Abstract
Carbon dioxide (CO2) is a non-toxic, abundant and recoverable source of carbon monoxide. Despite its thermodynamically stable and kinetically inert nature, research on CO2 utilisation is ongoing. CO2-based aryne reactions, crucial for synthesising ortho-substituted benzoic acids and their cyclisation products, have garnered significant attention, and multi-component reactions (MCRs) involving CO2, aryne and nucleophilic reagents have been extensively studied. This review highlights recent advancements in CO2 capture reactions utilising phenylalkyne reactive intermediates. Mechanistic insights into these reactions are provided together with prospects for further development in this field.
1. Introduction
Carbon dioxide (CO2) provides a non-toxic, abundant and recyclable C1 source and has broad application prospects in organic synthesis. However, the approach by which CO2 can be activated and utilised remains challenging due to its thermodynamic stability and kinetic inertness [1,2]. During the past few decades, various organic transformations of CO2 have been developed [3,4,5,6,7]. Examples of metal-catalyzed [8], photocatalyzed [9] and electrocatalyzed [10,11,12] effective promotion of CO2 conversion have been widely reported.
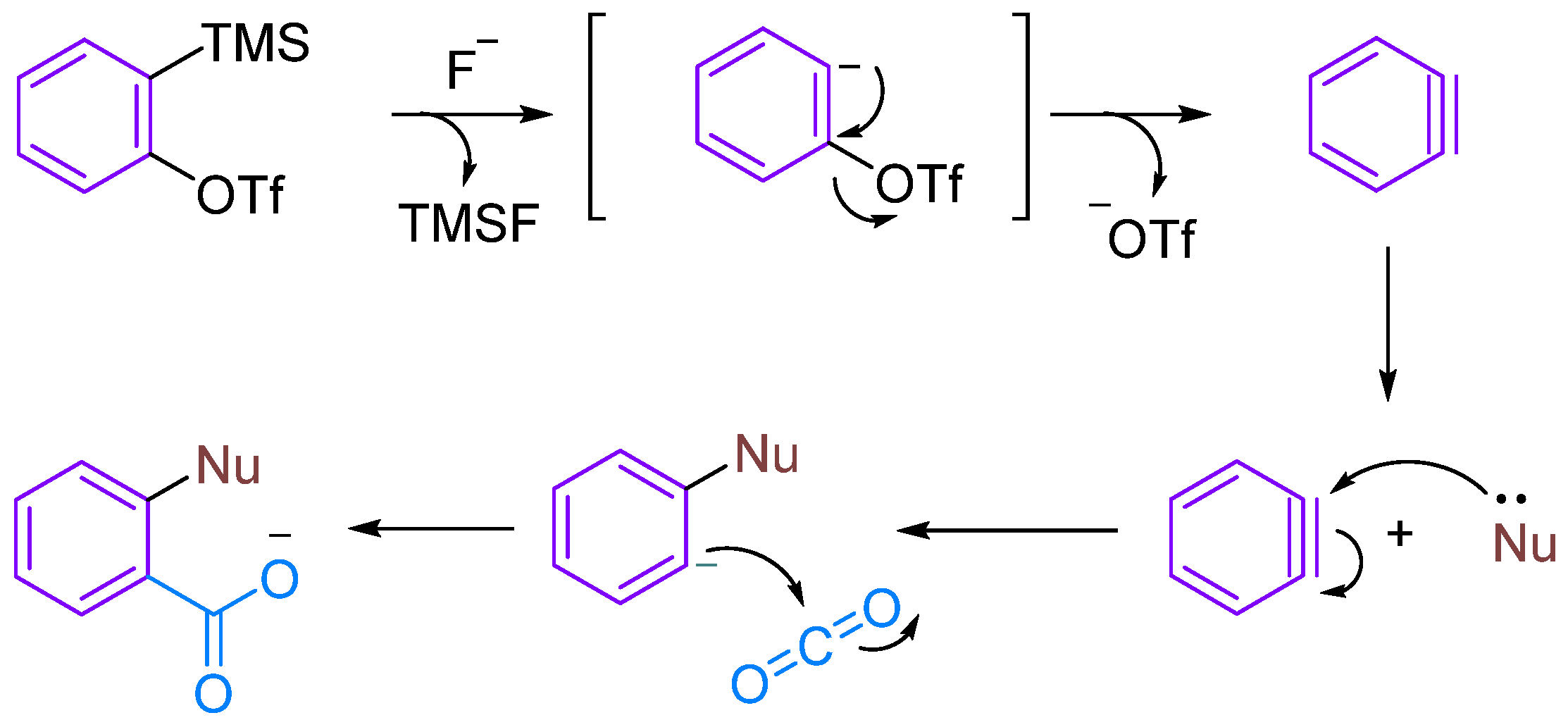
Arynes are highly reactive organic intermediates possessing a triple bond within a molecule intermediate between a double bond and a triple bond. One π-bond belongs to the aromatic system, whereas the other π-bond is formed by the lateral overlap of two sp2 orbitals in the plane of the benzene ring [13,14]. The ring strain induced by the formal triple bond on a six-membered ring results in the lowering of its LUMO (lowest unoccupied molecular orbital), leading to a small energy gap between the HOMO (highest occupied molecular orbital) and LUMO, making arynes excellent electrophilic reagents [15,16,17]. Kobayashi’s group [18] first reported 2-(trimethylsilyl)phenyl trifluoromethanesulfonate as a precursor for phenylalkynes in 1983. Under the action of fluoride ions, the trimethylsilyl group dissociates, resulting in the formation of phenylalkyne precursors.
Although CO2 is thermodynamically stable and kinetically inert, it exhibits certain electrophilicity due to the electron-withdrawing action of oxygen atoms. Therefore, aryne can undergo MCRs under certain conditions, such as when nucleophilic reagents attack to form amphipathic ions that can trap CO2 (Figure 1). In this review, we summarise the MCRs of aromatic alkynes with CO2 in combination with nucleophilic reagents, discuss the reaction mechanisms underlying this class of reactions and provide an outlook on future developments.
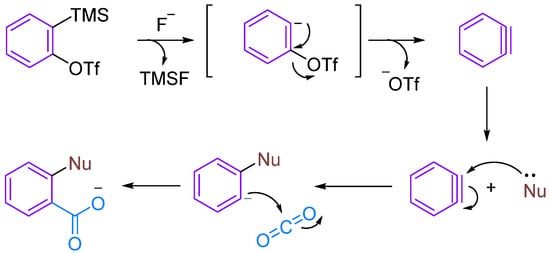
Figure 1.
The reaction between Kobayashi benzyne precursor and CO2.
2. Nucleophilic Addition Reactions of Aromatic Alkynes Involving CO2
2.1. Nucleophilic Addition Reactions of Aromatic Alkynes with N-Compounds Involving CO2
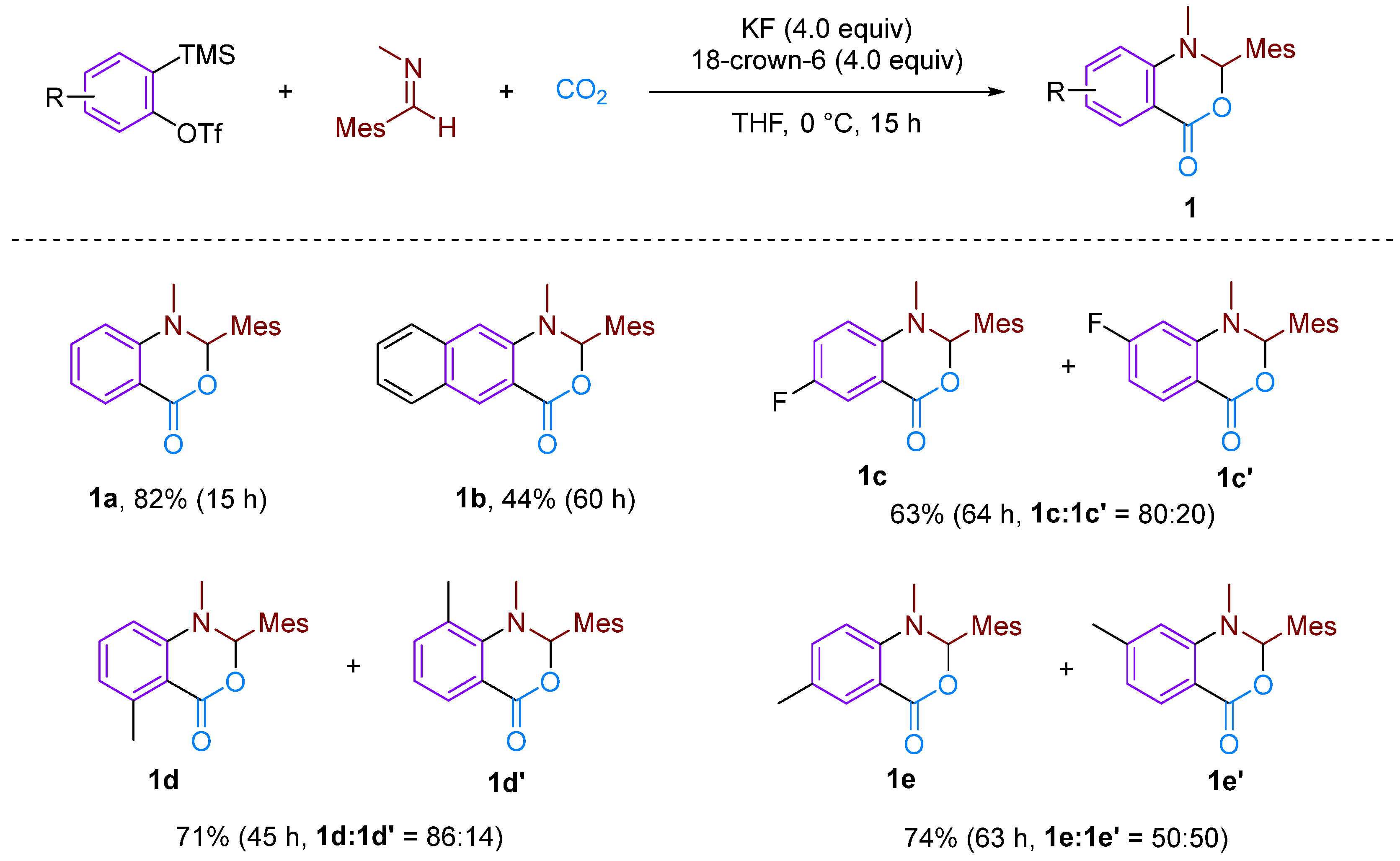
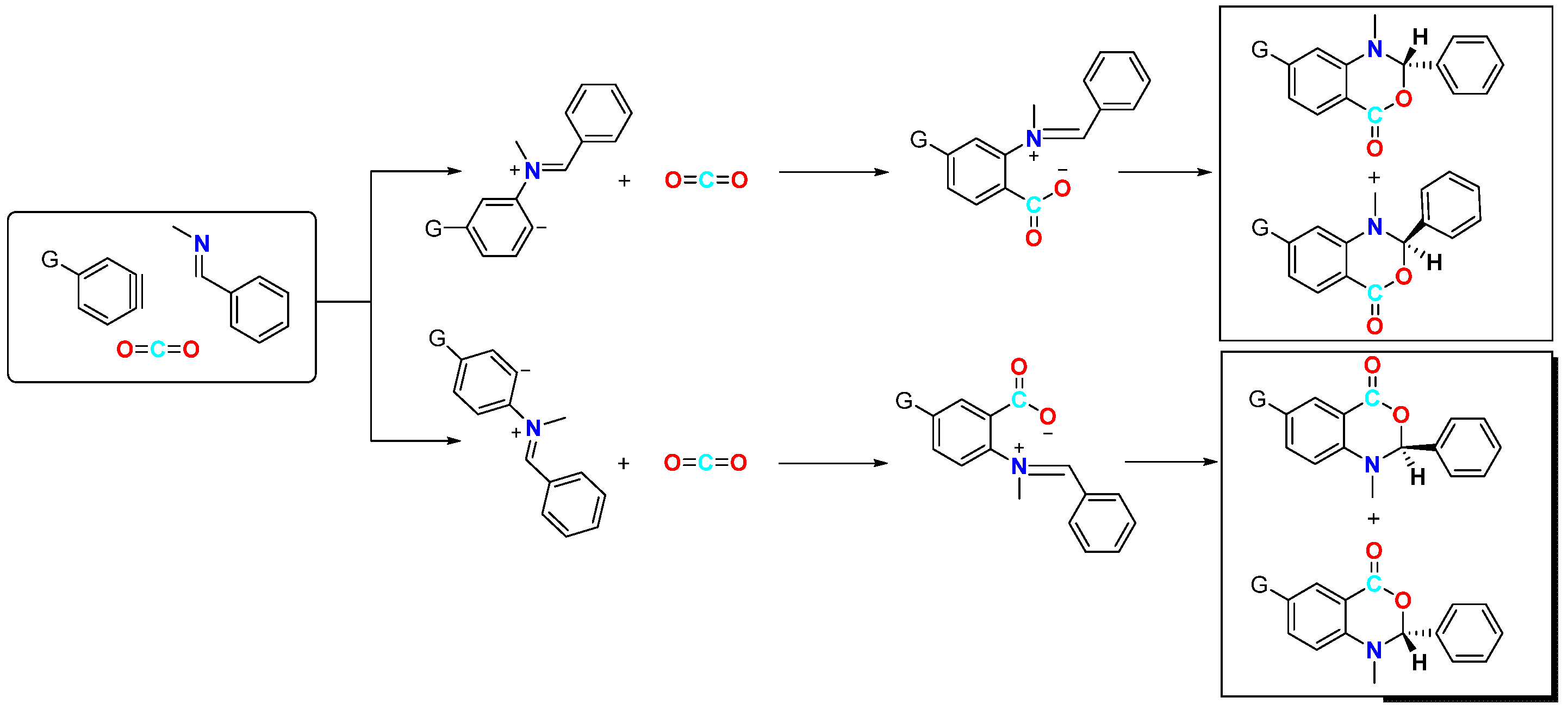
