The Synergetic Reduction of the Condensation Degree of Dissolved Lignin (DL) during the Refining Process of Wheat Straw Biomass Based on the MA/O3 System
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of MA/O3 on the Removal of Components of Wheat Straw
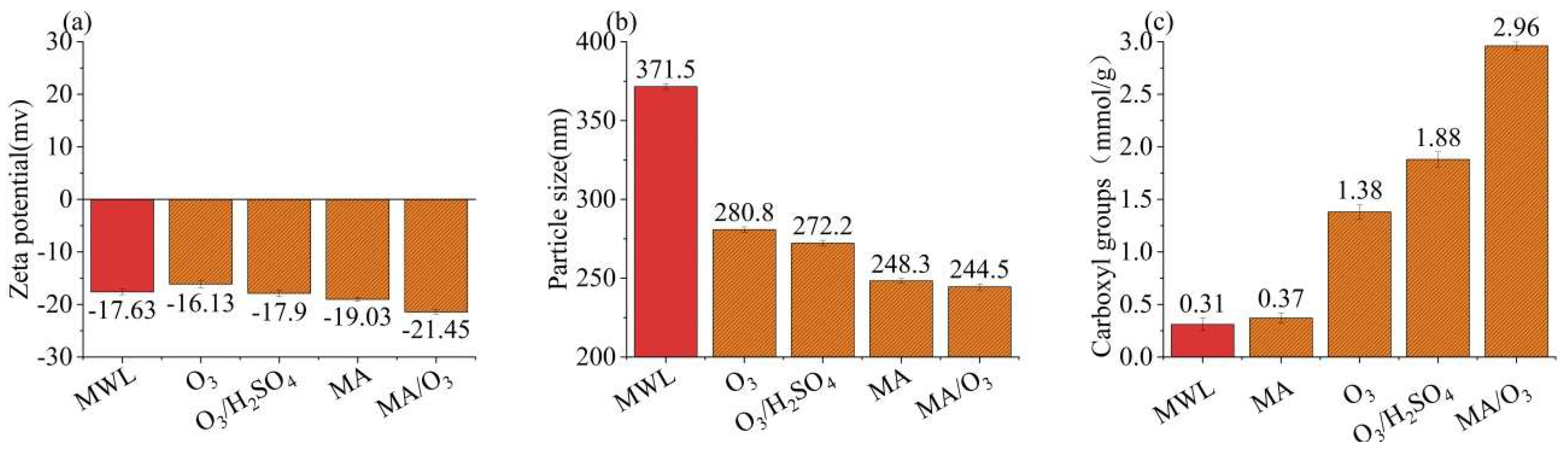
2.2. Particle Size, Zeta Potential, and Carboxyl Content of Lignin
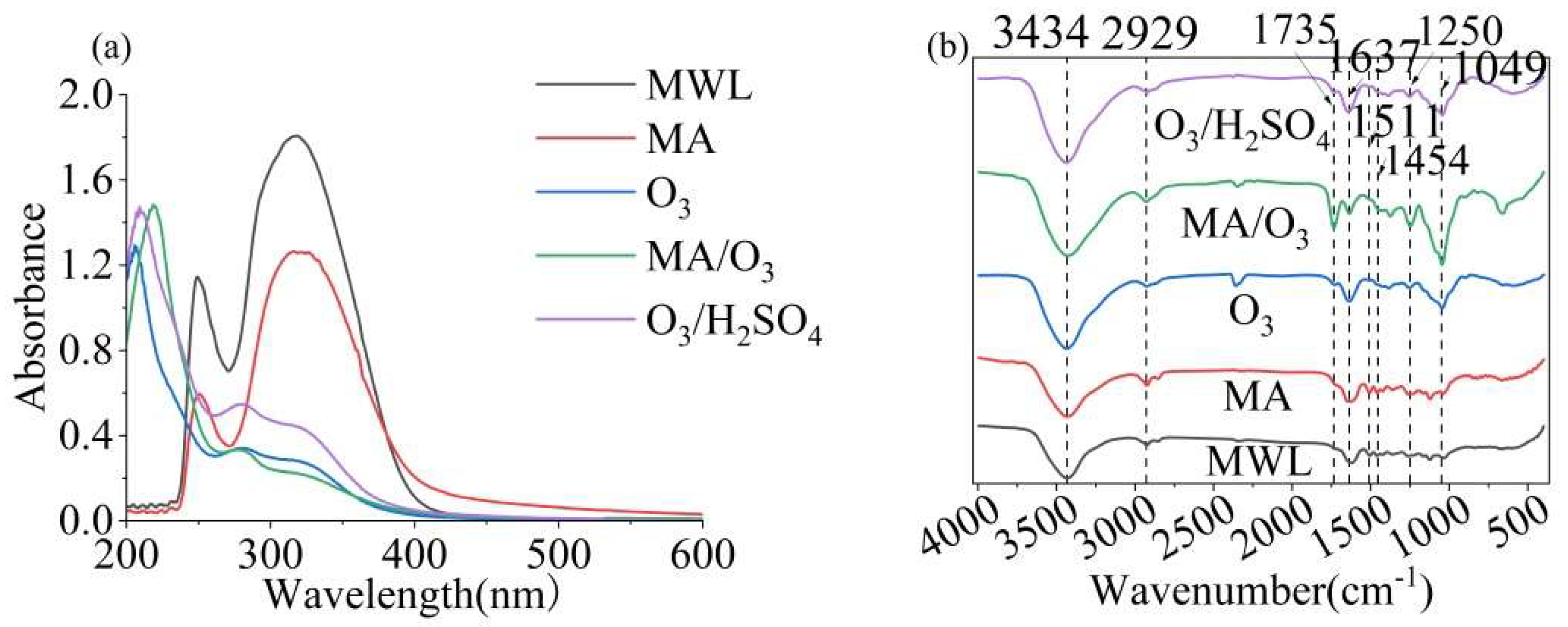
2.3. UV and FT-IR Spectra Analysis
2.4. Thermal Stability Analysis
2.5. Molecular Weight Analysis
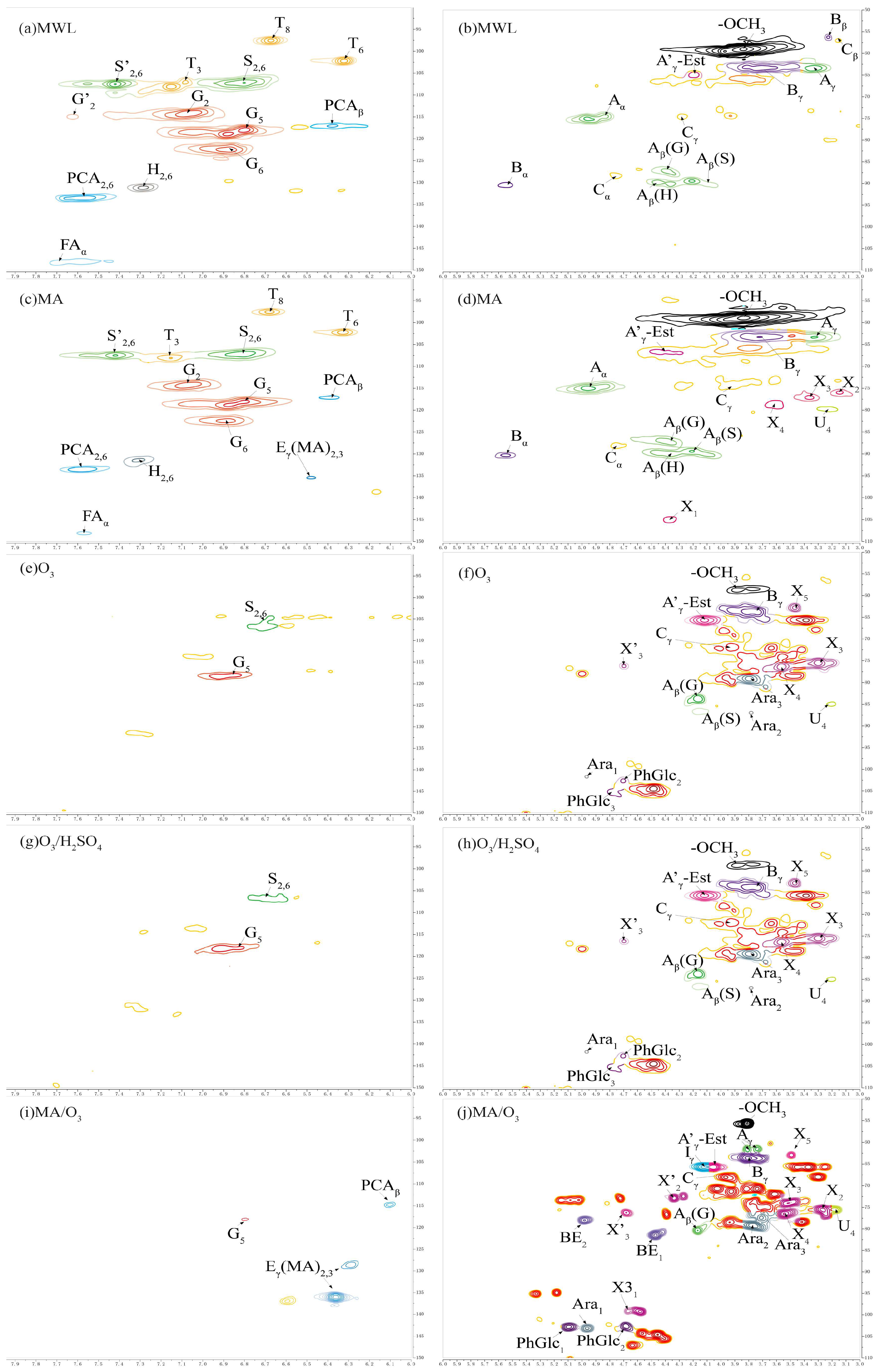
2.6. 2D-HSQC-NMR Spectra Analysis
2.6.1. Lignin Cross-Signals
2.6.2. Carbohydrate Cross-Signals
3. Materials and Methods
3.1. Materials
3.2. Pretreatment Procedure with MA/O3
3.3. Chemical Composition
3.4. Analytical Procedures
3.5. Lignin Structure Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jafari-Petroudy, S.R.; Resalati, H.; Rezayati-Charani, P. Newsprint from Soda Bagasse Pulp in Admixture with Hardwood CMP Pulp. Bioresources 2011, 6, 2483–2491. [Google Scholar] [CrossRef]
- Sarwar, M.; Khan, M.A.; Mahr-un, N. Nitrogen retention and chemical composition of urea treated wheat straw ensiled with organic acids or fermentable carbohydrates. Asian-Australas. J. Anim. Sci. 2003, 16, 1583–1592. [Google Scholar] [CrossRef]
- Taranenko, A.; Kulyk, M.; Galytska, M.; Taranenko, S.; Rozhko, I. Dynamics of soil organic matter in Panicum virgatum sole crops and intercrops. Zemdirb.-Agric. 2021, 108, 255–262. [Google Scholar] [CrossRef]
