Atomistic Modeling of Quaternized Chitosan Head Groups: Insights into Chemical Stability and Ion Transport for Anion Exchange Membrane Applications
Abstract
1. Introduction
2. Results and Discussion
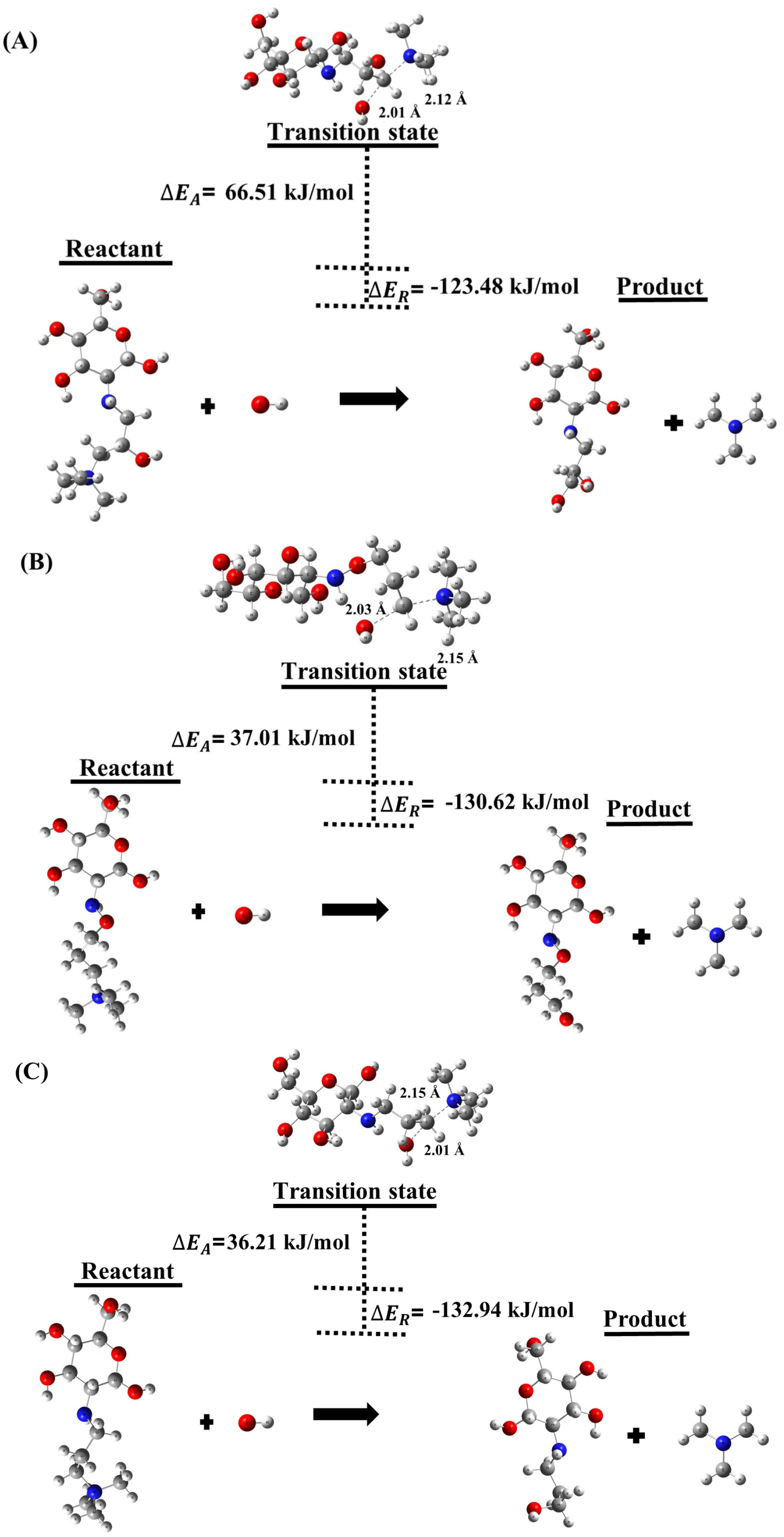
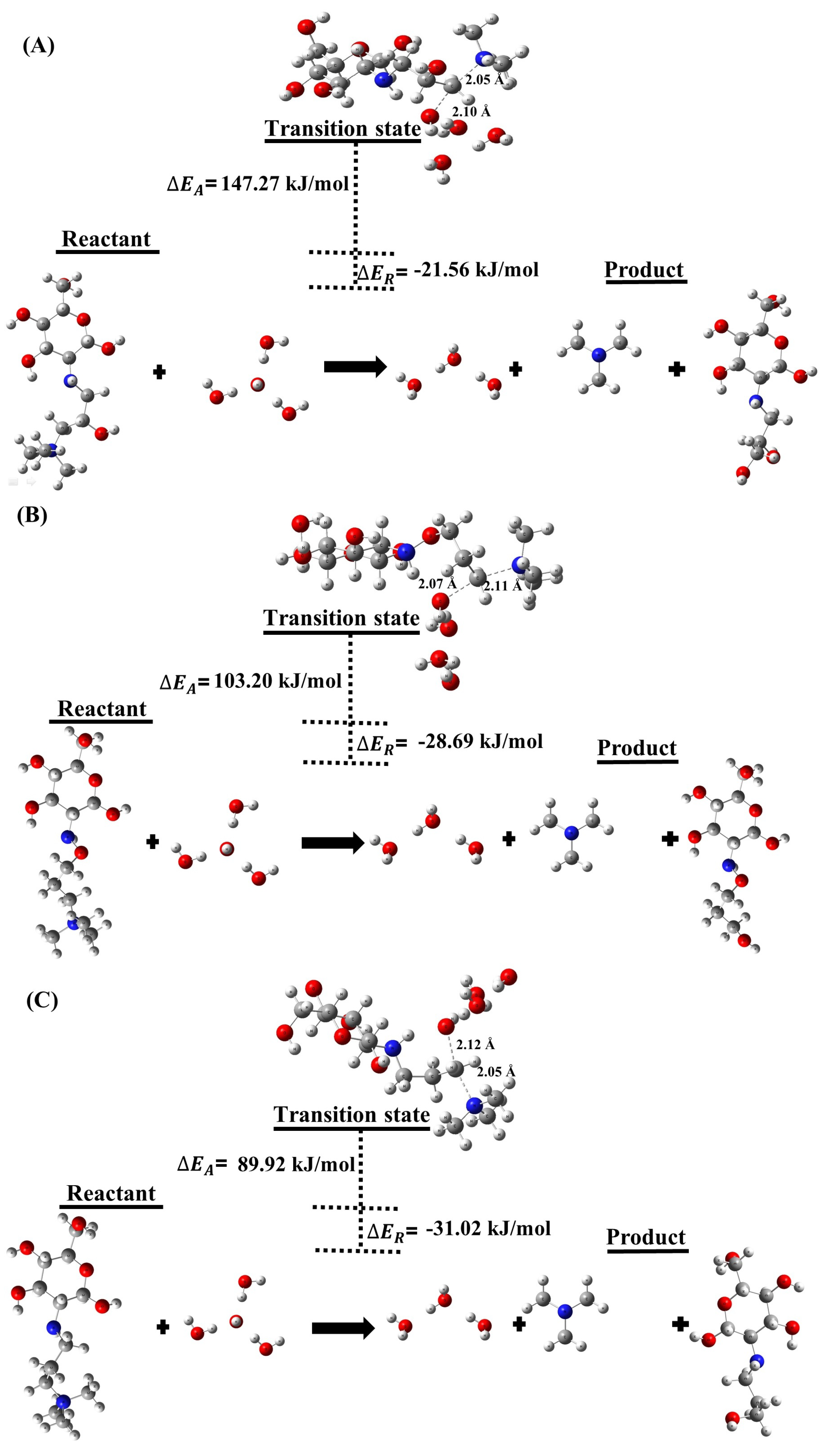
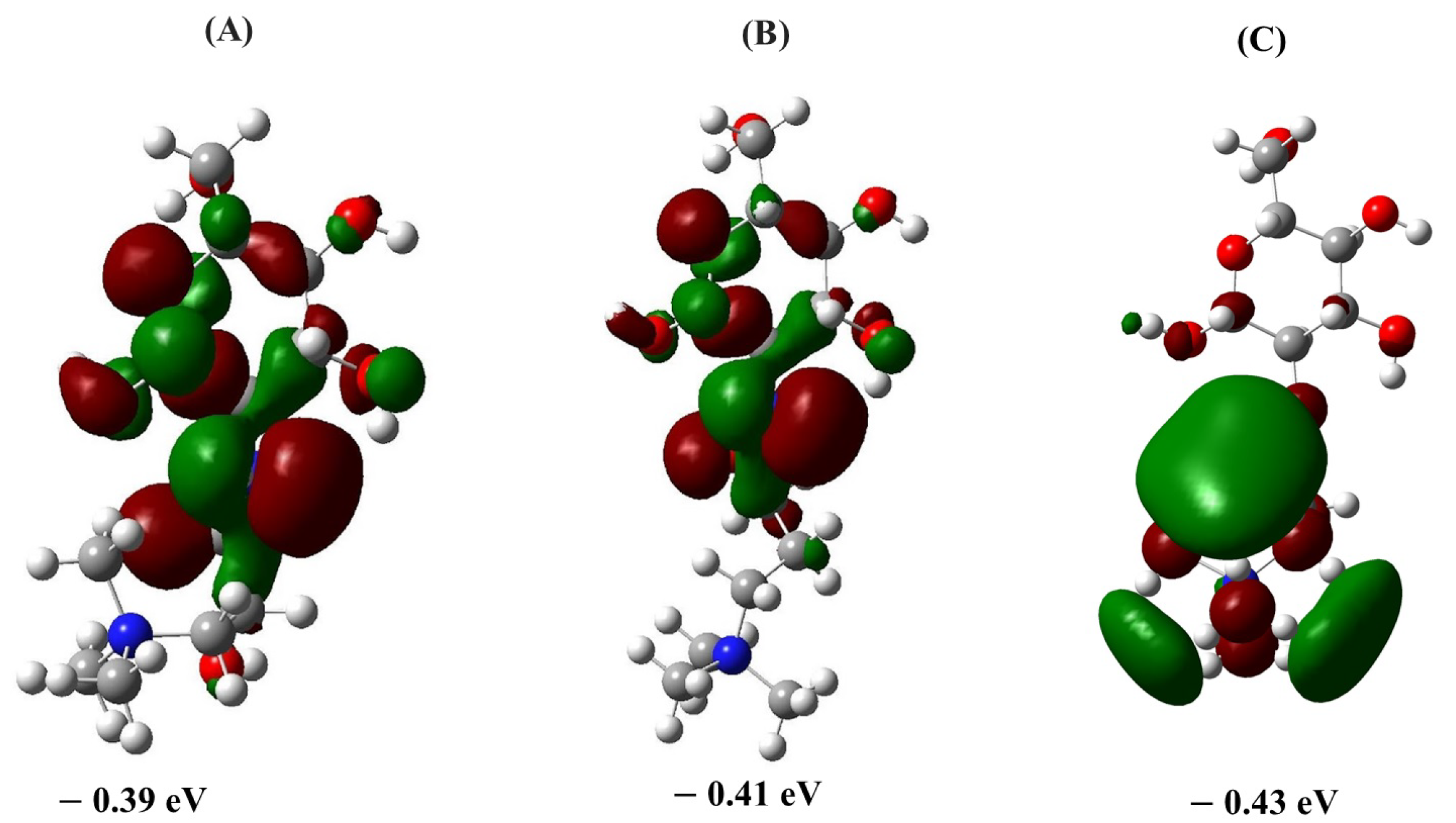
2.1. DFT Results
2.1.1. Degradation Reactions
2.1.2. LUMO Distribution and Energies
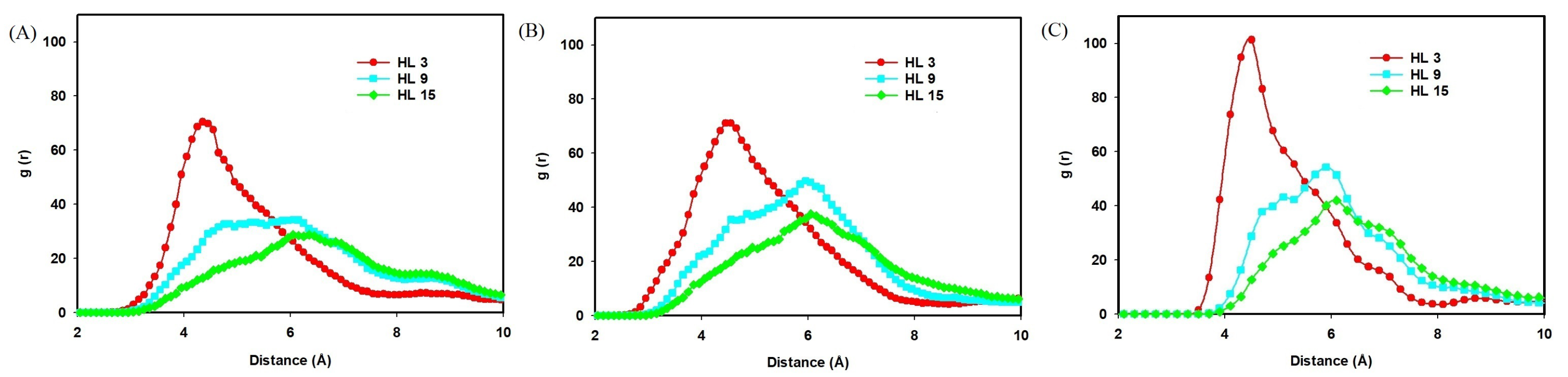
2.2. Classical All-Atom MD Results
2.2.1. Effect of Hydration Level
2.2.2. Effect of Temperature
3. Methodology
3.1. Computational Models
3.2. DFT Calculations
3.3. Classical All-Atom MD Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AEM | Anion Exchange Membrane |
| AEMFCs | Anion Exchange Membrane Fuel Cells |
| AFCs | Alkaline Fuel Cells |
| BSSE | Basis set superposition error |
| DFT | Density Functional Theory |
| Reaction energy | |
| Activation energy | |
| Binding energy | |
| DMFCs | Direct Methanol Fuel Cells |
| EFCs | Enzymatic Fuel Cells |
| HL | Hydration level |
| Hydroxide ion | |
| LUMO | Lowest unoccupied molecular orbital |
| MCFCs | Molten Carbonate Fuel Cells |
| MD | Molecular dynamics |
| MSD | Mean square displacement |
| Nucleophilic substitution | |
| PAFCs | Phosphoric Acid Fuel Cells |
| PCM | Polarizable continuum model |
| PEMFCs | Proton Exchange Membrane Fuel Cells |
| QCS | Quaternized chitosan |
| QA | Quaternary ammonium |
| RDF | Radial distribution function |
| SOFCs | Solid Oxide Fuel Cells |
References
- Dekel, D.R. Review of cell performance in anion exchange membrane fuel cells. J. Power Sources 2018, 375, 158–169. [Google Scholar] [CrossRef]
