α-Carbonyl Rh-Carbenoid Initiated Cascade Assembly of Diazobarbiturates with Alkylidene Pyrazolones for Synthesis of Spirofuropyrimidines
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Procedure for Cascade Assembly Reaction
3.2. Gram-Scale Synthesis of Compound 3a
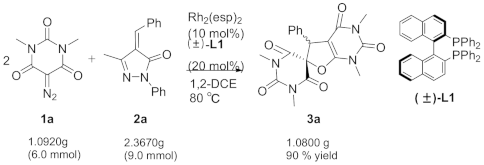
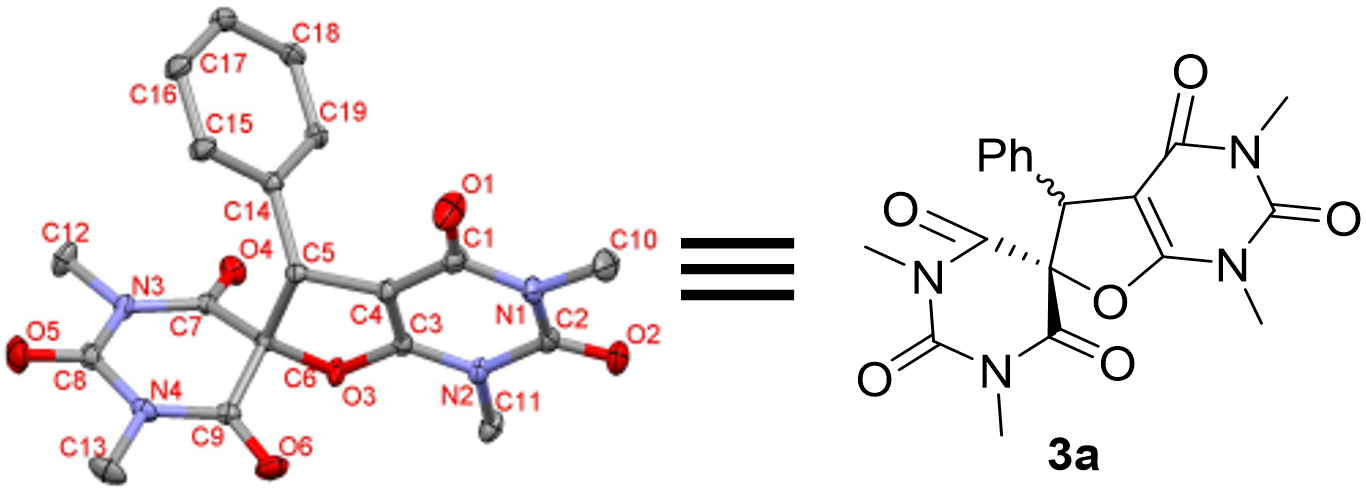
3.3. Characterization of Product 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Clercq, E. Highly potent and selective inhibition of varicella-zoster virus replication by bicyclic furo [2,3-d]pyrimidine nucleoside analogues. Med. Res. Rev. 2003, 23, 253–274. [Google Scholar] [CrossRef] [PubMed]
- Dutta, L.; Sharma, M.; Bhuyan, P.J. Regioisomeric synthesis of dihydrofuro [2,3-d]pyrimidines in a diastereoselective manner involving nitrogen ylides in one-pot three-component reaction. Tetrahedron 2016, 72, 6654–6660. [Google Scholar] [CrossRef]
- Olyaei, A.; Sadeghpour, M. Review on synthetic approaches towards barbituric acids-based furo [2,3-d] pyrimidines. J. Heterocycl. Chem. 2023, 60, 1838–1863. [Google Scholar] [CrossRef]
- Sayed, H.H.; Abbas, H.A.; Morsi, E.M.; Amr Ael, G.; Abdelwahad, N.A. Antimicrobial activity of some synthesized glucopyranosyl-pyrimidine carbonitrile and fused pyrimidine systems. Acta Pharm. 2010, 60, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Romeo, R.; Giofre, S.V.; Garozzo, A.; Bisignano, B.; Corsaro, A.; Chiacchio, M.A. Synthesis and biological evaluation of furopyrimidine N,O-nucleosides. Bioorg. Med. Chem. 2013, 21, 5688–5693. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.I.; Lee, S.H.; Cheong, C.S. A facile synthesis of some substituted furopyrimidine derivatives. J. Heterocycl. Chem. 2009, 43, 1129–1133. [Google Scholar] [CrossRef]
- Kaczmarek, R.; Twardy, D.J.; Olson, T.L.; Korczynski, D.; Andrei, G.; Snoeck, R.; Dolot, R.; Wheeler, K.A.; Dembinski, R. Extension of furopyrimidine nucleosides with 5-alkynyl substituent: Synthesis, high fluorescence, and antiviral effect in the absence of free ribose hydroxyl groups. Eur. J. Med. Chem. 2021, 209, 112884. [Google Scholar] [CrossRef]
- Han, J.; Kaspersen, S.J.; Nervik, S.; Norsett, K.G.; Sundby, E.; Hoff, B.H. Chiral 6-aryl-furo[2,3-d]pyrimidin-4-amines as EGFR inhibitors. Eur. J. Med. Chem. 2016, 119, 278–299. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Das, U. Studies in Pyrimidine-Annelated Heterocycles1 by Tandem Cyclization: Regioselective Synthesis of [6,6]Pyranopyran by Intramolecular [1,6] Michael Addition. J. Org. Chem. 1998, 63, 9997–10000. [Google Scholar] [CrossRef]
- Gangjee, A.; Li, W.; Yang, J.; Kisliuk, R.L. Design, Synthesis, and Biological Evaluation of Classical and Nonclassical 2-Amino-4-oxo-5-substituted-6-methylpyrrolo[3,2-d]pyrimidines as Dual Thymidylate Synthase and Dihydrofolate Reductase Inhibitors. J. Med. Chem. 2008, 51, 68–76. [Google Scholar] [CrossRef]
- Gangjee, A.; Vidwans, A.; Elzein, E.; McGuire, J.J.; Queener, S.F.; Kisliuk, R.L. Synthesis, Antifolate, and Antitumor Activities of Classical and Nonclassical 2-Amino-4-oxo-5-substituted-pyrrolo [2,3-d]pyrimidines. J. Med. Chem. 2001, 44, 1993–2003. [Google Scholar] [CrossRef]
