Bismuth(III)-Catalyzed Regioselective Selenation of Indoles with Diaryl Diselenides: Synthesis of 3-Selanylindoles
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. General Information
4.2. General Procedure for the Synthesis of Calcogenated Indoles
4.3. Characterization Data of Novel Compounds
4.3.1. 3-(4-Trifluoromethylphenyl)selanyl-1-methyl-1H-indole (3ae)
4.3.2. 1-Methyl-3-(2-methylphenyl)selanyl-1H-indole (3af)
4.3.3. 3-(2-Benzothienyl)selanyl-1-methyl-1H-indole (3ai)
4.3.4. 1,5-Dimethyl-3-phenylselanyl-1H-indole (3ca)
4.3.5. 5-Chloro-1-methyl-3-phenylselanyl-1H-indole (3da)
4.3.6. 1-Methyl-3-phenylselanyl-1H-indole-5-carbonitrile (3fa)
4.3.7. 1,4-Dimethyl-3-phenylselanyl-1H-indole (3ga)
4.3.8. 1,6-Dimethyl-3-phenylselanyl-1H-indole (3ha)
4.3.9. 1,7-Dimethyl-3-phenylselanyl-1H-indole (3ia)
4.4. Single-Crystal X-ray Diffraction Experiment of 3aa
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gandeepan, P.; Müller, T.; Zell, D.; Cera, G.; Warratz, S.; Ackermann, L. 3d Transition Metals for C–H Activation. Chem. Rev. 2019, 119, 2192–2452. [Google Scholar] [CrossRef] [PubMed]
- Rampon, D.S.; Luz, E.Q.; Lima, D.B.; Balaguez, R.A.; Schneider, P.H.; Alves, D. Transition Metal Catalysed Direct Selanylation of Arenes and Heteroarenes. Dalton Trans. 2019, 48, 9851–9905. [Google Scholar] [CrossRef]
- Hellwig, P.S.; Peglow, T.J.; Penteado, F.; Bagnoli, L.; Perin, G.; Lenardão, E.J. Recent Advances in the Synthesis of Selenophenes and Their Derivatives. Molecules 2020, 25, 5907. [Google Scholar] [CrossRef] [PubMed]
- Jose, D.E.; Kanchana, U.S.; Mathew, T.V.; Anilkumar, G. Recent Developments and Perspectives in the C-Se Cross Coupling Reactions. Curr. Org. Chem. 2020, 24, 1230–1262. [Google Scholar] [CrossRef]
- Sonawane, A.D.; Sonawane, R.A.; Ninomiya, M.; Koketsu, M. Diorganyl Diselenides: A Powerful Tool for the Construction of Selenium Containing Scaffolds. Dalton Trans. 2021, 50, 12764–12790. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Li, Z.; Bi, L.; Fan, L.; Zhang, P. Recent Advances in Organic Synthesis Applying Elemental Selenium. Tetrahedron 2022, 112, 132752. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Ananikov, V.P. Transition-Metal-Catalyzed C–S, C–Se, and C–Te Bond Formations via Cross-Coupling and Atom-Economic Addition Reactions. Achievements and Challenges. Chem. Rev. 2022, 122, 16110–16293. [Google Scholar] [CrossRef] [PubMed]
- Ranu, B.C.; Adak, L.; Mukherjee, N.; Ghosh, T. Benign-Metal-Catalyzed Carbon–Carbon and Carbon–Heteroatom Bond Formation. Synlett 2023, 34, 601–621. [Google Scholar] [CrossRef]
