Experimental and Theoretical Studies on Indigo-Dye-Modified Conjugated Polymers
Abstract
:1. Introduction
2. Results and Discussion
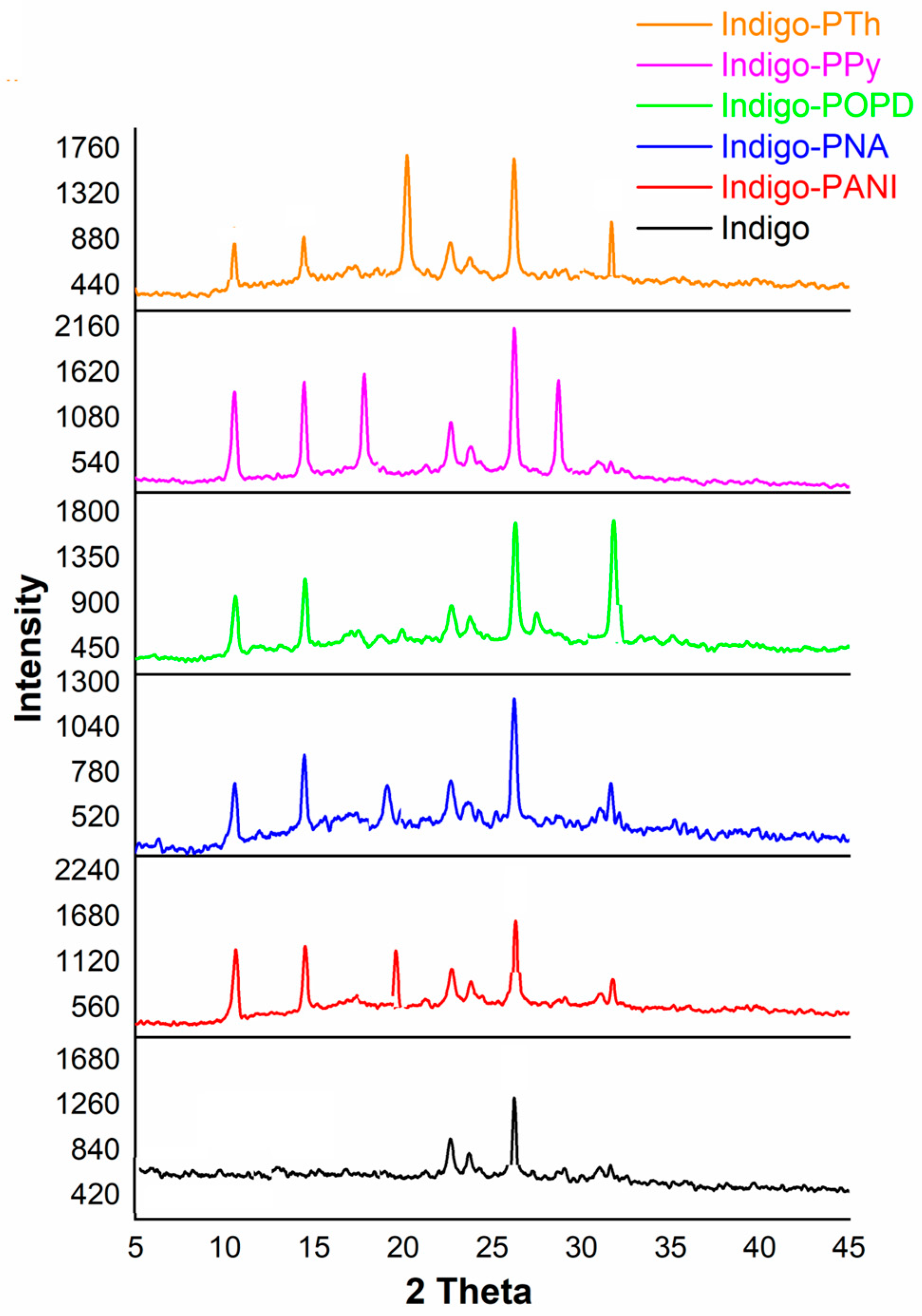
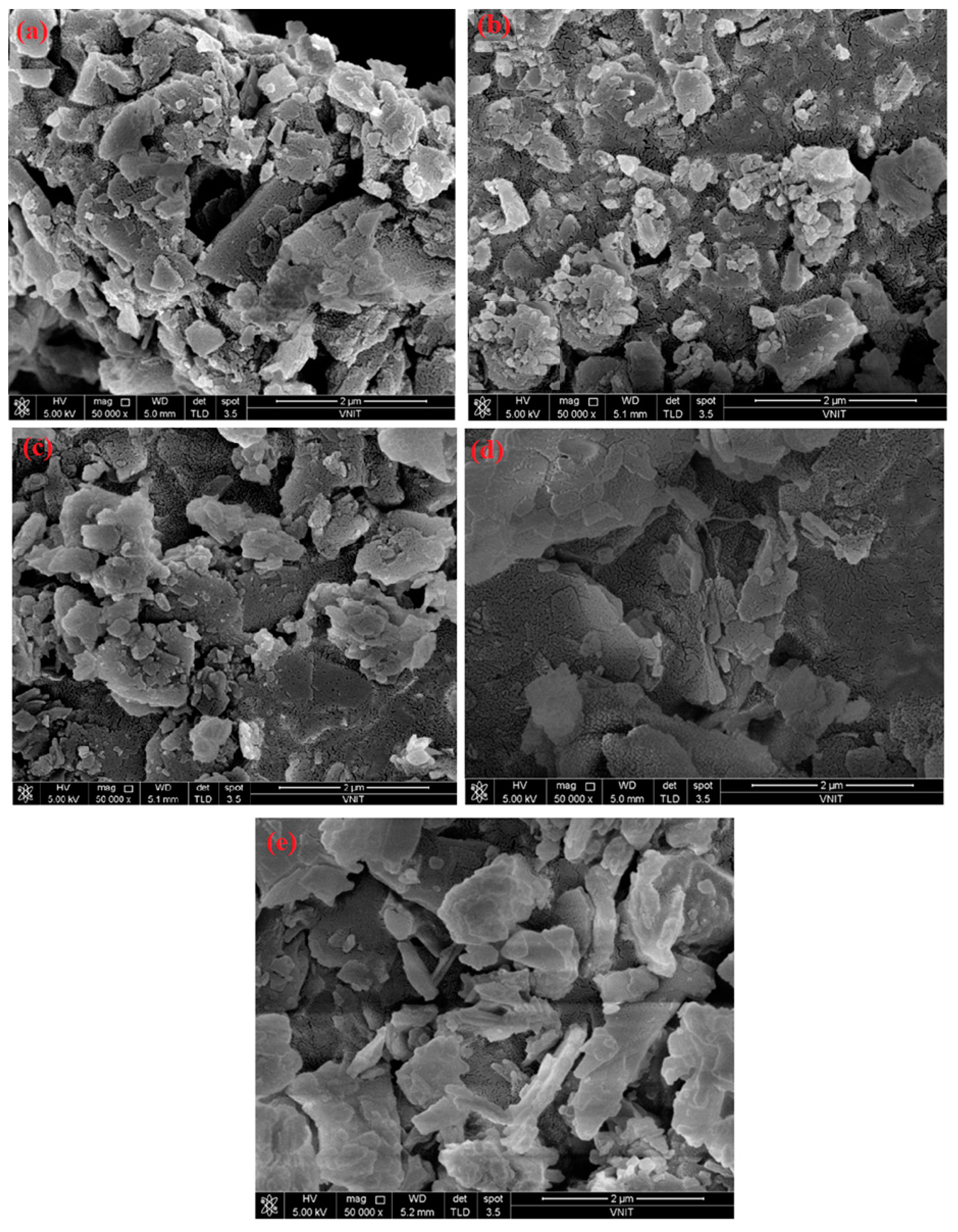
2.1. Morphological Studies via XRD and SEM Analysis
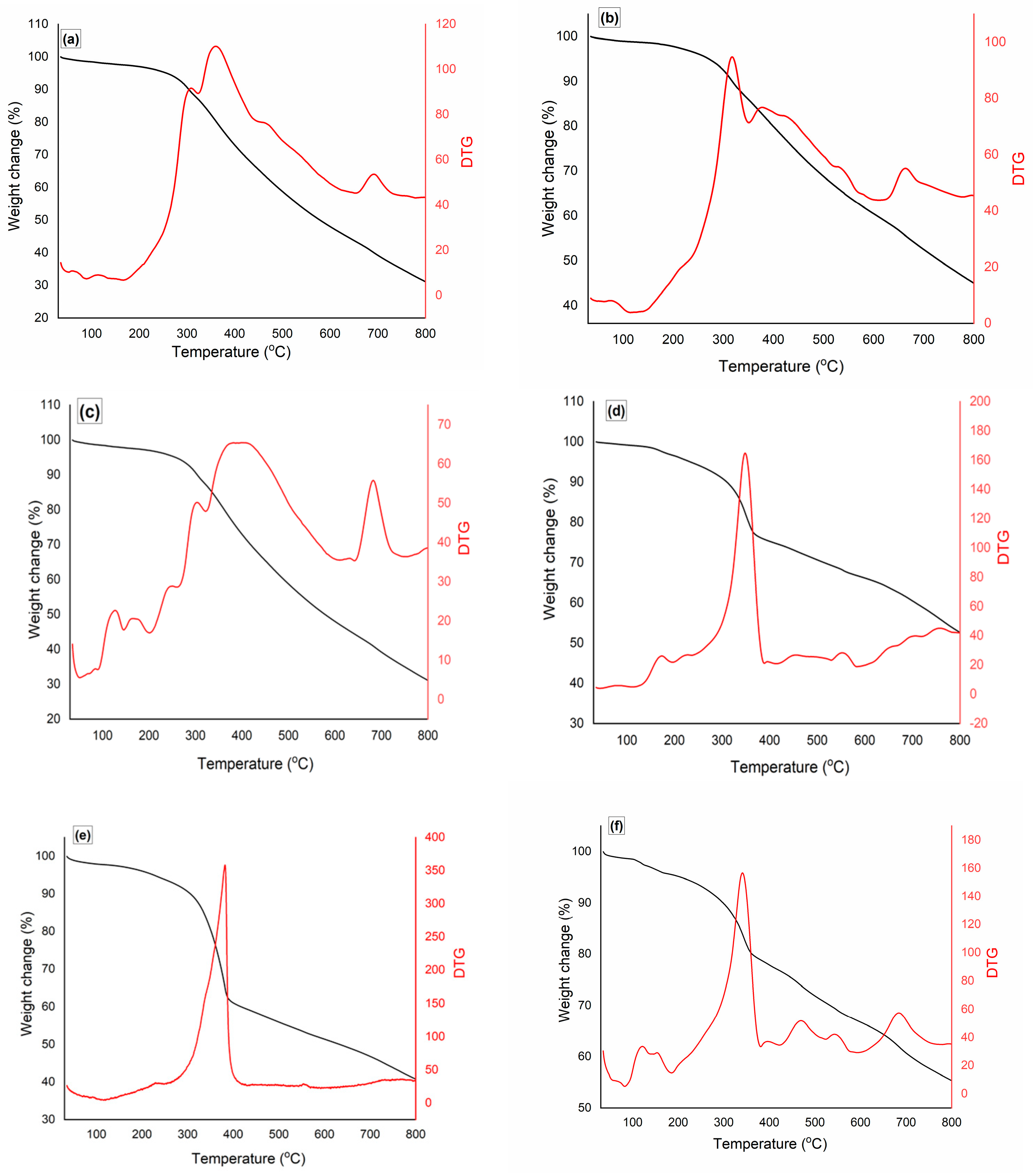
2.2. Thermogravimetric Analysis
2.3. Fluorescence Studies
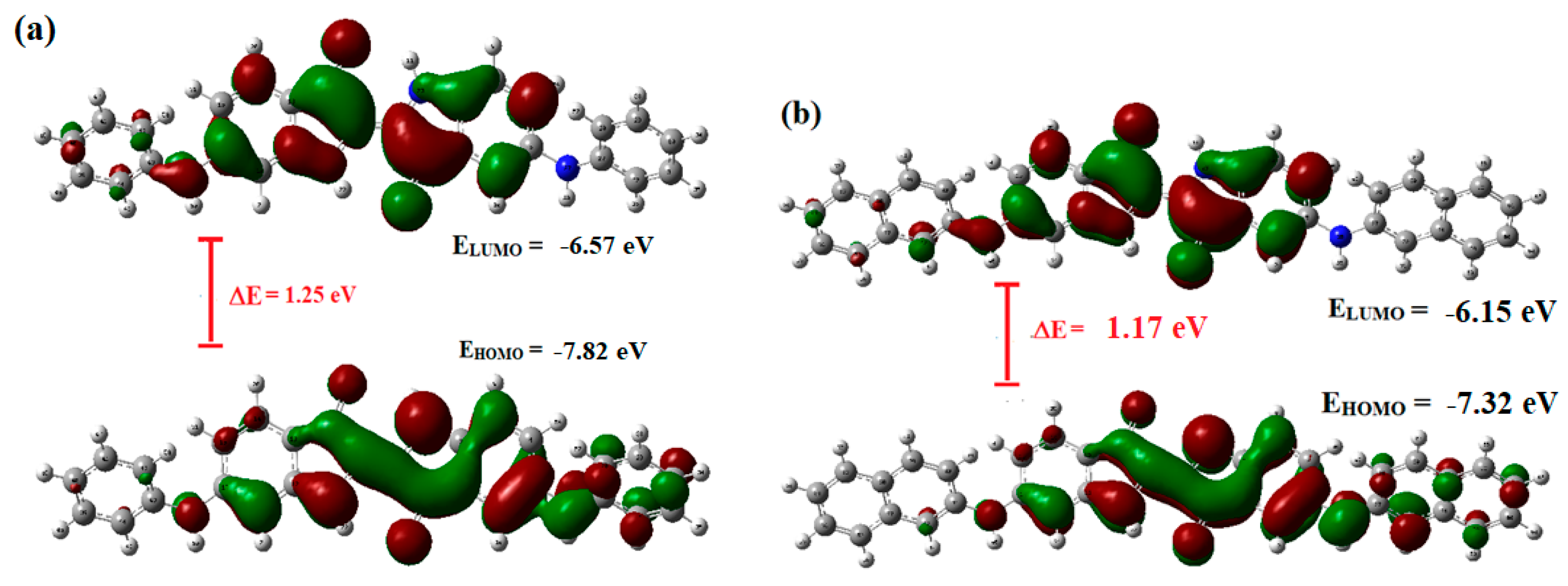
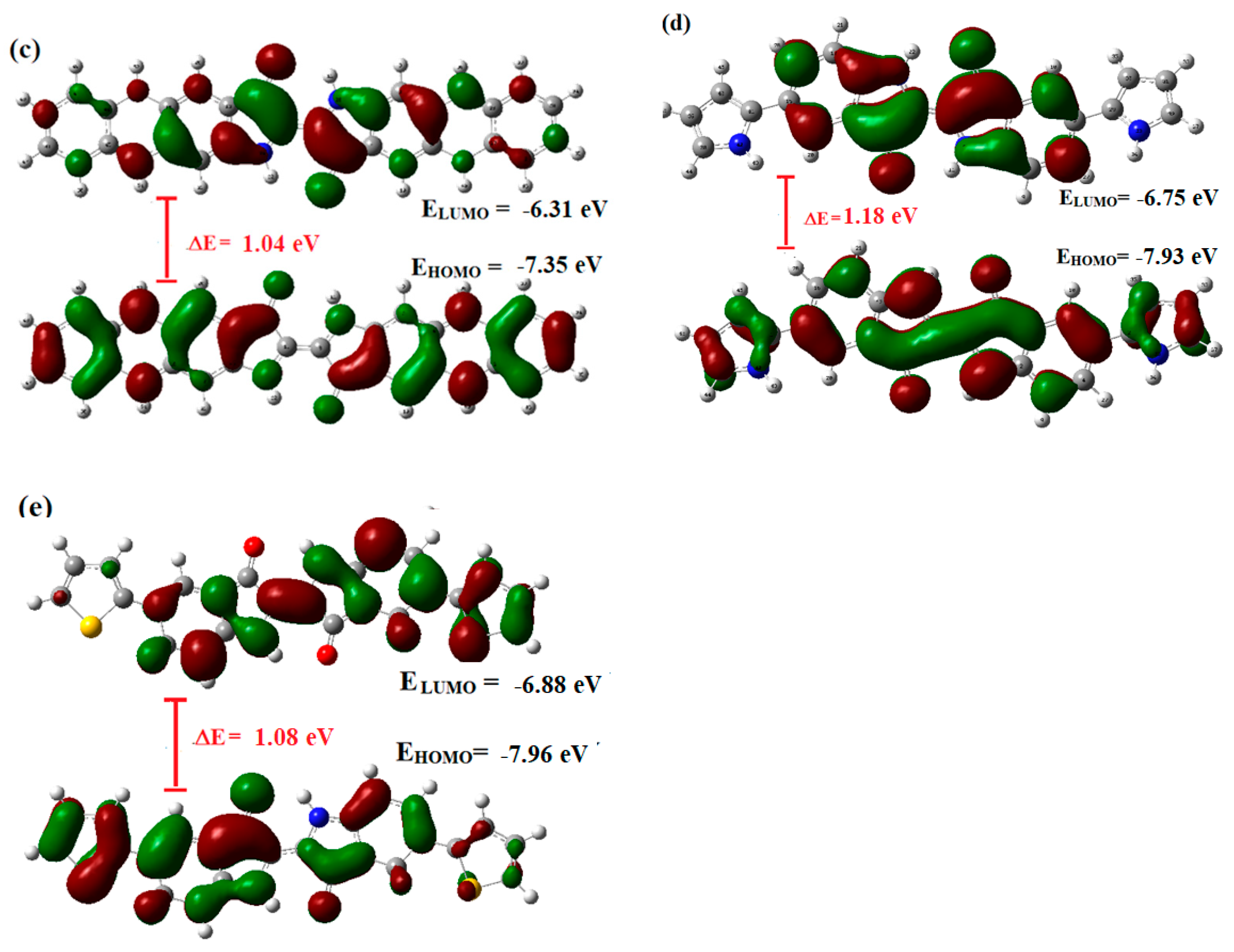
2.4. Comaprison of Experimental Data with Computational Studies to Confirm the Proposed Structures of Indigo-Dye-Modified Oligomers Using DFT: Optimized Geometries and Frontier Molecular Orbitals
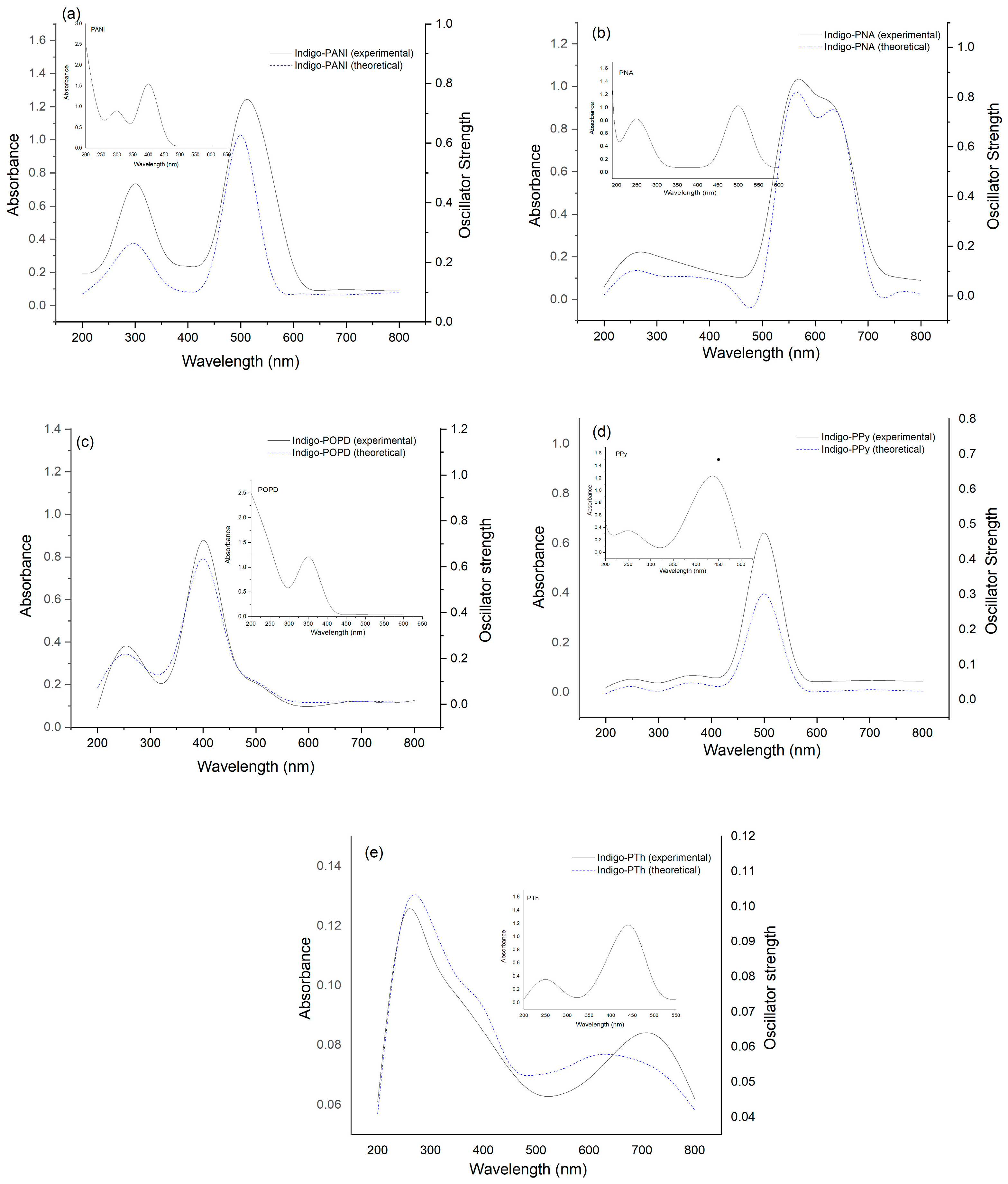
2.5. Comparison of Expermental and Theoretical UV–Visible Spectra
2.6. Comparison of Experimental and Computational Vibrational Spectra
3. Conclusions
4. Experimental Section
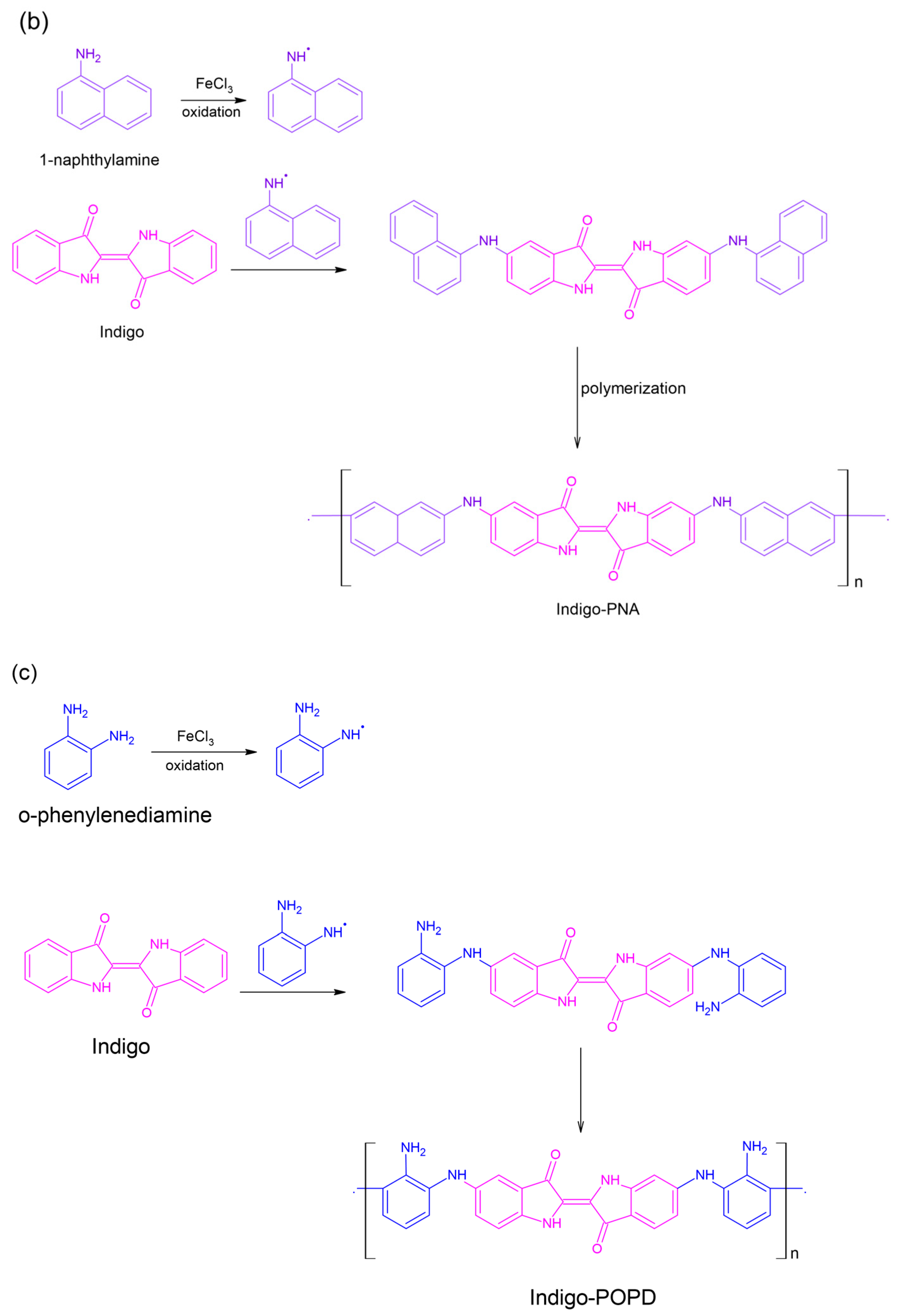
