Heterogeneous Iron-Based Catalysts for Organic Transformation Reactions: A Brief Overview
Abstract
1. Introduction
2. C–H Activation Reaction
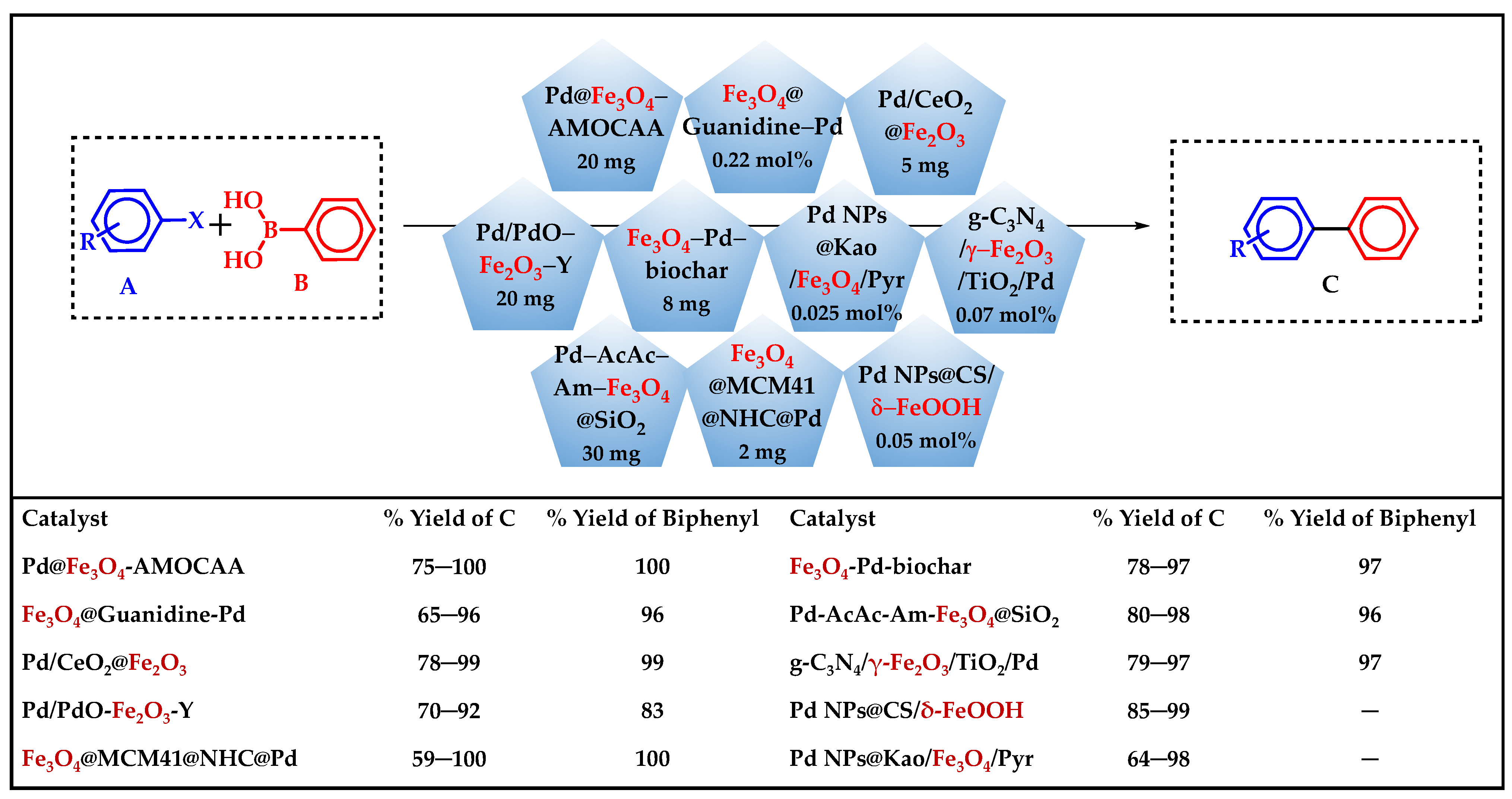
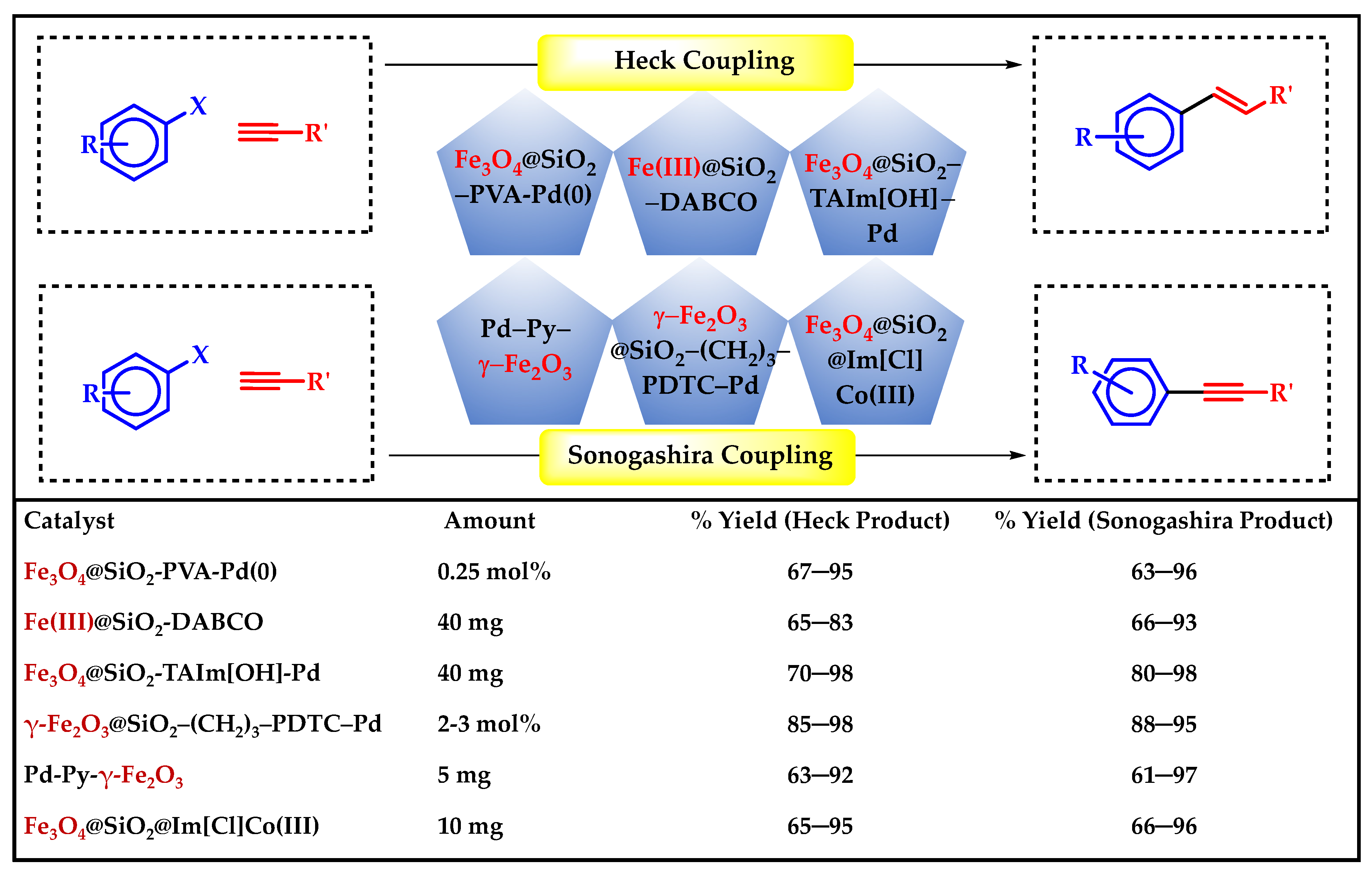
3. C–C Coupling Reactions
4. C–N Bond Formation Reactions
5. Oxidation Reactions
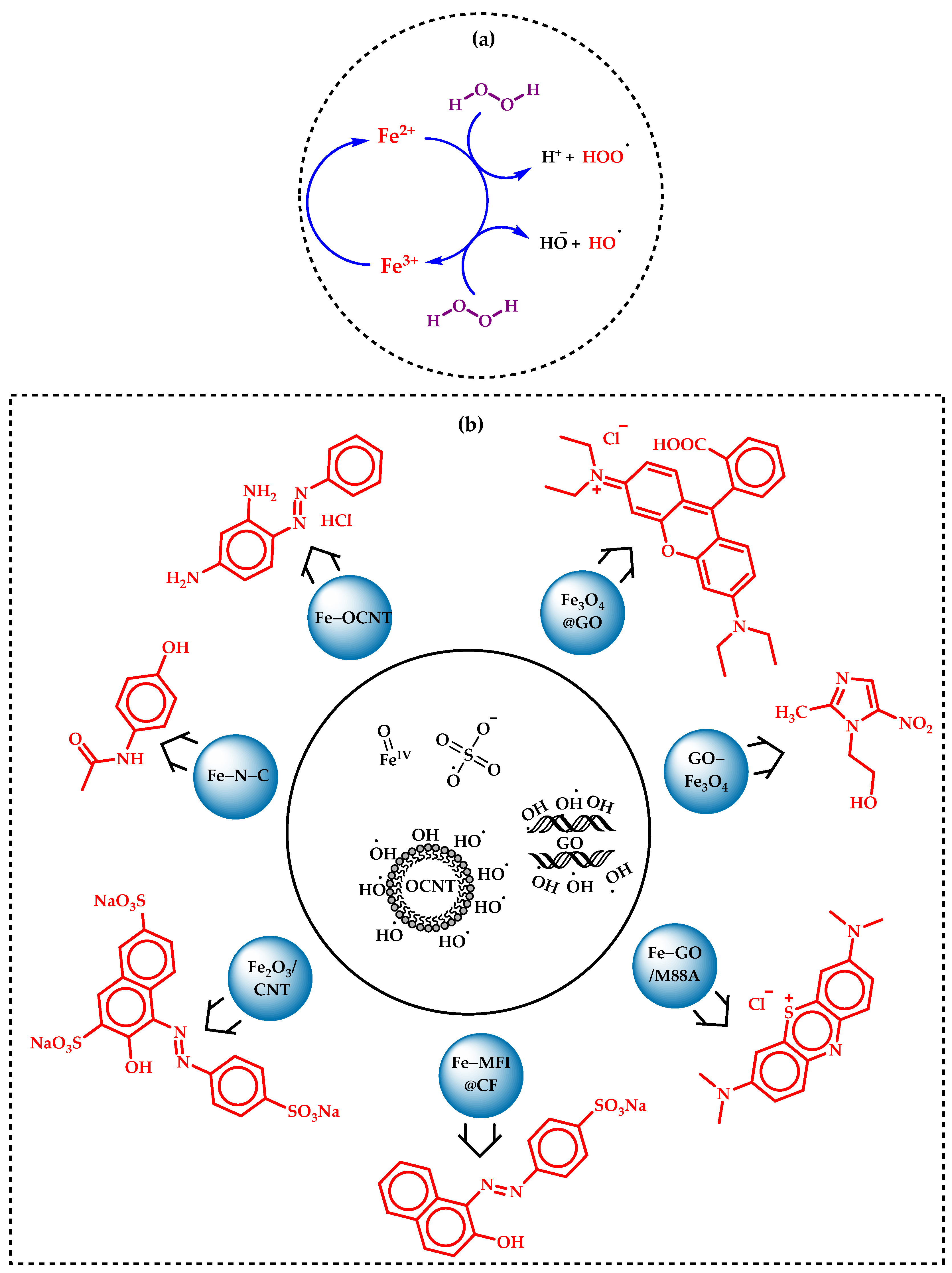
5.1. Advanced Oxidation Process by Fenton’s Reaction
5.2. Oxidation of Benzyl Alcohols
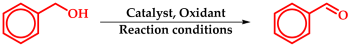 | ||||||
|---|---|---|---|---|---|---|
| Entry | Catalyst | Oxidant | % Conv. (BA) | % Sel. (BAL) | % Yield (BAL) | Ref. |
| 1 | Pd-Fe@TiO2 | H2O2 | 97 | 100 | − | [118] |
| 2 | NiFe2O4 NPs | TBHP | 85 | 100 | − | [120] |
| 3 | Fe2O3-HNT | O2, H2O2 | 88, 92 | 100, 100 | − | [121] |
| 4 | SA-Fe/Nx-C | O2 | 92 | 97 | − | [122] |
| 5 | Fe3O4@Cu2O | air | − | 99 | 99 | [126] |
| 6 | α-Fe2O3@Au | air | − | 98 | 96 | [127] |
| Catalyst | Oxidant | Reactant | Product(s) | % Conv. [a] | % Yield | % Sel. [b] |
|---|---|---|---|---|---|---|
| Fe–N/C | O2 (1 atm) |  |  | 99 | 91 | 91 |
 |  | 70 | 17 | 12 | ||
 | 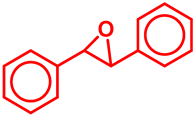 | 78 | 67 | 52 | ||
 |  | 79 | 96 | 76 | ||
| Fe/PMA @CIN-1 | H2O2 (1 mmol) | 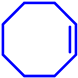 |  | 88 | − | 99 |
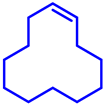 |  | 58 | − | 99 |
5.3. Epoxidation Reactions
5.4. Hydroxylation Reactions
5.5. Oxidative Coupling Reactions
6. Epoxide Ring Opening Reactions
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tietze, L.F. Domino Reactions in Organic Synthesis. Chem. Rev. 1996, 96, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Norbert, H. Photochemical Reactions as Key Steps in Organic Synthesis. Chem. Rev. 2008, 108, 1052–1103. [Google Scholar]
- Xiao, J.; Liu, X.; Pan, L.; Shi, C.; Zhang, X.; Zou, J.J. Heterogeneous Photocatalytic Organic Transformation Reactions Using Conjugated Polymers-Based Materials. ACS Catal. 2020, 10, 12256–12283. [Google Scholar] [CrossRef]
- Vekariya, R.L. A Review of Ionic Liquids: Applications Towards Catalytic Organic Transformations. J. Mol. Liq. 2017, 227, 44–60. [Google Scholar] [CrossRef]
- Chng, L.L.; Erathodiyil, N.; Ying, J.Y. Nanostructured Catalysts for Organic Transformations. Acc. Chem. Res. 2013, 46, 1825–1837. [Google Scholar] [CrossRef]
- Li, Z.; Brouwer, C.; He, C. Gold-Catalyzed Organic Transformations. Chem. Rev. 2008, 108, 3239–3265. [Google Scholar] [CrossRef]
- Cheng, W.M.; Shang, R. Transition Metal-Catalyzed Organic Reactions Under Visible Light: Recent Developments and Future Perspectives. ACS Catal. 2020, 10, 9170–9196. [Google Scholar] [CrossRef]
- Lyons, T.W.; Sanford, M.S. Palladium-Catalyzed Ligand-Directed C–H Functionalization Reactions. Chem. Rev. 2010, 110, 1147–1169. [Google Scholar] [CrossRef]
- Labinger, J.A. Platinum-Catalyzed C–H Functionalization. Chem. Rev. 2017, 117, 8483–8496. [Google Scholar] [CrossRef]
- Naota, T.; Takaya, H.; Murahashi, S.I. Ruthenium-Catalyzed Reactions for Organic Synthesis. Chem. Rev. 1998, 98, 2599–2660. [Google Scholar] [CrossRef]
- Rana, S.; Biswas, J.P.; Paul, S.; Paik, A.; Maiti, D. Organic Synthesis with the Most Abundant Transition Metal–Iron: From Rust to Multitasking Catalysts. Chem. Soc. Rev. 2021, 50, 243–472. [Google Scholar] [PubMed]
- Bauer, I.; Knolker, H.J. Iron Catalysis in Organic Synthesis. Chem. Rev. 2015, 115, 3170–3387. [Google Scholar] [PubMed]
- Zhu, F.; Lu, G.P.; Wang, F.; Ren, E.; Yu, Y.; Lin, Y. Iron Catalyzed Organic Reactions in Water: A “Nature-Like” Synthesis. Curr. Opin. Green Sustain. Chem. 2023, 40, 100754. [Google Scholar] [CrossRef]
- Pellissier, H. Recent Developments in Enantioselective Iron-Catalyzed Transformations. Coord. Chem. Rev. 2019, 386, 1–31. [Google Scholar] [CrossRef]
- Sun, C.L.; Li, B.J.; Shi, Z.J. Direct C− H Transformation via Iron Catalysis. Chem. Rev. 2011, 111, 1293–1314. [Google Scholar] [CrossRef]
- Chughtai, A.H.; Ahmad, N.; Younus, H.A.; Laypkov, A.; Verpoort, F. Metal–Organic Frameworks: Versatile Heterogeneous Catalysts for Efficient Catalytic Organic Transformations. Chem. Soc. Rev. 2015, 44, 6804–6849. [Google Scholar] [CrossRef]
- Kumar, P.; Tomar, V.; Kumar, D.; Joshi, R.K.; Nemiwal, M. Magnetically Active Iron Oxide Nanoparticles for Catalysis of Organic Transformations: A Review. Tetrahedron 2022, 106, 132641. [Google Scholar] [CrossRef]
- Pouran, S.R.; Raman, A.A.A.; Daud, W.M.A.W. Review on the Application of Modified Iron Oxides as Heterogeneous Catalysts in Fenton Reactions. J. Clean. Prod. 2014, 64, 24–35. [Google Scholar] [CrossRef]
- Malhotra, N.; Lee, J.S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflores, O.B.; Ger, T.R.; Hsiao, C.D. Potential Toxicity of Iron Oxide Magnetic Nanoparticles: A Review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef]
- Rusevova, K.; Kopinke, F.D.; Georgi, A. Nano-Sized Magnetic Iron Oxides as Catalysts for Heterogeneous Fenton-Like Reactions—Influence of Fe(II)/Fe(III) Ratio on Catalytic Performance. J. Hazard. Mater. 2012, 241, 433–440. [Google Scholar] [CrossRef]
- Elahi, N.; Rizwan, M. Progress and Prospects of Magnetic Iron Oxide Nanoparticles in Biomedical Applications: A review. Artif. Organs 2021, 45, 1272–1299. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Céspedes, S.; Betori, R.C.; Cismesia, M.A.; Kirsch, J.K.; Yang, Q. Heterogeneous Catalysis for Cross-Coupling Reactions: An Underutilized Powerful and Sustainable Tool in the Fine Chemical Industry? Org. Process Res. Dev. 2021, 25, 740–753. [Google Scholar] [CrossRef]