- Brummer, V.; Jurena, T.; Hlavacek, V.; Omelkova, J.; Bebar, L.; Gabriel, P.; Stehlik, P. Enzymatic hydrolysis of pretreated waste paper—Source of raw material for production of liquid biofuels. Bioresour. Technol. 2014, 152, 543–547. [Google Scholar] [CrossRef]
- Cekmecelioglu, D.; Uncu, O.N. Kinetic modeling of enzymatic hydrolysis of pretreated kitchen wastes for enhancing bioethanol production. Waste Manag. 2013, 33, 735–739. [Google Scholar] [CrossRef]
- Lai, C.H.; Yang, C.D.; Jia, Y.; Xu, X.; Wang, K.; Yong, Q. Lignin fractionation to realize the comprehensive elucidation of structure-inhibition relationship of lignins in enzymatic hydrolysis. Bioresour. Technol. 2022, 355, 127255. [Google Scholar] [CrossRef]
- Li, K.Y.; Zhong, W.; Li, P.H.; Ren, J.P.; Jiang, K.J.; Wu, W.J. Antibacterial mechanism of lignin and lignin-based antimicrobial materials in different fields. Int. J. Biol. Macromol. 2023, 252, 126281. [Google Scholar] [CrossRef]
- Ma, Y.L.; Dai, J.X.; Wu, L.L.; Fang, G.Z.; Guo, Z.H. Enhanced anti-ultraviolet, anti-fouling and anti-bacterial polyelectrolyte membrane of polystyrene grafted with trimethyl quaternary ammonium salt modified lignin. Polymer 2017, 114, 113–121. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Kiriazis, A.; Hynninen, V.; Liu, Z.H.; Bauleth-Ramos, T.; Rahikkala, A.; Correia, A.; Kohout, T.; Sarmento, B.; et al. In vitro evaluation of biodegradable lignin-based nanoparticles for drug delivery and enhanced antiproliferation effect in cancer cells. Biomaterials 2017, 121, 97–108. [Google Scholar] [CrossRef]
- Frangville, C.; Rutkevicius, M.; Richter, A.P.; Velev, O.D.; Stoyanov, S.D.; Paunov, V.N. Fabrication of Environmentally Biodegradable Lignin Nanoparticles. Chemphyschem 2012, 13, 4235–4243. [Google Scholar] [CrossRef]