- Mugesh, G.; du Mont, W.-W.; Sies, H. Chemistry of Biologically Important Synthetic Organoselenium Compounds. Chem. Rev. 2001, 101, 2125–2179. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and Organotellurium Compounds: Toxicology and Pharmacology. Chem. Rev. 2004, 104, 6255–6285. [Google Scholar] [CrossRef]
- Sarma, B.K.; Mugesh, G. Thiol Cofactors for Selenoenzymes and Their Synthetic Mimics. Org. Biomol. Chem. 2008, 6, 965–974. [Google Scholar] [CrossRef]
- Nogueira, C.W.; Rocha, J.B.T. Toxicology and Pharmacology of Selenium: Emphasis on Synthetic Organoselenium Compounds. Arch. Toxicol. 2011, 85, 1313–1359. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Pérez, M.; Ali, W.; Marć, M.A.; Handzlik, J.; Domínguez-Álvarez, E. Selenides and Diselenides: A Review of Their Anticancer and Chemopreventive Activity. Molecules 2018, 23, 628. [Google Scholar] [CrossRef] [PubMed]
- Chuai, H.; Zhang, S.-Q.; Bai, H.; Li, J.; Wang, Y.; Sun, J.; Wen, E.; Zhang, J.; Xin, M. Small Molecule Selenium-Containing Compounds: Recent Development and Therapeutic Applications. Eur. J. Med. Chem. 2021, 223, 113621. [Google Scholar] [CrossRef]
- Guan, Q.; Han, C.; Zuo, D.; Zhai, M.; Li, Z.; Zhang, Q.; Zhai, Y.; Jiang, X.; Bao, K.; Wu, Y.; et al. Synthesis and Evaluation of Benzimidazole Carbamates Bearing Indole Moieties for Antiproliferative and Antitubulin Activities. Eur. J. Med. Chem. 2014, 87, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Xu, J.; Wang, Z.; Qi, H.; Xu, Q.; Bai, Z.; Zhang, Q.; Bao, K.; Wu, Y.; Zhang, W. 3-(3,4,5-Trimethoxyphenylselenyl)-1H-indoles and Their Selenoxides as Combretastatin A-4 Analogs: Microwave-Assisted Synthesis and Biological Evaluation. Eur. J. Med. Chem. 2015, 90, 184–194. [Google Scholar] [CrossRef]
- Wen, Z.; Li, X.; Zuo, D.; Lang, B.; Wu, Y.; Jiang, M.; Ma, H.; Bao, K.; Wu, Y.; Zhang, W. Ultrasound-Promoted Two-Step Synthesis of 3-Arylselenylindoles and 3-Arylthioindoles as Novel Combretastatin A-4 Analogues. Sci. Rep. 2016, 6, 23986. [Google Scholar] [CrossRef] [PubMed]
- Casaril, A.M.; Ignasiak, M.T.; Chuang, C.Y.; Vieira, B.; Padilha, N.B.; Carroll, L.; Lenardão, E.J.; Savegnago, L.; Davies, M.J. Selenium-Containing Indolyl Compounds: Kinetics of Reaction with Inflammation-Associated Oxidants and Protective Effect against Oxidation of Extracellular Matrix Proteins. Free Radic. Biol. Med. 2017, 113, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Vieira, B.M.; Thurow, S.; da Costa, M.; Casaril, A.M.; Domingues, M.; Schumacher, R.F.; Perin, G.; Alves, D.; Savegnago, L.; Lenardão, E.J. Ultrasound-Assisted Synthesis and Antioxidant Activity of 3-Selanyl-1H-indole and 3-Selanylimidazo [1,2-a]pyridine Derivatives. Asian J. Org. Chem. 2017, 6, 1635–1646. [Google Scholar] [CrossRef]
