Versatile Activated Carbon Fibers Derived from the Cotton Fibers Used as CO2 Solid-State Adsorbents and Electrode Materials
Abstract
:1. Introduction
2. Results and Discussion
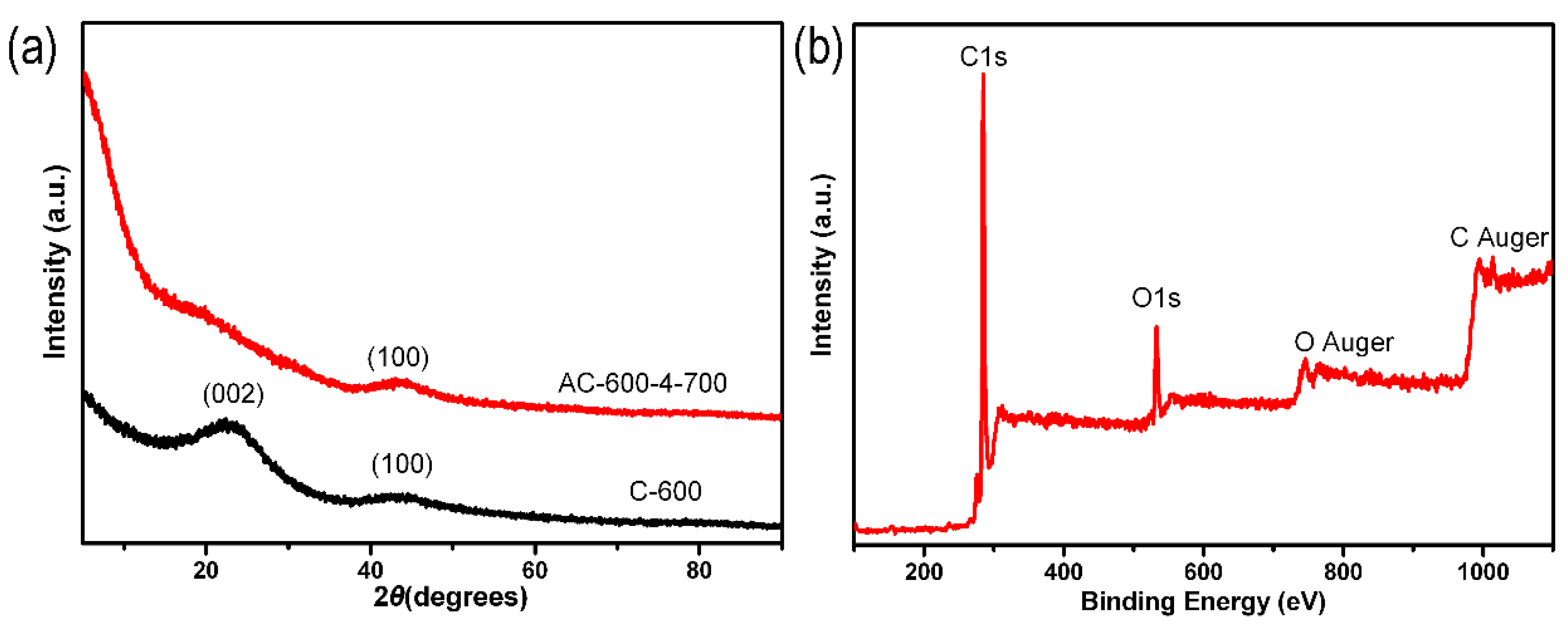
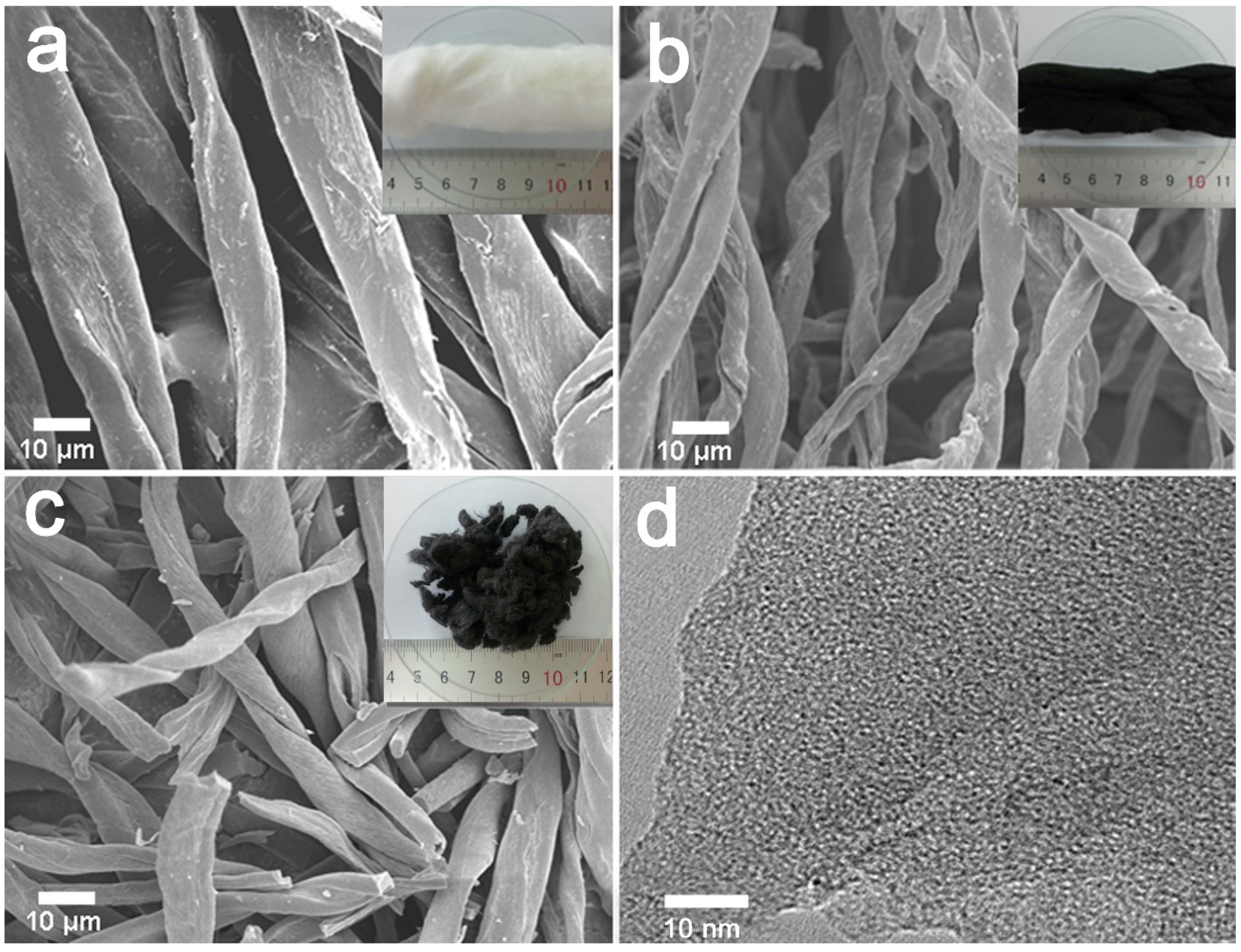
2.1. Sample Structure
2.2. CO2 Capture Capacity
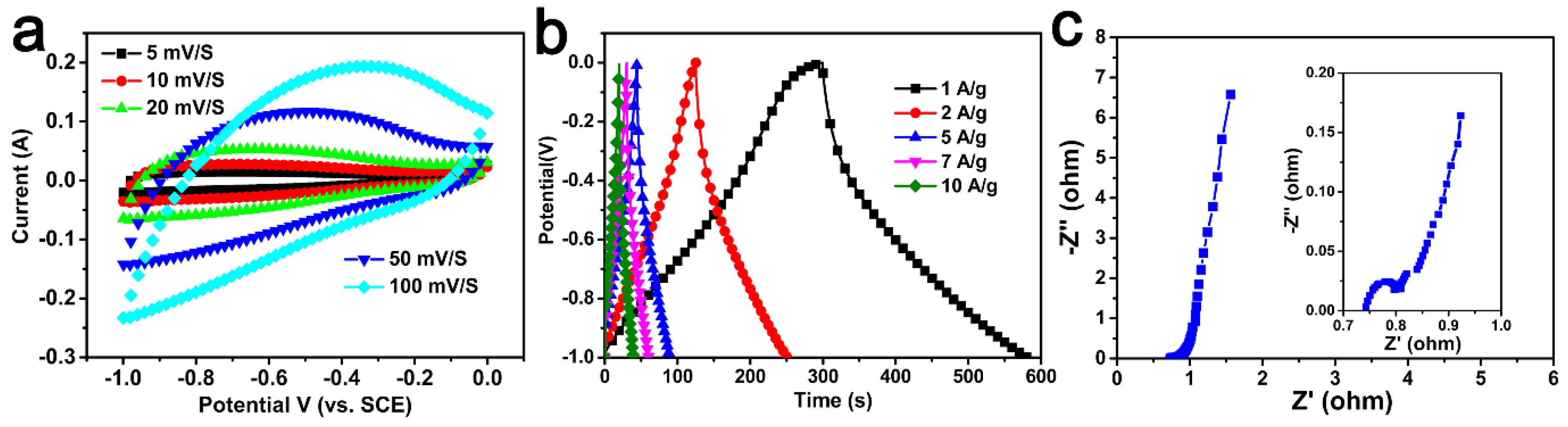
2.3. Electrochemical Studies
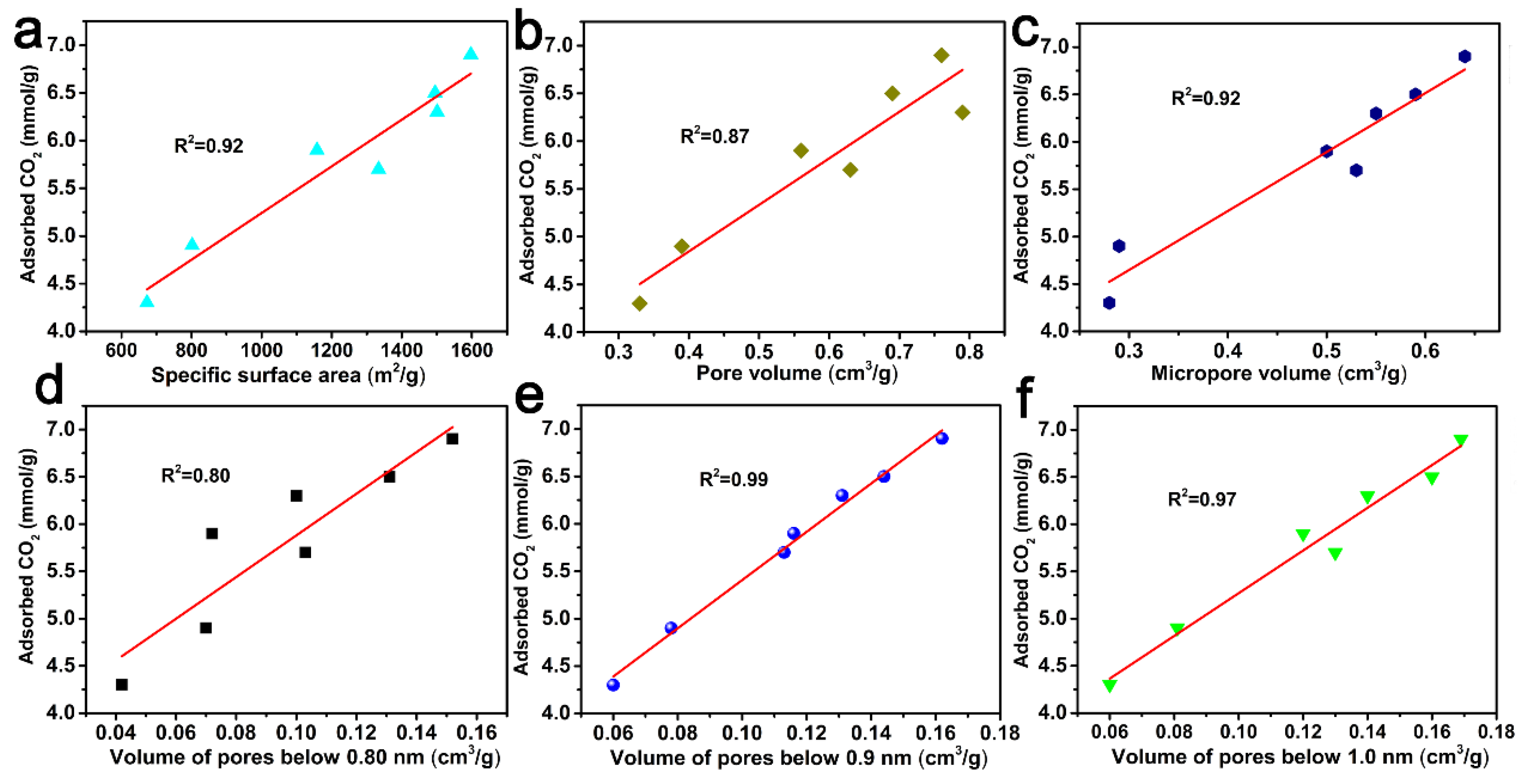
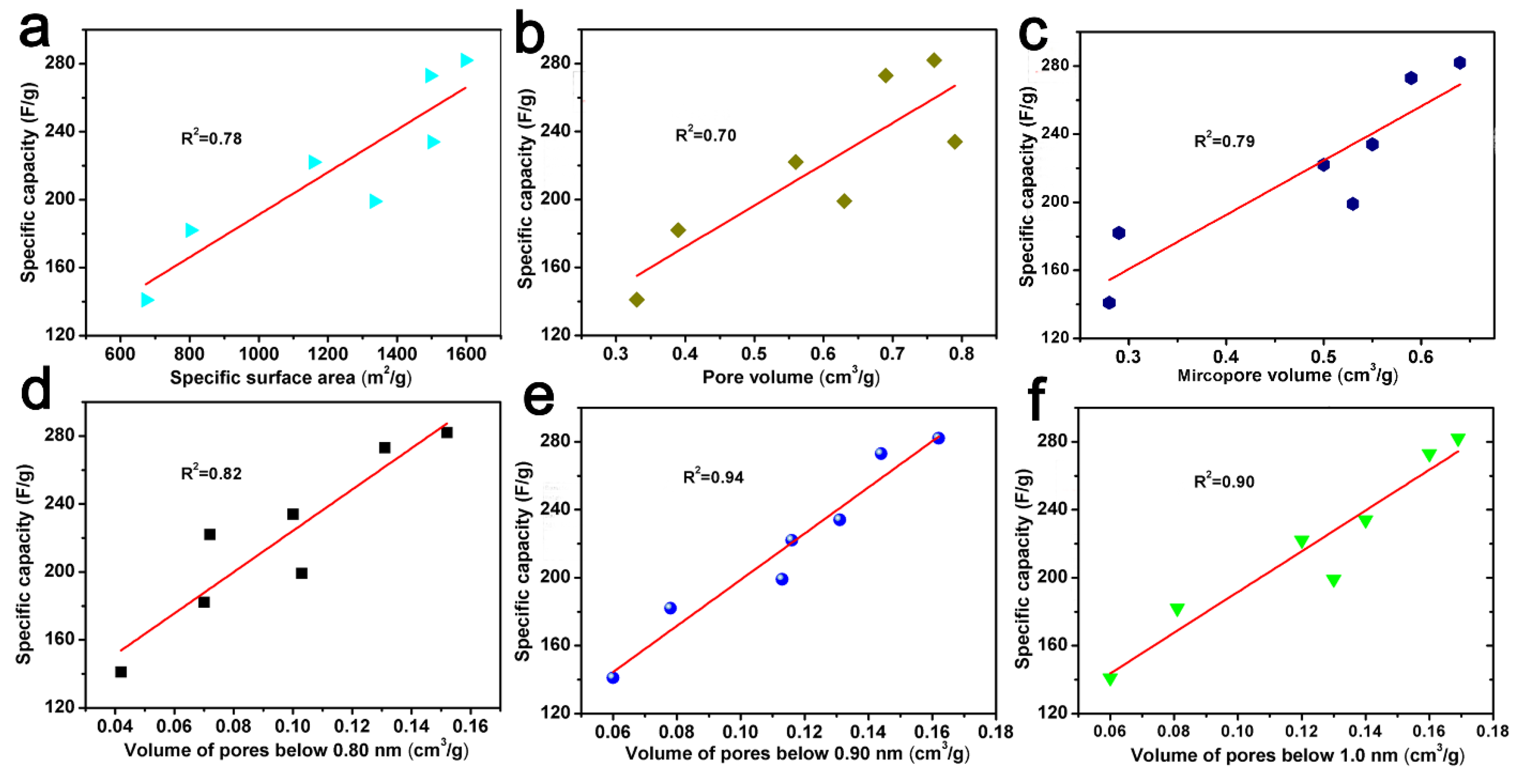
2.4. The Relationship between Pore Volume with a Specific Range of Pores and the CO2 Adsorption Capacity or Specific Capacity of ACF Samples
3. Materials and Methods
3.1. Materials
3.2. ACFs Material Preparation
3.3. Characterizations of ACFs Material
3.4. CO2 Capture Measurement
3.5. Electrochemical Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dissanayake, P.D.; You, S.M.; Igalavithana, A.D.; Xia, Y.F.; Bhatnagar, A.; Gupta, S.; Kua, H.W.; Kim, S.; Kwon, J.H.; Tsang DC, W.; et al. Biochar-based adsorbents for carbon dioxide capture: A critical review. Renew. Sustain. Energy Rev. 2020, 119, 109582. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, W.; Wang, R.Q.; Gonzalez-Diaz, A.; Rojas-Michaga, M.F.; Michailos, S.; Pourkashanian, M.; Zhang, X.J.; Font-Palma, C. Sorption direct air capture with CO2 utilization. Prog. Energy Combust. Sci. 2023, 95, 101069. [Google Scholar] [CrossRef]
- Kim, C.; Talapaneni, S.N.; Dai, L. Porous carbon materials for CO2 capture, storage and electrochemical conversion. Mater. Rep. Energy 2023, 3, 100199. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, Q.; Zhao, Y.; Chen, T.; Wang, J.; Gui, L.; Lu, C. Recent progress and future directions of biomass-derived hierarchical porous carbon: Designing, preparation, and supercapacitor applications. Energy Fuels 2023, 37, 3523–3554. [Google Scholar] [CrossRef]
- Yuan, C.; Xu, H.; El-khodary, S.A.; Ni, G.; Esakkimuthu, S.; Zhong, S.; Wang, S. Recent advances and challenges in biomass-derived carbon materials forsupercapacitors: A review. Fuel 2024, 362, 130795. [Google Scholar] [CrossRef]