In 2006, Hiroto Yoshida et al. first reported the CO2-involved nucleophilic cyclisation of three-component aromatic alkynes (Figure 2) for synthesising six-membered heterocyclic benzoxazinones [19]. They observed that the reaction of a newly prepared aryne with N-(2,4,6-trimethylbenzylidene)-methylamine under a CO2 atmosphere formed a three-component coupling product, benzoxazinone, with a high yield (82%). This reaction was found to occur more efficiently with electron-rich and neutral imines, resulting in higher yields of the coupling product. However, the reaction failed to occur with less nucleophilic imines and imines with larger spatial steric hindrance such as N-t-Bu and N-Ph imine. The reaction mechanism involves attacking the imine on the phenyl alkyne intermediate, resulting in the formation of an amphiphilic intermediate. Subsequently, CO2 undergoes electrophilic addition with this amphiphilic anion, forming a carboxylate anion, which ultimately yields the target product. In addition to simple benzene, other substituted aromatics are suitable for this reaction; furthermore, a certain regioselectivity is observed for asymmetrically substituted aromatics. This regioselectivity is attributed to the electronic and spatial effects caused by substituent groups on the arylalkane. Sabet-Sarvestani’s group conducted further mechanistic studies of this reaction [20] and suggested that the reaction is most likely initiated by the nucleophilic attack of the imine (Figure 3).
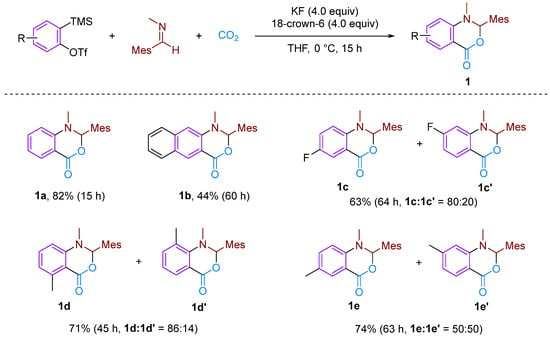
Figure 2.
CO2-involved three-component coupling reaction between arynes and N-(2,4,6-trimethylbenzylidene)methylamine.
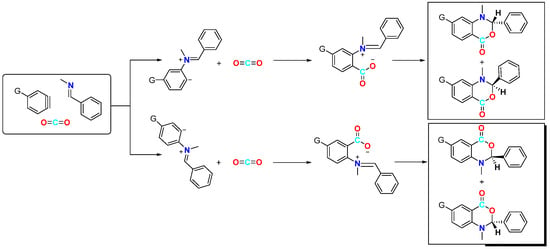
Figure 3.
Mechanistic studies of CO2-involved three-component coupling reaction between arynes and N-(2,4,6-trimethylbenzylidene)methylamine.
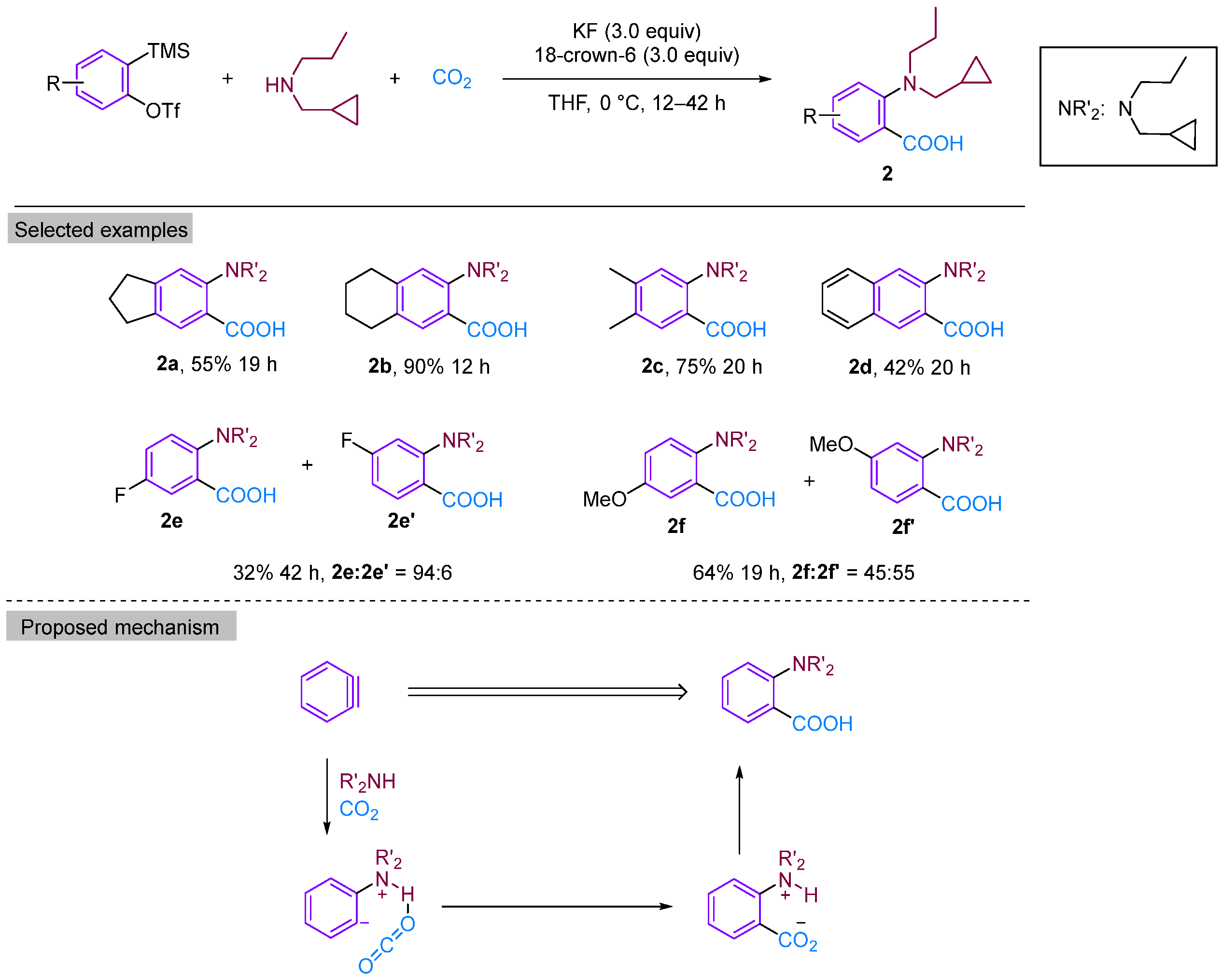
In 2008, Yoshida’s group reported a three-component coupling reaction using a neutral nucleophilic reagent instead of an imine as the substrate [21]. This reaction resulted in the formation of dipropylamine with benzyne to form ortho-aminobenzoic acid derivatives under a CO2 atmosphere (Figure 4). They explored a range of secondary amine substrates and obtained moderate to high yields of neighbouring amino acids, with the exception of tetrandrine. Secondary amines were found to possess greater stability than imines. Additionally, the use of secondary amines allows for increased structural diversity, leading to a significant expansion in the scope of aromatic ring substrates. According to the proposed reaction mechanism, the electrophilic attack by a secondary amine on phenylalkynes generates amphipathic ions, which are subsequently captured by CO2 to produce o-aminobenzoic acid through proton migration. The high selectivity of the three-component coupling reaction relative to other amine arylation reactions may be attributed to the formation of intramolecular hydrogen bonds between amine-H and CO2, which considerably enhances the electrophilicity of CO2. Furthermore, the Lewis basicity of nitrogen effectively inhibits the competition between hydrogen protons and CO2, thereby avoiding the formation of protonated products.