- de Albuquerque, T.L.; Cavalcante, V.G.C.; Rocha, W.D.; de Macedo, A.C.; Rocha, M.V.P. Hydrogels based on lignin extracted from cashew apple bagasse and its application in antimicrobial wound dressings. Int. J. Biol. Macromol. 2024, 262, 130169. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, X.; Ma, X.; Cui, M.; Wang, X.; Chen, J.; Zhu, J.; Chen, J. Antimicrobial Nonisocyanate Polyurethane Foam Derived from Lignin for Wound Healing. ACS Appl. Bio Mater. 2024, 7, 1301–1310. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Cárcamo-Martínez, A.; Stewart, S.A.; Donnelly, R.F.; Larrañeta, E.; Borrega, M. Lignin for pharmaceutical and biomedical applications—Could this become a reality? Sustain. Chem. Pharm. 2020, 18, 100320. [Google Scholar] [CrossRef]
- Norouzi, M.; Rafienia, M.; Hosseini, S. Characterization and biological evaluation of new PLGA/fibrin/lignin biocomposite electrospun scaffolds. Phys. Scr. 2023, 98, 095506. [Google Scholar] [CrossRef]
- Kandil, H.; Ekram, B.; Abo-Zeid, M.A.M. Cytocompatibility of MG-63 osteosarcoma cells on chitosan/hydroxyapatite/lignin hybrid composite scaffold in vitro. Biomed. Mater. 2023, 18, 015002. [Google Scholar] [CrossRef]
- Winters, C.; Carsi, M.; Sanchis, M.J.; Culebras, M.; Collins, M.N. On the design of lignin reinforced acrylic acid/hyaluronic acid adhesive hydrogels with conductive PEDOT:HA nanoparticles. Int. J. Biol. Macromol. 2024, 273, 133093. [Google Scholar] [CrossRef]