- Janeba, Z.; Balzarini, J.; Andrei, G.; Snoeck, R.; Clercq, E.D.; Robins, M.J. Synthesis and Biological Evaluation of Acyclic 3-[(2-Hydroxyethoxy)methyl] Analogues of Antiviral Furo- and Pyrrolo[2,3-d]pyrimidine Nucleosides. J. Med. Chem. 2005, 48, 4690–4696. [Google Scholar] [CrossRef] [PubMed]
- Vereshchagin, A.N.; Elinson, M.N.; Dorofeeva, E.O.; Zaimovskaya, T.A.; Stepanov, N.O.; Gorbunov, S.V.; Belyakov, P.A.; Nikishin, G.I. Electrocatalytic and chemical assembling of N,N′-dialkylbarbituric acids and aldehydes: Efficient cascade approach to the spiro-[furo[2,3-d]pyrimidine-6,5′-pyrimidine]-2,2′,4,4′,6′-(1′H,3H,3′H)-pentone framework. Tetrahedron 2012, 68, 1198–1206. [Google Scholar] [CrossRef]
- Teimouri, M.B.; Akbari-Moghaddam, P.; Motaghinezhad, M. Urotropine–bromine promoted synthesis of functionalized oxaspirotricyclic furopyrimidines via a domino Knoevenagel condensation/Michael addition/α-bromination/Williamson cycloetherification sequence in water. Tetrahedron 2013, 69, 6804–6809. [Google Scholar] [CrossRef]
- Mohammadi Ziarani, G.; Aleali, F.; Lashgari, N. Recent applications of barbituric acid in multicomponent reactions. RSC Adv. 2016, 6, 50895–50922. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Stepanov, N.O.; Belyakov, P.A.; Nikishin, G.I. Cascade assembly of N,N′-dialkylbarbituric acids and aldehydes: A simple and efficient one-pot approach to the substituted 1,5-dihydro-2H,2′H-spiro(furo[2,3-d]pyrimidine-6,5′-pyrimidine)-2,2′,4,4′,6′(1′H,3H,3′H)-pentone framework. Tetrahedron Lett. 2010, 51, 6598–6601. [Google Scholar] [CrossRef]
- Elinson, M.N.; Merkulova, V.M.; Ilovaisky, A.I.; Nikishin, G.I. Cascade Assembling of Isatins and Barbituric Acids: Facile and Efficient Way to 2′′H-Dispiro[indole-3,5′-furo[2,3-d]pyrimidine-6′,5′′-pyrimidine]-2,2′,2′′,4′,4′′,6′′-(1H,1′H,1′′H,3′H,3′′H)-hexone Scaffold. J. Heterocycl. Chem. 2013, 50, 1236–1241. [Google Scholar] [CrossRef]
- Teimouria, M.B.; Moghaddamb, P.A. Molecular iodine-catalysed tandem synthesis of oxospirotricyclic furopyrimidines in water. J. Chem. Res. 2016, 40, 196–198. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Mi, Y.H.; Zhao, H.W.; Song, X.Q.; Li, J.T.; Zhang, F. Rh(II)-Catalyzed Homocoupling/[4+1] Cycloaddition Cascade of Diazobarbiturates with Diazopyrazolones to Prepare Spirobarbiturates. Adv. Synth. Catal. 2024, 366, 2596–2601. [Google Scholar] [CrossRef]
- Yin, Z.; He, Y.; Chiu, P. Application of (4+3) cycloaddition strategies in the synthesis of natural products. Chem. Soc. Rev. 2018, 47, 8881–8924. [Google Scholar] [CrossRef]
- Xia, Y.; Qiu, D.; Wang, J. Transition-Metal-Catalyzed Cross-Couplings through Carbene Migratory Insertion. Chem. Rev. 2017, 117, 13810–13889. [Google Scholar] [CrossRef] [PubMed]
- Roose, T.R.; Verdoorn, D.S.; Mampuys, P.; Ruijter, E.; Maes, B.U.W.; Orru, R.V.A. Transition metal-catalysed carbene- and nitrene transfer to carbon monoxide and isocyanides. Chem. Soc. Rev. 2022, 51, 5842–5877. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.Z.; Goh, J.; Maraswami, M.; Jia, Z.; Tian, J.S.; Loh, T.P. Recent Advances in Alkenyl sp2 C-H and C-F Bond Functionalizations: Scope, Mechanism, and Applications. Chem. Rev. 2022, 122, 17479–17646. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, J. Gold-catalyzed transformations of alpha-diazocarbonyl compounds: Selectivity and diversity. Chem. Soc. Rev. 2016, 45, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.; Miel, H.; Ring, A.; Slattery, C.N.; Maguire, A.R.; McKervey, M.A. Modern Organic Synthesis with alpha-Diazocarbonyl Compounds. Chem. Rev. 2015, 115, 9981–10080. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.P.; Duffy, R.; Ratnikov, M.; Zhou, L. Catalytic Carbene Insertion into C-H Bonds. Chem. Rev. 2010, 110, 704–724. [Google Scholar] [CrossRef]