- Chen, H.; Tao, R.; Bang, K.T.; Shao, M.; Kim, Y. Anion Exchange Membranes for Fuel Cells: State-of-the-Art and Perspectives. Adv. Energy Mater. 2022, 12, 2200934. [Google Scholar] [CrossRef]
- Li, F.; Chan, S.H.; Tu, Z. Recent Development of Anion Exchange Membrane Fuel Cells and Performance Optimization Strategies: A Review. Chem. Rec. 2024, 24, e202300067. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, M.A.; Kohl, P.A.; Kucernak, A.R.; Mustain, W.E.; Nijmeijer, K.; Scott, K.; et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Wan, Y.; Peppley, B.; Creber, K.A.; Bui, V.T. Anion-exchange membranes composed of quaternized-chitosan derivatives for alkaline fuel cells. J. Power Sources 2010, 195, 3785–3793. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Nam, S.Y. A Review of Recent Chitosan Anion Exchange Membranes for Polymer Electrolyte Membrane Fuel Cells. Membranes 2022, 12, 1265. [Google Scholar] [CrossRef]
- Ma, J.; Sahai, Y. Chitosan biopolymer for fuel cell applications. Carbohydr. Polym. 2013, 92, 955–975. [Google Scholar] [CrossRef]
- Myrzakhmetov, B.; Akhmetova, A.; Bissenbay, A.; Karibayev, M.; Pan, X.; Wang, Y.; Bakenov, Z.; Mentbayeva, A. Review: Chitosan-based biopolymers for anion-exchange membrane fuel cell application. R. Soc. Open Sci. 2023, 10, 230843. [Google Scholar] [CrossRef]
- Maurya, S.; Shin, S.H.; Kim, Y.; Moon, S.H. A review on recent developments of anion exchange membranes for fuel cells and redox flow batteries. Rsc Adv. 2015, 5, 37206–37230. [Google Scholar] [CrossRef]
- Arges, C.G.; Zhang, L. Anion exchange membranes’ evolution toward high hydroxide ion conductivity and alkaline resiliency. ACS Appl. Energy Mater. 2018, 1, 2991–3012. [Google Scholar] [CrossRef]