- Nazir, G.; Rehman, A.; Hussain, S.; Mahmood, Q.; Fteiti, M.; Heo, K.; Ikram, M.; Din, M.A.U. Towards a sustainable conversion of biomass/biowaste to porous carbons for CO2 adsorption: Recent advances, current challenges, and future directions. Green Chem. 2023, 25, 4941–4980. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Z.; Gong, W.; Xu, F.; Song, X.; He, X.; Fan, M. Electrospun carbon nanofibers and their applications in several areas. ACS Omega 2023, 8, 22316–22330. [Google Scholar] [CrossRef]
- Bai, J.; Huang, J.; Yu, Q.; Demir, M.; Kilic, M.; Altay, B.N.; Hu, X.; Wang, L. N-doped porous carbon derived from macadamia nut shell for CO2 adsorption. Fuel Process. Technol. 2023, 249, 107854. [Google Scholar] [CrossRef]
- Wu, R.; Bao, A. Preparation of cellulose carbon material from cow dung and its CO2 adsorption performance. J. CO2 Util. 2023, 68, 102377. [Google Scholar] [CrossRef]
- Tang, Z.; Gao, J.; Zhang, Y.; Du, Q.; Feng, D.; Dong, H.; Peng, Y.; Zhang, T.; Xie, M. Ultra-microporous biochar-based carbon adsorbents by a facile chemical activation strategy for high-performance CO2 adsorption. Fuel Process. Technol. 2023, 241, 107613. [Google Scholar] [CrossRef]
- Wu, C.; Liu, J.; Wang, Y.; Zhao, Y.; Li, G.; Zhang, G. KCl-assisted activation of macadamia nut shell-derived carbon: Unveiling enhanced pore structure, adsorption and supercapacitor performance. Sep. Purif. Technol. 2024, 329, 125188. [Google Scholar] [CrossRef]
- Bai, J.; Huang, J.; Yu, Q.; Demir, M.; Gecit, F.H.; Altay, B.N.; Wang, L.; Hu, X. One-pot synthesis of self S-doped porous carbon for efficient CO2 adsorption. Fuel Process. Technol. 2023, 244, 107700. [Google Scholar] [CrossRef]
- Jin, Y.; Xu, Q.; Zheng, F.; Lu, J. Enhancement in CO2 adsorption by zeolite synthesized from co-combustion ash of coal and rice husk modified with lithium ion. J. Energy Inst. 2023, 110, 101348. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Sustainable porous carbons with a superior performance for CO2 capture. Energy Environ. Sci. 2011, 4, 1765–1771. [Google Scholar] [CrossRef]