- Requejo, M.M.; Perez, P.J. Coinage Metal Catalyzed C-H Bond Functionalization of Hydrocarbons. Chem. Rev. 2008, 108, 3379–3394. [Google Scholar] [CrossRef]
- Yadagiri, D.; Anbarasan, P. Catalytic Functionalization of Metallocarbenes Derived from alpha-Diazocarbonyl Compounds and Their Precursors. Chem. Rev. 2021, 21, 3872–3883. [Google Scholar]
- Sebastian, D.; Satishkumar, S.; Pradhan, P.; Yang, L.; Lakshman, M.K. General Approach to N(6),C5′-Difunctionalization of Adenosine. J. Org. Chem. 2022, 87, 18–39. [Google Scholar] [CrossRef]
- Luo, X.; Chen, G.; He, L.; Huang, X. Amination of Diazocarbonyl Compounds: N-H Insertion under Metal-Free Conditions. J. Org. Chem. 2016, 81, 2943–2949. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tian, X.; Huang, J.; Qian, Y.; Xu, X.; Kang, Z.; Hu, W. Enantioselective Propargylation of Oxonium Ylide with alpha-Propargylic-3-Indolymethanol: Access to Chiral Propargylic Indoles. Org. Lett. 2022, 24, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Kidonakis, M.; Stratakis, M. Au Nanoparticle-Catalyzed Insertion of Carbenes from alpha-Diazocarbonyl Compounds into Hydrosilanes. Org. Lett. 2018, 20, 4086–4089. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Yu, S.; Liu, Z.; Zhang, Y. Rh-Catalyzed Coupling of Acrylic/Benzoic Acids with alpha-Diazocarbonyl Compounds: An Alternative Route for alpha-Pyrones and Isocoumarins. Org. Lett. 2022, 24, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Yu, S.; Liu, Z.; Xu, Z.; Zhang, Y. Synthesis of Furans via Rhodium(III)-Catalyzed Cyclization of Acrylic Acids with alpha-Diazocarbonyl Compounds. J. Org. Chem. 2022, 87, 11979–11988. [Google Scholar] [CrossRef]
- Guo, Y.; Empel, C.; Pei, C.; Atodiresei, I.; Fallon, T.; Koenigs, R.M. Photochemical Cyclopropanation of Cyclooctatetraene and (Poly-)unsaturated Carbocycles. Org. Lett. 2020, 22, 5126–5130. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Liu, X.; Feng, X. Asymmetric Catalytic Rearrangements with alpha-Diazocarbonyl Compounds. Acc. Chem. Res. 2022, 55, 415–428. [Google Scholar] [CrossRef]
- Chen, X.; Wang, M.; Zhang, X.; Fan, X. Rh(III)-Catalyzed Cascade Reactions of Sulfoxonium Ylides with alpha-Diazocarbonyl Compounds: An Access to Highly Functionalized Naphthalenones. Org. Lett. 2019, 21, 2541–2545. [Google Scholar] [CrossRef]
- Alavi, S.; Lin, J.B.; Grover, H.K. Copper-Catalyzed Annulation of Indolyl alpha-Diazocarbonyl Compounds Leads to Structurally Rearranged Carbazoles. Org. Lett. 2021, 23, 5559–5564. [Google Scholar] [CrossRef] [PubMed]
- Zhai, C.; Xing, D.; Jing, C.; Zhou, J.; Wang, C.; Wang, D.; Hu, W. Facile synthesis of 3-aryloxindoles via bronsted acid catalyzed Friedel-Crafts alkylation of electron-rich arenes with 3-diazooxindoles. Org. Lett. 2014, 16, 2934–2937. [Google Scholar] [CrossRef]
- Yu, X.; Yu, S.; Xiao, J.; Wan, B.; Li, X. Rhodium(III)-catalyzed azacycle-directed intermolecular insertion of arene C-H bonds into alpha-diazocarbonyl compounds. J. Org. Chem. 2013, 78, 5444–5452. [Google Scholar] [CrossRef]