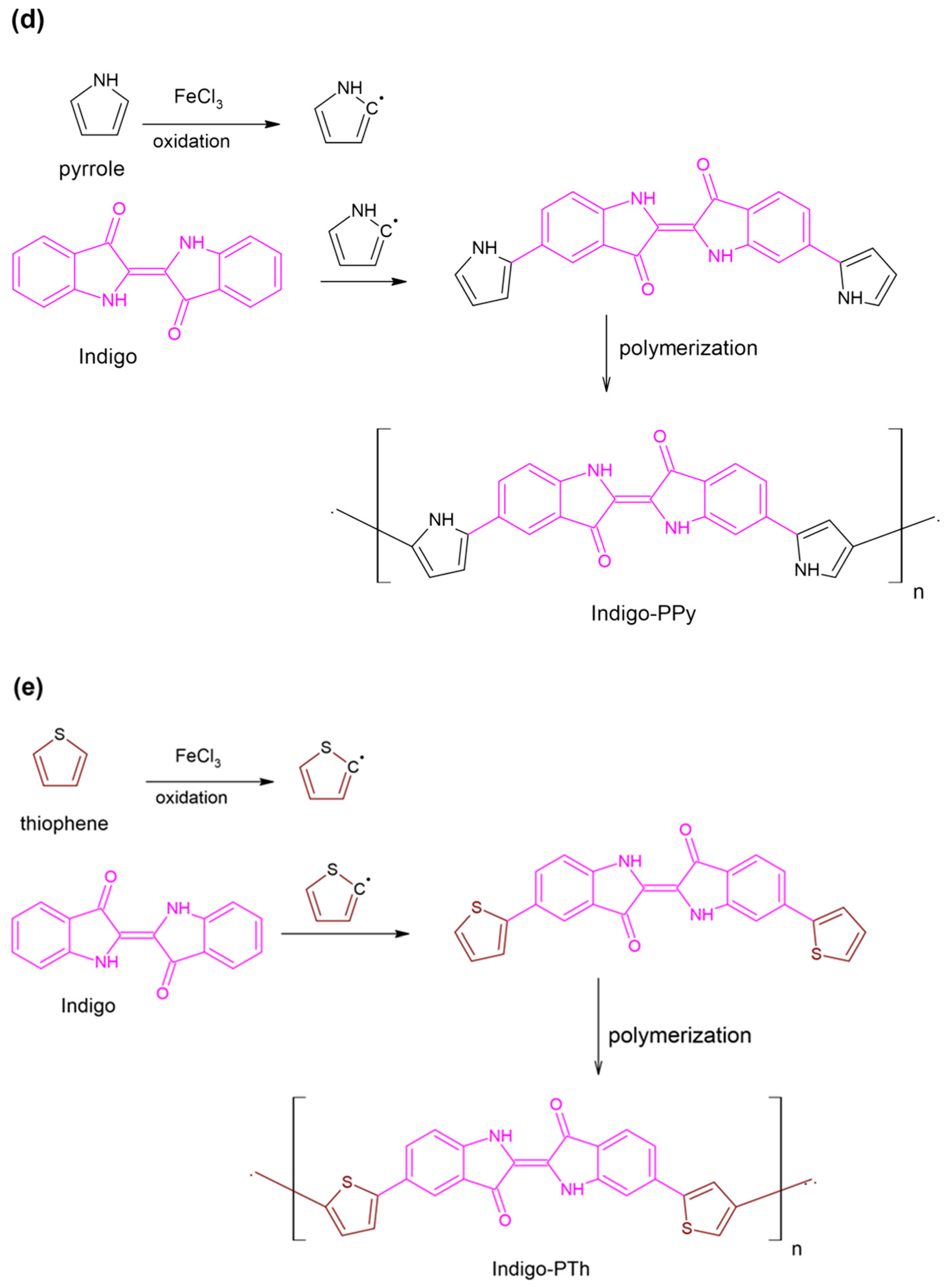
4.1. Synthesis of Indigo-Dye-Incorporated Polymers
4.2. Characterization
4.2.1. Spectral Analysis
4.2.2. Morphological Analysis
4.2.3. Thermal Analysis
4.2.4. DFT and TD-DFT Calculations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beygisangchin, M.; Rahid, S.A.; Shafie, S.; Sadrolhosseini, A.R.; Lim, H.N. Preparations, Properties, and Applications of Polyaniline and Polyaniline Thin Films—A Review. Polymers 2021, 13, 2003. [Google Scholar] [CrossRef] [PubMed]
- Bekkar, F.; Bettahar, F.; Moreno, I.; Meghabar, R.; Hamadouche, M.; Hernáez, E.; Vilela, J.L.V.; Rubio, L.R. Polycarbazole and Its Derivatives: Synthesis and Applications. A Review of the Last 10 Years. Polymers 2020, 12, 2227. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.K. A Review on Self-Assembly Driven Optoelectronic Properties of Polythiophene-Peptide and Polythiophene-Polymer Conjugates. Langmuir 2024, 40, 9385–9405. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Kar, A.K. Morphological and Optical Properties of Polypyrrole Nanoparticles Synthesized by Variation of Monomer to Oxidant Ratio. Mater. Today Proceed. 2019, 18, 1072–1076. [Google Scholar] [CrossRef]
- Chung, C.Y.; Wen, T.C.; Gopalan, A. In situ UV-Vis spectroelectrochemical studies to identify electrochromic sites in poly (1-naphthylamine) modified by diphenylamine. Spectrochim. Acta Part A: Mol. Biomol. Spectroscopy 2004, 60, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Zoromba, M.S.; Alshehri, A.A.; Al-Hossainy, A.F.; Abdel-Aziz, M.H. Doped-poly (anthranilic acid-co-o-phenylene diamine) thin film for optoelectronic applications. Opt. Mater. 2021, 111, 110621. [Google Scholar] [CrossRef]
- Garg, S.; Goel, N. Optoelectronic applications of conjugated organic polymers: Influence of donor/acceptor groups through density functional studies. J. Phys. Chem. C 2022, 126, 9313–9323. [Google Scholar] [CrossRef]
- Abel, S.B.; Yslas, E.I.; Rivarola, C.R.; Barbero, C.A. Synthesis of polyaniline (PANI) and functionalized polyaniline (F-PANI) nanoparticles with controlled size by solvent displacement method. Application in fluorescence detection and bacteria killing by photothermal effect. Nanotechnol. 2018, 29, 125604. [Google Scholar] [CrossRef]
- Saha, S.; Baker, G.L. Surface-tethered conjugated polymers created via the grafting-from approach. J. Appl. Polym. Sci. 2015, 132, 41363–41369. [Google Scholar] [CrossRef]
- Kotal, M.; Thakur, A.K.; Bhowmick, A.K. Polyaniline–carbon nanofiber composite by a chemical grafting approach and its supercapacitor application. ACS Appl. Mater. Interfaces 2013, 5, 8374–8386. [Google Scholar] [CrossRef]