- Srivastava, A.; Kaur, H.; Pahuja, H.; Rangarajan, T.M.; Varma, R.S.; Pasricha, S. Optimal Exploitation of Supported Heterogenized Pd Nanoparticles for C-C Cross-Coupling Reactions. Coord. Chem. Rev. 2024, 507, 215763. [Google Scholar] [CrossRef]
- Cera, G.; Ackermann, L. Iron-Catalyzed C–H Functionalization Processes. In Ni-and Fe-Based Cross-Coupling Reactions; Springer: Berlin/Heidelberg, Germany, 2017; pp. 191–224. [Google Scholar]
- Gopalaiah, K. Chiral Iron Catalysts for Asymmetric Synthesis. Chem. Rev. 2013, 113, 3248–3296. [Google Scholar] [CrossRef] [PubMed]
- Quintard, A.; Constantieux, T.; Rodriguez, J. An Iron/Amine-Catalyzed Cascade Process for the Enantioselective Functionalization of Allylic Alcohols. Angew. Chem. 2013, 125, 13121. [Google Scholar] [CrossRef]
- Roy, S.D.; Das, K.C.; Dhar, S.S. Conventional to Green Synthesis of Magnetic Iron Oxide Nanoparticles; Its Application as Catalyst, Photocatalyst and Toxicity: A Short Review. Inorg. Chem. Commun. 2021, 134, 109050. [Google Scholar] [CrossRef]
- Liu, Y.; You, T.; Wang, H.X.; Tang, Z.; Zhou, C.Y.; Che, C.M. Iron-And Cobalt-Catalyzed C (Sp3)–H Bond Functionalization Reactions and Their Application in Organic Synthesis. Chem. Soc. Rev. 2020, 49, 5310–5358. [Google Scholar] [CrossRef]
- Zhang, R.K.; Chen, K.; Huang, X.; Wohlschlager, L.; Renata, H.; Arnold, F.H. Enzymatic Assembly of Carbon–Carbon Bonds via Iron-Catalysed Sp3 C–H Functionalization. Nature 2019, 565, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Rogge, T.; Kaplaneris, N.; Chatani, N.; Kim, J.; Chang, S.; Punji, B.; Schafer, L.L.; Musaev, D.G.; Wencel-Delord, J.; Roberts, C.A.; et al. C–H Activation. Nat. Rev. Methods Prim. 2021, 1, 43. [Google Scholar] [CrossRef]
- Ouyang, X.H.; Li, Y.; Song, R.J.; Hu, M.; Luo, S.; Li, J.H. Intermolecular Dialkylation of Alkenes with Two Distinct C (Sp3)-H Bonds Enabled by Synergistic Photoredox Catalysis and Iron Catalysis. Sci. Adv. 2019, 5, 9839. [Google Scholar] [CrossRef]
- Bettoni, L.; Seck, C.; Mbaye, M.D.; Gaillard, S.; Renaud, J.L. Iron-Catalyzed Tandem Three-Component Alkylation: Access to α-Methylated Substituted Ketones. Org. Lett. 2019, 21, 3057–3061. [Google Scholar] [CrossRef]
- Lemmens, V.; Janssens, K.; Baestaens, W.J.; Bugaev, A.L.; Van Velthoven, N.; Adriaensen, K.; Marquez, C.; Henrion, M.; De Vos, D.E. An Iron-Loaded Metal–Organic Framework (CAU-27-Fe) As Effective Heterogeneous Catalyst for The Direct C–H Amination of Ethers Using NH-Heterocycles. J. Catal. 2024, 434, 115516. [Google Scholar] [CrossRef]
- Liu, Y.; You, T.; Wang, T.T.; Che, C.M. Iron-Catalyzed C–H Amination and Its Application in Organic Synthesis. Tetrahedron 2019, 75, 130607. [Google Scholar] [CrossRef]
- Du, Y.; Tang, J.J.; Wang, Y.; Hu, J.; Chen, C.; Xiong, Z.; Li, Y.; Fan, J.; Bao, M.; Yu, X. Visible-Light-Driven Iron-Catalyzed Intermolecular Benzylic C (sp3)–H Amination with 1, 2, 3, 4-Tetrazoles. Org. Lett. 2024, 26, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Gao, P.; Zhang, S.; Jia, X.; Wang, M.; Yuan, Y. Iron (iii)-catalyzed direct C–H radical amination of (hetero) arenes. Org. Chem. Front. 2021, 8, 5440–5445. [Google Scholar] [CrossRef]
- Doan, S.H.; Tran, C.B.; Cao, A.L.; Le, N.T.; Phan, N.T. A New Pathway to 2-Arylbenzoxazoles And 2-Arylbenzothiazoles via One-Pot Oxidative Cyclization Reactions Under Iron-Organic Framework Catalysis. Catal. Lett. 2019, 149, 2053–2063. [Google Scholar] [CrossRef]
- Zhou, Y.; Ni, J.; Lyu, Z.; Li, Y.; Wang, T.; Cheng, G.J. Mechanism and Reaction Channels of Iron-Catalyzed Primary Amination of Alkenes by Hydroxylamine Reagents. ACS Catal. 2023, 13, 1863–1874. [Google Scholar] [CrossRef]
- Shang, R.; Ilies, L.; Nakamura, E. Iron-catalyzed C–H bond activation. Chem. Rev. 2017, 117, 9086–9139. [Google Scholar] [CrossRef] [PubMed]
- Doba, T.; Shang, R.; Nakamura, E. Iron-Catalyzed C–H Activation for Heterocoupling and Copolymerization of Thiophenes with Enamines. J. Am. Chem. Soc. 2022, 144, 21692–21701. [Google Scholar] [CrossRef]
- Yoshikai, N.; Nakamura, E. Theoretical Studies on Diastereo-and Enantioselective Rhodium-Catalyzed Cyclization of Diazo Compound via Intramolecular C–H Bond Insertion. Adv. Synth. Catal. 2003, 345, 1159–1171. [Google Scholar] [CrossRef]
- Nakamura, N.; Tajima, Y.; Sakai, K. Direct Phenylation of Isoxazoles Using Palladium Catalysts. Synthesis of 4-Phenylmuscimol. Heterocycles 1982, 17, 235. [Google Scholar] [CrossRef]
- Nakamura, E.; Yoshikai, N. Low-Valent Iron-Catalyzed C–C Bond Formation–Addition, Substitution, and C–H Bond Activation. J. Org. Chem. 2010, 75, 6061–6067. [Google Scholar] [CrossRef] [PubMed]
- Loup, J.; Dhawa, U.; Pesciaioli, F.; Wencel-Delord, J.; Ackermann, L. Enantioselective C─H Activation with Earth-Abundant 3d Transition Metals. Angew. Chem. Int. Ed. 2019, 58, 12803–12818. [Google Scholar] [CrossRef]
- Ackermann, L. Metalla-Electrocatalyzed C–H Activation by Earth-Abundant 3d Metals and Beyond. Acc. Chem. Res. 2019, 53, 84–104. [Google Scholar] [CrossRef] [PubMed]
- Messinis, A.M.; Finger, L.H.; Hu, L.; Ackermann, L. Allenes for Versatile Iron-Catalyzed C–H Activation by Weak O-Coordination: Mechanistic Insights by Kinetics, Intermediate Isolation, and Computation. J. Am. Chem. Soc. 2020, 142, 13102–13111. [Google Scholar] [CrossRef]
- Ackermann, L. Carboxylate-Assisted Transition-Metal-Catalyzed C–H Bond Functionalizations: Mechanism and Scope. Chem. Rev. 2011, 11, 1315–1345. [Google Scholar] [CrossRef] [PubMed]
- Gandeepan, P.; Muller, T.; Zell, D.; Cera, G.; Warratz, S.; Ackermann, L. 3d Transition Metals for C–H Activation. Chem. Rev. 2018, 119, 2192–2452. [Google Scholar] [CrossRef]