- Rao, C.; Mai, S.; Song, Q. Rh(ii)/phosphine-cocatalyzed synthesis of dithioketal derivatives from diazo compounds through simultaneous construction of two different C-S bonds. Chem. Commun. 2018, 54, 5964–5967. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Wang, S. Chemodivergent Synthesis of Oxazoles and Oxime Ethers Initiated by Selective C-N/C-O Formation of Oximes and Diazo Esters. Org. Lett. 2021, 23, 8549–8553. [Google Scholar] [CrossRef] [PubMed]
- Lou, Q.X.; Niu, Y.; Qi, Z.C.; Yang, S.D. Ir(III)-Catalyzed C-H Functionalization of Triphenylphosphine Oxide toward 3-Aryl Oxindoles. J. Org. Chem. 2020, 85, 14527–14536. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Yang, X.; Xie, F.; Li, X. Divergent Access to 1-Naphthols and Isocoumarins via Rh(III)-Catalyzed C-H Activation Assisted by Phosphonium Ylide. Org. Lett. 2017, 19, 3410–3413. [Google Scholar] [CrossRef]
- Happy, S.; Junaid, M.; Yadagiri, D. Reactivity of quinone methides with carbenes generated from alpha-diazocarbonyl compounds and related compounds. Chem. Commun. 2023, 59, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Zhao, Y.; Li, H.; Zheng, Y.; Lian, P.; Wan, X. [3 + 3]-Cycloaddition of alpha-Diazocarbonyl Compounds and N-Tosylaziridines: Synthesis of Polysubstituted 2 H-1,4-Oxazines through Synergetic Catalysis of AgOTf/Cu(OAc)2. Org. Lett. 2019, 21, 2356–2359. [Google Scholar] [CrossRef]
- DeAngelis, A.; Dmitrenko, O.; Fox, J.M. Rh-catalyzed intermolecular reactions of cyclic alpha-diazocarbonyl compounds with selectivity over tertiary C-H bond migration. J. Am. Chem. Soc. 2012, 134, 11035–11043. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Xu, X.; Wojtas, L.; Kim, M.M.; Zhang, X.P. Regioselective synthesis of multisubstituted furans via metalloradical cyclization of alkynes with alpha-diazocarbonyls: Construction of functionalized alpha-oligofurans. J. Am. Chem. Soc. 2012, 134, 19981–19984. [Google Scholar] [CrossRef]
- Chen, Z.S.; Duan, X.H.; Zhou, P.X.; Ali, S.; Luo, J.Y.; Liang, Y.M. Palladium-catalyzed divergent reactions of alpha-diazocarbonyl compounds with allylic esters: Construction of quaternary carbon centers. Angew. Chem. Int. Ed. 2012, 124, 1399–1403. [Google Scholar] [CrossRef]
- Austeri, M.; Rix, D.; Zeghida, W.; Lacour, J. CpRu-Catalyzed O-H Insertion and Condensation Reactions of α-Diazocarbonyl Compounds. Org. Lett. 2011, 13, 1394–1397. [Google Scholar] [CrossRef]
- Zhou, Q.; Song, X.; Zhang, X.; Fan, X. Synthesis of Spiro[benzo[d][1,3]oxazine-4,4′-isoquinoline]s via [4+1+1] Annulation of N-Aryl Amidines with Diazo Homophthalimides and O2. Org. Lett. 2022, 24, 1280–1285. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xu, Y.; Zhang, X.; Fan, X. Selective Synthesis of Pyrazolonyl Spirodihydroquinolines or Pyrazolonyl Spiroindolines under Aerobic or Anaerobic Conditions. Org. Lett. 2022, 24, 9473–9478. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, M.; Lu, P.; Wang, Y. Rh(III)-Catalyzed Reaction of 4-Diazoisochroman-3-imines with (2-Formylaryl)boronic Acids To Access a Straightforward Construction of 5H-Isochromeno[3,4-c]isoquinolines. J. Org. Chem. 2023, 88, 13544–13552. [Google Scholar] [CrossRef]