- Pedroso, G.J.; Costa, D.M.S.; Felipe Kokuszi, L.T.; da Silva, E.B.V.; Cavalcante, M.F.O.; Junca, E.; Moraes, C.A.O.; Pich, C.T.; de Lima, V.R.; Saba, S.; et al. Selenylated Indoles: Synthesis, Effects on Lipid Membrane Properties and DNA Cleavage. New J. Chem. 2023, 47, 2719–2726. [Google Scholar] [CrossRef]
- Silveira, C.C.; Mendes, S.R.; Wolf, L.; Martins, G.M.; von Mühlen, L. Efficient Synthesis of 3-Selanyl- and 3-Sulfanylindoles Employing Trichloroisocyanuric Acid and Dichalcogenides. Tetrahedron 2012, 68, 10464–10469. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Yan, J. Selective Synthesis of 3-Selanylindoles from Indoles and Diselenides Using IK/mCPBA System. Appl. Organomet. Chem. 2017, 31, e3864. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Zhang, Y.-Q.; Zhou, C.-F.; Jiang, Y.-Q.; Xu, Y.; Zeng, X.; Liu, G.-Q. Iodine Pentoxide-Mediated Oxidative Selenation and Seleno/Thiocyanation of Electron-Rich Arenes. Org. Biomol. Chem. 2022, 20, 5463–5469. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.L.; Azeredo, J.B.; Fiorentin, B.L.; Braga, A.L. Synthesis of 3-Selenylindoles under Ecofriendly Conditions. Eur. J. Org. Chem. 2015, 2015, 5070–5074. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, Y.; Song, Z.; Liang, G. An Efficient t-BuOK Promoted C3-Chalcogenylation of Indoles with Dichalcogenides. Org. Biomol. Chem. 2018, 16, 4958–4962. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yi, R.; Zeng, C.; Cui, Y.; Xu, X.; Wang, X.-Q.; Li, N. CsOH-Promoted Regiospecific Sulfenylation, Selenylation, and Telluration of Indoles in H2O. Synlett 2023, 34, 124–132. [Google Scholar]
- Zhang, Q.-B.; Ban, Y.-L.; Yuan, P.-F.; Peng, S.-J.; Fang, J.-G.; Wu, L.-Z.; Liu, Q. Visible-Light-Mediated Aerobic Selenation of (Hetero)Arenes with Diselenides. Green Chem. 2017, 19, 5559–5563. [Google Scholar] [CrossRef]
- Kumaraswamy, G.; Ramesh, V.; Gangadhar, M.; Vijaykumar, S. Catalyst and Sensitizer-Free Visible-Light-Induced C(sp2)−H Chalcogenation of Arenes/Heteroarenes with Dichalcogenides. Asian J. Org. Chem. 2018, 7, 1689–1697. [Google Scholar] [CrossRef]
- Saba, S.; Rafique, J.; Franco, M.S.; Schneider, A.R.; Espíndola, L.; Silva, D.O.; Braga, A.L. Rose Bengal Catalysed Photo-Induced Selenylation of Indoles, Imidazoles and Arenes: A Metal Free Approach. Org. Biomol. Chem. 2018, 16, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Rathore, V.; Kumar, S. Visible-Light-Induced Metal and Reagent-Free Oxidative Coupling of sp2 C–H Bonds with Organo-Dichalcogenides: Synthesis of 3-Organochalcogenyl Indoles. Green Chem. 2019, 21, 2670–2676. [Google Scholar] [CrossRef]