- Mo, J.; Messinis, A.M.; Li, J.; Warratz, S.; Ackermann, L. Chelation-Assisted Iron-Catalyzed C–H Activations: Scope and Mechanism. Acc. Chem. Res. 2023, 57, 10–22. [Google Scholar] [CrossRef]
- Cattani, S.; Cera, G. Modern Organometallic C–H Functionalizations with Earth-Abundant Iron Catalysts: An Update. Chem. Asian J. 2024, 19, e202300897. [Google Scholar] [CrossRef]
- Zhang, L.; Liardet, L.; Luo, J.; Ren, D.; Grätzel, M.; Hu, X. Photo-electrocatalytic arene C–H amination. Nat. Catal. 2019, 2, 366–373. [Google Scholar] [CrossRef]
- Devarajan, N.; Suresh, P. Iron-MOF-Catalyzed Domino Cyclization and Aromatization Strategy for the Synthesis of 2,4-Diarylquinolines. Asian J. Org. Chem. 2020, 9, 437–444. [Google Scholar] [CrossRef]
- To, T.A.; Vo, Y.H.; Nguyen, H.T.; Ha, P.T.; Doan, S.H.; Doan, T.L.; Li, S.; Le, H.V.; Tu, T.N.; Phan, N.T. Iron-Catalyzed One-Pot Sequential Transformations: Synthesis of Quinazolinones via Oxidative Csp3H Bond Activation Using a New Metal-Organic Framework as Catalyst. J. Catal. 2019, 370, 11–20. [Google Scholar] [CrossRef]
- Doan, S.H.; Tran, N.K.; Pham, P.H.; Nguyen, V.H.; Nguyen, N.N.; Ha, P.T.; Li, S.; Le, H.V.; Le, N.T.; Tu, T.N.; et al. A New Synthetic Pathway to Triphenylpyridines via Cascade Reactions Utilizing a New Iron-Organic Framework as a Recyclable Heterogeneous Catalyst. Eur. J. Org. Chem. 2019, 2019, 2382–2389. [Google Scholar] [CrossRef]
- Motokura, K.; Ozawa, N.; Sato, R.; Manaka, Y.; Chun, W.J. Porous FeO(OH) Dispersed on Mg-Al Hydrotalcite Surface for One-Pot Synthesis of Quinoline Derivatives. ChemCatChem 2021, 13, 2915–2921. [Google Scholar] [CrossRef]
- Mashhoori, M.S.; Sandaroos, R. New Ecofriendly Heterogeneous Nano-Catalyst for the Synthesis of 1-Substituted and 5-Substituted 1 H-Tetrazole Derivatives. Sci. Rep. 2022, 12, 15364. [Google Scholar] [CrossRef] [PubMed]
- Waghchaure, R.H.; Jagdale, B.S.; Koli, P.B.; Adole, V.A. Nano 5% Fe–ZnO: A highly efficient and recyclable heterogeneous solid nano catalyst for the Biginelli reaction. J Indian Chem. Soc. 2022, 99, 100468. [Google Scholar] [CrossRef]
- Sánchez-Velandia, J.E.; Villa, A.L. Isomerization of α-and β-Pinene Epoxides Over Fe or Cu Supported MCM-41 and SBA-15 Materials. Appl. Catal. A Gen. 2019, 580, 17–27. [Google Scholar] [CrossRef]
- Fürstner, A.; Leitner, A.; Méndez, M.; Krause, H. Iron-Catalyzed Cross-Coupling Reactions. J. Am. Chem. Soc. 2002, 124, 13856–13863. [Google Scholar] [CrossRef] [PubMed]
- Czaplik, W.M.; Mayer, M.; Cvengroš, J.; von Wangelin, A.J. Coming of Age: Sustainable Iron-Catalyzed Cross-Coupling Reactions. ChemSusChem 2009, 2, 396–417. [Google Scholar] [CrossRef]
- Cassani, C.; Bergonzini, G.; Wallentin, C.J. Active Species and Mechanistic Pathways in Iron-Catalyzed C–C Bond-Forming Cross-Coupling Reactions. ACS Catal. 2016, 6, 1640–1648. [Google Scholar] [CrossRef]
- Jang, S.; Hira, S.A.; Annas, D.; Song, S.; Yusuf, M.; Park, J.C.; Park, S.; Park, K.H. Recent Novel Hybrid Pd–Fe3O4 Nanoparticles as Catalysts for Various C–C Coupling Reactions. Processes 2019, 7, 422. [Google Scholar] [CrossRef]
- Hegde, S.; Nizam, A.; Vijayan, A. Furaldehyde-Based Magnetic Supported Palladium Nanoparticles as an Efficient Heterogeneous Catalyst for Mizoroki–Heck Cross-Coupling Reaction. New J. Chem. 2024, 48, 1121–1129. [Google Scholar] [CrossRef]
- Sonawane, S.A.; Mhaldar, P.M.; Chhowala, T.N.; Pore, D.M. Novel Palladium Tagged Ferrite Nanoparticle Supported Ionic Liquid Phase Catalyst for The Efficient Copper-Free Sonogashira Coupling. J. Mol. Struct. 2022, 1269, 133729. [Google Scholar] [CrossRef]
- Sardarian, A.R.; Eslahi, H.; Esmaeilpour, M. Green, Cost-Effective and Efficient Procedure for Heck and Sonogashira Coupling Reactions Using Palladium Nanoparticles Supported on Functionalized Fe3O4@SiO2 by Polyvinyl Alcohol as a Highly Active, Durable and Reusable Catalyst. Appl. Organomet. Chem. 2019, 33, e4856. [Google Scholar] [CrossRef]
- Akay, S.; Baran, T.; Kayan, B.; Kalderis, D. Assessment of a Pd–Fe3O4-Biochar Nanocomposite as a Heterogeneous Catalyst for the Solvent-Free Suzuki-Miyaura Reaction. Mater. Chem. Phys. 2021, 259, 124176. [Google Scholar] [CrossRef]
- Tamoradi, T.; Daraie, M.; Heravi, M.M. Synthesis of Palladated Magnetic Nanoparticle (Pd@ Fe3O4/AMOCAA) as an Efficient and Heterogeneous Catalyst for Promoting Suzuki and Sonogashira Cross-Coupling Reactions. Appl. Organmet. Chem. 2020, 34, e5538. [Google Scholar] [CrossRef]
- Halligudra, G.; Paramesh, C.C.; Mudike, R.; Ningegowda, M.; Rangappa, D.; Shivaramu, P.D. PdII on Guanidine-Functionalized Fe3O4 Nanoparticles as an Efficient Heterogeneous Catalyst for Suzuki–Miyaura Cross-Coupling and Reduction of Nitroarenes in Aqueous Media. ACS Omega 2021, 6, 34416–34428. [Google Scholar] [CrossRef] [PubMed]
- Bora, T.J.; Hazarika, N.; Gour, N.K.; Lee, S.; Park, Y.B.; Biswas, S.; Devi, A.; Bania, K.K. Low-Palladium-Content Iron (III) Nanocatalyst Supported on Zeolite-NaY for C–Cl Bond Activation. ACS Appl. Nano Mater. 2023, 6, 17972–17985. [Google Scholar] [CrossRef]
- Akkoc, M.; Buğday, N.; Altın, S.; Yaşar, S. Magnetite@ MCM-41 Nanoparticles as Support Material for Pd-N-Heterocyclic Carbene Complex: A Magnetically Separable Catalyst for Suzuki–Miyaura Reaction. Appl. Organomet. Chem. 2021, 35, e6233. [Google Scholar] [CrossRef]
- Çalışkan, M.; Baran, T. Decorated Palladium Nanoparticles on Chitosan/δ-FeOOH Microspheres: A Highly Active and Recyclable Catalyst for Suzuki Coupling Reaction and Cyanation of Aryl Halides. Int. J. Biol. Macromol. 2021, 174, 120–133. [Google Scholar] [CrossRef]