- Wang, K.; Sun, Y.; Li, B.; Zhang, X.; Fan, X. Expeditious Synthesis of Spiroindoline Derivatives via Tandem C(sp2)-H and C(sp3)-H Bond Functionalization of N-Methyl-N-nitrosoanilines. Org. Lett. 2024, 26, 3091–3096. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.W.; Yoshikai, N. Copper-Catalyzed Coupling of 2-Siloxy-1-alkenes and Diazocarbonyl Compounds: Approach to Multisubstituted Furans, Pyrroles, and Thiophenes. J. Org. Chem. 2016, 81, 5566–5573. [Google Scholar] [CrossRef] [PubMed]
- Kantin, G.; Dar’in, D.; Krasavin, M. RhII-Catalyzed Cycloaddition of α-Diazo Homophthalimides and Nitriles Delivers Oxazolo[5,4-c]isoquinolin-5(4H)-one Scaffold. Eur. J. Org. Chem. 2018, 2018, 4857–4859. [Google Scholar] [CrossRef]
- Inyutina, A.; Kantin, G.; Dar In, D.; Krasavin, M. Diastereoselective Formal [5 + 2] Cycloaddition of Diazo Arylidene Succinimides-Derived Rhodium Carbenes and Aldehydes: A Route to 2-Benzoxepines. J. Org. Chem. 2021, 86, 13673–13683. [Google Scholar] [CrossRef]
- Huang, Z.; He, Y.; Wang, L.; Li, J.; Xu, B.H.; Zhou, Y.G.; Yu, Z. Copper-Catalyzed [4+1] Annulation of Enaminothiones with Indoline-Based Diazo Compounds. J. Org. Chem. 2022, 87, 4424–4437. [Google Scholar] [CrossRef]
- He, X.; Liu, K.; Yan, S.; Wang, Y.; Jiang, Y.; Zhang, X.; Fan, X. Synthesis of 1,7-Fused Indolines Tethered with Spiroindolinone Based on C-H Activation Strategy with Air as a Sustainable Oxidant. J. Org. Chem. 2024, 89, 1880–1897. [Google Scholar] [CrossRef]
- Guranova, N.I.; Dar’in, D.; Kantin, G.; Novikov, A.S.; Bakulina, O.; Krasavin, M. Rh(II)-Catalyzed Spirocyclization of alpha-Diazo Homophthalimides with Cyclic Ethers. J. Org. Chem. 2019, 84, 4534–4542. [Google Scholar] [CrossRef]
- Gecht, M.; Kantin, G.; Dar’in, D.; Krasavin, M. A novel approach to biologically relevant oxazolo[5,4-d]pyrimidine-5,7-diones via readily available diazobarbituric acid derivatives. Tetrahedron Lett. 2019, 60, 151120. [Google Scholar] [CrossRef]
- Best, D.; Burns, D.J.; Lam, H.W. Direct Synthesis of 5-Aryl Barbituric Acids by Rhodium(II)-Catalyzed Reactions of Arenes with Diazo Compounds. Angew. Chem. Int. Ed. 2015, 54, 7410–7413. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lee, Y.R. Efficient Synthesis of Spirobarbiturates and Spirothiobarbiturates Bearing Cyclopropane Rings by Rhodium(II)-Catalyzed Reactions of Cyclic Diazo Compounds. Bull. Korean Chem. Soc. 2013, 34, 1735–1740. [Google Scholar] [CrossRef]
- Torán, R.; Miguélez, R.; Sanz-Marco, A.; Vila, C.; Pedro, J.R.; Blay, G. Asymmetric Addition and Cycloaddition Reactions with Ylidene-Five-Membered Heterocycles. Adv. Synth. Catal. 2021, 23, 5196–5234. [Google Scholar] [CrossRef]
- Zheng, K.; Liu, X.; Feng, X. Recent Advances in Metal-Catalyzed Asymmetric 1,4-Conjugate Addition (ACA) of Nonorganometallic Nucleophiles. Chem. Rev. 2018, 118, 7586–7656. [Google Scholar] [CrossRef]
- Zhan, G.; Du, W.; Chen, Y.C. Switchable divergent asymmetric synthesis via organocatalysis. Chem. Soc. Rev. 2017, 46, 1675–1692. [Google Scholar] [CrossRef] [PubMed]
- CCDC 2309187 (3a) contains the Supplementary Crystallographic Data for this Paper. These Data can be Obtained Free of Charge from The Cambridge Crystallographic Data Center. X-ray Single Crystal Structures of 3a was shown with Thermal Ellipsoils Shown at the 50% Probability Level. Available online: http://www.ccdc.camac.uk/data_request/cif (accessed on 1 April 2023).