- Lemir, I.D.; Castro-Godoy, W.D.; Heredia, A.A.; Schmidt, L.C.; Argüello, J.E. Metal- and Photocatalyst-Free Synthesis of 3-Selenylindoles and Asymmetric Diarylselenides Promoted by Visible Light. RSC Adv. 2019, 9, 22685–22694. [Google Scholar] [CrossRef] [PubMed]
- Heredia, A.A.; Soria-Castro, S.M.; Castro-Godoy, W.D.; Lemir, I.D.; López-Vidal, M.; Bisogno, F.R.; Argüello, J.E.; Oksdath-Mansilla, G. Multistep Synthesis of Organic Selenides under Visible Light Irradiation: A Continuous-Flow Approach. Org. Process Res. Dev. 2020, 24, 540–545. [Google Scholar] [CrossRef]
- Huang, Q.; Peng, X.; Li, H.; He, H.; Liu, L. Visible-Light-Induced, Graphene Oxide-Promoted C3-Chalcogenylation of Indoles Strategy under Transition-Metal-Free Conditions. Molecules 2022, 27, 772. [Google Scholar] [CrossRef] [PubMed]
- Quadros, G.T.; de Medeiros, S.P.; de Oliveira, C.A.; Rambo, M.W.; Abenante, L.; Lenardão, E.J.; Penteado, F. Benzeneseleninic Acids (BSA) and Photocatalysis: An Alternative Duo for the Synthesis of 3-Selanylindoles. Asian J. Org. Chem. 2023, 12, e202300517. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Jiang, H.; Sun, L. Convenient Synthesis of Selenyl-Indoles via Iodide Ion-Catalyzed Electrochemical C–H Selenation. Chem. Commun. 2018, 54, 8781–8784. [Google Scholar] [CrossRef] [PubMed]
- Meirinho, A.G.; Pereira, V.F.; Martins, G.M.; Saba, S.; Rafique, J.; Braga, A.L.; Mendes, S.R. Electrochemical Oxidative C(sp2)–H Bond Selenylation of Activated Arenes. Eur. J. Org. Chem. 2019, 2019, 6465–6469. [Google Scholar] [CrossRef]
- Fang, X.-L.; Tang, R.-Y.; Zhong, P.; Li, J.-H. Iron-Catalyzed Sulfenylation of Indoles with Disulfides Promoted by a Catalytic Amount of Iodine. Synthesis 2009, 24, 4183–4189. [Google Scholar]
- Vieira, B.M.; Thurow, S.; Brito, J.S.; Perin, G.; Alves, D.; Jacob, R.G.; Santi, C.; Lenardão, E.J. Sonochemistry: An Efficient Alternative to the Synthesis of 3-Selanylindoles Using CuI as Catalyst. Ultrason. Sonochem. 2015, 27, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Céspedes, S.; Ferry, A.; Candish, L.; Glorius, F. Heterogeneously Catalyzed Direct C-H Thiolation of Heteroarenes. Angew. Chem. Int. Ed. 2015, 54, 5772–5776. [Google Scholar] [CrossRef] [PubMed]
- Luz, E.Q.; Seckler, D.; Araújo, J.S.; Angst, L.; Lima, D.B.; Rios, E.A.M.; Ribeiro, R.R.; Rampon, D.S. Fe(III)-Catalyzed Direct C3 Chalcogenylation of Indole: The Effect of Iodide Ions. Tetrahedron 2019, 75, 1258–1266. [Google Scholar] [CrossRef]