- Sheikh, S.; Nasseri, M.A.; Chahkandi, M.; Reiser, O.; Allahresani, A. Dendritic structured Palladium Complexes: Magnetically Retrievable, Highly Efficient Heterogeneous Nanocatalyst for Suzuki and Heck Cross-Coupling Reactions. RSC Adv. 2022, 12, 8833–8840. [Google Scholar] [CrossRef]
- Vibhute, S.P.; Mhaldar, P.M.; Shejwal, R.V.; Pore, D.M. Magnetic nanoparticles-supported palladium catalyzed Suzuki-Miyaura cross coupling. Tetrahedron Lett. 2020, 61, 151594. [Google Scholar] [CrossRef]
- Baran, T.; Nasrollahzadeh, M. Facile Fabrication of Magnetically Separable Palladium Nanoparticles Supported on Modified Kaolin as a Highly Active Heterogeneous Catalyst for Suzuki Coupling Reactions. J. Phys. Chem. Solids 2020, 146, 109566. [Google Scholar] [CrossRef]
- Liu, B.; Yan, Z.; Xu, T.; Li, C.; Gao, R.; Hao, H.; Bai, J. Co-Construction of Oxygen Vacancies and Heterojunctions on CeO2 via One-Step Fe Doping for Enhanced Photocatalytic Activity in Suzuki Reaction. Chem. Eng. J. 2022, 442, 136226. [Google Scholar] [CrossRef]
- Jahanshahi, R.; Khazaee, A.; Sobhani, S.; Sansano, J.M. gC3N4/γ-Fe2O3/TiO2/Pd: A New Magnetically Separable Photocatalyst for Visible-Light-Driven Fluoride-Free Hiyama and Suzuki–Miyaura Cross-Coupling Reactions at Room Temperature. New J. Chem. 2020, 44, 11513–11526. [Google Scholar] [CrossRef]
- Adam, M.S.S.; Ullah, F.; Makhlouf, M.M. Hybrid Organic-Inorganic Cu(II) Iminoisonicotine@TiO2@Fe3O4 Heterostructure as Efficient Catalyst for Cross-Couplings. J Am. Ceram. Soc. 2020, 103, 4632–4653. [Google Scholar] [CrossRef]
- Deepa, M.; Selvarasu, U.; Kalaivani, K.; Parasuraman, K. Recyclable Heterogeneous Iron Supported on Imidazolium Ionic Liquid Catalysed Palladium and Copper-Free Heck Reaction. J. Organomet. Chem. 2021, 954, 122073. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Abolfathi, P.; Tavangar-Rizi, Z. Iron-catalyzed cross-coupling reaction: Heterogeneous palladium and copper-free Heck and Sonogashira cross-coupling reactions catalyzed by a reusable Fe (III) complex. Appl. Organomet. Chem. 2018, 32, e4353. [Google Scholar] [CrossRef]
- Min, Q.; Miao, P.; Chu, D.; Liu, J.; Qi, M.; Kazemnejadi, M. Introduction of a Recyclable Basic Ionic Solvent with Bis-(NHC) Ligand Property and the Possibility of Immobilization on Magnetite for Ligand-And Base-Free Pd-Catalyzed Heck, Suzuki and Sonogashira Cross-Coupling Reactions in Water. Catal. Lett. 2021, 151, 3030–3047. [Google Scholar] [CrossRef]
- Sobhani, S.; Esmaeilzadeh-Soleimani, S. Immobilized Palladium-Pyridine Complex On γ-Fe2O3 Magnetic Nanoparticles as a New Magnetically Recyclable Heterogeneous Catalyst for Heck, Suzuki and Copper-Free Sonogashira Reactions. Org. Chem. Res. 2019, 5, 10–24. [Google Scholar]
- Tashrifi, Z.; Bahadorikhalili, S.; Lijan, H.; Ansari, S.; Hamedifar, H.; Mahdavi, M. Synthesis and Characterization of γ-Fe2O3@SiO2–(CH2)3–PDTC–Pd Magnetic Nanoparticles: A New and Highly Active Catalyst for the Heck/Sonogashira Coupling Reactions. New J. Chem. 2019, 43, 8930–8938. [Google Scholar] [CrossRef]
- Kazemnejadi, M.; Rezazadeh, Z.; Nasseri, M.A.; Allahresani, A.; Esmaeilpour, M. Imidazolium Chloride-Co (III) Complex Immobilized on Fe3O4@SiO2 as a Highly Active Bifunctional Nanocatalyst for the Copper-, Phosphine-, and Base-Free Heck and Sonogashira Reactions. Green Chem. 2019, 21, 1718–1734. [Google Scholar] [CrossRef]
- Gebbink, R.J.K.; Moret, M.E. (Eds.) Non-Noble Metal Catalysis: Molecular Approaches and Reactions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Muci, A.R.; Buchwald, S.L. Practical palladium catalysts for CN and CO bond formation. In Cross-Coupling Reactions: A Practical Guide; Springer: Berlin/Heidelberg, Germany, 2002; pp. 131–209. [Google Scholar]
- Sun, Y.; Tang, H.; Chen, K.; Hu, L.; Yao, J.; Shaik, S.; Chen, H. Two-State Reactivity in Low-Valent Iron-Mediated C–H Activation and the Implications for Other First-Row Transition Metals. J. Am. Chem. Soc. 2016, 138, 3715–3730. [Google Scholar] [CrossRef]
- Aoki, Y.; Toyoda, T.; Kawasaki, H.; Takaya, H.; Sharma, A.K.; Morokuma, K.; Nakamura, M. Iron-Catalyzed Chemoselective C− N Coupling Reaction: A Protecting-Group-Free Amination of Aryl Halides Bearing Amino or Hydroxy Groups. Asian J. Org. Chem. 2020, 9, 372–376. [Google Scholar] [CrossRef]
- Kaviani, N.; Behrouz, S.; Jafari, A.A.; Rad, M.N.S. Functionalization of Fe3O4@SiO2 Nanoparticles with Cu (I)-Thiosemicarbazone Complex as a Robust and Efficient Heterogeneous Nanocatalyst for N-Arylation of N-Heterocycles with Aryl Halides. RSC Adv. 2023, 13, 30293–30305. [Google Scholar] [CrossRef] [PubMed]
- Chahkamali, F.O.; Sobhani, S.; Sansano, J.M. Water-Dispersible Pd–N-Heterocyclic Carbene Complex Immobilized on Magnetic Nanoparticles as a New Heterogeneous Catalyst for Fluoride-Free Hiyama, Suzuki–Miyaura and Cyanation Reactions in Aqueous Media. Catal. Lett. 2022, 152, 2650–2668. [Google Scholar] [CrossRef]
- Abdollahi-Alibeik, M.; Ramazani, Z. Synthesis, Characterization and Application of Magnetic Mesoporous Fe3O4@ Fe-Cu/MCM-41 as Efficient and Recyclable Nanocatalyst for the Buchwald-Hartwig CN Cross-Coupling Reaction. J. Chem. Sci. 2022, 134, 77. [Google Scholar] [CrossRef]
- Nithya, K.; Anbarasan, R.; Anbuselvan, N.; Vasantha, V.S.; Suresh, D.; Amali, A.J. Heterogenization of Cobalt on Nanostructured Magnetic Covalent Triazine Framework: Effective Catalyst for Buchwald-Hartwig N-Arylation, Reduction, and Oxidation Reactions. ACS Appl. Nano Mater. 2024, 7, 9554–9564. [Google Scholar] [CrossRef]