- Dekel, D.R.; Amar, M.; Willdorf, S.; Kosa, M.; Dhara, S.; Diesendruck, C.E. Effect of water on the stability of quaternary ammonium groups for anion exchange membrane fuel cell applications. Chem. Mater. 2017, 29, 4425–4431. [Google Scholar] [CrossRef]
- Dekel, D.R.; Willdorf, S.; Ash, U.; Amar, M.; Pusara, S.; Dhara, S.; Srebnik, S.; Diesendruck, C.E. The critical relation between chemical stability of cations and water in anion exchange membrane fuel cells environment. J. Power Sources 2018, 375, 351–360. [Google Scholar] [CrossRef]
- Wan, Y.; Peppley, B.; Creber, K.A.; Bui, V.T.; Halliop, E. Quaternized-chitosan membranes for possible applications in alkaline fuel cells. J. Power Sources 2008, 185, 183–187. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Q.L.; Zhang, Q.G.; Zhu, A.M. Synthesis and characterization of cross-linked quaternized poly (vinyl alcohol)/chitosan composite anion exchange membranes for fuel cells. J. Power Sources 2008, 183, 447–453. [Google Scholar] [CrossRef]
- Liao, G.M.; Yang, C.C.; Hu, C.C.; Pai, Y.L.; Lue, S.J. Novel quaternized polyvinyl alcohol/quaternized chitosan nano-composite as an effective hydroxide-conducting electrolyte. J. Membr. Sci. 2015, 485, 17–29. [Google Scholar] [CrossRef]
- Karibayev, M.; Myrzakhmetov, B.; Kalybekkyzy, S.; Wang, Y.; Mentbayeva, A. Binding and Degradation Reaction of Hydroxide Ions with Several Quaternary Ammonium Head Groups of Anion Exchange Membranes Investigated by the DFT Method. Molecules 2022, 27, 2686. [Google Scholar] [CrossRef]