- Rios, E.A.M.; Gomes, C.M.B.; Silvério, G.L.; Luz, E.Q.; Ali, S.; D’Oca, C.d.R.M.; Albach, B.; Campos, R.B.; Rampon, D.S. Silver-Catalyzed Direct Selanylation of Indoles: Synthesis and Mechanistic Insights. RSC Adv. 2023, 13, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Benchawan, T.; Maneewong, J.; Saeeng, R. Selective Synthesis of 3-Chalcogenylindoles via Silver-Catalyzed Direct Chalcogenation of Indoles with Dichalcogenides. ChemistrySelect 2023, 8, e202301988. [Google Scholar] [CrossRef]
- Azeredo, J.B.; Godoi, M.; Martins, G.M.; Silveira, C.C.; Braga, A.L. A Solvent- and Metal-Free Synthesis of 3-Chacogenyl-Indoles Employing DMSO/I2 as an Eco-Friendly Catalytic Oxidation System. J. Org. Chem. 2014, 79, 4125–4130. [Google Scholar] [CrossRef] [PubMed]
- Rafique, J.; Saba, S.; Franco, M.S.; Bettanin, L.; Schneider, A.R.; Silva, L.T.; Braga, A.L. Direct, Metal-free C(sp2)−H Chalcogenation of Indoles and Imidazopyridines with Dichalcogenides Catalysed by KIO3. Chem. Eur. J. 2018, 24, 4173–4180. [Google Scholar] [CrossRef] [PubMed]
- Menezes, J.R.; Gularte, M.M.; dos Santos, F.C.; Roehrs, J.A.; Azeredo, J.B. Synthesis of 3-Chalcogenyl-Indoles Mediated by the Safer Reagent Urea-Hydrogen Peroxide (UHP). Tetrahedron Lett. 2023, 120, 154446. [Google Scholar] [CrossRef]
- Bhunia, S.K.; Das, P.; Jana, R. Atom-Economical Selenation of Electron-Rich Arenes and Phosphonates with Molecular Oxygen at Room Temperature. Org. Biomol. Chem. 2018, 16, 9243–9250. [Google Scholar] [CrossRef] [PubMed]
- Leonard, N.M.; Wieland, L.C.; Mohan, R.S. Applications of Bismuth(III) Compounds in Organic Synthesis. Tetrahedron 2002, 58, 8373–8397. [Google Scholar] [CrossRef]
- Gaspard-Iloughmane, H.; Le Roux, C. Bismuth(III) Triflate in Organic Synthesis. Eur. J. Org. Chem. 2004, 2004, 2517–2532. [Google Scholar] [CrossRef]
- Bothwell, J.M.; Krabbe, S.W.; Mohan, R.S. Applications of Bismuth(III) Compounds in Organic Synthesis. Chem. Soc. Rev. 2011, 40, 4649–4707. [Google Scholar] [CrossRef] [PubMed]
- Ondet, P.; Lemière, G.; Duñach, E. Cyclisations Catalysed by Bismuth(III) Triflate. Eur. J. Org. Chem. 2017, 2017, 761–780. [Google Scholar] [CrossRef]
- Raţ, C.I.; Soran, A.; Varga, R.A.; Silvestru, C. C–H Bond Activation Mediated by Inorganic and Organometallic Compounds of Main Group Metals. Adv. Organomet. Chem. 2018, 70, 233–311. [Google Scholar]