- Zhang, W.; Veisi, H.; Sharifi, R.; Salamat, D.; Karmakar, B.; Hekmati, M.; Hemmati, S.; Zangeneh, M.M.; Zhang, Z.; Su, Q. Fabrication of Pd NPs on Pectin-Modified Fe3O4 NPs: A Magnetically Retrievable Nanocatalyst for Efficient C–C and C–N Cross Coupling Reactions and an Investigation of its Cardiovascular Protective Effects. Int. J. Biol. Macromol. 2020, 160, 1252–1262. [Google Scholar] [CrossRef]
- Rouzifar, M.; Sobhani, S.; Farrokhi, A.; Sansano, J.M. Fe-MIL-101 Modified by Isatin-Schiff-Base-Co: A Heterogeneous Catalyst for C–C, C–O, C–N, and C–P Cross Coupling Reactions. New J. Chem. 2021, 45, 19963–19976. [Google Scholar] [CrossRef]
- Dubey, A.V.; Kumar, A.V. A Bio-Inspired Magnetically Recoverable Palladium Nanocatalyst for the Ullmann Coupling reaction of Aryl halides and Arylboronic acids In Aqueous Media. Appl. Organomet. Chem. 2020, 34, e5570. [Google Scholar] [CrossRef]
- Fekri, S.; Mansoori, Y.; Esquivel, D.; Navarro, M.A. A New Bis-(NHC)-P (II) Complex Supported on Magnetic Mesoporous Silica: An Efficient Pd (II) Catalyst for the Selective Buchwald-Hartwig Monoarylation of Ammonia. ChemistrySelect 2023, 8, e202204378. [Google Scholar] [CrossRef]
- Hemmati, S.; Ahany Kamangar, S.; Yousefi, M.; Hashemi Salehi, M.; Hekmati, M. Cu (I)-Anchored Polyvinyl Alcohol Coated-Magnetic Nanoparticles as Heterogeneous Nanocatalyst in Ullmann-Type C–N Coupling Reactions. Appl. Organomet. Chem. 2020, 34, e5611. [Google Scholar] [CrossRef]
- Ding, Q.; Yu, Y.; Huang, F.; Zhang, L.; Zheng, J.G.; Xu, M.; Baell, J.B.; Huang, H. A Reusable CNT-Supported Single-Atom Iron Catalyst for the Highly Efficient Synthesis of C–N Bonds. Chem. Eur. J. 2020, 26, 4592–4598. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Shan, H.; Neumann, H.; Lu, G.P.; Beller, M. Efficient Iron Single-Atom Catalysts for Selective Ammoxidation of Alcohols to Nitriles. Nat. Commun. 2022, 13, 1848. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Darcel, C. Iron-Catalyzed Hydrogen Transfer Reduction of Nitroarenes with Alcohols: Synthesis of Imines and AZA Heterocycles. J. Org. Chem. 2020, 86, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Podyacheva, E.; Afanasyev, O.I.; Vasilyev, D.V.; Chusov, D. Borrowing Hydrogen Amination Reactions: A Complex Analysis of Trends and Correlations of the Various Reaction Parameters. ACS Catal. 2022, 12, 7142–7198. [Google Scholar] [CrossRef]
- Coutanceau, C.; Brimaud, S.; Lamy, C.; Léger, J.M.; Dubau, L.; Rousseau, S.; Vigier, F. Review of Different Methods for Developing Nanoelectrocatalysts for the Oxidation of Organic Compounds. Electrochim. Acta 2008, 53, 6865–6880. [Google Scholar] [CrossRef]
- Xiong, L.; Tang, J. Strategies and Challenges on Selectivity of Photocatalytic Oxidation of Organic Substances. Adv. Energy Mater. 2021, 11, 2003216. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical Review of Advanced Oxidation Processes in Organic Wastewater Treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Y.; He, D.; Pan, X. Application of Iron-Based Materials in Heterogeneous Advanced Oxidation Processes for Wastewater Treatment: A Review. Chem. Eng. J. 2021, 407, 127191. [Google Scholar] [CrossRef]
- Liu, J.; Peng, C.; Shi, X. Preparation, characterization, and applications of Fe-based catalysts in advanced oxidation processes for organics removal: A review. Environ. Pollut. 2022, 293, 118565. [Google Scholar] [CrossRef] [PubMed]
- Esteves, B.M.; Morales-Torres, S.; Maldonado-Hódar, F.J.; Madeira, L.M. Fitting Biochars and Activated Carbons from Residues of the Olive Oil Industry as Supports of Fe-Catalysts for the Heterogeneous Fenton-Like Treatment of Simulated Olive Mill Wastewater. Nanomaterials 2020, 10, 876. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Duan, X.; Shang, Y.; Gao, B.; Xu, X. Engineered Carbon Supported Single Iron Atom Sites and Iron Clusters from Fe-Rich Enteromorpha for Fenton-Like Reactions via Nonradical Pathways. Appl. Catal. B Environ. 2021, 287, 119963. [Google Scholar] [CrossRef]
- Madihi-Bidgoli, S.; Asadnezhad, S.; Yaghoot-Nezhad, A.; Hassani, A. Azurobine Degradation Using Fe2O3@Multi-Walled Carbon Nanotube Activated Peroxymonosulfate (PMS) Under UVA-LED Irradiation: Performance, Mechanism and Environmental Application. J. Environ. Chem. Eng. 2021, 9, 106660. [Google Scholar] [CrossRef]
- Chen, L.; Wang, S.; Yang, Z.; Qian, J.; Pan, B. Selective Interfacial Oxidation of Organic Pollutants in Fenton-Like System Mediated by Fe(III)-Adsorbed Carbon Nanotubes. Appl. Catal. B Environ. 2021, 292, 120193. [Google Scholar] [CrossRef]
- Xie, A.; Cui, J.; Yang, J.; Chen, Y.; Lang, J.; Li, C.; Yan, Y.; Dai, J. Graphene oxide/Fe (III)-Based Metal-Organic Framework Membrane for Enhanced Water Purification Based on Synergistic Separation and Photo-Fenton Processes. Appl. Catal. B Environ. 2020, 264, 118548. [Google Scholar] [CrossRef]
- Pervez, M.N.; He, W.; Zarra, T.; Naddeo, V.; Zhao, Y. New Sustainable Approach for The Production of Fe3O4/Graphene Oxide-Activated Persulfate System for Dye Removal in Real Wastewater. Water 2020, 12, 733. [Google Scholar] [CrossRef]
- Görmez, F.; Görmez, Ö.; Gözmen, B.; Kalderis, D. Degradation of Chloramphenicol and Metronidazole by Electro-Fenton Process Using Graphene Oxide-Fe3O4 as Heterogeneous Catalyst. J. Environ. Chem. Eng. 2019, 7, 102990. [Google Scholar] [CrossRef]
- Le, T.X.H.; Drobek, M.; Bechelany, M.; Motuzas, J.; Julbe, A.; Cretin, M. Application of Fe-MFI Zeolite Catalyst in Heterogeneous Electro-Fenton Process for Water Pollutants Abatement. Micropor. Mesopor. Mater. 2019, 278, 64–69. [Google Scholar]
- Chan-Thaw, C.E.; Savara, A.; Villa, A. Selective Benzyl Alcohol Oxidation Over Pd Catalysts. Catalysts 2018, 8, 431. [Google Scholar] [CrossRef]