- Albright, H.; Davis, A.J.; Gomez-Lopez, J.L.; Vonesh, H.L.; Quach, P.K.; Lambert, T.H.; Schindler, C.S. Carbonyl–Olefin Metathesis. Chem. Rev. 2021, 15, 9359–9406. [Google Scholar] [CrossRef]
- Hoveyda, A.H.; Qin, C.; Sui, X.Z.; Liu, Q.; Li, X.; Nikbakht, A. Taking Olefin Metathesis to the Limit: Stereocontrolled Synthesis of Trisubstituted Alkenes. Acc. Chem. Res. 2023, 18, 2426–2446. [Google Scholar] [CrossRef]
- Lozano-Vila, A.M.; Monsaert, S.; Bajek, A.; Verpoort, F. Ruthenium-Based Olefin Metathesis Catalysts Derived from Alkynes. Chem. Rev. 2010, 110, 4865–4909. [Google Scholar] [CrossRef]
- Kumar, V.; Scilabra, P.; Politzer, P.; Terraneo, G.; Daolio, A.; Fernandez-Palacio, F.; Murray, J.S.; Resnati, G. Tetrel and Pnictogen Bonds Complement Hydrogen and Halogen Bonds in Framing the Interactional Landscape of Barbituric Acids. Cryst. Growth Des. 2020, 21, 642–652. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Yuan, C.; Wang, G.P.; Zhu, S.F.; Wu, Y.; Wang, B.; Sun, Z.; Xiao, Y.; Zhou, Q.L.; et al. Enantioselective Synthesis of Spirobarbiturate-Cyclohexenes through Phosphine-Catalyzed Asymmetric [4 + 2] Annulation of Barbiturate-Derived Alkenes with Allenoates. Org. Lett. 2016, 18, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Temprado, M.; Roux, M.V.; Ros, F.; Notario, R.; Segura, M.; Chickos, J.S. Thermophysical Study of Several Barbituric Acid Derivatives by Differential Scanning Calorimetry (DSC). J. Chem. Eng. Data 2010, 56, 263–268. [Google Scholar] [CrossRef]
- Fakhraian, H.; Nafari, Y. Preparative, mechanistic and tautomeric investigation of 1-phenyl and 1-methyl derivative of 3-methyl-5-pyrazolone. J. Chem. Sci. 2021, 133, 40. [Google Scholar] [CrossRef]
- Tonga, M. Tunable optical properties of push-pull chromophores: End group effect. Tetrahedron Lett. 2020, 61, 152205. [Google Scholar] [CrossRef]
- Yu, J.; Xu, J.; Li, J.; Jin, Y.; Xu, W.; Yu, Z.; Lv, Y. A continuous-flow procedure for the synthesis of 4-Benzylidene-pyrazol-5-one derivatives. J. Flow Chem. 2018, 8, 29–34. [Google Scholar] [CrossRef]
- Aljohani, F.A.; El-Hag, M.; El-Manawaty, M.A. An Efficient One-pot Synthesis of Certain StereoselectiveSpiro [pyrazole-4,5′-isoxazoline]-5-one Derivatives: In vitro Evaluation of Antitumor Activities, Molecular Docking and In silico ADME Predictions. Chem. Res. Chin. Univ. 2022, 38, 1073–1082. [Google Scholar] [CrossRef]
- Khairnar, P.V.; Su, Y.H.; Edukondalu, A.; Lin, W. Enantioselective Synthesis of Spiropyrazolone-Fused Cyclopenta[c]chromen-4-ones Bearing Five Contiguous Stereocenters via [3+2] Cycloaddition. J. Org. Chem. 2021, 86, 12326–12335. [Google Scholar] [CrossRef]