- Singh, G.; Kim, I.Y.; Lakhi, K.S.; Srivastava, P.; Naidu, R.; Vinu, A. Single step synthesis of activated bio-carbons with a high surface area and their excellent CO2 adsorption capacity. Carbon 2017, 116, 448–455. [Google Scholar] [CrossRef]
- Serafin, J.; Srenscek-Nazzal, J.; Kaminska, A.; Paszkiewicz, O.; Michalkiewicz, B. Management of surgical mask waste to activated carbons for CO2 capture. J CO2 Util. 2022, 59, 101970. [Google Scholar] [CrossRef]
- Vazhayal, L.; Wilson, P.; Prabhakaran, K. Utilization of waste aquatic weeds for the sustainable production of nitrogen doped nanoporous carbon for CO2 capture. Mater. Today Proc. 2021, 52, 2315–2321. [Google Scholar] [CrossRef]
- Singh, G.; Bahadur, R.; Mee, L.J. Nanoporous activated biocarbons with high surface areas from alligator weed and their excellent performance for CO2 capture at both low and high pressures. Chem. Eng. J. 2021, 406, 126787. [Google Scholar] [CrossRef]
- Ismail, I.S.; Singh, G.; Smith, P. Oxygen functionalized porous activated biocarbons with high surface area derived from grape marc for enhanced capture of CO2 at elevated-pressure. Carbon 2020, 160, 113–124. [Google Scholar] [CrossRef]
- Kiełbasa, K.; Bayar, Ş.; Varol, E.A.; Sreńscek-Nazzal, J.; Bosacka, M.; Miądlicki, P.; Serafin, J.; Wróbel, R.J.; Michalkiewicz, B. Carbon dioxide adsorption over activated carbons produced from molasses using H2SO4, H3PO4, HCl, NaOH, and KOH as Activating Agents. Molecules 2022, 27, 7467. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, W.; Shen, D.; Zhang, H.; Wang, Z. Preparation of porous carbon materials from black liquor lignin and its utilization as CO2 adsorbents. J. Energy Inst. 2023, 107, 101179. [Google Scholar] [CrossRef]
- Rahimi, V.; Ferreiro-Salgado, A.; Gómez-Díaz, D.; Freire, M.S.; González-Álvarez, J. Evaluating the performance of carbon-based adsorbents fabricated from renewable biomass precursors for post-combustion CO2 capture. Sep. Purif. Technol. 2024, 344, 127110. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.; Zhan, W.; Zhang, D.; Liu, Y.; Xu, Y.; Wu, Z. Enhancing CO2 capture with K2CO3-activated carbon derived from peanut shell. Biomass Bioenergy 2024, 183, 107148. [Google Scholar] [CrossRef]