- McCullough, L.A.; Matyjaszewski, K. Conjugated conducting polymers as components in block copolymer systems. Mol. Cryst. Liq. Cryst. 2010, 521, 1–55. [Google Scholar] [CrossRef]
- Riaz, U.; Ashraf, S.M.; Fatima, T.; Jadoun, S. Tuning the spectral, morphological and photophysical properties of sonochemically synthesized poly (carbazole) using acid Orange, fluorescein and rhodamine 6G. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2017, 173, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Ali, R.; Ashraf, S.M.; Rub, A.; Riaz, U. Experimental and computational studies of novel Sudan-I dye modified conjugated oligomers: Efficient 1O2 generation and antileishmanial characteristics. Mater. Sci. Eng. B 2021, 265, 114993. [Google Scholar] [CrossRef]
- Singh, N.; Arish, M.; Kumar, P.; Rub, A.; Riaz, U. Experimental and theoretical studies of novel azo benzene functionalized conjugated polymers: In-vitro antileishmanial activity and bioimaging. Sci. Rep. 2020, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Aazam, E.S.; Riaz, U. Experimental and Computational Studies of Azo Dye-Modified Luminol Oligomers: Potential Application in Lithium Ion Sensing. ACS Omega 2021, 6, 27833–27841. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, H.; Nakajima, H.; Kojima, T.; Yamamoto, T. Preparation and chemical properties of π-conjugated polymers containing indigo unit in the main chain. Materials 2014, 7, 2030–2043. [Google Scholar] [CrossRef]
- Voss, G.; Drechsler, M.; Eller, S.; Gradzielski, M.; Gunzelmann, D.; Mondal, S.; van Smaalen, S.; Voertler, C.S. Polymeric colorants: Statistical copolymers of indigo building blocks with defined structures. Helv. Chim. Acta 2019, 92, 2675–2697. [Google Scholar] [CrossRef]