- Ferguson, L.N. The synthesis of aromatic aldehydes. Chem. Rev. 1946, 38, 227–254. [Google Scholar] [CrossRef]
- Jacquot, C.; Middelkoop, V.; Köckritz, A.; Pohar, A.; Bienert, R.; Kellici, S.; Bărăgău, I.A.; Venezia, B.; Gavriilidis, A.; Likozar, B.; et al. 3D Printed Catalytic Reactors for Aerobic Selective Oxidation of Benzyl Alcohol into Benzaldehyde in Continuous Multiphase Flow. Sustain. Mater. Technol. 2021, 30, e00329. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, J.; Wang, D.; Pu, Y.; Wang, J.X.; Chen, J.F. Metal-free catalytic oxidation of benzylic alcohols for benzaldehyde. React. Chem. Eng. 2019, 4, 507–515. [Google Scholar] [CrossRef]
- Crombie, C.M.; Lewis, R.J.; Taylor, R.L.; Morgan, D.J.; Davies, T.E.; Folli, A.; Murphy, D.M.; Edwards, J.K.; Qi, J.; Jiang, H.; et al. Enhanced Selective Oxidation of Benzyl Alcohol via in Situ H2O2 Production Over Supported Pd-Based Catalysts. ACS Catal. 2021, 11, 2701–2714. [Google Scholar] [CrossRef]
- Das, B.; Baruah, M.J.; Sharma, M.; Sarma, B.; Karunakar, G.V.; Satyanarayana, L.; Roy, S.; Bhattacharyya, P.K.; Borah, K.K.; Bania, K.K. Self pH Regulated Iron (II) Catalyst for Radical Free Oxidation of Benzyl Alcohols. Appl. Catal. A Gen. 2020, 589, 117292. [Google Scholar] [CrossRef]
- Iraqui, S.; Kashyap, S.S.; Rashid, M.H. NiFe2O4 Nanoparticles: An Efficient and Reusable Catalyst for the Selective Oxidation of Benzyl Alcohol to Benzaldehyde Under Mild Conditions. Nanoscale Adv. 2020, 2, 5790–5802. [Google Scholar] [CrossRef]
- Baruah, M.J.; Bora, T.J.; Dutta, R.; Roy, S.; Guha, A.K.; Bania, K.K. Fe (III) Superoxide Radicals in Halloysite Nanotubes for Visible-Light-Assisted Benzyl Alcohol Oxidation and Oxidative C-C Coupling of 2-Naphthol. Mol. Catal. 2021, 515, 111858. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, J.; Shen, W. Atomically Dispersed Feδ+ Anchored on Nitrogen-Rich Carbon for Enhancing Benzyl Alcohol Oxidation Through Mott-Schottky Effect. Appl. Catal. B Environ. 2021, 292, 120195. [Google Scholar] [CrossRef]
- Correia, L.M.; Kuznetsov, M.L.; Alegria, E.C. Core–Shell Catalysts for Conventional Oxidation of Alcohols: A Brief Review. Catalysts 2023, 13, 1137. [Google Scholar] [CrossRef]
- Sun, L.; Zhan, W.; Shang, J.; Chen, G.; Wang, S.; Chen, Y.; Long, Z. Carbon-Encapsulated Fe3O4 for Catalyzing the Aerobic Oxidation of Benzyl Alcohol and Benzene. React. Kinet. Mech. Catal. 2019, 126, 1055–1065. [Google Scholar] [CrossRef]
- Dabiri, M.; Nikbakht, R.; Movahed, S.K. Palladium Nanoparticle Supported on Core-Shell Feox@Nitrogen-Doped Carbon Cubes and Their Photocatalytic Activities in Selective Oxidation of Alcohols and Ullmann Homocoupling in One Reaction System. Mater. Chem. Phys. 2021, 258, 123908. [Google Scholar] [CrossRef]
- Xu, B.; Senthilkumar, S.; Zhong, W.; Shen, Z.; Lu, C.; Liu, X. Magnetic Core–Shell Fe3O4@Cu2O and Fe3O4@Cu2O–Cu Materials as Catalysts for Aerobic Oxidation of Benzylic Alcohols Assisted by TEMPO and N-Methylimidazole. RSC Adv. 2020, 10, 26142–26150. [Google Scholar] [CrossRef]
- Paul, A.; Bhuyan, B.; Dhar, S.S. Study of Core–Shell α-Fe2O3@Au Nanohybrid and Their High Catalytic Performances in Aerial Oxidation of Benzyl Alcohols. Chem. Eng. Commun. 2020, 207, 1185–1195. [Google Scholar] [CrossRef]
- Zheng, Z.; Han, F.; Xing, B.; Han, X.; Li, B. Synthesis of Fe3O4@CdS@CQDs Ternary Core–Shell Heterostructures as A Magnetically Recoverable Photocatalyst for Selective Alcohol Oxidation Coupled with H2O2 Production. J. Colloid Interface Sci. 2022, 624, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Malko, D.; Guo, Y.; Jones, P.; Britovsek, G.; Kucernak, A. Heterogeneous Iron Containing Carbon Catalyst (Fe-N/C) for Epoxidation with Molecular Oxygen. J. Catal. 2019, 370, 357–363. [Google Scholar] [CrossRef]
- Yu, D.; Gao, W.; Xing, S.; Lian, L.; Zhang, H.; Wang, X. Fe-Doped H3PMo12O40 Immobilized on Covalent Organic Frameworks (Fe/PMA@ Cofs): A Heterogeneous Catalyst for the Epoxidation of Cyclooctene with H2O2. RSC Adv. 2019, 9, 4884–4891. [Google Scholar] [CrossRef]
- Rayati, S.; Nafarieh, P. A Practical Innovative Method for Highly Selective Oxidation of Alkenes and Alkanes Using Fe (III) And Mn (III) Porphyrins Supported onto Multi-Wall Carbon Nanotubes as Reusable Heterogeneous Catalysts. Appl. Organomet. Chem. 2019, 33, e4789. [Google Scholar] [CrossRef]
- Zarnegaryan, A.; Dehbanipour, Z. Iron (II) Complex Supported on Graphene Nanosheet: An Efficient and Heterogeneous Catalyst for Epoxidation of Alkenes. Appl. Surf. Sci. Adv. 2021, 4, 100074. [Google Scholar] [CrossRef]
- Mitra, M.; Cusso, O.; Bhat, S.S.; Sun, M.; Cianfanelli, M.; Costas, M.; Nordlander, E. Highly Enantioselective Epoxidation of Olefins by H2O2 Catalyzed by A Non-Heme Fe (Ii) Catalyst of A Chiral Tetradentate Ligand. Dalton Trans. 2019, 48, 6123–6131. [Google Scholar] [CrossRef]
- Solé-Daura, A.; Zhang, T.; Fouilloux, H.; Robert, C.; Thomas, C.M.; Chamoreau, L.M.; Carbó, J.J.; Proust, A.; Guillemot, G.; Poblet, J.M. Catalyst Design for Alkene Epoxidation by Molecular Analogues of Heterogeneous Titanium-Silicalite Catalysts. ACS Catal. 2020, 10, 4737–4750. [Google Scholar] [CrossRef]
- Li, W.; Wu, G.; Hu, W.; Dang, J.; Wang, C.; Weng, X.; Da Silva, I.; Manuel, P.; Yang, S.; Guan, N.; et al. Direct Propylene Epoxidation with Molecular Oxygen Over Cobalt-Containing Zeolites. J. Am. Chem. Soc. 2022, 144, 4260–4268. [Google Scholar] [CrossRef]