- Zhao, C.; Shi, K.; He, G.; Gu, Q.; Ru, Z.; Yang, L.; Zhong, G. NHC-Catalyzed Asymmetric Formal [4 + 2] Annulation to Construct Spirocyclohexane Pyrazolone Skeletons. Org. Lett. 2019, 21, 7943–7947. [Google Scholar] [CrossRef]
- Sheibani, H.; Babaie, M. Three-Component Reaction to Form 1,4-Dihydropyrano [2,3-c]-pyrazol-5-yl Cyanides. Synth. Commun. 2009, 40, 257–265. [Google Scholar] [CrossRef]
- Awasthi, A.; Yadav, P.; Kumar, V.; Tiwari, D.K. α-Amino Acids Mediated C–C Double Bonds Cleavage in Diastereoselective Synthesis of Aza-Spirocyclic Pyrazolones. Adv. Synth. Catal. 2020, 362, 4378–4383. [Google Scholar] [CrossRef]
- Shindalkar, S.S.; Madje, B.R.; Hangarge, R.V.; Patil, P.T.; Dongare, M.K. Borate Zirconia Mediated Knoevenagel Condensation Reaction in Water. J. Korean Chem. Soc. 2005, 49, 377–380. [Google Scholar] [CrossRef]


 | |||
|---|---|---|---|
| Entry | [M] [f] | Time (h) | Yield [b] (%) |
| 1 [c] | Rh2(OAc)4 | 6 | 40 |
| 2 [d] | Rh2(OAc)4 | 6 | 65 |
| 3 | Rh2(OAc)4 | 6 | 75 |
| 4 | Ph3PAuCl | 6 | NR [e] |
| 5 | (CH3CN)4·CuBF4 | 6 | NR [e] |
| 6 | DPPE·NiCl2 | 8 | NR [e] |
| 7 | DPPE·PdCl2 | 8 | NR [e] |
| 8 | Pd(DPPE)2 | 8 | NR [e] |
| 9 | Pd2(dba)3 | 6 | trace |
| 10 | (F3CSO2)NAg | 6 | trace |
| 11 | Ru(OAc)3 | 6 | 21 |
| 12 | [Rh(COD)2]BF4 | 6 | NR [e] |
| 13 | [Rh3O(OAc)6(H2O)3]OAc | 6 | NR [e] |
| 14 | Rh2(esp)2 | 6 | 93 |
| 15 [g] | Rh2(esp)2 | 6 | 25 |
| 16 [h] | Rh2(esp)2 | 6 | 16 |
| 17 [i] | Rh2(esp)2 | 6 | trace |
| 18 [j] | Rh2(esp)2 | 6 | NR [e] |
 | |||||
|---|---|---|---|---|---|
| Entry | Ligand | Solvent | Time (h) | Yield [b] (%) | ee [d] (%) |
| 1 | - | 1,2-DCE | 6 | trace | - |
| 2 | (±)-L2 | 1,2-DCE | 6 | NR [c] | - |
| 3 | (±)-L3 | 1,2-DCE | 6 | 45 | - |
| 4 | (±)-L4 | 1,2-DCE | 6 | 67 | - |
| 5 | dppf | 1,2-DCE | 6 | 68 | - |
| 6 | dppb | 1,2-DCE | 6 | 77 | - |
| 7 | (±)-L5 | 1,2-DCE | 8 | 82 | - |
| 8 | (±)-L1 | 1,2-DCE | 6 | 93 | - |
| 9 | (±)-L1 | PhCF3 | 6 | NR [c] | - |
| 10 | (±)-L1 | HFIP | 6 | NR [c] | - |
| 11 | (±)-L1 | C6F6 | 6 | NR [c] | - |
| 12 | (±)-L1 | CHCl3 | 6 | NR [c] | - |
| 13 | (S)-L2 | 1,2-DCE | 6 | 45 | 0 |
| 14 | (R, R)-L3 | 1,2-DCE | 6 | 67 | 0 |
| 15 | (R)-L5 | 1,2-DCE | 6 | 93 | 0 |
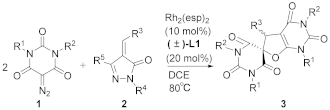 | |||||
|---|---|---|---|---|---|
| Entry | 1 (R1, R2) | 2 (R3, R4, R5) | 3 | Time (h) | Yield [b] (%) |
| 1 | 1a (Me, Me) | 2a (Ph, Ph, Me) | 3a | 6 | 93 |
| 2 | 1a (Me, Me) | 2b (Ph, Me, Me) | 3a | 6 | 86 |
| 3 | 1a (Me, Me) | 2c (Ph, Me, H) | 3a | 6 | 96 |
| 4 | 1a (Me, Me) | 2d (Ph, Ph, Ph) | 3a | 6 | 57 |
| 5 | 1a (Me, Me) | 2e (Me, Ph, Me) | 3b | 6 | 38 |
| 6 | 1a (Me, Me) | 2f (Priso, Ph, Me) | - | 6 | NR [c] |
| 7 | 1a (Me, Me) | 2g  | - | 6 | NR [c] |