- Fallon, K.J.; Bronstein, H. Indolonaphthyridine: A versatile chromophore for organic electronics inspired by natural indigo dye. Acc. Chem. Res. 2021, 54, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Cao, Y.; Fan, Y.; Liu, C.J.; Yuan, S.C.; Pei, J. High-performance air-stable organic field-effect transistors: Isoindigo-based conjugated polymers. J. Am. Chem. Soc. 2011, 133, 6099–6101. [Google Scholar] [CrossRef]
- Guo, C.; Sun, B.; Quinn, J.; Yan, Z.; Li, Y. Synthesis and properties of indigo based donor–acceptor conjugated polymers. J. Mater. Chem. C 2014, 2, 4289–4296. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Scherer, N.F. Ultrafast dynamics of polarons in conductive polyaniline: Comparison of primary and secondary doped forms. J. Phys. Chem. B. 2008, 112, 15576–15587. [Google Scholar] [CrossRef] [PubMed]




| Polymers | Peak Position (nm) | Area | Quantum Yield |
|---|---|---|---|
| Indigo-PANI | 500 | 8.8 × 104 | 3.2 × 10−4 |
| Indigo-PNA | 510 | 2.2 × 106 | 6.2 × 10−3 |
| Indigo-POPD | 550 | 4.6 × 106 | 5.3 × 10−3 |
| Indigo-PPy | 480 | 1.8 × 105 | 4.0 × 10−4 |
| Indigo-PTh | 650 | 8.0 × 104 | 1.6 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Douglas, T.; Singh, N.; Riaz, U. Experimental and Theoretical Studies on Indigo-Dye-Modified Conjugated Polymers. Molecules 2024, 29, 3200. https://doi.org/10.3390/molecules29133200
Douglas T, Singh N, Riaz U. Experimental and Theoretical Studies on Indigo-Dye-Modified Conjugated Polymers. Molecules. 2024; 29(13):3200. https://doi.org/10.3390/molecules29133200
Chicago/Turabian StyleDouglas, Tionna, Neetika Singh, and Ufana Riaz. 2024. "Experimental and Theoretical Studies on Indigo-Dye-Modified Conjugated Polymers" Molecules 29, no. 13: 3200. https://doi.org/10.3390/molecules29133200