- Zhang, N.; Huo, J.; Yang, B.; Ruan, X.; Zhang, X.; Bao, J.; Qi, W.; He, G. Understanding of imidazolium group hydration and polymer structure for hydroxide anion conduction in hydrated imidazolium-g-PPO membrane by molecular dynamics simulations. Chem. Eng. Sci. 2018, 192, 1167–1176. [Google Scholar] [CrossRef]
- Dubey, V.; Maiti, A.; Daschakraborty, S. Predicting the solvation structure and vehicular diffusion of hydroxide ion in an anion exchange membrane using nonreactive molecular dynamics simulation. Chem. Phys. Lett. 2020, 755, 137802. [Google Scholar] [CrossRef]
- Luo, X.; Liu, H.; Bae, C.; Tuckerman, M.E.; Hickner, M.A.; Paddison, S.J. Mesoscale Simulations of Quaternary Ammonium-Tethered Triblock Copolymers: Effects of the Degree of Functionalization and Styrene Content. J. Phys. Chem. C 2020, 124, 16315–16323. [Google Scholar] [CrossRef]
- Munoz-Santiburcio, D.; Marx, D. Confinement-controlled aqueous chemistry within nanometric slit pores: Focus review. Chem. Rev. 2021, 121, 6293–6320. [Google Scholar] [CrossRef]
- Zelovich, T.; Tuckerman, M.E. OH− and H3O+ Diffusion in Model AEMs and PEMs at Low Hydration: Insights from Ab Initio Molecular Dynamics. Membranes 2021, 11, 355. [Google Scholar] [CrossRef]
- Zelovich, T.; Long, Z.; Hickner, M.; Paddison, S.J.; Bae, C.; Tuckerman, M.E. Ab initio molecular dynamics study of hydroxide diffusion mechanisms in nanoconfined structural mimics of anion exchange membranes. J. Phys. Chem. C 2019, 123, 4638–4653. [Google Scholar] [CrossRef]
- Wan, Y.; Creber, K.A.; Peppley, B.; Bui, V.T. Ionic conductivity of chitosan membranes. Polymer 2003, 44, 1057–1065. [Google Scholar] [CrossRef]
- Muller-Plathe, F. Permeation of polymers—a computational approach. Acta Polym. 1994, 45, 259–293. [Google Scholar] [CrossRef]