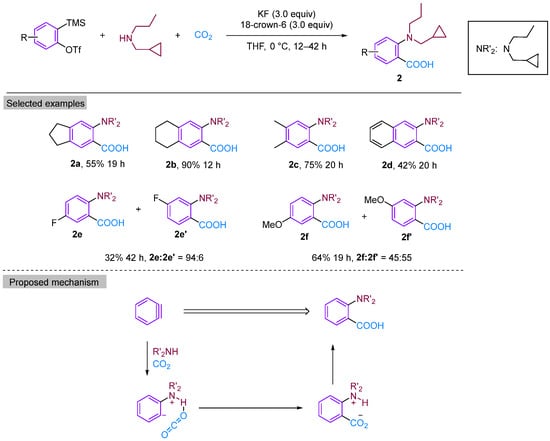
Figure 4.
CO2-involved three-component coupling reaction between arynes and dipropylamine.
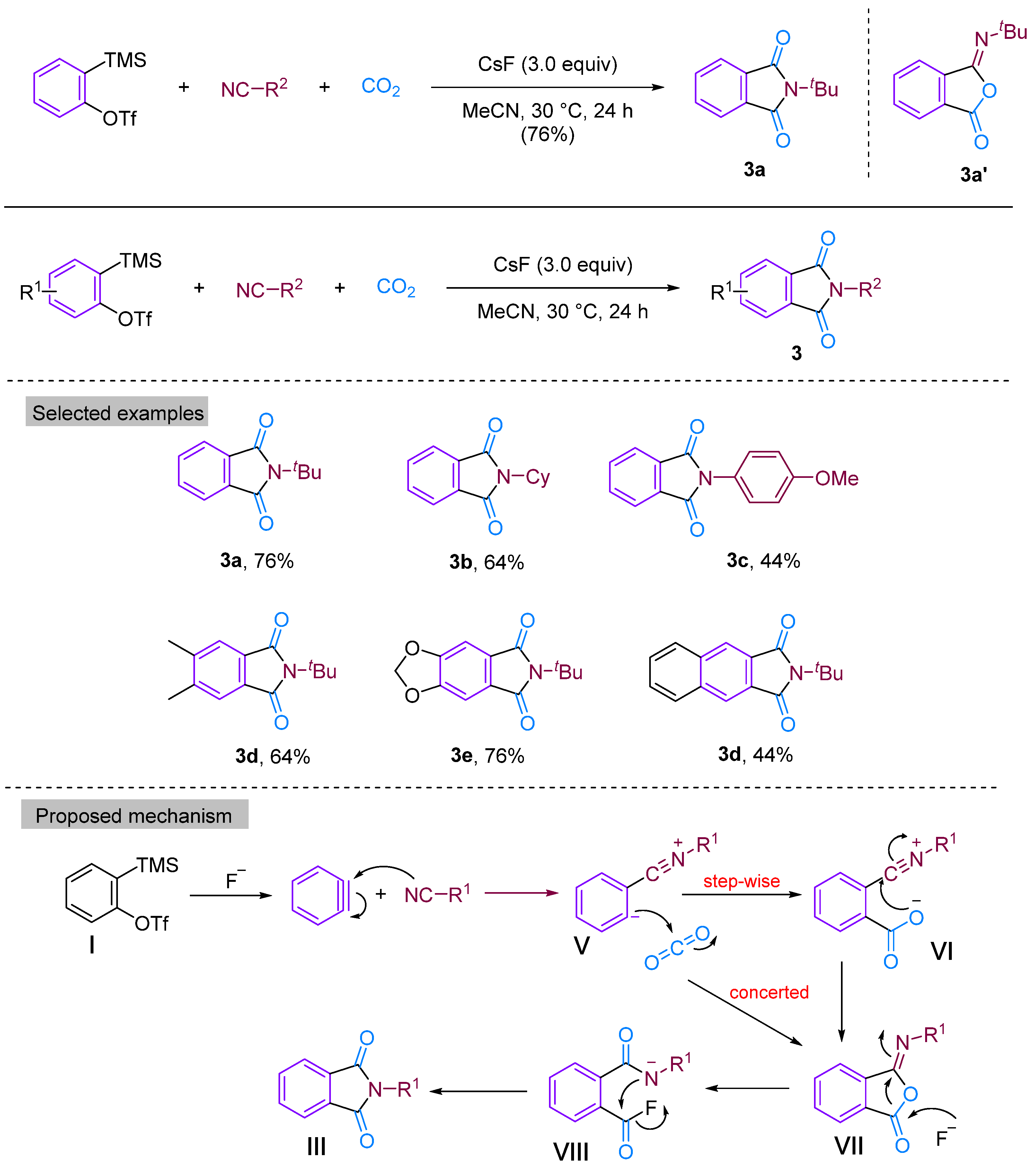
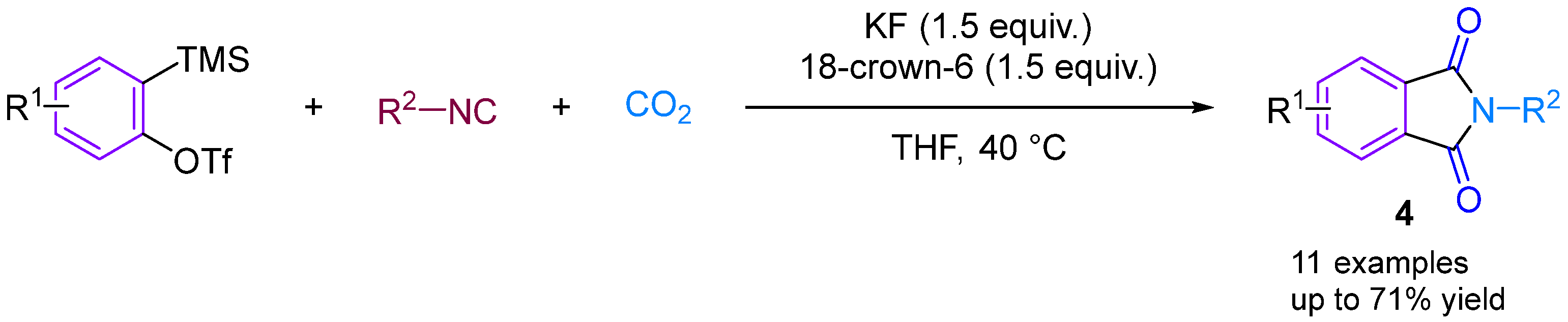
In 2014, Kaicharla’s group reported a CO2-involved three-component coupling reaction between phenylalkynes and isocyanides, resulting in the production of N-substituted phthalimides [22]. Although they expected an iminoisobenzofuranone derivative 3a due to the interception of the 1:1 adduct between aryne and isocyanide with CO2, a different final product was formed, namely N-tert-butylphthalimide 3a′, but still achieved a high yield of 76% (Figure 5). The reaction proceeds by the nucleophilic addition of isocyanide to the aryl hydrocarbon formed in situ from precursor I, leading to the generation of 1,3-amphiphilic intermediate V. This nucleophilic aryl anionic intermediate V can then progressively combine with the electrophilic carbonyl group of CO2 to form the amphiphilic ion VI, which subsequently closes the ring to generate the iminoisobenzofuranone derivative VII. However, the aryl anion V and CO2 can also undergo addition and cyclisation to form VII via a synergistic reaction, with the subsequent fluorination-induced ring-opening of VII generating the acidic fluorine intermediate VIII, which is further cyclised to give the phthalic acid imine derivative III (Figure 5). Previous studies have documented and elucidated the rearrangement of isoimine VII to imine III and a fluoride ion-induced rearrangement of isoimine VII to phthalimide III, which further confirms the authors’ claim [23,24]. In 2015, Yi’s group [25] also obtained phthalimide derivatives (Figure 6) under different conditions, but these derivatives differed from those produced by Kaicharla’s group in terms of their substrate suitability.
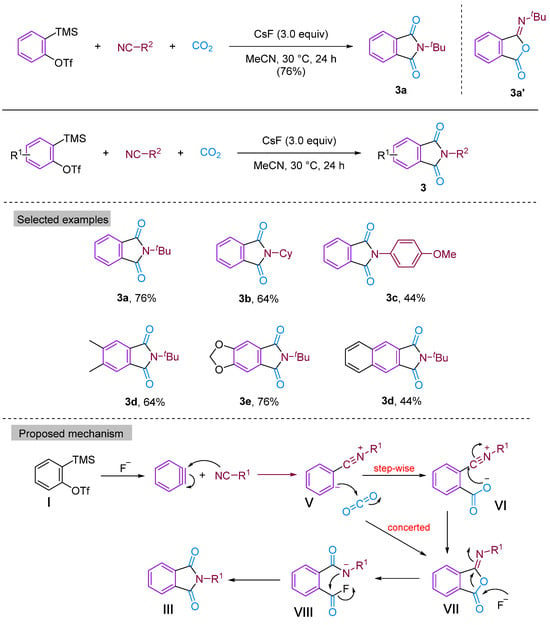
Figure 5.
CO2-involved three-component coupling reaction between arynes and isocyanides (Kaicharla’s group).
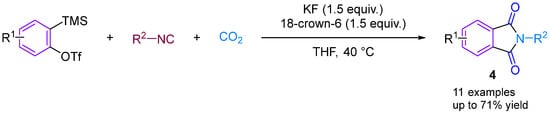
Figure 6.
CO2-involved three-component coupling reaction between arynes and isocyanides (Yi’s group).