- Xiong, W.; Gu, X.K.; Zhang, Z.; Chai, P.; Zang, Y.; Yu, Z.; Li, D.; Zhang, H.; Liu, Z.; Huang, W. Fine Cubic Cu2O Nanocrystals as Highly Selective Catalyst for Propylene Epoxidation with Molecular Oxygen. Nat. Commun. 2021, 12, 5921. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, S.; Zhao, H.; Li, A.; Luo, L.; Guo, L. Subnano-FeOx Clusters Anchored in an Ultrathin Amorphous Al2O3 Nanosheet for Styrene Epoxidation. ACS Catal. 2021, 11, 11542–11550. [Google Scholar] [CrossRef]
- Xiong, Y.; Sun, W.; Xin, P.; Chen, W.; Zheng, X.; Yan, W.; Zheng, L.; Dong, J.; Zhang, J.; Wang, D.; et al. Gram-Scale Synthesis of High-Loading Single-Atomic-Site Fe Catalysts for Effective Epoxidation of Styrene. Adv. Mater. 2020, 32, 2000896. [Google Scholar] [CrossRef]
- Pó, R.; Cardi, N. Synthesis of Syndiotactic Polystyrene: Reaction Mechanisms and Catalysis. Prog. Polym. Sci. 1996, 21, 47–88. [Google Scholar] [CrossRef]
- Niu, C.; Du, K.; Xu, Z.; Li, Z.; Li, T.; Wang, R. Mechanical properties of epoxy resin composites modified by epoxy styrene-butadiene latex. J. Appl. Polymer Sci. 2023, 140, e54002. [Google Scholar] [CrossRef]
- Wu, L.; Wang, X.; Li, B. Porous Core–Shell Fe3O4@ CuSiO3 Microsphere as Effective Catalyst for Styrene Epoxidation. Appl. Surf. Sci. 2022, 591, 153158. [Google Scholar] [CrossRef]
- Liu, J.; Meng, R.; Li, J.; Jian, P.; Wang, L.; Jian, R. Achieving High-Performance for Catalytic Epoxidation of Styrene with Uniform Magnetically Separable CoFe2O4 Nanoparticles. Appl. Catal. B Environ. 2019, 254, 214–222. [Google Scholar] [CrossRef]
- Sheng, B.; Deng, C.; Li, Y.; Xie, S.; Wang, Z.; Sheng, H.; Zhao, J. In situ hydroxylation of a single-atom iron catalyst for preferential 1O2 production from H2O2. Acs Catal. 2022, 12, 14679–14688. [Google Scholar] [CrossRef]
- ElMetwally, A.E.; Eshaq, G.; Yehia, F.Z.; Al-Sabagh, A.M.; Kegnæs, S. Iron Oxychloride as an Efficient Catalyst for Selective Hydroxylation of Benzene to Phenol. ACS Catal. 2018, 8, 10668–10675. [Google Scholar] [CrossRef]
- Yan, Y.; Wu, J.; Hu, G.; Gao, C.; Guo, L.; Chen, X.; Liu, L.; Song, W. Current State and Future Perspectives of Cytochrome P450 Enzymes for C–H And C=C Oxygenation. Synth. Systems Biotechnol. 2022, 7, 887–899. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, H.; Cai, H.; Zhang, J.; Gong, X.; Han, W. Iron-Catalyzed Arene C–H Hydroxylation. Science 2021, 374, 77–81. [Google Scholar] [CrossRef]
- Vega, G.; Quintanilla, A.; Belmonte, M.; Casas, J.A. Kinetic Study of Phenol Hydroxylation by H2O2 In 3D Fe/Sic Honeycomb Monolithic Reactors: Enabling the Sustainable Production of Dihydroxybenzenes. Chem. Eng. J. 2022, 428, 131128. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, S.; Gao, Y.; Huang, L.; Yang, C.; Hou, Y.; Zhang, J. Unique functionalities of carbon shells coating on ZnFe2O4 for enhanced photocatalytic hydroxylation of benzene to phenol. Appl. Catal. B Environ. 2022, 304, 120999. [Google Scholar] [CrossRef]
- Mishra, S.; Bal, R.; Dey, R.K. Heterogeneous Recyclable Copper Oxide Supported on Activated Red Mud as An Efficient and Stable Catalyst for the One Pot Hydroxylation of Benzene to Phenol. Mol. Catal. 2021, 499, 111310. [Google Scholar] [CrossRef]
- Salazar-Aguilar, A.D.; Vega, G.; Casas, J.A.; Vega-Díaz, S.M.; Tristan, F.; Meneses-Rodríguez, D.; Belmonte, M.; Quintanilla, A. Direct Hydroxylation of Phenol to Dihydroxybenzenes by H2O2 and Fe-Based Metal-Organic Framework Catalyst at Room Temperature. Catalysts 2020, 10, 172. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Wang, F.; Zhai, Y.; Cui, X.; Lv, G.; Jiang, T.; Hu, J. Synergistic Effect Between Fe and Cu Species on Mesoporous Silica for Hydroxylation of Benzene to Phenol. Ind. Eng. Chem. Res. 2021, 60, 8386–8395. [Google Scholar] [CrossRef]
- Yue, M.; Jiang, X.; Zhang, H.; Zhang, S.; Xue, T.; Li, Y. Quasi-solid-phase synthesis of Fe-MFI zeolites by using Fe-containing zeolite seed sol for hydroxylation of benzene with H2O2. Micropor. Mesopor. Mater. 2020, 294, 109891. [Google Scholar] [CrossRef]
- Xiao, P.; Osuga, R.; Wang, Y.; Kondo, J.N.; Yokoi, T. Bimetallic Fe–Cu/beta zeolite catalysts for direct hydroxylation of benzene to phenol: Effect of the sequence of ion exchange for Fe and Cu cations. Catal. Sci. Technol. 2020, 10, 6977–6986. [Google Scholar] [CrossRef]
- Lu, E.; Wu, J.; Yang, B.; Yu, D.; Yu, Z.; Hou, Y.; Zhang, J. Selective hydroxylation of benzene to phenol over Fe nanoparticles encapsulated within N-doped carbon shells. ACS Appl. Nano Mater. 2020, 3, 9192–9199. [Google Scholar] [CrossRef]
- Zeng, L.; Liang, H.; An, P.; Yu, D.; Yang, C.; Hou, Y.; Zhang, J. Carbon Encapsulated Bimetallic FeCo Nanoalloys for One-Step Hydroxylation of Benzene to Phenol. Appl. Catal. A Gen. 2022, 633, 118499. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, J.; Lei, A. Recent Advances in Electrochemical Oxidative Cross-Coupling with Hydrogen Evolution Involving Radicals. Chem. Soc. Rev. 2021, 50, 10058–10086. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yuan, J.; Gao, M.; Tang, S.; Li, W.; Shi, R.; Lei, A. Oxidative Coupling Between Two Hydrocarbons: An Update of Recent C–H Functionalizations. Chem. Rev. 2015, 115, 12138–12204. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Liu, C.; Lei, A. Transition-metal catalyzed oxidative cross-coupling reactions to form C–C bonds involving organometallic reagents as nucleophiles. Chem. Soc. Rev. 2011, 40, 2761–2776. [Google Scholar] [CrossRef]
- Oheix, E.; Herrero, C.; Moutet, J.; Rebilly, J.N.; Cordier, M.; Guillot, R.; Bourcier, S.; Banse, F.; Sénéchal-David, K.; Auffrant, A. FeIII and FeII Phosphasalen Complexes: Synthesis, Characterization, and Catalytic Application for 2-Naphthol Oxidative Coupling. Chem. Eur. J. 2020, 26, 13634–13643. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Recent Advances in Asymmetric Oxidative Coupling of 2-Naphthol and Its Derivatives. Chirality 2010, 22, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, N.V.; Bryliakov, K.P. Transition metal catalyzed aerobic asymmetric coupling of 2-naphthols. Mini-Rev. Org. Chem. 2019, 16, 392–398. [Google Scholar] [CrossRef]