- Wan, Y.; Creber, K.A.; Peppley, B.; Bui, V.T. Synthesis, characterization and ionic conductive properties of phosphorylated chitosan membranes. Macromol. Chem. Phys. 2003, 204, 850–858. [Google Scholar] [CrossRef]
- Akhmetova, A.; Myrzakhmetov, B.; Wang, Y.; Bakenov, Z.; Mentbayeva, A. Development of Quaternized Chitosan Integrated with Nanofibrous Polyacrylonitrile Mat as an Anion-Exchange Membrane. ACS Omega 2022, 7, 45371–45380. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Creber, K.A.; Peppley, B.; Bui, V.T. Ionic conductivity and related properties of crosslinked chitosan membranes. J. Appl. Polym. Sci. 2003, 89, 306–317. [Google Scholar] [CrossRef]
- Tomasino, E.; Mukherjee, B.; Ataollahi, N.; Scardi, P. Water Uptake in an Anion Exchange Membrane Based on Polyamine: A First-Principles Study. J. Phys. Chem. B 2022, 126, 7418–7428. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wang, Y.; Zhang, Y.; Zhang, W.; You, W. Alkaline-Stable Anion-Exchange Membranes with Barium [2.2. 2] Cryptate Cations: The Importance of High Binding Constants. Angew. Chem. 2023, 135, e202217742. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Mennucci, B. Polarizable continuum model. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 386–404. [Google Scholar] [CrossRef]
- Gill, P.M.; Johnson, B.G.; Pople, J.A.; Frisch, M.J. The performance of the Becke—Lee—Yang—Parr (B—LYP) density functional theory with various basis sets. Chem. Phys. Lett. 1992, 197, 499–505. [Google Scholar] [CrossRef]