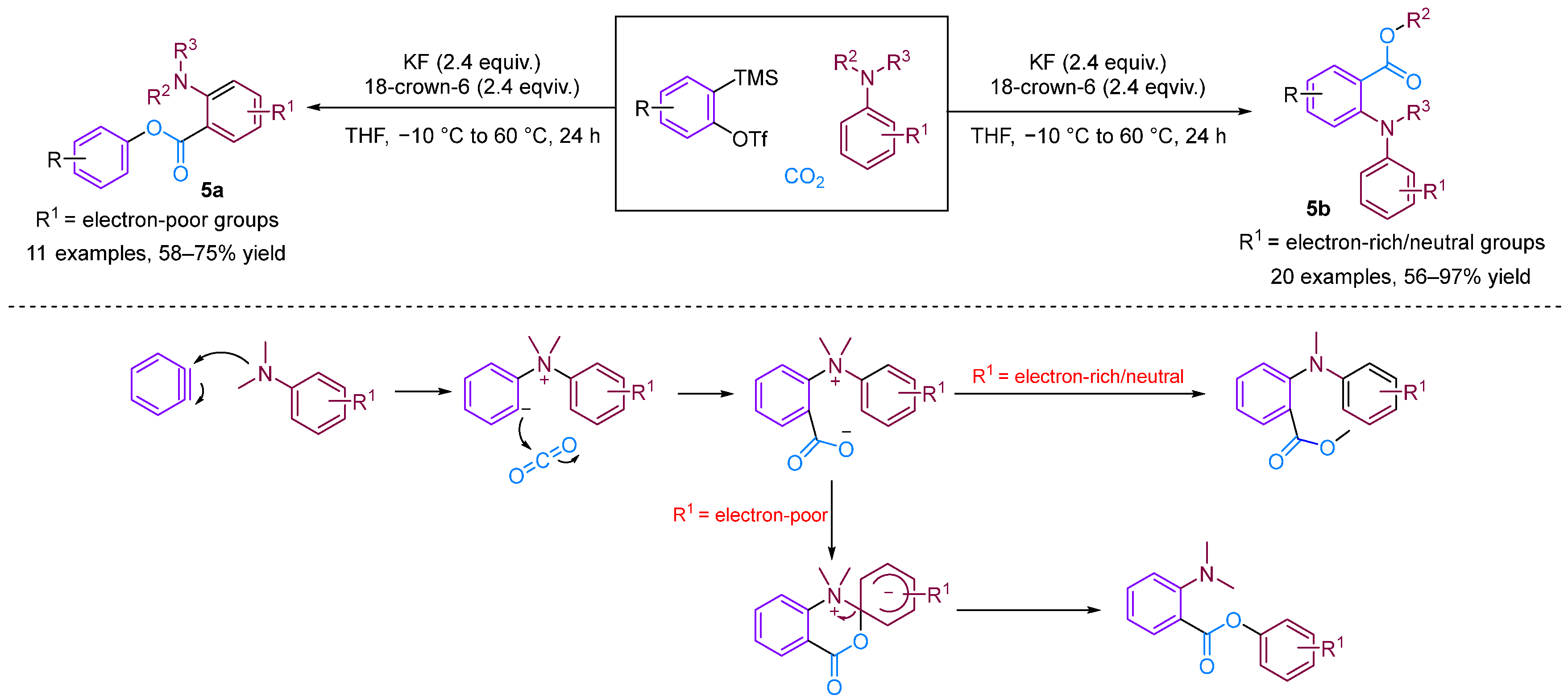
The Bhojgude group reported transition-metal-free MCC involving arynes [26], aromatic tertiary amines and CO2 (Figure 7). Interestingly, this reaction exhibits switchable selectivity depending on the electronic nature of the aromatic amines used. When using amines bearing electron-releasing/neutral groups as the nucleophilic trigger, the reaction afforded 2-arylamino benzoates via nitrogen to oxygen alkyl group migration. However, using electron-deficient amines in the reaction led to the production of 2-aminoaryl benzoates via the aryl-to-aryl amino group migration, resembling a Smiles rearrangement [27].
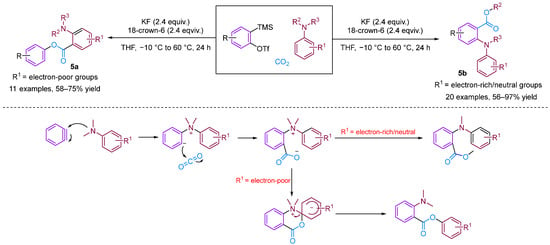
Figure 7.
CO2-involved three-component coupling reaction between arynes and aromatic tertiary amines.
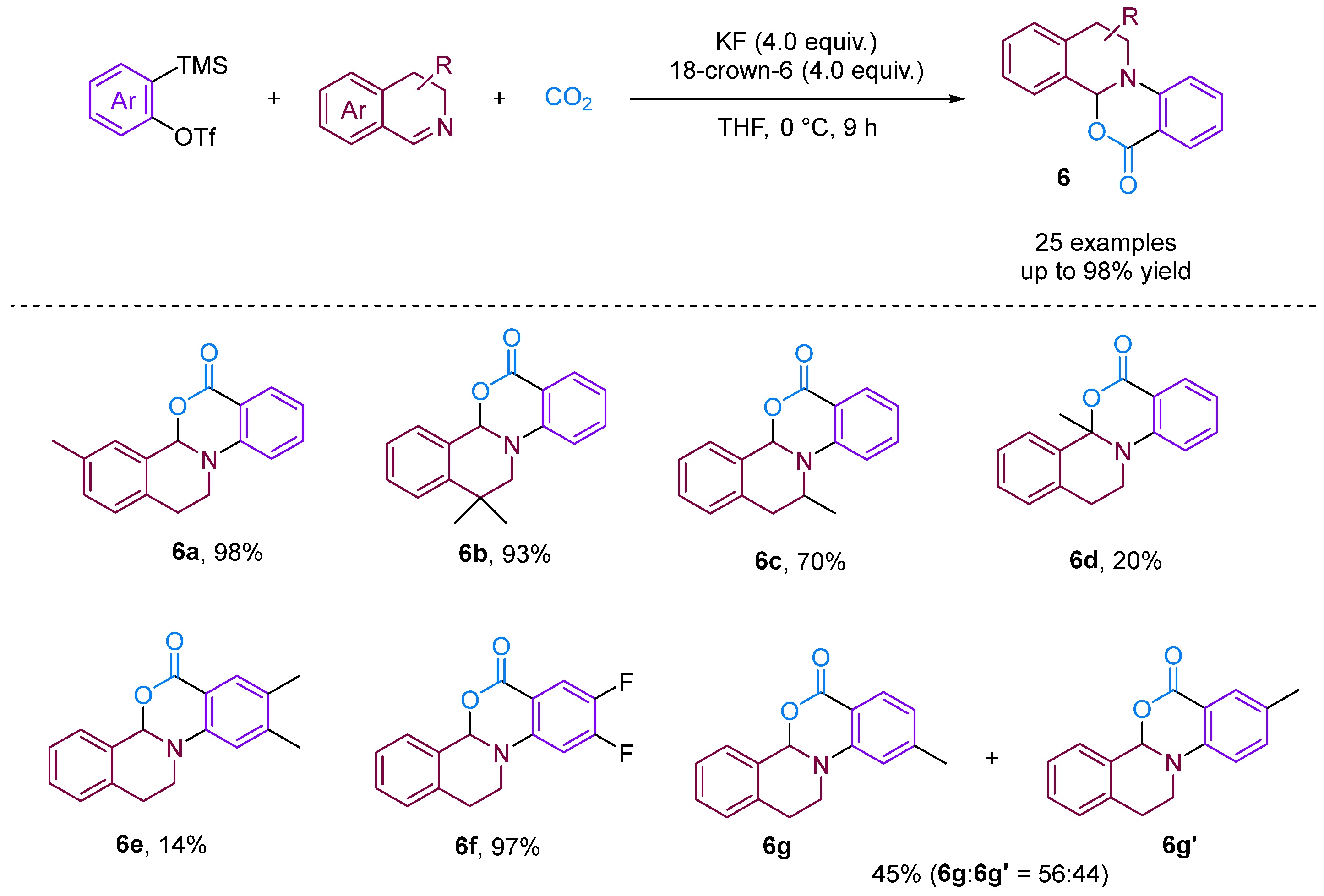
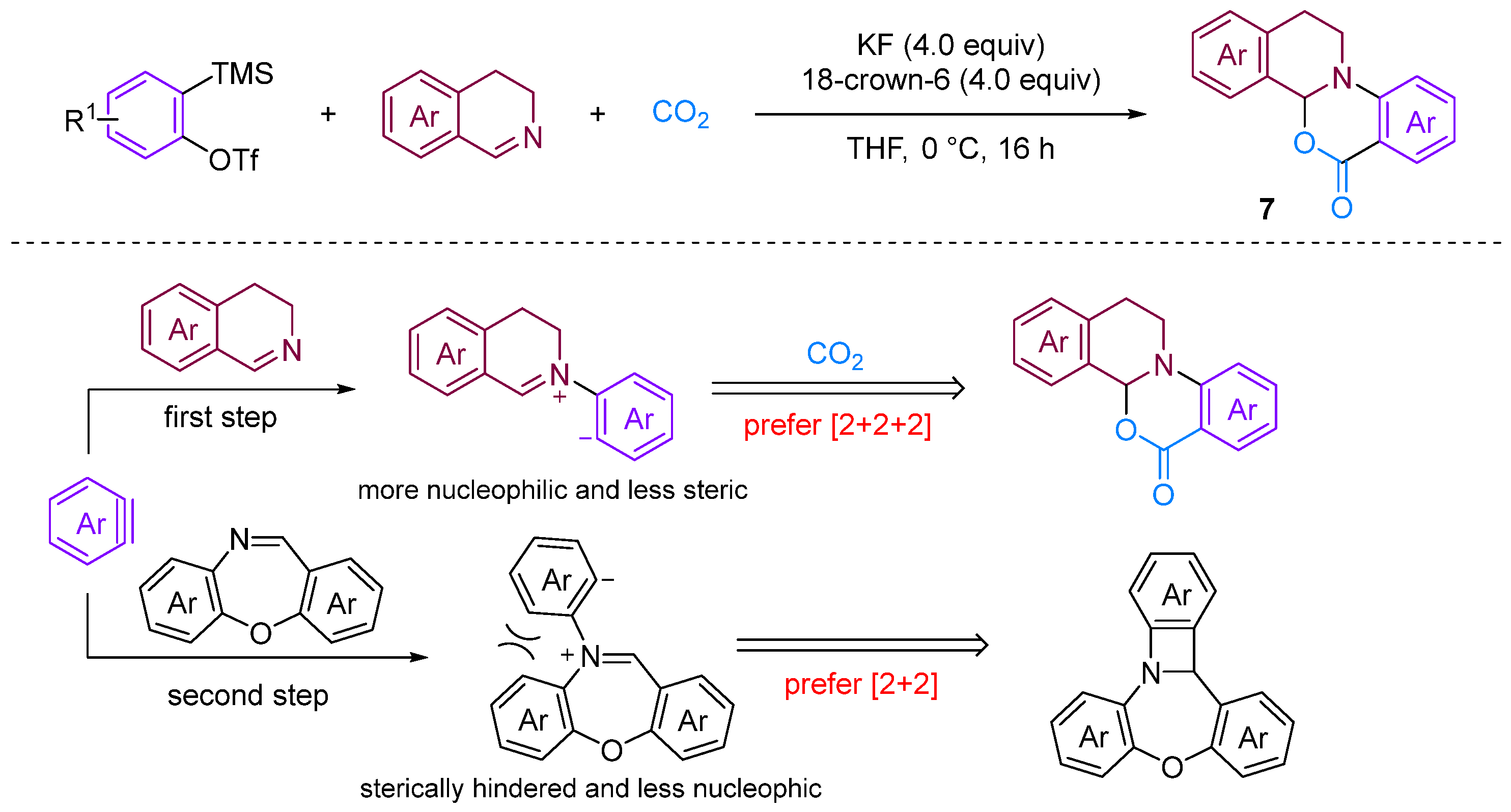
Chen and Xu [28] developed a formal [2 + 2 + 2] annulation of 3,4-dihydroisoquinolines, CO2 and arynes for the efficient synthesis of tetrahydroisoquinoline-fused polycyclic heterocycles (Figure 8). Their method demonstrated the advantages of convergent synthesis and the utilisation of CO2 and readily available starting materials; furthermore, this transformation has been successfully scaled up to the gram scale and the resulting annuladducts can be converted into a series of valuable azapolyheterocyclic derivatives. The generation of electron-rich dihydroisoquinolines was found to have superior reactivity. Notably, the transformation efficiency of electron-deficient 3-methoxy benzyne was significantly higher than that of electron-rich 3-methyl benzyne, reflecting the special regioselectivity of the reaction. The observed preference for reactions with electron-rich dihydroisoquinolines and electron-deficient aryne suggests that the rate-limiting step is likely the nucleophilic reaction between imines and benzynes. Notably, when employing dibenzo [1,4]oxazepines as the reactant for the cyclisation reaction with aryne in the presence of CO2, [2 + 2], annulation was obtained; this differs remarkably from a previous work in which aryne and CO2 with 3,4-dihydroisoquinolines, a representative of N-alkyl cyclic imines, was found to deliver exclusively [2 + 2 + 2] annulation products [29]. In [2 + 2] annulation, the Hammett analyses revealed that the nucleophilic attack by imines on benzynes (the first step, negative ρ) and attack by phenyl anions on iminium species (the second step, positive ρ) influence the reaction rate (Figure 9). Based on the incentive verification experiment, the author suggests that the steric and nucleophilic factors controlled the annuloselectivity. Reactivity analysis revealed that the electron-rich and -deficient steric N-alkyl cyclic imines and electron-deficient benzynes favour [2 + 2 + 2] annulation, while the electron-deficient and bulky N-aryl cyclic imines prefer [2 + 2] annulation [28].