- Serafin, J.; Dziejarski, B.; Vendrell, X.; Kiełbasa, K.; Michalkiewicz, B. Biomass waste fern leaves as a material for a sustainable method of activated carbon production for CO2 capture. Biomass Bioenergy 2023, 175, 106880. [Google Scholar] [CrossRef]
- Kim, M.J.; Choi, S.W.; Kim, H.; Mun, S.; Lee, K.B. Simple synthesis of spent coffee ground-based microporous carbons using K2CO3 as an activation agent and their application to CO2 capture. Chem. Eng. J. 2020, 397, 125404. [Google Scholar] [CrossRef]
- Muhammad, R.; Nah, Y.-C.; Oh, H. Spider silk-derived nanoporous activated carbon fiber for CO2 capture and CH4 and H2 storage. J. CO2 Util. 2023, 69, 102401. [Google Scholar] [CrossRef]
- Wang, P.; Lang, J.; Xu, S.; Wang, X. Nitrogen-containing activated carbon fibers derived from silk fibers for CO2 capture. Mater. Lett. 2015, 152, 145–147. [Google Scholar] [CrossRef]
- Wang, S.; Sun, W.; Yang, D.-S.; Yang, F. Conversion of soybean waste to sub-micron porous-hollow carbon spheres for supercapacitor via a reagent and template-free route. Mater. Today Energy 2019, 13, 50–55. [Google Scholar] [CrossRef]
- Yu, M.; Han, Y.; Li, J.; Wang, L. CO2-activated porous carbon derived from cattail biomass for removal of malachite green dye and application as supercapacitors. Chem. Eng. J. 2017, 317, 493–502. [Google Scholar]
- Li, Y.; Sun, Y.; Li, H.; Sun, M.; Shen, J.; Wang, S. High nitrogen-oxygen dual-doped three-dimensional hierarchical porous carbon network derived from Eriocheir sinensis for advanced supercapacitors. Energy 2023, 270, 126942. [Google Scholar] [CrossRef]
- Jiang, C.; Yakaboylu, G.A.; Yumak, T.; Zondle, J.W.; Sabolsky, E.M.; Wang, J. Activated carbons prepared by indirect and direct CO2 activation of lignocellulosic biomass for supercapacitor electrodes. Renew. Energy 2020, 155, 38–52. [Google Scholar] [CrossRef]
- Chen, W.; Li, K.; Xia, M.; Chen, Y.; Yang, H.; Chen, Z.; Chen, X.; Chen, H. Influence of NH3 concentration on biomass nitrogen-enriched pyrolysis. Bioresour. Technol. 2018, 263, 350–357. [Google Scholar] [CrossRef]