- De Castro, E.; Jorge, F. Accurate universal Gaussian basis set for all atoms of the periodic table. Chem. Phys. 1998, 108, 5225–5229. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H.; et al. G16_C01. Gaussian 16, Revision C. 01; Gaussian, Inc.: Wallingford, CT, USA, 2016; Volume 248. [Google Scholar]
- Zoete, V.; Cuendet, M.A.; Grosdidier, A.; Michielin, O. SwissParam: A fast force field generation tool for small organic molecules. J. Comput. Chem. 2011, 32, 2359–2368. [Google Scholar] [CrossRef]
- MacKerell, A.D., Jr.; Banavali, N.; Foloppe, N. Development and current status of the CHARMM force field for nucleic acids. Biopolym. Orig. Res. Biomol. 2000, 56, 257–265. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Han, K.W.; Ko, K.H.; Abu-Hakmeh, K.; Bae, C.; Sohn, Y.J.; Jang, S.S. Molecular dynamics simulation study of a polysulfone-based anion exchange membrane in comparison with the proton exchange membrane. J. Phys. Chem. C 2014, 118, 12577–12587. [Google Scholar] [CrossRef]
- Ewald, P.P. Die Berechnung optischer und elektrostatischer Gitterpotentiale. Ann. Phys. 1921, 369, 253–287. [Google Scholar] [CrossRef]
- Evans, D.J.; Holian, B.L. The nose–hoover thermostat. J. Chem. Phys. 1985, 83, 4069–4074. [Google Scholar] [CrossRef]
- Berendsen, H.J.C. Transport properties computed by linear response through weak coupling to a bath. In Computer Simulation in Materials Science: Interatomic Potentials, Simulation Techniques and Applications; Springer: Dordrecht, The Netherlands, 1991; pp. 139–155. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD–Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Karibayev, M.; Bekeshov, D.; Myrzakhmetov, B.; Kalybekkyzy, S.; Wang, Y.; Bakenov, Z.; Mentbayeva, A. Effect of Hydration on the Intermolecular Interaction of Various Quaternary Ammonium Based Head Groups with Hydroxide Ion of Anion Exchange Membrane Studied at the Molecular Level. Eurasian-Chem.-Technol. J. 2023, 25, 89–102. [Google Scholar] [CrossRef]