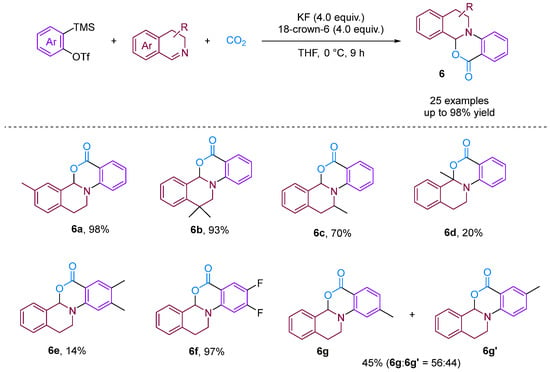
Figure 8.
CO2-involved three-component coupling reaction between arynes and 3,4-dihydroisoquinolines.
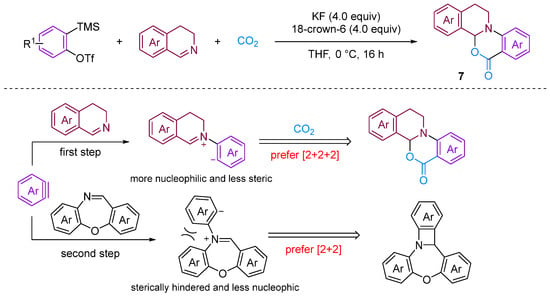
Figure 9.
The steric and nucleophilic factors controlled the annuloselectivity.
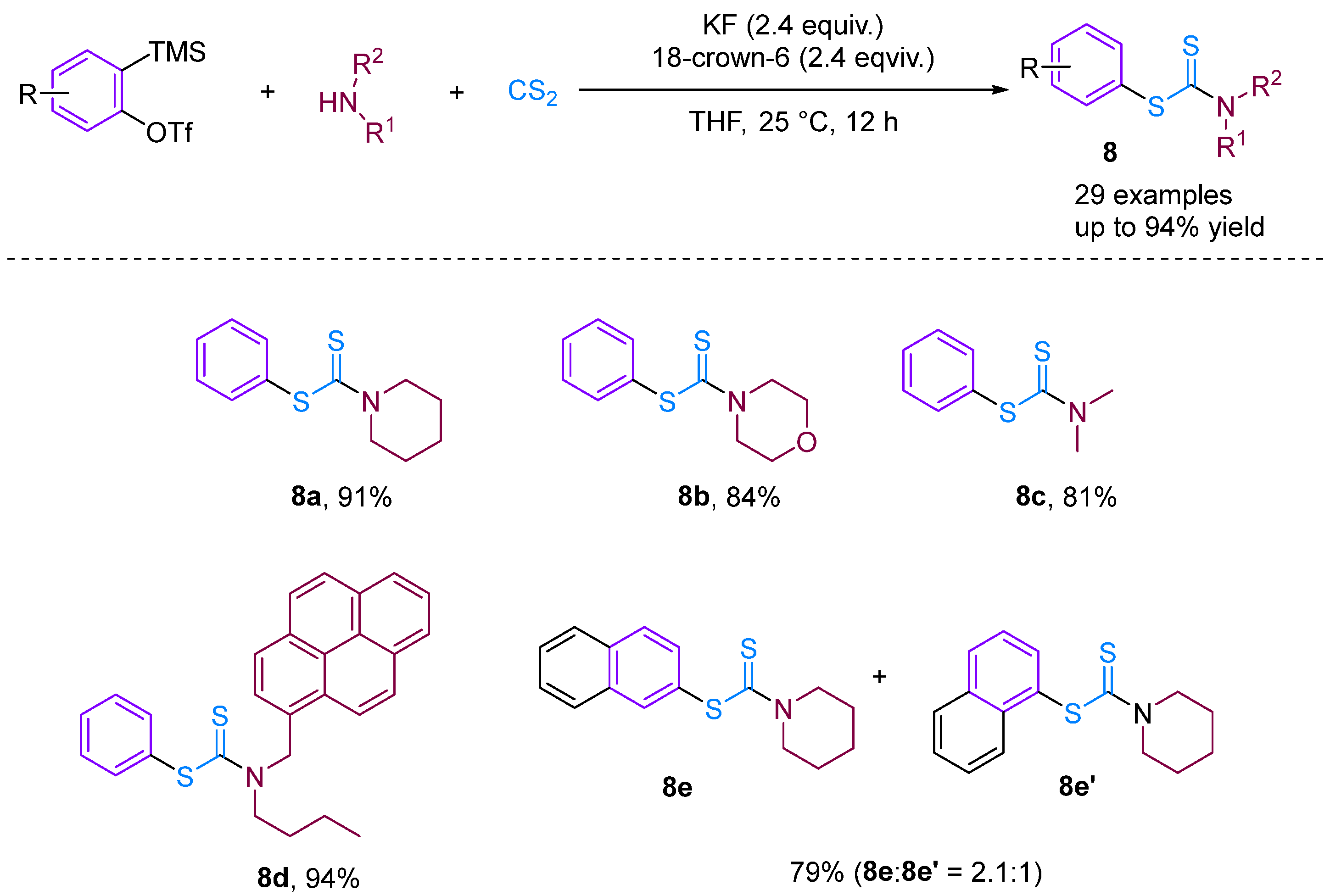
Recently, based on previous work, Bhojgude, Jindal and co-workers reported three-component coupling involving arynes [30], aliphatic amines and CS2 leading to the formation of S-aryl dithiocarbamates in high yield (Figure 10). Contrary to known aryne MCC involving amines and CO2, the present reaction proceeds via the initial addition of amines to CS2 followed by trapping with arynes to obtain the desired products. Detailed experimental and density functional theory studies have demonstrated the addition mechanism involved. Moreover, using 3-triflyloxybenzyne, a four-component coupling with the incorporation of THF was also observed.
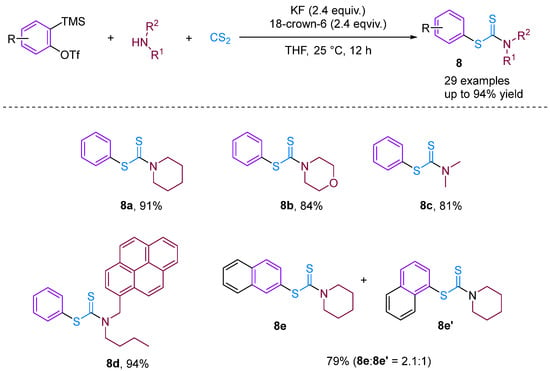
Figure 10.
CS2-involved three-component coupling reaction between arynes and aliphatic amines.
2.2. Nucleophilic Addition Reactions of Aromatic Alkynes with O-Containing Compounds Involving CO2
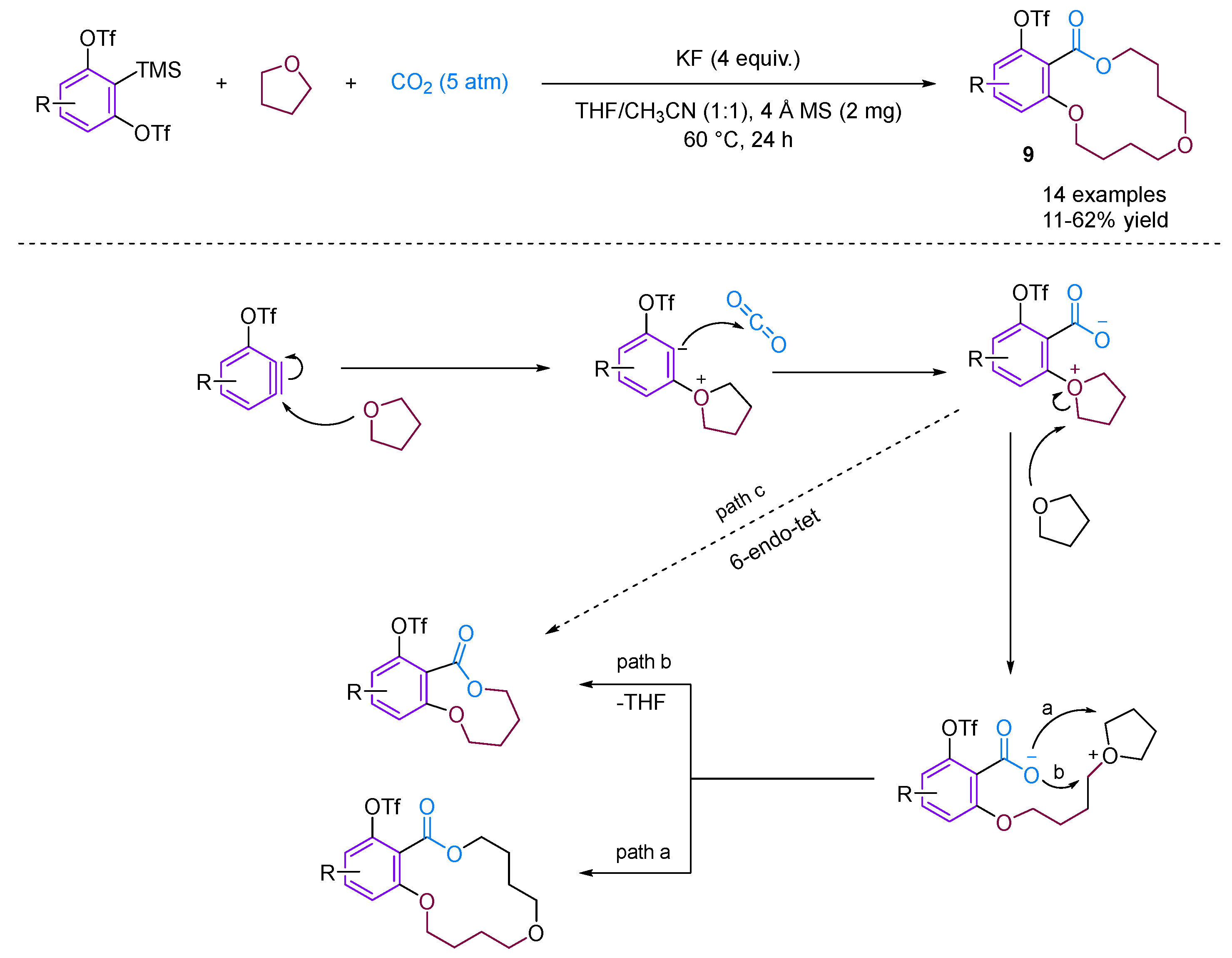
Recently, Qi, Jiang and their team developed a straightforward method for synthesizing a variety of structurally intriguing and valuable 14-membered macrocyclic lactones [31]. This is achieved through a carboxylative macrocyclization of 3-triflyloxybenzynes with CO2 and THF, while the novel [2 + 2 + 5 + 5] coupling reaction proceeds under transition metal-free conditions, forming one new C–C bond and three new C–O bonds in a single step (Figure 11).