- Prasankumar, T.; Salpekar, D.; Bhattacharyya, S.; Manoharan, K.; Yadav, R.M.; Mata MA, C.; Miller, K.A.; Vajtai, R.; Jose, S.; Roy, S.; et al. Biomass derived hierarchical porous carbon for supercapacitor application and dilute stream CO2 capture. Carbon 2022, 199, 249–257. [Google Scholar] [CrossRef]
- Khan, A.; Senthil, R.A.; Pan, J.; Osman, S.; Sun, Y.; Shu, X. A new biomass derived rod-like porous carbon from tea-waste as inexpensive and sustainable energy material for advanced supercapacitor application. Electrochim. Acta 2020, 335, 135588. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Chen, C.; Zhu, Z. Low-cost, high-performance supercapacitor based on activated carbon electrode materials derived from baobab fruit shells. J. Colloid Interface Sci. 2019, 538, 308–319. [Google Scholar] [CrossRef]
- Tan, Y.; Xu, Z.; He, L.; Li, H. Three-dimensional high graphitic porous biomass carbon from dandelion flower activated by K2FeO4 for supercapacitor electrode. J. Storage Mater. 2022, 52, 104889. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, S.-C.; Sun, G.-T.; Kang, K.; Zhu, M.-Q.; Geng, Z.-C. Comparison of activated carbons prepared by one-step and two-step chemical activation process based on cotton stalk for supercapacitors application. Energy 2021, 215, 119144. [Google Scholar] [CrossRef]
- Khan, A.; Senthil, R.A.; Pan, J.; Sun, Y.; Liu, X. Hierarchically porous biomass carbon derived from natural withered rose flowers as high-performance material for advanced supercapacitors. Batter. Supercaps 2020, 3, 731–737. [Google Scholar] [CrossRef]
- Guo, F.; Jiang, X.; Jia, X.; Liang, S.; Qian, L.; Rao, Z. Synthesis of biomass carbon electrode materials by bimetallic activation for the application in supercapacitors. J. Electroanal. Chem. 2019, 844, 105–115. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Jaroniec, M. Importance of small micropores in CO2 capture by phenolic resin-based activated carbon spheres. J. Mater. Chem. A 2013, 1, 112–116. [Google Scholar] [CrossRef]
- Presser, V.; McDonough, J.; Yeonb, S.-H.; Gogotsi, Y. Effect of pore size on carbon dioxide sorption by carbide derived carbon. Energy Environ. Sci. 2011, 4, 3059–3066. [Google Scholar] [CrossRef]