- Wu, L.Y.; Usman, M.; Liu, W.B. Enantioselective Iron/Bisquinolyldiamine Ligand-Catalyzed Oxidative Coupling Reaction of 2-Naphthols. Molecules 2020, 25, 852. [Google Scholar] [CrossRef]
- Horibe, T.; Nakagawa, K.; Hazeyama, T.; Takeda, K.; Ishihara, K. An Enantioselective Oxidative Coupling Reaction of 2-Naphthol Derivatives Catalyzed by Chiral Diphosphine Oxide–Iron (II) Complexes. Chem. Commun. 2019, 55, 13677–13680. [Google Scholar] [CrossRef]
- Deshpande, N.; Parulkar, A.; Joshi, R.; Diep, B.; Kulkarni, A.; Brunelli, N.A. Epoxide Ring Opening with Alcohols Using Heterogeneous Lewis Acid Catalysts: Regioselectivity and Mechanism. J. Catal. 2019, 370, 46–54. [Google Scholar] [CrossRef]
- Tadiello, L.; Gandini, T.; Stadler, B.M.; Tin, S.; Jiao, H.; de Vries, J.G.; Pignataro, L.; Gennari, C. Regiodivergent Reductive Opening of Epoxides by Catalytic Hydrogenation Promoted by a (Cyclopentadienone) Iron Complex. ACS Catal. 2021, 12, 235–246. [Google Scholar] [CrossRef]
- Meng, Y.; Taddeo, F.; Aguilera, A.F.; Cai, X.; Russo, V.; Tolvanen, P.; Leveneur, S. The Lord of the Chemical Rings: Catalytic Synthesis of Important Industrial Epoxide Compounds. Catalysts 2021, 11, 765. [Google Scholar] [CrossRef]
- Cao, H.; Liu, S.; Wang, X. Environmentally Benign Metal Catalyst for The Ring-Opening Copolymerization of Epoxide and CO2: State-Of-the-Art, Opportunities, and Challenges. Green Chem. Eng. 2022, 3, 111–124. [Google Scholar] [CrossRef]
- Della Monica, F.; Buonerba, A.; Capacchione, C. Homogeneous Iron Catalysts in The Reaction of Epoxides with Carbon Dioxide. Adv. Synth. Catal. 2019, 361, 265–282. [Google Scholar] [CrossRef]
- Das, S.; Asefa, T. Epoxide Ring-Opening Reactions with Mesoporous Silica-Supported Fe (III) Catalysts. ACS Catal. 2011, 1, 502–510. [Google Scholar] [CrossRef]
- Liu, W.; Li, W.; Spannenberg, A.; Junge, K.; Beller, M. Iron-Catalysed Regioselective Hydrogenation of Terminal Epoxides to Alcohols Under Mild Conditions. Nat. Catal. 2019, 2, 523–528. [Google Scholar] [CrossRef]
- Shi, J.; Wang, S.; Wang, M.; Wang, X.; Li, W. Robust Salen-Typed Ce-Mofs Supported Fe (III) Catalyst Fabricated by Metalloligand Strategy for Catalytic Epoxides with Alcohols. Mol. Catal. 2022, 533, 112764. [Google Scholar] [CrossRef]
- Li, Z.; Yan, Y.; Liu, M.; Qu, Z.; Yue, Y.; Mao, T.; Zhao, S.; Liu, M.; Lin, Z. Robust Ring-Opening Reaction via Asymmetrically Coordinated Fe Single Atoms Scaffolded by Spoke-Like Mesoporous Carbon Nanospheres. Proc. Natl. Acad. Sci. USA 2023, 120, e2218261120. [Google Scholar] [CrossRef]
- Reis, N.V.; Deacy, A.C.; Rosetto, G.; Durr, C.B.; Williams, C.K. Heterodinuclear Mg (II) M (II)(M=Cr, Mn, Fe, Co, Ni, Cu and Zn) complexes for the ring opening copolymerization of carbon dioxide/epoxide and anhydride/epoxide. Chem. Eur. J. 2022, 28, e202104198. [Google Scholar] [CrossRef]
- Nagarjun, N.; Concepcion, P.; Dhakshinamoorthy, A. MIL-101 (Fe) as an Active Heterogeneous Solid Acid Catalyst for the Regioselective Ring Opening of Epoxides by Indoles. Mol. Catal. 2020, 482, 110628. [Google Scholar] [CrossRef]
- Shi, X.L.; Sun, B.; Hu, Q.; Chen, Y.; Duan, P. Fiber-Supported Fe (III) Complex Catalyst in Spinning Basket Reactor for Cleaner Ring-Opening of Epoxides with Alcohols. Green Chem. 2019, 21, 3573–3582. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Y.; Zhang, F.; Jiang, P.; Gao, W.; Cong, R.; Yang, T. Ring-Opening Hydration of Epoxides into Diols with a Low Water–Epoxide Ratio Catalyzed by a Fe-Incorporated Octahedra-Based Molecular Sieve. J. Phys. Chem. C 2021, 125, 13291–13303. [Google Scholar] [CrossRef]

 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Amount (mg) | Temp. (°C) | Time (h) | % Sel. (Phe.) |
| 1 | Fe-MFI-Zeolite | 100 | 60 | 2 | 97 |
| 2 | Fe-Cu/Beta Zeolite | 50 | 60 | 6 | 96 |
| 3 | Cu-Fe/SBA-15 | 30 | 60 | 3 | 93 |
| 4 | Fe-NC | 30 | 60 | 4 | 95 |
| 5 | CuO-Fe2O3/Fe3O4 | 50 | 40 | 24 | 85 |
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Catalyst | Amount | Oxidant | Temp. | Time (h) | % Yield [a] | % ee [b] | Subs. [c] |
| 1 | Fe2O3-HNT | 30 mg | O2, H2O2 | RT | 2, 1 | 89, 72 | − | 1 |
| 2 | Fe(OTf)2-ligand | 5 mol% | TBHP | −20–10 °C | 60 | 77–98 | 6–70 | 19 |
| 3 | Fe(ClO)4-ligand | 10 mol% | O2 | 50 °C | 5–18 | 51–99 | 12–62 | 12 |
| 4 | [Fe(salan)-ν-(OH)]2 | 4 mM | O2 | 60 °C | 72 | 87 | − | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baruah, M.J.; Dutta, R.; Zaki, M.E.A.; Bania, K.K. Heterogeneous Iron-Based Catalysts for Organic Transformation Reactions: A Brief Overview. Molecules 2024, 29, 3177. https://doi.org/10.3390/molecules29133177
Baruah MJ, Dutta R, Zaki MEA, Bania KK. Heterogeneous Iron-Based Catalysts for Organic Transformation Reactions: A Brief Overview. Molecules. 2024; 29(13):3177. https://doi.org/10.3390/molecules29133177
Chicago/Turabian StyleBaruah, Manash J., Rupjyoti Dutta, Magdi E. A. Zaki, and Kusum K. Bania. 2024. "Heterogeneous Iron-Based Catalysts for Organic Transformation Reactions: A Brief Overview" Molecules 29, no. 13: 3177. https://doi.org/10.3390/molecules29133177
APA StyleBaruah, M. J., Dutta, R., Zaki, M. E. A., & Bania, K. K. (2024). Heterogeneous Iron-Based Catalysts for Organic Transformation Reactions: A Brief Overview. Molecules, 29(13), 3177. https://doi.org/10.3390/molecules29133177