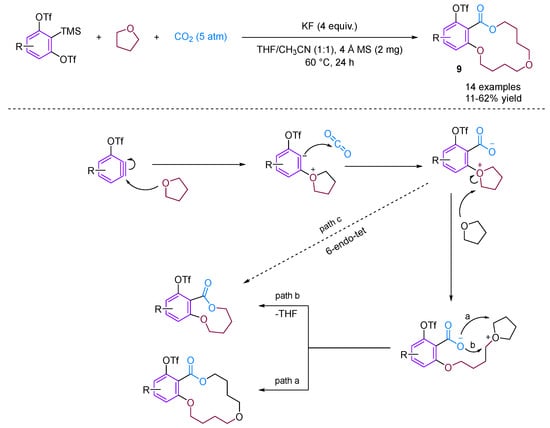
Figure 11.
CO2-involved three-component coupling reaction between arynes and tetrahydrofuran.
2.3. Nucleophilic Addition Reactions of Aromatic Alkynes with P-Containing Compounds Involving CO2
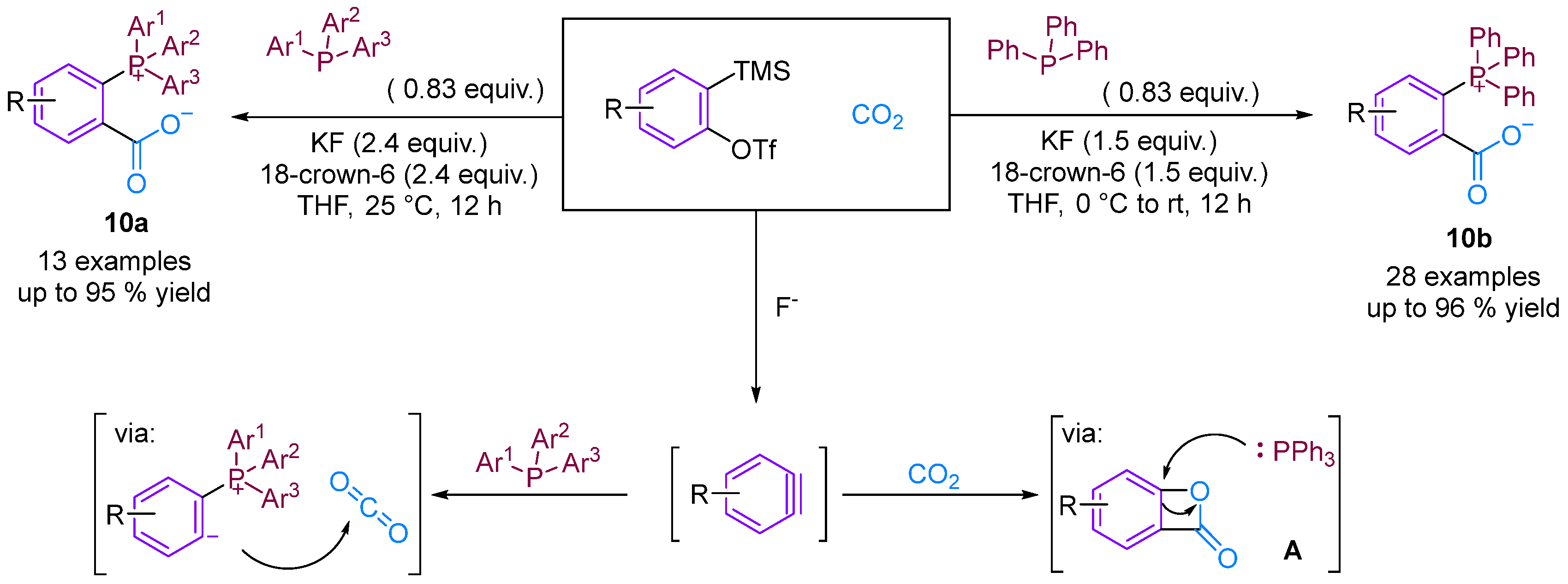
Considering the potential application of phosphonium salts as ligands, reagents, ionic liquids and organo-catalysts in organic synthesis, zwitterionic phosphonium benzoates developed using the aryne strategy are likely to have important applications [32,33]. Bhojgude, Roy and co-workers reported the triggering of transition metal-free aryne three-component coupling by phosphines using CO2 as the third component [34]. The reaction afforded zwitterionic phosphonium benzoates under mild and facile simple conditions instead of the envisioned benzooxaphosphol-3(1H)-ones. Mechanistically, in most cases, the initial phosphine–aryne zwitterion was trapped by CO2 to form carboxylates in high yield. He, Cai and colleagues prepared the corresponding zwitterionic phosphonium benzoates using a similar method [35]. However, He’s group views the reaction mechanism differently, proposing that the reaction occurs through two mechanisms. The first is similar to that reported by Bhojgude, and the second is via the [2 + 2] cycloaddition of benzyne and CO2 to generate intermediate A, which subsequently undergoes a ring-opening reaction with triphenylphosphine to produce the final product. Control experiments indicate the possibility of forming intermediate A (Figure 12).
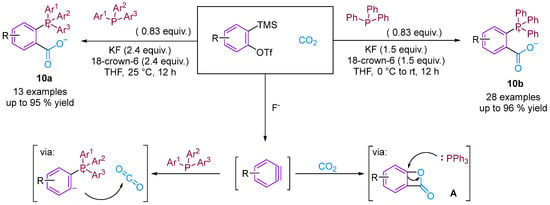
Figure 12.
CO2-involved three-component coupling reaction between arynes and phosphines.
2.4. Nucleophilic Addition Reactions of Aromatic Alkynes with Halogens Involving CO2
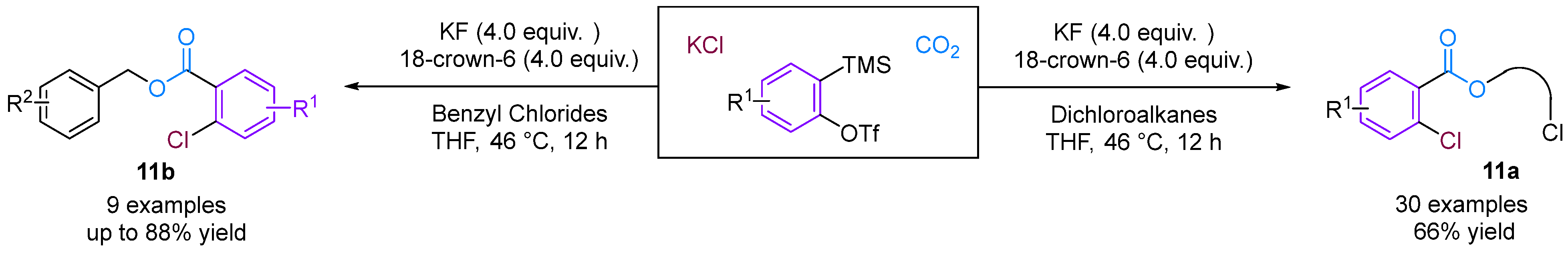
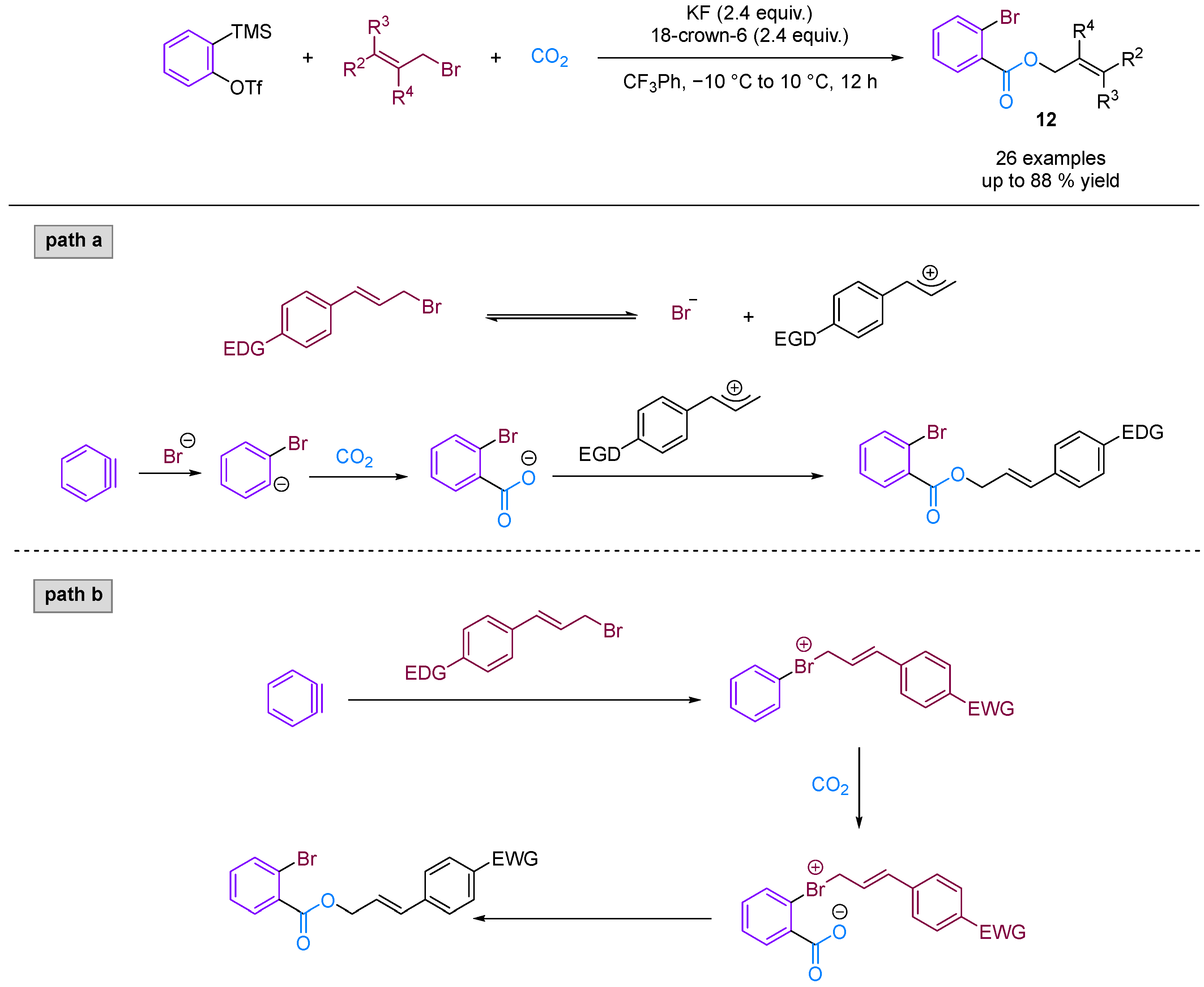
Jiang and co-workers [36] developed a novel three-phase four-component coupling reaction (3P-4CR) using KCl, arynes, chloroalkanes and CO2, thereby providing a facile method for synthesising different types of o-chloro benzoates (Figure 13). The reaction involves the formation of three different new C–Cl, C–C and C–O bonds in a one-pot fashion. In addition, the chloro and ester groups of the products can be alternatively utilised for diverse transformations. Building on this innovative work, Jiang and colleagues [37] developed an unprecedented three-component coupling involving arynes, allyl bromides and CO2, providing efficient and facile access to structurally diverse ortho-brominated aryl esters (Figure 14). Distinct from their conventional role as electrophiles in organic synthesis, organic bromides serve as nucleophiles in this reaction, affording a new approach to MCRs involving aryne intermediates. Additionally, the Hammett analyses suggested that two reaction mechanisms were present (path a and path b), depending on the electronic nature of the cinnamyl bromides used in the reaction.