- Vix-Guterl, C.; Frackowiak, E.; Jurewicz, K.; Friebe, M.; Parmentierc, J.; Béguin, F. Electrochemical energy storage in ordered porous carbon materials. Carbon 2005, 43, 1293–1302. [Google Scholar] [CrossRef]
- Li, Q.; Lu, T.; Wang, L.; Pang, R.; Shao, J.; Liu, L.; Hu, X. Biomass based N-doped porous carbons as efficient CO2 adsorbents and high-performance supercapacitor electrodes. Sep. Purif. Technol. 2021, 275, 119204. [Google Scholar] [CrossRef]
- Lu, W.; Si, Y.; Zhao, C.; Chen, T.; Li, C.; Zhang, C.; Wang, K. Biomass-derived carbon applications in the field of supercapacitors: Progress and prospects. Chem. Eng. J. 2024, 495, 153311. [Google Scholar] [CrossRef]
- Li, C.; Yan, L.; Wang, M.; Kong, J.; Bao, W.; Chang, L. Synthesis strategies and applications for pitch-based anode: From industrial by-products to power sources. Chem. Rec. 2022, 23, e202200216. [Google Scholar] [CrossRef]
- Xing, L.-A.; Yang, F.; Zhong, X.; Liu, Y.; Lu, H.; Guo, Z.; Lv, G.; Yang, J.; Yuan, A.; Pan, J. Ultra-microporous cotton fiber-derived activated carbon by a facile one-step chemical activation strategy for efficient CO2 adsorption. Sep. Purif. Technol. 2023, 324, 124470. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-based supercapacitors produced by activation of graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef]
- Vinodh, R.; Sangeetha, D. Carbon supported silver (Ag/C) electrocatalysts for alkaline membrane fuel cells. J. Mater. Sci. 2012, 47, 852–859. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Wang, K.-P.; Teng, H.A. simplified preparation of mesoporous carbon and the examination of the carbon accessibility for electric double layer formation. Carbon 2005, 43, 559–566. [Google Scholar] [CrossRef]
- Alhamed, Y.A.; Bamufleh, H.S. Theoretical study of the transesterification of triglycerides to biodiesel fuel. Fuel 2009, 88, 87–91. [Google Scholar] [CrossRef]
- Chmiola, J.; Largeot, C.; Taberna, P.-L.; Simon, P.; Gogotsi, Y. Desolvation of ions in subnanometer pores and its effect on capacitance and double-layer theory. Angew. Chem. Int. Ed. 2008, 47, 3392. [Google Scholar] [CrossRef]