- Nhung, L.T.T.; Kim, I.Y.; Yoon, Y.S. Quaternized chitosan-based anion exchange membrane composited with quaternized poly (vinylbenzyl chloride)/polysulfone blend. Polymers 2020, 12, 2714. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.C.; Chuang, F.S.; Tsen, W.C.; Kuo, T.W. Quaternized chitosan/functionalized carbon nanotubes composite anion exchange membranes. J. Appl. Polym. Sci. 2019, 136, 47778. [Google Scholar] [CrossRef]

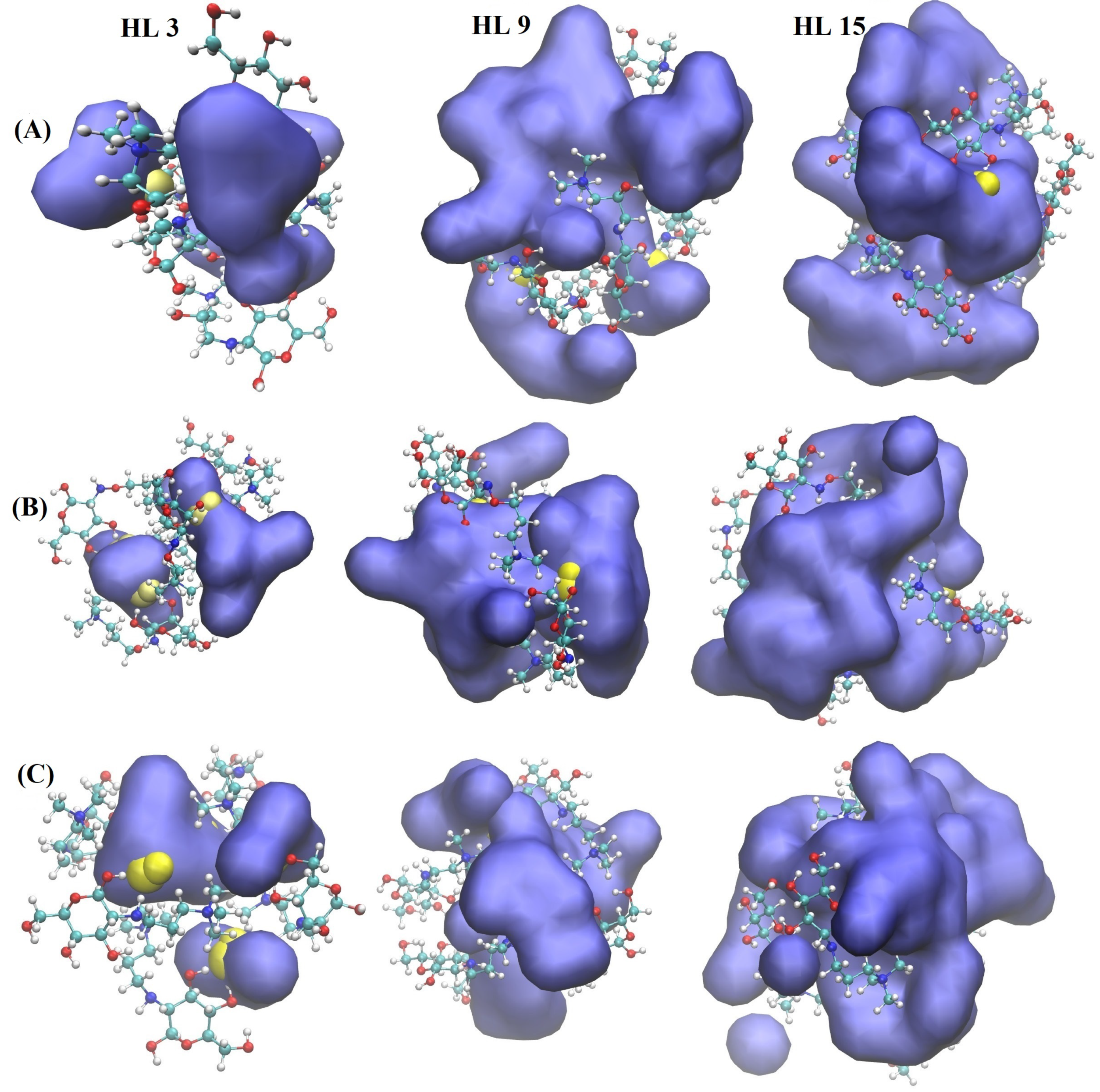


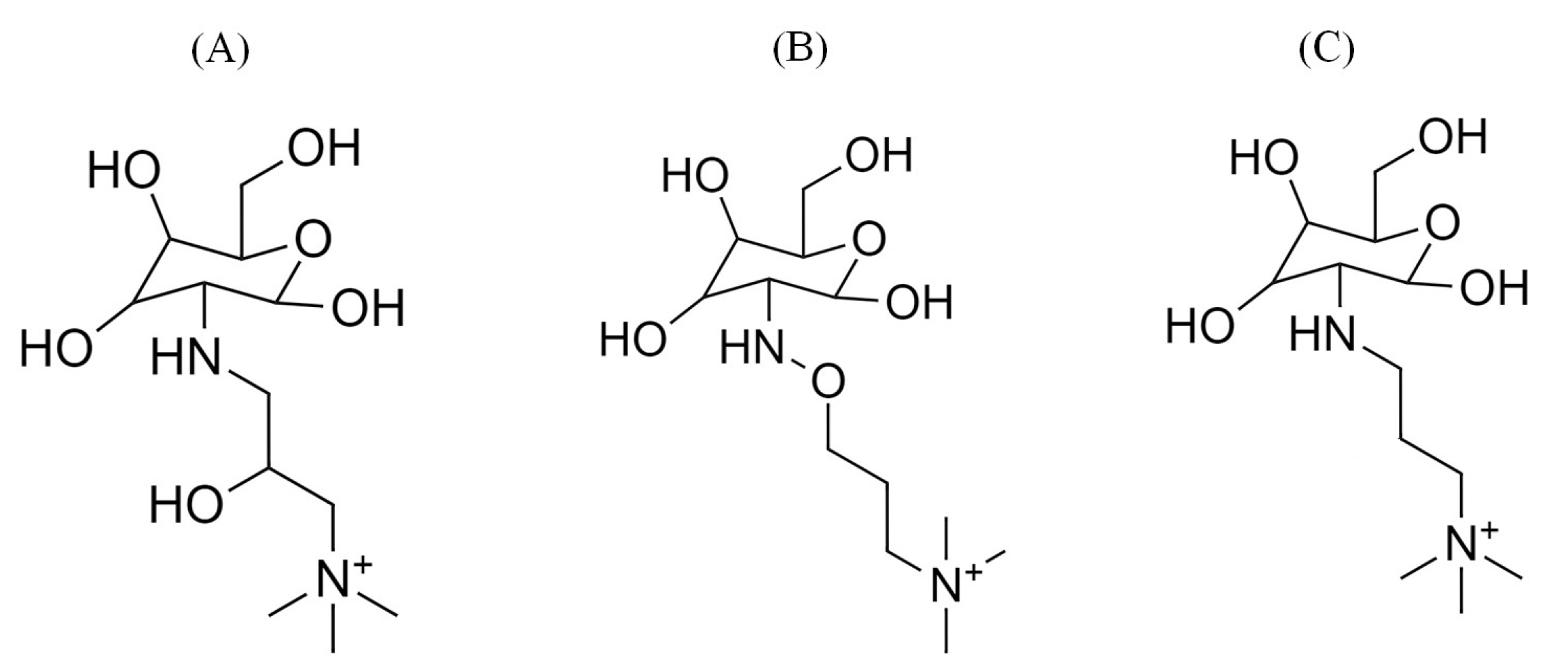
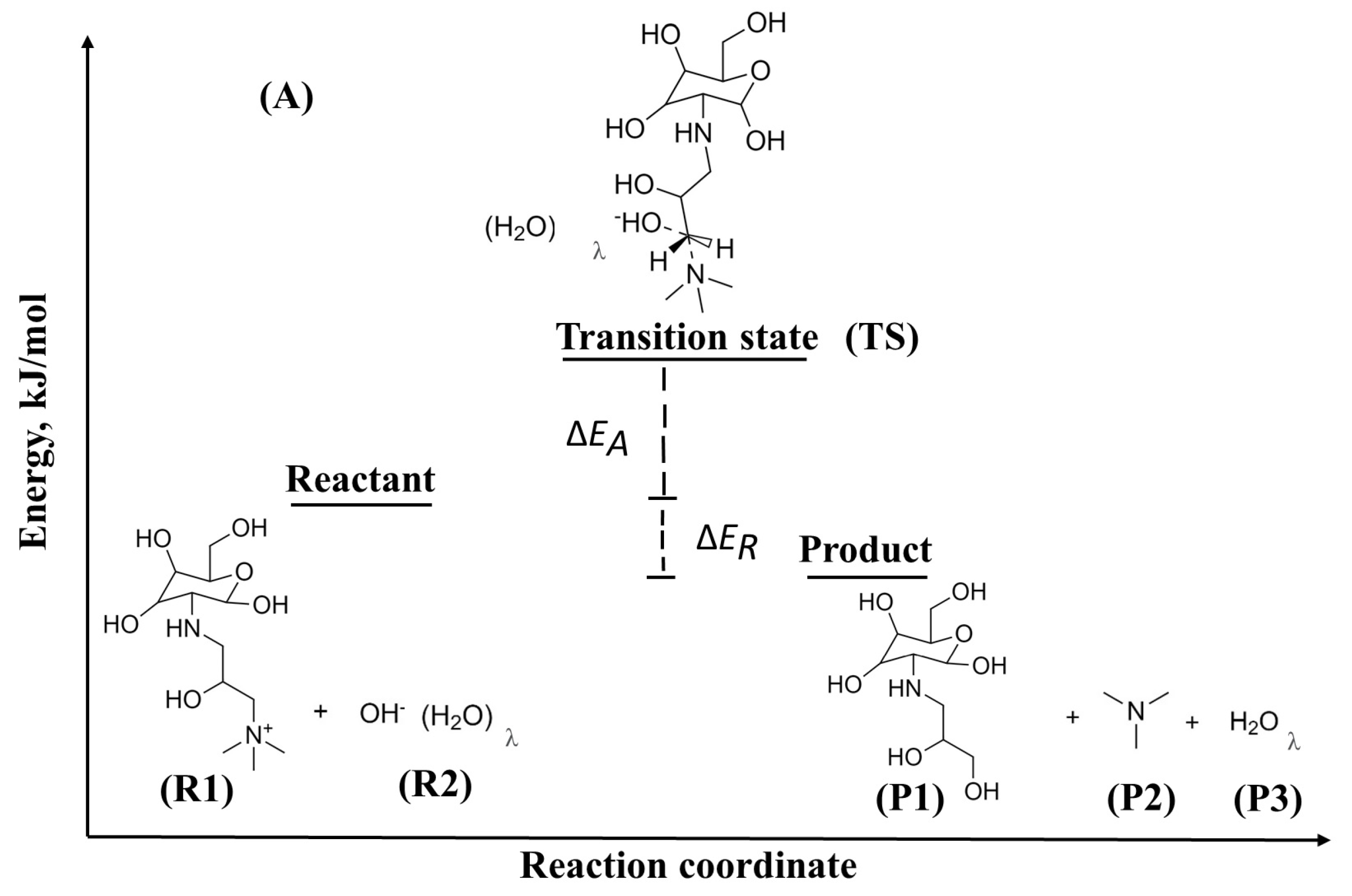
| QCS | BSSE | ||
|---|---|---|---|
| (A) | −123.48 | 15.12 | 66.51 |
| (B) | −130.62 | 16.11 | 37.01 |
| (C) | −132.94 | 14.72 | 36.21 |
| QCS | BSSE | ||
|---|---|---|---|
| (A) | −21.56 | 11.47 | 147.27 |
| (B) | −28.69 | 8.38 | 103.20 |
| (C) | −31.02 | 8.47 | 89.92 |
| D, , (SE) | HL Values | |||
|---|---|---|---|---|
| 3 | 9 | 15 | ||
| (A) | 0.011 (0.004) | 0.017 (0.005) | 0.027 (0.003) | |
| (B) | 0.011 (0.003) | 0.016 (0.006) | 0.026 (0.002) | |
| (C) | 0.011 (0.001) | 0.015 (0.002) | 0.026 (0.002) | |
| D, , (SE) | HL Values | |||
|---|---|---|---|---|
| 3 | 9 | 15 | ||
| (A) | 0.20 (0.07) | 0.40 (0.06) | 0.46 (0.04) | |
| (B) | 0.18 (0.04) | 0.39 (0.05) | 0.43 (0.06) | |
| (C) | 0.13 (0.03) | 0.39 (0.02) | 0.42 (0.05) | |
| D, , (SE) | Temperature Values (K) | |||
|---|---|---|---|---|
| 298 | 330 | 350 | ||
| (A) | 0.011 (0.004) | 0.023 (0.003) | 0.027 (0.003) | |