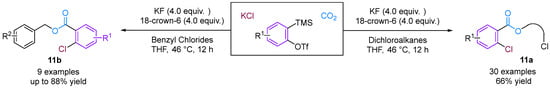
Figure 13.
CO2-involved 3P-4CR between KCl, arynes and chloroalkanes.
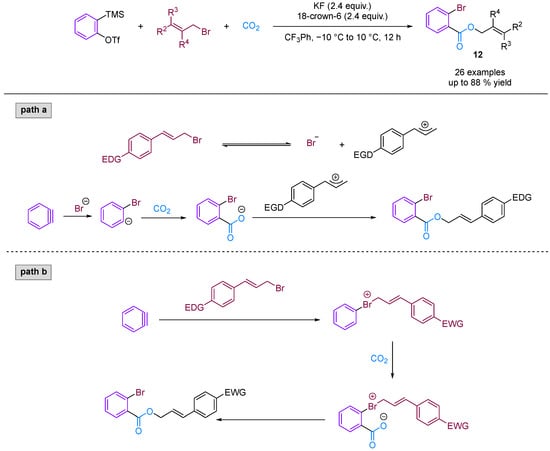
Figure 14.
CO2-involved three-component coupling reaction between arynes and allyl bromides.
2.5. Nucleophilic Addition Reactions of Aromatic Alkynes with Terminal Alkynes Involving CO2
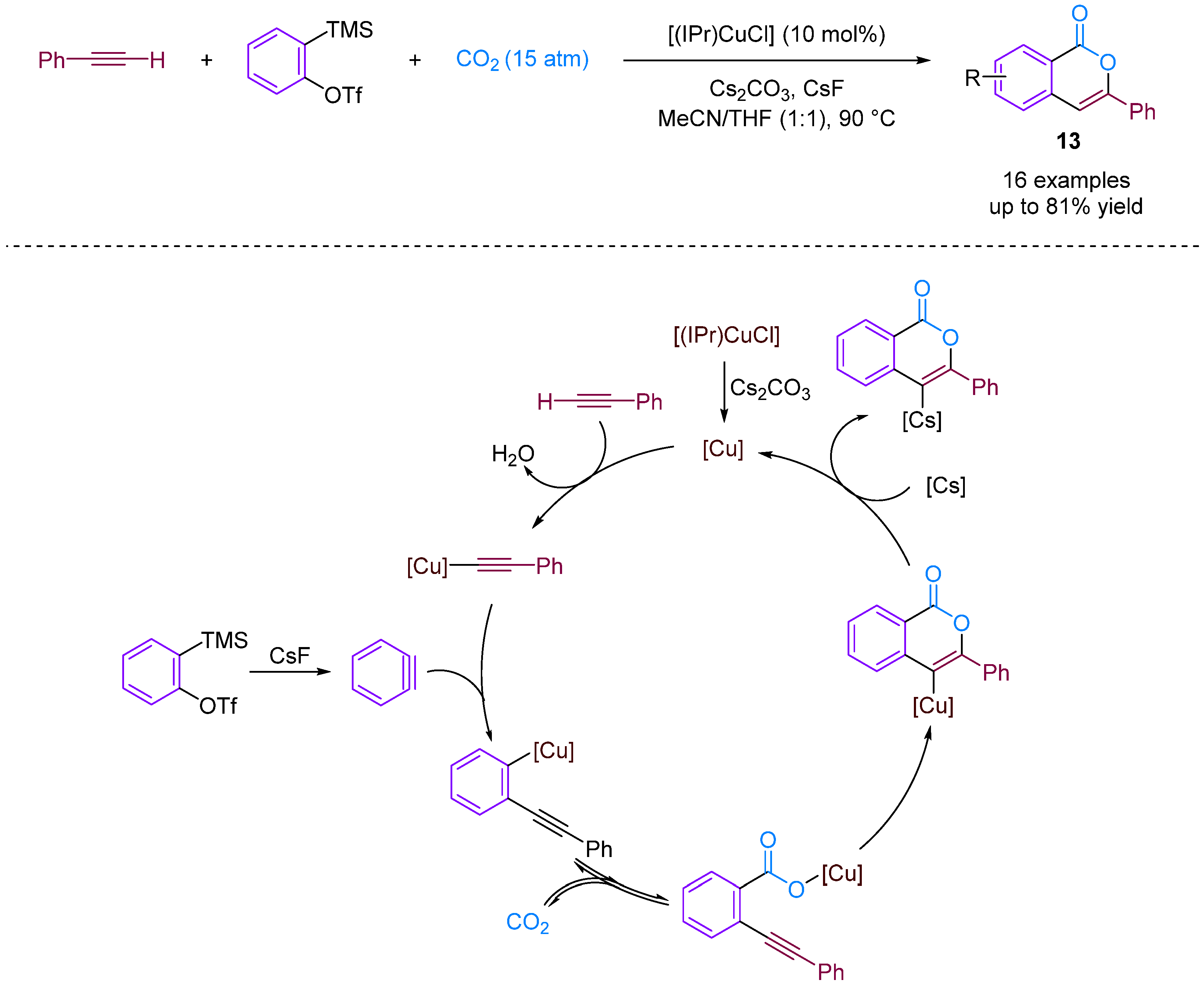
Kobayashi and colleagues (2014) presented the synthesis of isocoumarins using the three-component couplings of arynes, terminal alkynes and CO2 catalysed by an NHC–copper complex [38]. Isocoumarins are important lactones found in natural products and exhibit various biological activities. Moreover, new synthetic strategies are being developed to prepare these materials efficiently, such as the intramolecular cyclisation of ortho-alkynylbenzoic acid derivatives; however, this requires a multistep synthetic route [39]. A copper-catalysed carboxylation reaction of terminal alkynes with CO2 successfully produced isocoumarins. N-heterocyclic carbene–copper complexes were found to facilitate multiple transformations in a multi-component reaction. The reaction involved the generation of copper carboxylate and isocoumarins. Furthermore, the synthetic utility of the reaction was demonstrated at the gram scale. The reaction mechanism involves the formation of reactive intermediates leading to desired products (Figure 15).
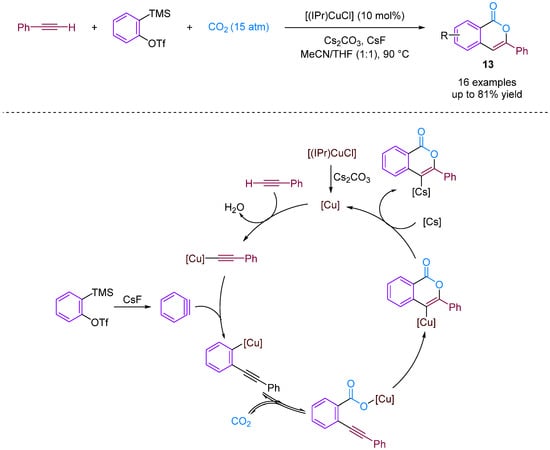
Figure 15.
CO2-involved three-component coupling reaction between arynes and terminal alkynes catalysed by an NHC–copper complex.
3. Conclusions
CO2-involved aryne reactions typically proceed under very mild conditions, requiring minimal strong bases and often relatively low reaction temperatures. This gentle conversion of carbon dioxide, which is a relatively stable compound, is highly significant for translating into carbonyl compounds. In addition, employing various nucleophilic reagents renders it convenient to obtain benzoic acids or cyclisation products with varying substitution patterns, playing a crucial role in pharmaceutical and material synthesis.
In terms of substrate selection, despite the considerable progress, few nucleophilic reagents are currently available for attack, most of which are N-containing compounds or their derivatives. The reason for this is twofold. On the one hand, nitrogen atoms possess strong nucleophilicity, allowing them to rapidly attack in situ benzyne intermediates and proceed with subsequent reactions. A more critical factor is that common nucleophilic reagents (such as alcohols and phenols) generate protons in situ during the reaction, which severely hinders the further involvement of carbon dioxide in the reaction and may lead to the formation of large amounts of protonated by-products if not handled appropriately. However, N-containing compounds (such as amine compounds) can, to some extent, bind the formed protons due to their Lewis basicity, thereby affording the target product in higher yield, as demonstrated in the reaction shown in Figure 4. Therefore, effectively trapping the generated protons in the reaction system is crucial for the success of similar reactions. Furthermore, the efficient implementation of such transformations can be achieved through substrate design to facilitate ring-closing reactions after addition.