- Takasawa, R.; Jona, A.; Inoue, M.; Azuma, M.; Akahane, H.; Ueno, Y.; Nakagawa, Y.; Chimori, R.; Mano, Y.; Murata, Y.; et al. Triphenylbismuth Dichloride Inhibits Human Glyoxalase I and Induces Cytotoxicity in Cultured Cancer Cell Lines. J. Toxicol. Sci. 2022, 47, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Ohki, H.; Wada, M.; Akiba, K.Y. Bismuth Trichloride as a New Efficient Catalyst in the Aldol Reaction. Tetrahedron Lett. 1988, 29, 4719–4722. [Google Scholar] [CrossRef]
- Wada, M.; Takeichi, E.; Matsumoto, T. Bismuth Trichloride as a New Efficient Catalyst in the Aldol Reaction and the Michael Reaction. Bull. Chem. Soc. Jpn. 1991, 64, 990–994. [Google Scholar] [CrossRef]
- Ollevier, T.; Lavie-Compin, G. An Efficient Method for the Ring Opening of Epoxides with Aromatic Amines Catalyzed by Bismuth Trichloride. Tetrahedron Lett. 2002, 43, 7891–7893. [Google Scholar] [CrossRef]
- De, S.K.; Gibbs, R.A. Bismuth(III) Chloride-Catalyzed Direct Deoxygenative Allylation of Substituted Benzylic Alcohols with Allyltrimethylsilane. Tetrahedron Lett. 2005, 46, 8345–8350. [Google Scholar] [CrossRef]
- Sabitha, G.; Reddy, E.V.; Maruthi, C.; Yadav, J.S. Bismuth(III) Chloride-Catalyzed Intramolecular Hetero-Diels–Alder Reactions: A Novel Synthesis of Hexahydrodibenzo[b,h][1,6]Naphthyridines. Tetrahedron Lett. 2002, 43, 1573–1575. [Google Scholar] [CrossRef]
- Sabitha, G.; Reddy, E.V.; Yadav, J.S.; Rama Krishna, K.V.S.; Ravi Sankar, A. Stereoselective Synthesis of Octahydro-3bH-[1,3]dioxolo [4″,5″:4′,5′]furo [2′,3′:5,6]pyrano [4,3-b]quinolines via Intramolecular Hetero-Diels–Alder Reactions Catalyzed by Bismuth(III) Chloride. Tetrahedron Lett. 2002, 43, 4029–4032. [Google Scholar] [CrossRef]
- Li, Z.; Wei, C.; Chen, L.; Varma, R.S.; Li, C.-J. Three-Component Coupling of Aldehyde, Alkyne, and Amine Catalyzed by Silver in Ionic Liquid. Tetrahedron Lett. 2004, 45, 2443–2446. [Google Scholar] [CrossRef]
- Li, H.; Zeng, H.-Y.; Shao, H.-W. Bismuth(III) Chloride-Catalyzed One-Pot Mannich Reaction: Three-Component Synthesis of β-Amino Carbonyl Compounds. Tetrahedron Lett. 2009, 50, 6858–6860. [Google Scholar] [CrossRef]
- Wu, F.; Huang, W.; Yiliqi; Yang, J.; Gu, Y. Relay Catalysis of Bismuth Trichloride and Byproduct Hydrogen Bromide Enables the Synthesis of Carbazole and Benzo[α]carbazoles from Indoles and α-Bromoacetaldehyde Acetals. Adv. Synth. Catal. 2018, 360, 3318–3330. [Google Scholar] [CrossRef]
- Wu, Z.; Feng, X.-X.; Wang, Q.-D.; Yun, J.-J.; Rao, W.; Yang, J.-M.; Shen, Z.-L. Bismuth Trichloride-Catalyzed Oxy-Michael Addition of Water and Alcohol to α,β-Unsaturated Ketones. Chin. Chem. Lett. 2020, 31, 1297–1300. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Feng, B.; Liang, J.; You, G.; Liu, X.; Xian, L. Bi(III)-Catalyzed Aminooxygenation of Propargyl Amidines to Synthesize 2-Fluoroalkyl Imidazole-5-carbaldehydes and Their Decarbonylations. Chem. Commun. 