| (B) | 0.011 (0.003) | 0.023 (0.003) | 0.026 (0.003) | |
| (C) | 0.011 (0.001) | 0.016 (0.002) | 0.026 (0.003) | |
| D, (SE) | Temperature Values (K) | |||
|---|---|---|---|---|
| 298 | 330 | 350 | ||
| (A) | 0.20 (0.07) | 1.54 (0.04) | 2.86 (0.25) | |
| (B) | 0.18 (0.04) | 1.38 (0.06) | 1.92 (0.20) | |
| (C) | 0.13 (0.03) | 0.44 (0.05) | 0.84 (0.09) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karibayev, M.; Myrzakhmetov, B.; Bekeshov, D.; Wang, Y.; Mentbayeva, A. Atomistic Modeling of Quaternized Chitosan Head Groups: Insights into Chemical Stability and Ion Transport for Anion Exchange Membrane Applications. Molecules 2024, 29, 3175. https://doi.org/10.3390/molecules29133175
Karibayev M, Myrzakhmetov B, Bekeshov D, Wang Y, Mentbayeva A. Atomistic Modeling of Quaternized Chitosan Head Groups: Insights into Chemical Stability and Ion Transport for Anion Exchange Membrane Applications. Molecules. 2024; 29(13):3175. https://doi.org/10.3390/molecules29133175
Chicago/Turabian StyleKaribayev, Mirat, Bauyrzhan Myrzakhmetov, Dias Bekeshov, Yanwei Wang, and Almagul Mentbayeva. 2024. "Atomistic Modeling of Quaternized Chitosan Head Groups: Insights into Chemical Stability and Ion Transport for Anion Exchange Membrane Applications" Molecules 29, no. 13: 3175. https://doi.org/10.3390/molecules29133175
APA StyleKaribayev, M., Myrzakhmetov, B., Bekeshov, D., Wang, Y., & Mentbayeva, A. (2024). Atomistic Modeling of Quaternized Chitosan Head Groups: Insights into Chemical Stability and Ion Transport for Anion Exchange Membrane Applications. Molecules, 29(13), 3175. https://doi.org/10.3390/molecules29133175