In terms of mechanistic studies, computational evidence has demonstrated that amphipathic ions generated in the nucleophilic attack of aryne by nucleophilic reagents serve as effective CO2 trappers. However, mechanistic studies on cycloaddition reactions involving CO2 remain relatively underexplored. Furthermore, achieving regioselective control over aryl ring substitution substrates is crucial. Moreover, various reactive intermediates have garnered significant interest for CO2 activation and should form the focus of future studies.
Overall, CO2-involved aryne reactions have many promising applications for synthesising diverse and meaningful molecular backbones, provided that challenges such as substrate selection, mechanistic understanding and regioselective control are addressed effectively.
Author Contributions
Conceptualization, Z.Z. and S.G.; writing—original draft preparation, S.G.; writing—review and editing, S.G., X.X., H.S., Y.L. and J.L.; supervision, Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Sichuan Province (No. 2022NSFSCO200), Sichuan Science and Technology Program (No. MZGC20230100).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sakakura, T.; Choi, J.C.; Yasuda, H. Transformation of Carbon Dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef]
- Louie, J. Transition Metal Catalyzed Reactions of Carbon Dioxide and Other Heterocumulenes. Curr. Org. Chem. 2005, 9, 605–623. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, Y.; Zhang, Q.F.; Yu, D.G. Visible Light-driven Carboxylation with CO2. Chem. J. Chin. Univ. 2022, 43, 20220255. [Google Scholar] [CrossRef]
- Asare Bediako, B.B.; Qian, Q.; Han, B. Synthesis of C2+ Chemicals from CO2 and H2 via C–C Bond Formation. Acc. Chem. Res. 2021, 54, 2467–2476. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Jiang, Y.X.; Zhang, Z.; Gui, Y.Y.; Zhou, X.Y.; Yu, D.G. CO2 = CO + [O]: Recent advances in carbonylation of C–H bonds with CO2. Chem. Commun. 2020, 56, 8355–8367. [Google Scholar] [CrossRef]
- Arshadi, S.; Banaei, A.; Ebrahimiasl, S.; Monfared, A.; Vessally, E. Solvent-free incorporation of CO2 into 2-oxazolidinones: A review. RSC Adv. 2019, 9, 19465–19482. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, L.; Jackstell, R.; Beller, M. Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 2015, 6, 5933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, X.L.; Xie, X.M.; Gao, T.Y.; Qin, J.; Li, J.J.; Feng, C.; Yu, D.G. Iron-promoted carbonylation–rearrangement of α-aminoaryl-tethered alkylidenecyclopropanes with CO2: Facile synthesis of quinolinofurans. Chin. Chem. Lett. 2024, 110056. [Google Scholar] [CrossRef]
- Cai, B.; Cheo, H.W.; Liu, T.; Wu, J. Light-Promoted Organic Transformations Utilizing Carbon-Based Gas Molecules as Feedstocks. Angew. Chem. Int. Ed. Engl. 2021, 60, 18950–18980. [Google Scholar] [CrossRef]
- Shi, Y.; Xia, C.; Huang, Y.; He, L. Electrochemical Approaches to Carbonylative Coupling Reactions. Chem. Asian J. 2021, 16, 2830–2841. [Google Scholar] [CrossRef]
- Nielsen, D.U.; Hu, X.-M.; Daasbjerg, K.; Skrydstrup, T. Chemically and electrochemically catalysed conversion of CO2 to CO with follow-up utilization to value-added chemicals. Nat. Catal. 2018, 1, 244–254. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, S.; Chen, C.; Peng, Q.; Wang, D.; Li, Y. Single-Atom Catalysts: Synthetic Strategies and Electrochemical Applications. Joule 2018, 2, 1242–1264. [Google Scholar] [CrossRef]
- Aped, P.; Allinger, N.L. A molecular mechanics study of cyclopropanes within the MM2 and MM3 force fields. J. Am. Chem. Soc. 1992, 114, 1–16. [Google Scholar] [CrossRef]
- Berry, R.S.; Clardy, J.; Schafer, M.E. Benzyne. J. Am. Chem. Soc. 1964, 86, 2738–2739. [Google Scholar] [CrossRef]
- Shi, J.; Li, L.; Li, Y. o-Silylaryl Triflates: A Journey of Kobayashi Aryne Precursors. Chem. Rev. 2021, 121, 3892–4044. [Google Scholar] [CrossRef]
- Bhojgude, S.S.; Bhunia, A.; Biju, A.T. Employing Arynes in Diels–Alder Reactions and Transition-Metal-Free Multicomponent Coupling and Arylation Reactions. Acc. Chem. Res. 2016, 49, 1658–1670. [Google Scholar] [CrossRef]
- Rondan, N.G.; Domelsmith, L.N.; Houk, K.N.; Bowne, A.T.; Levin, R.H. ChemInform Abstract: The Relative Rates of Electron-Rich and Electron-Deficient Alkene Cycloadditions to Benzyne. Enhanced Electrophilicity as a Consequence of Alkyne Bending Distortions. Chem. Informationsdienst 1979, 10, 3237. [Google Scholar] [CrossRef]
- Himeshima, Y.; Sonoda, T.; Kobayashi, H. Fluoride-induced 1,2-elimination of o-trimethylsilylphenyl triflate to benzyne under mild conditions. Chem. Lett. 1983, 12, 1211–1214. [Google Scholar] [CrossRef]
- Yoshida, H.; Hiroyuki, F.; Ohshita, J.; Kunai, A. CO2 Incorporation Reaction Using Arynes: Straightforward Access to Benzoxazinone. J. Am. Chem. Soc. 2006, 128, 11040–11041. [Google Scholar] [CrossRef]
- Sabet-Sarvestani, H.; Eshghi, H.; Izadyar, M. Substituent effects and mechanism studies in CO2 transformation to benzoxazinone derivatives as worthwhile N-containing heterocycles: Insight from Density functional theory. Int. J. Quantum Chem. 2021, 121, e26784. [Google Scholar] [CrossRef]
- Yoshida, H.; Takami, M.; Morishita, T.; Ohshita, J. Direct Access to Anthranilic Acid Derivatives via CO2 Incorporation Reaction Using Arynes. Org. Lett. 2008, 10, 3845–3847. [Google Scholar] [CrossRef] [PubMed]
- Kaicharla, T.; Thangaraj, M.; Biju, A.T. Practical Synthesis of Phthalimides and Benzamides by a Multicomponent Reaction Involving Arynes, Isocyanides, and CO2/H2O. Org. Lett. 2014, 16, 1728–1731. [Google Scholar] [CrossRef] [PubMed]
- Yoshihiko, I.; Toshiro, K.; Syotaro, F.; Junichiro, S.; Takeo, S. Reaction of 2-Halo-1-Olefin-1-Carboxylic Acid with Cu2O and Isonitrile. Bull. Chem. Soc. Jpn. 1975, 48, 115–117. [Google Scholar] [CrossRef]
- Sauers, C.K.R.; Howard, M. Carbon-13 and proton magnetic resonance study of syn-anti isomerism in maleisoimides and succinisoimides. J. Am. Chem. Soc. 1973, 95, 7731–7736. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, S.Y.; Ji, S.J. Synthesis of phthalimides through 1,3-dipolar cycloaddition of CO2 with isocyanides and arynes. Tetrahedron 2015, 71, 2768–2771. [Google Scholar] [CrossRef]
- Bhojgude, S.S.; Roy, T.; Gonnade, R.G.; Biju, A.T. Substrate-Controlled Selectivity Switch in the Three-Component Coupling Involving Arynes, Aromatic Tertiary Amines, and CO2. Org. Lett. 2016, 18, 5424–5427. [Google Scholar] [CrossRef]
- Snape, T.J. A truce on the Smiles rearrangement: Revisiting an old reaction—The Truce–Smiles rearrangement. Chem. Soc. Rev. 2008, 37, 2452–2458. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, K.; Meng, Y.; Xu, J.; Chen, N. Aryne and CO2 based formal [2 + 2 + 2] annulation to access tetrahydroisoquinoline-fused benzoxazinones. Org. Biomol. Chem. 2023, 21, 6892–6897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liu, S.; Wang, L.; Liu, X.; Xu, J.; Chen, N. Annuloselectivity of Dibenzoxazepines and Arynes in the Presence of CO2: [2 + 2] vs [2 + 2 + 2] Annulation. ChemistrySelect 2024, 9, e202304458. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Deswal, S.; Manoj, N.; Jindal, G.; Biju, A.T. Aryne Three-Component Coupling Involving CS2 for the Synthesis of S-Aryl Dithiocarbamates. Org. Lett. 2021, 23, 9083–9088. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, H.; Qi, C. Macrocyclization of carbon dioxide with 3-triflyloxybenzynes and tetrahydrofuran: Straightforward access to 14-membered macrolactones. Chem. Commun. 2024, 60, 6639–6642. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, H.; Guo, H. Recent Advances in Phosphonium Salt Catalysis. Adv. Synth. Catal. 2021, 363, 2023–2036. [Google Scholar] [CrossRef]
- Olivier-Bourbigou, H.; Magna, L.; Morvan, D. Ionic liquids and catalysis: Recent progress from knowledge to applications. Appl. Catal. A Gen. 2010, 373, 1–56. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Raju, A.; Gaykar, R.N.; Gonnade, R.G.; Roy, T.; Biju, A.T. Rapid Synthesis of Zwitterionic Phosphonium Benzoates by a Three-Component Coupling Involving Phosphines, Arynes and CO2. Chem. Asian J. 2020, 15, 2203–2207. [Google Scholar] [CrossRef]
- Xie, P.; Yang, S.; Guo, Y.; Cai, Z.; Dai, B.; He, L. Multicomponent Reaction of Phosphines, Benzynes, and CO2: Facile Synthesis of Stable Zwitterionic Phosphonium Inner Salts. J. Org. Chem. 2020, 85, 8872–8880. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Y.; Xiong, W.; Cen, J.; Wang, L.; Cheng, R.; Qi, C.; Wu, W. A Three-Phase Four-Component Coupling Reaction: Selective Synthesis of o-Chloro Benzoates by KCl, Arynes, CO2, and Chloroalkanes. Org. Lett. 2018, 21, 345–349. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, W.; Cen, J.; Yan, W.; Wu, Y.; Qi, C.; Wu, W.; Jiang, H. Direct bromocarboxylation of arynes using allyl bromides and carbon dioxide. Chem. Commun. 2019, 55, 12304–12307. [Google Scholar] [CrossRef] [PubMed]
- Yoo, W.J.; Nguyen, T.V.Q.; Kobayashi, S. Synthesis of Isocoumarins through Three-Component Couplings of Arynes, Terminal Alkynes, and Carbon Dioxide Catalyzed by an NHC–Copper Complex. Angew. Chem. Int. Ed. Engl. 2014, 53, 10213–10217. [Google Scholar] [CrossRef]
- Li, X.; Chianese, A.R.; Vogel, T.; Crabtree, R.H. Intramolecular Alkyne Hydroalkoxylation and Hydroamination Catalyzed by Iridium Hydrides. Org. Lett. 2005, 7, 5437–5440. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).