2020, 56, 6400–6403. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.-T.; Zhao, C.; Wang, D.-R.; Wu, G.-C.; Chen, G.-S.; Chen, S.-J.; Ren, H.; Deng, D.-S.; Xu, Y.-B.; Hu, X.-W.; et al. BiCl3-Mediated Tandem Cyclization of Tryptamine-Derived Ynamide: Concise Synthesis of Pentacyclic Spiroindolines and Tricyclic Indole Derivatives. Adv. Synth. Catal. 2022, 364, 890–896. [Google Scholar] [CrossRef]
- Malik, P.; Joseph, D.; Chakraborty, D. BiCl3-catalyzed Carbon–Carbon Cross-Coupling of Organoboronic Acids with Aryl Iodides. Appl. Organometal. Chem. 2013, 27, 519–522. [Google Scholar] [CrossRef]
- Riyaz, M.A.B.; Swu, T. Bismuth-catalyzed N-Arylation of 2-Aminobenzimidazole and Phosphorylation of Substituted Coumarins via C-H Functionalization. ChemistrySelect 2022, 7, e202203281. [Google Scholar] [CrossRef]
- Zhang, J.Z. Interfacial Charge Carrier Dynamics of Colloidal Semiconductor Nanoparticles. J. Phys. Chem. B 2000, 104, 7239–7253. [Google Scholar] [CrossRef]
- Ünlü, F.; Deo, M.; Mathur, S.; Kirchartz, T.; Kulkarni, A. Bismuth-Based Halide Perovskite and Perovskite-Inspired Light Absorbing Materials for Photovoltaics. J. Phys. D Appl. Phys. 2022, 55, 113002. [Google Scholar] [CrossRef]
- Komatsu, N.; Uda, M.; Suzuki, H. Bismuth(III) Halides and Sulfate as Highly Efficient Catalyst for the Sulfenylation of Carbonyl and Related Compounds1. Synlett 1995, 9, 984–986. [Google Scholar] [CrossRef]
- Cunha, S.; Rodrigues, M.T., Jr. The First Bismuth(III)-Catalyzed Guanylation of Thioureas. Tetrahedron Lett. 2006, 47, 6955–6956. [Google Scholar] [CrossRef]
- Bailey, A.D.; Baru, A.R.; Tasche, K.K.; Mohan, R.S. Environmentally Friendly Organic Synthesis Using Bismuth Compounds: Bismuth(III) Iodide Catalyzed Deprotection of Acetals in Water. Tetrahedron Lett. 2008, 49, 691–694. [Google Scholar] [CrossRef]
- Adonin, S.A.; Peresypkina, E.V.; Sokolov, M.N.; Korolkov, I.V.; Fedin, V.P. Polyoxomolybdate-Supported Bismuth Trihalides [Mo8O26(BiX3)2]4– (X = Cl, Br, I): Syntheses and Study of Polymorphism. Inorg. Chem. 2014, 53, 6886–6892. [Google Scholar] [CrossRef] [PubMed]
- Wedal, J.C.; Ziller, J.W.; Evans, W.J. Expanding Bismuth Trihalide Coordination Chemistry with Trimethyltriazacyclohexane and Trimethyltriazacyclononane. Inorg. Chem. 2022, 61, 11766–11774. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Dong, Z.; Zhang, P.; Xing, W.; Li, L. Direct Selenation of Imidazoheterocycles and Indoles with Selenium Powder in a Copper-Catalyzed Three-Component One-Pot System. Tetrahedron Lett. 2018, 59, 2554–2558. [Google Scholar] [CrossRef]