| 8 | 1a (Me, Me) | 2h (2-thiophenyl, Ph, Me) | - | 6 | NR [c] |
| 9 | 1a (Me, Me) | 2i (5-benzofuranyl, Ph, Me) | 3c | 6 | 56 |
| 10 | 1b (cyclohexyl, cyclohexyl) | 2c (Ph, Me, H) | - | 6 | NR [c] |
| 11 | 1c (Bn, Bn) | 2c (Ph, Me, H) | 3d | 6 | 88 |
| 12 | 1d (Et, Et) | 2c (Ph, Me, H) | 3e | 6 | 91 |
| 13 | 1e (Priso, Priso) | 2c (Ph, Me, H) | 3f | 6 | 47 |
| 14 | 1f (Butert, Butert) | 2c (Ph, Me, H) | - | 6 | NR [c] |
| 15 | 1g (4-MeC6H4, 4-MeC6H4) | 2c (Ph, Me, H) | - | 6 | NR [c] |
| 16 | 1a (Me, Me) | 2j (4-MeC6H4, Ph, Me) | 3g | 3 | 68 |
| 17 | 1a (Me, Me) | 2k (4-BrC6H4, Ph, Me) | 3h | 3 | 66 |
| 18 | 1a (Me, Me) | 2l (3-ClC6H4, Ph, Me) | 3i | 3 | 72 |
| 19 | 1a (Me, Me) | 2m (2-naphthyl, Ph, Me) | 3j | 6 | 78 |
| 20 | 1a (Me, Me) | 2n (4- F3CC6H4, Ph, Me) | 3k | 6 | 80 |
| 21 | 1a (Me, Me) | 2o (4-MeOC6H4, Ph, Me) | 3l | 3 | 48 |
| 22 | 1a (Me, Me) | 2p (4-O2NC6H4, Ph, Me) | 3m | 6 | 62 |
| 23 | 1a (Me, Me) | 2q (2-BrC6H4, Ph, Me) | 3n | 6 | 66 |
| 24 | 1a (Me, Me) | 2r (3-MeC6H4, Ph, Me) | 3o | 6 | 90 |
| 25 | 1d (Et, Et) | 2j (4-MeC6H4, Ph, Me) | 3p | 6 | 82 |
| 26 | 1d (Et, Et) | 2k (4-BrC6H4, Ph, Me) | 3q | 6 | 95 |
| 27 | 1d (Et, Et) | 2l (3-ClC6H4, Ph, Me) | 3r | 6 | 93 |
| 28 | 1d (Et, Et) | 2m (2-naphthyl, Ph, Me) | 3s | 6 | 80 |
| 29 | 1d (Et, Et) | 2o (4-MeOC6H4, Ph, Me) | 3t | 6 | 65 |
| 30 | 1d (Et, Et) | 2p (4-O2NC6H4, Ph, Me) | 3u | 6 | 90 |
| 31 | 1d (Et, Et) | 2q (2-BrC6H4, Ph, Me) | 3v | 6 | 63 |
| 32 | 1d (Et, Et) | 2r (3-MeC6H4, Ph, Me) | 3w | 6 | 73 |
| 33 | 1h (Me,Bn) | 2a (Ph, Ph, Me) | - | 6 | NR [c] |
| 34 | 1h (Me, Bn) | 2c (Ph, Me, H) | - | 6 | NR [c] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Mi, Y.-H.; Wang, K.; Zhao, H.-W. α-Carbonyl Rh-Carbenoid Initiated Cascade Assembly of Diazobarbiturates with Alkylidene Pyrazolones for Synthesis of Spirofuropyrimidines. Molecules 2024, 29, 3178. https://doi.org/10.3390/molecules29133178
Zhang Y, Mi Y-H, Wang K, Zhao H-W. α-Carbonyl Rh-Carbenoid Initiated Cascade Assembly of Diazobarbiturates with Alkylidene Pyrazolones for Synthesis of Spirofuropyrimidines. Molecules. 2024; 29(13):3178. https://doi.org/10.3390/molecules29133178
Chicago/Turabian StyleZhang, Yue, Yu-Hang Mi, Kuo Wang, and Hong-Wu Zhao. 2024. "α-Carbonyl Rh-Carbenoid Initiated Cascade Assembly of Diazobarbiturates with Alkylidene Pyrazolones for Synthesis of Spirofuropyrimidines" Molecules 29, no. 13: 3178. https://doi.org/10.3390/molecules29133178
APA StyleZhang, Y., Mi, Y.-H., Wang, K., & Zhao, H.-W. (2024). α-Carbonyl Rh-Carbenoid Initiated Cascade Assembly of Diazobarbiturates with Alkylidene Pyrazolones for Synthesis of Spirofuropyrimidines. Molecules, 29(13), 3178. https://doi.org/10.3390/molecules29133178