- Thommes, M.; Morlay, C.; Ahmad, R.; Joly, J.P. Assessing surface chemistry and pore structure of active carbons by a combination of physisorption (H2O, Ar, N2, CO2), XPS and TPD-MS. Adsorption 2011, 17, 653–661. [Google Scholar] [CrossRef]
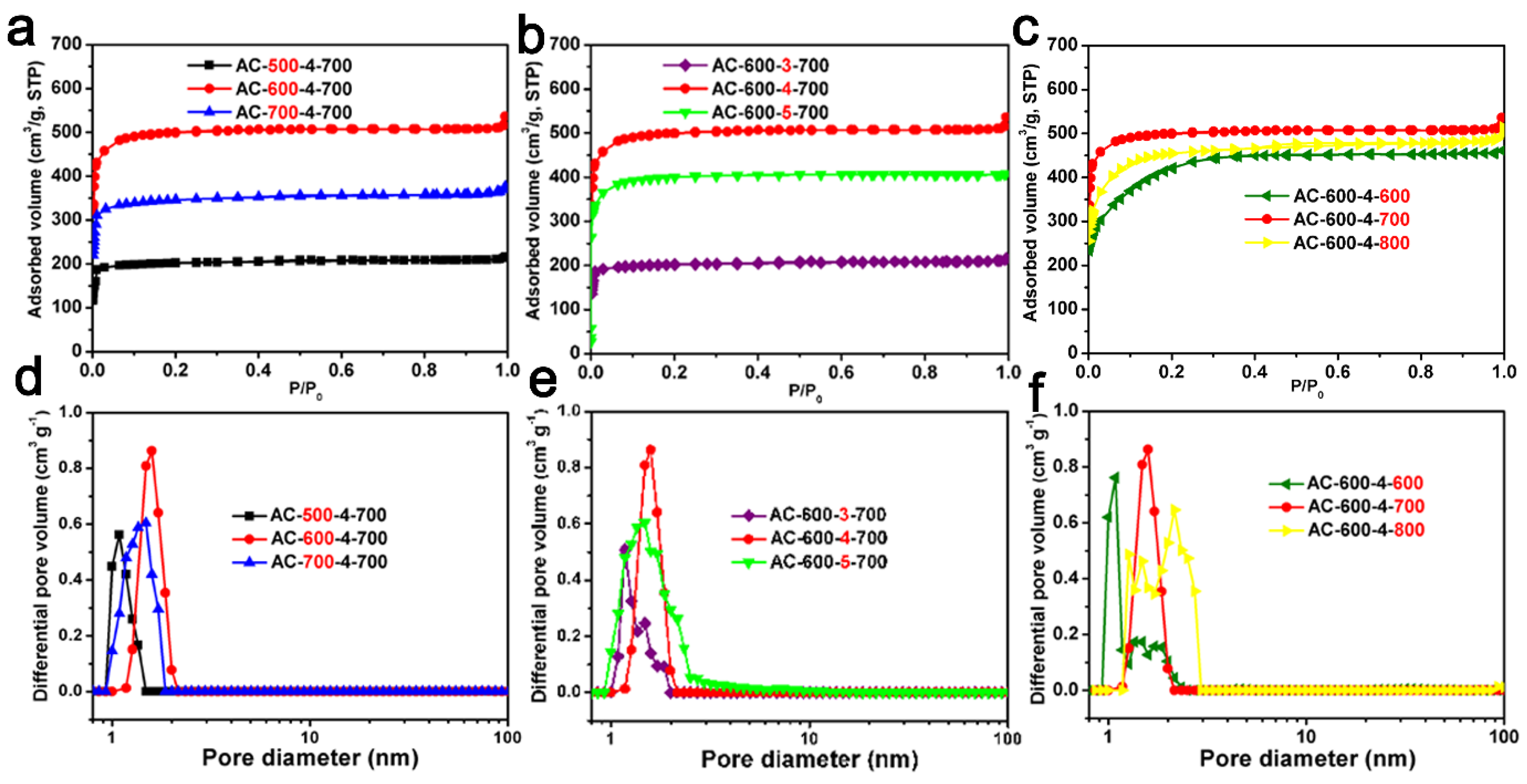
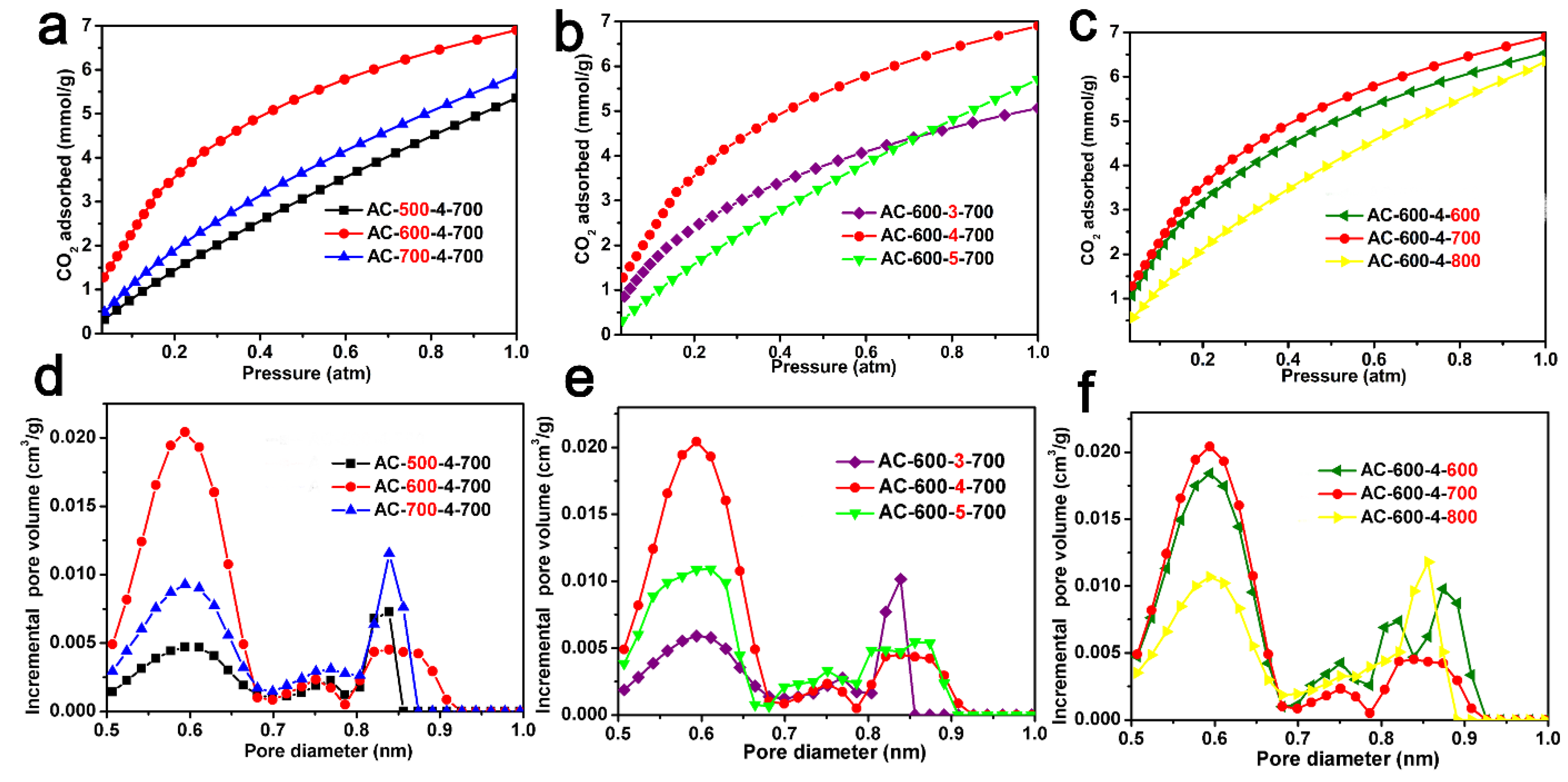

| Sample | SSA (m2/g) a | Pore Volume a (cm3/g) | Micropore Volume b (cm3/g) | CO2 Uptake 0 °C (mmol/g) | CO2 Uptake 25 °C (mmol/g) | Volume of Pores c ≤1.0 nm (cm3/g) | Volume of Pores c ≤0.90 nm (cm3/g) | Volume of Pores c ≤0.80 (cm3/g) | Specific Capacity |
|---|---|---|---|---|---|---|---|---|---|
| AC-500-4-700 | 673 | 0.33 | 0.28 | 4.3 | 2.8 | 0.060 | 0.060 | 0.042 | 141 |
| AC-600-4-700 | 1597 | 0.76 | 0.64 | 6.9 | 4.6 | 0.169 | 0.162 | 0.152 | 282 |
| AC-700-4-700 | 1158 | 0.56 | 0.50 | 5.9 | 4.0 | 0.120 | 0.116 | 0.072 | 222 |
| AC-600-3-700 | 802 | 0.39 | 0.29 | 4.9 | 3.6 | 0.081 | 0.078 | 0.070 | 182 |
| AC-600-5-700 | 1334 | 0.63 | 0.53 | 5.7 | 4.0 | 0.130 | 0.113 | 0.103 | 199 |
| AC-600-4-600 | 1495 | 0.69 | 0.59 | 6.5 | 4.4 | 0.160 | 0.144 | 0.131 | 273 |
| AC-600-4-800 | 1501 | 0.79 | 0.55 | 6.3 | 4.2 | 0.140 | 0.131 | 0.100 | 234 |
| Carbon Source | CO2 Adsorbed (mmol/g, 0 °C) | CO2 Adsorbed (mmol/g, 25 °C) | Ref. |
|---|---|---|---|
| Macadamia nut shell b | 6.6 | 4.4 | [8] |
| Cellulose carbon material from cow dung b | 4.6 | 3.3 | [9] |
| Biochar-based carbon b | 6.1 | 3.7 | [10] |
| Macadamia nut shell b | 6.5 | 4.1 | [11] |
| Petroleum coke b | 5.6 | 3.5 | [12] |
| Coal and rice husk b | 6.1 | 5.0 | [13] |
| Sawdust b | 6.6 | 4.8 | [14] |
| Arundo donax b | 6.3 | 3.6 | [15] |
| Waste surgical mask b | 3.9 | 2.6 | [16] |
| Water hyacinth b | 6.0 | 2.6 | [17] |
| Alligator weed b | 6.4 | 3.4 | [18] |
| Grape marc b | 6.7 | 3.9 | [19] |
| Molasses b | 5.2 | / | [20] |
| lignin-based b | 5.2 | 3.6 | [21] |
| Olive stone b | 6.1 | 3.8 | [22] |
| Peanut shells b | 5.7 | 3.7 | [23] |
| Fern leaves b | 6.8 | 3.6 | [24] |
