.3 | 1.36 |
| 12 | 18.6 | 129.9 | 132.8 | 2.18 | 17.7 | 125.0 | 131.3 | 4.80 |
| 16 | 19.2 | 128.8 | 126.8 | –1.61 | 18.0 | 127.1 | 125.3 | –1.41 |
| 20 | 19.2 | 111.8 | 120.7 | 7.39 | 17.7 | 110.0 | 119.4 | 7.84 |
| CPCMs | Mass Content of EG | Thermal Conductivity (W·m−1·k−1) | Literature |
|---|---|---|---|
| LA–SA/EG | 5% | 0.466 | [26] |
| 15% | 0.585 | ||
| CA–LA–PA/EG | 5% | 0.738 | [27] |
| CA–LA/EP/EG | 5% | 0.244 | [28] |
| CA–LA/EVM/EG | 5% | 0.253 | |
| LA–SA–FA–EA | 3% | 2.545 | [29] |
| 5% | 3.229 | ||
| 7% | 6.045 | ||
| CSOD/EG | 8.33% | 3.136 | [30] |
| CA–MA/EG | 5% | 0.2683 | This study |
| 8% | 0.3827 | ||
| 12% | 0.7656 | ||
| 16% | 1.1227 | ||
| 20% | 1.2130 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, D.; Xiao, S.; Liu, Y. The Effect of Expanded Graphite Content on the Thermal Properties of Fatty Acid Composite Materials for Thermal Energy Storage. Molecules 2024, 29, 3146. https://doi.org/10.3390/molecules29133146
Zhou D, Xiao S, Liu Y. The Effect of Expanded Graphite Content on the Thermal Properties of Fatty Acid Composite Materials for Thermal Energy Storage. Molecules. 2024; 29(13):3146. https://doi.org/10.3390/molecules29133146
Chicago/Turabian StyleZhou, Dongyi, Shuaizhe Xiao, and Yicai Liu. 2024. "The Effect of Expanded Graphite Content on the Thermal Properties of Fatty Acid Composite Materials for Thermal Energy Storage" Molecules 29, no. 13: 3146. https://doi.org/10.3390/molecules29133146
APA StyleZhou, D., Xiao, S., & Liu, Y. (2024). The Effect of Expanded Graphite Content on the Thermal Properties of Fatty Acid Composite Materials for Thermal Energy Storage. Molecules, 29(13), 3146. https://doi.org/10.3390/molecules29133146