- Lin, M.; Kang, L.; Gu, J.; Dai, L.; Tang, S.; Zhang, T.; Wang, Y.; Li, L.; Zheng, X.; Zhu, W.; et al. Heterogeneous Synergistic Catalysis by Ru-RuOx Nanoparticles for Se–Se Bond Activation. Nano Res. 2017, 10, 922–932. [Google Scholar] [CrossRef]
- Chen, J.; Hu, L.; Wang, H.; Tan, H. Iodine-Catalyzed Telluration of Indole Derivatives with Diarylditellurides for Synthesis of 3-Aryltellurylindoles. Chin. J. Org. Chem. 2019, 39, 2048–2052. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]


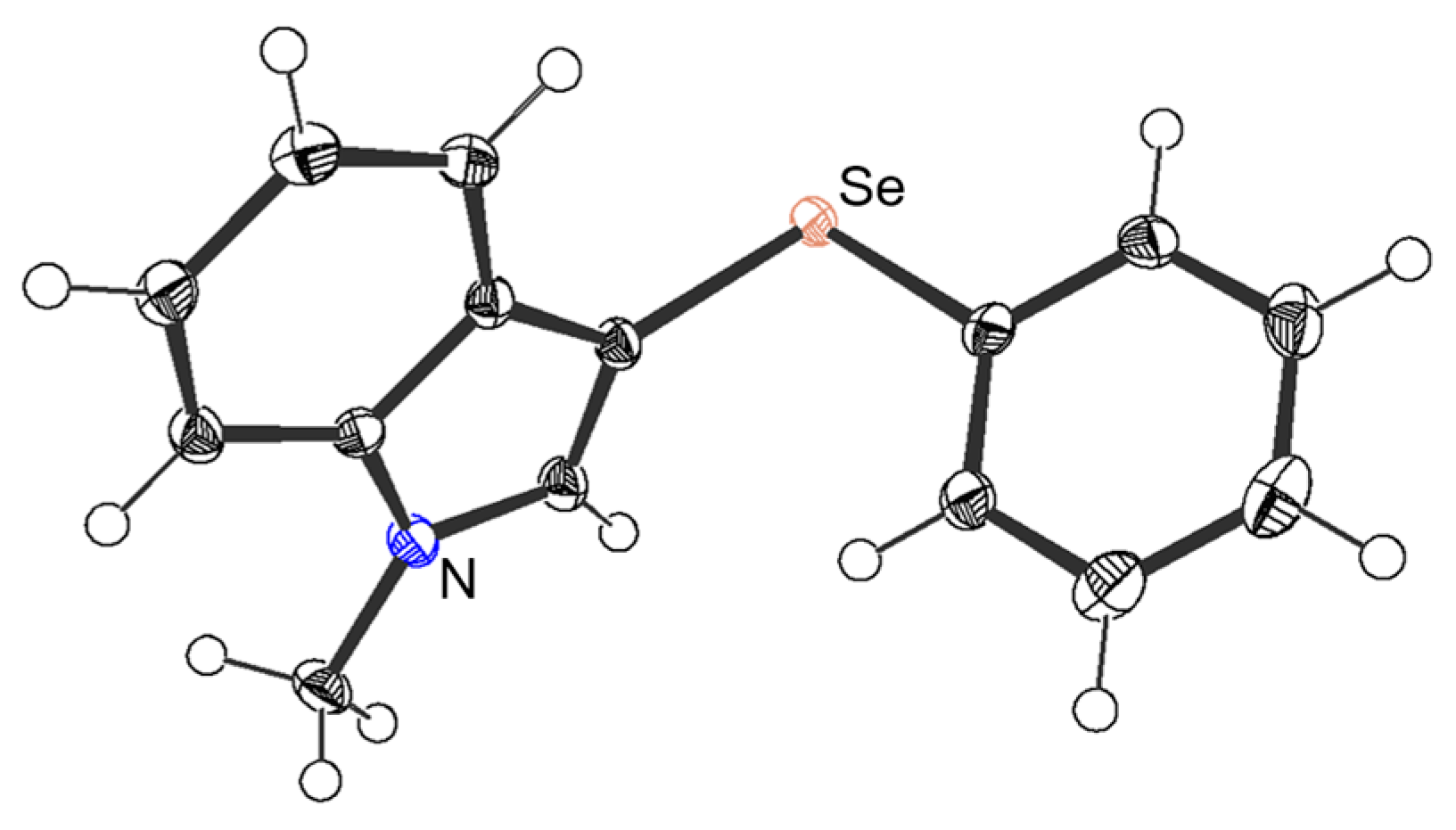

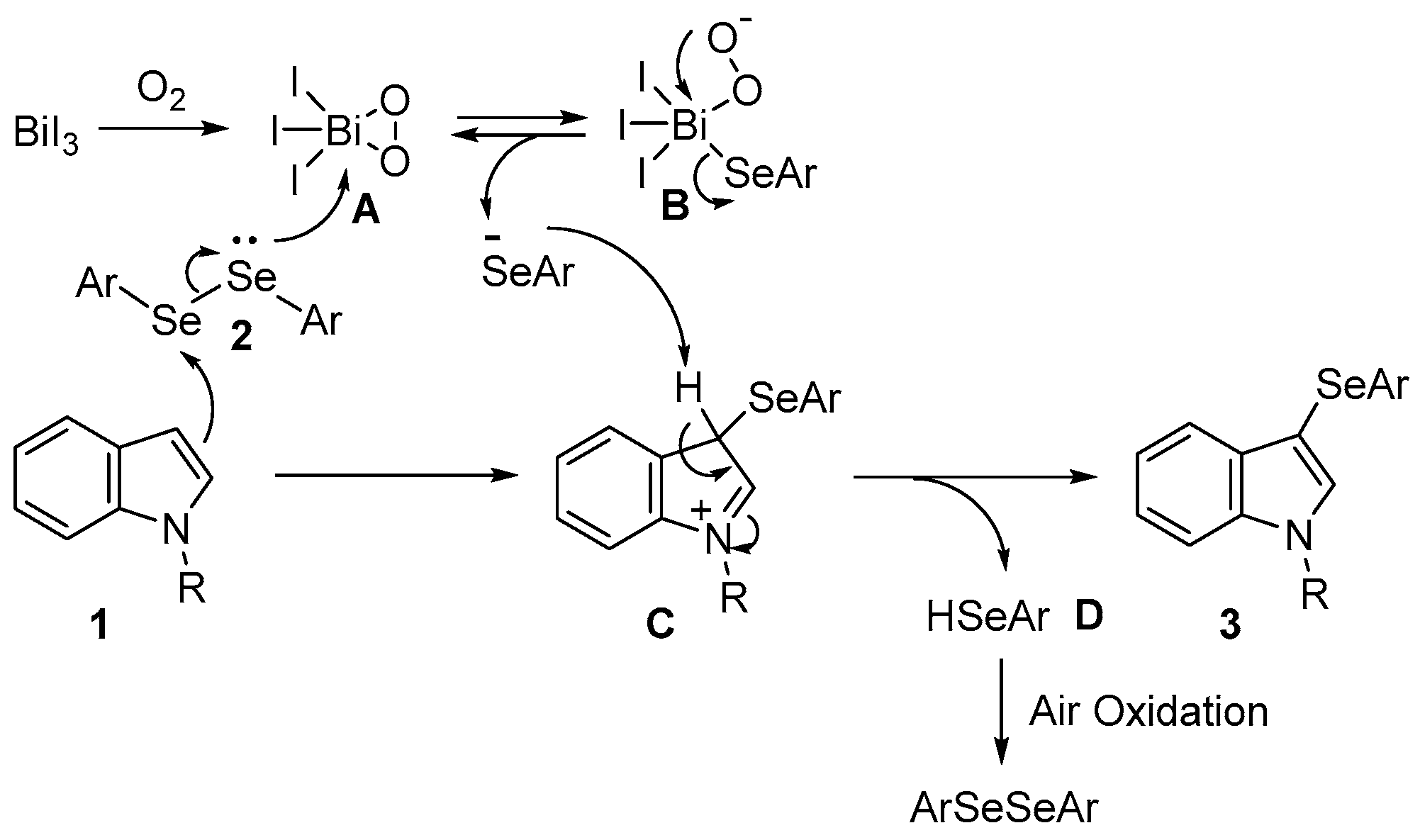
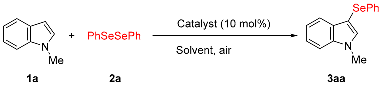 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Solvent | Temp. [°C] | Time [h] | Yield (%) [b] |
| 1 | BiCl3 | DMF | 100 | 24 | 85 |
| 2 | BiBr3 | DMF | 100 | 6 | 84 |
| 3 | BiI3 | DMF | 100 | 1 | 97 (91) [c] |
| 4 | BiF3 | DMF | 100 | 24 | 14 |
| 5 | Bi(OTf)3 | DMF | 100 | 24 | 77 |
| 6 | Bi(ONO2)3 | DMF | 100 | 24 | 49 |
| 7 | Ph3Bi | DMF | 100 | 24 | 2 |
| 8 | SbBr3 | DMF | 100 | 24 | 77 |
| 9 | SbI3 | DMF | 100 | 24 | 74 |
| 10 | AlCl3 | DMF | 100 | 24 | 11 |
| 11 | InCl3 | DMF | 100 | 24 | 25 |
| 12 | FeCl3 | DMF | 100 | 24 | 41 |
| 13 | I2 | DMF | 100 | 24 | 20 |
| 14 | BiI3 | DMSO | 100 | 2 | 89 |
| 15 | BiI3 | CH3CN | 80 | 24 | 45 |
| 16 | BiI3 | THF | 60 | 24 | 60 |
| 17 | BiI3 | MeOH | 60 | 24 | 51 |
| 18 | BiI3 | 1,2-DCE | 80 | 2 | 12 |
| 19 | BiI3 | Dioxane | 100 | 24 | 19 |
| 20 | BiI3 | Toluene | 100 | 24 | 12 |
| 21 | BiI3 | DMF | 60 | 8 | 89 |
| 22 [d] | BiI3 | DMF | 100 | 1 | 94 |
| 23 [e] | BiI3 | DMF | 100 | 24 | 9 |
| 24 [f] | BiI3 | DMF | 100 | 8 | 94 |
| 25 [g] | BiI3 | DMF | 100 | 24 | 92 |
| 26 [h] | BiI3 | DMF | 100 | 1 | 94 |
| 27 [i] | BiI3 | DMF | 100 | 1 | 96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumura, M.; Umeda, A.; Sumi, Y.; Aiba, N.; Murata, Y.; Yasuike, S. Bismuth(III)-Catalyzed Regioselective Selenation of Indoles with Diaryl Diselenides: Synthesis of 3-Selanylindoles. Molecules 2024, 29, 3227. https://doi.org/10.3390/molecules29133227
Matsumura M, Umeda A, Sumi Y, Aiba N, Murata Y, Yasuike S. Bismuth(III)-Catalyzed Regioselective Selenation of Indoles with Diaryl Diselenides: Synthesis of 3-Selanylindoles. Molecules. 2024; 29(13):3227. https://doi.org/10.3390/molecules29133227
Chicago/Turabian StyleMatsumura, Mio, Airi Umeda, Yuika Sumi, Naoki Aiba, Yuki Murata, and Shuji Yasuike. 2024. "Bismuth(III)-Catalyzed Regioselective Selenation of Indoles with Diaryl Diselenides: Synthesis of 3-Selanylindoles" Molecules 29, no. 13: 3227. https://doi.org/10.3390/molecules29133227