| Spent coffee grounds a | 7.2 | 4.5 | [25] |
| Silk fibers a | 7.0 | 4.8 | [27] |
| Cotton fibers | 6.9 | 4.6 | This work |
| Carbon Source | Electrolyte | Current Density (A/g) | Specific Capacitance (F/g) | Ref. |
|---|---|---|---|---|
| Soybean | 1 M Na2SO4 | 0.1 | 143 | [28] |
| Cattail | 6 M KOH | 0.5 | 127 | [29] |
| Eriocheir sinensis shells | 6 M KOH | 1 | 161 | [30] |
| Hybrid willow | 6 M KOH | 0.1 | 93 | [31] |
| Bamboo | 6 M KOH | 1 | 120 | [32] |
| Tasmanian blue gum tree bark | 1 M KOH | 1 | 212 | [33] |
| Tea-waste | 6 M KOH | 1 | 332 | [34] |
| Baobab fruit shells | 6 M KOH | 1 | 255 | [35] |
| Dandelion flower stem | 6 M KOH | 0.5 | 309 | [36] |
| Cotton stalk | 1 M H2SO4 | 0.2 | 338 | [37] |
| Rose flowers | 6 M KOH | 1 | 350 | [38] |
| Peanut shells | 1 M Na2SO4 | 1 | 247 | [39] |
| Cotton Fibers | 2 M KOH | 1 A/g | 282 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Liu, H.; Zhu, W.; Chen, W.; Wang, X.; Yang, L.; Yang, B.; Chen, Q.; Limao, C.; Cairang, Z. Versatile Activated Carbon Fibers Derived from the Cotton Fibers Used as CO2 Solid-State Adsorbents and Electrode Materials. Molecules 2024, 29, 3153. https://doi.org/10.3390/molecules29133153
Wang P, Liu H, Zhu W, Chen W, Wang X, Yang L, Yang B, Chen Q, Limao C, Cairang Z. Versatile Activated Carbon Fibers Derived from the Cotton Fibers Used as CO2 Solid-State Adsorbents and Electrode Materials. Molecules. 2024; 29(13):3153. https://doi.org/10.3390/molecules29133153
Chicago/Turabian StyleWang, Peiyu, Hang Liu, Wenting Zhu, Wanjun Chen, Xiangli Wang, Le Yang, Bao Yang, Qiong Chen, Cairang Limao, and Zhuoma Cairang. 2024. "Versatile Activated Carbon Fibers Derived from the Cotton Fibers Used as CO2 Solid-State Adsorbents and Electrode Materials" Molecules 29, no. 13: 3153. https://doi.org/10.3390/molecules29133153
APA StyleWang, P., Liu, H., Zhu, W., Chen, W., Wang, X., Yang, L., Yang, B., Chen, Q., Limao, C., & Cairang, Z. (2024). Versatile Activated Carbon Fibers Derived from the Cotton Fibers Used as CO2 Solid-State Adsorbents and Electrode Materials. Molecules, 29(13), 3153. https://doi.org/10.3390/molecules29133153