- Alaoui, C.H.; Rethoré, G.; Weiss, P.; Fatimi, A. Sustainable Biomass Lignin-Based Hydrogels: A Review on Properties, Formulation, and Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 13493. [Google Scholar] [CrossRef] [PubMed]
- Garlapati, V.K.; Chandel, A.K.; Kumar, S.P.J.; Sharma, S.; Sevda, S.; Ingle, A.P.; Pant, D. Circular economy aspects of lignin: Towards a lignocellulose biorefinery. Renew. Sustain. Energy Rev. 2020, 130, 109977. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, W.J.; Pang, B.; Sun, Z.H.; Lam, S.S.; Sonne, C.; Yuan, Q. Ultrastructural change in lignocellulosic biomass during hydrothermal pretreatment. Bioresour. Technol. 2021, 341, 125807. [Google Scholar] [CrossRef]
- Hsu, T.C.; Guo, G.L.; Chen, W.H.; Hwang, W.S. Effect of dilute acid pretreatment of rice straw on structural properties and enzymatic hydrolysis. Bioresour. Technol. 2010, 101, 4907–4913. [Google Scholar] [CrossRef]
- Chen, J.H.; Xu, J.K.; Huang, P.L.; Sun, R.C. Effect of alkaline pretreatment on the preparation of regenerated lignocellulose fibers from bamboo stem. Cellulose 2016, 23, 2727–2739. [Google Scholar] [CrossRef]
- Zakaria, S.M.; Idris, A.; Alias, Y. Lignin Extraction from Coconut Shell Using Aprotic Ionic Liquids. Bioresources 2017, 12, 5749–5774. [Google Scholar] [CrossRef]
- Zhang, X.M.; Yuan, T.Q.; Peng, F.; Xu, F.; Sun, R.C. Separation and Structural Characterization of Lignin from Hybrid Poplar Based on Complete Dissolution in DMSO/LiCl. Sep. Sci. Technol. 2010, 45, 2497–2506. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem.-Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Y.Y.; Xu, J.Y.; Guo, Y.Z.; Zhou, J.H.; Wang, X. Ni12P5/P-N-C Derived from Natural Single-Celled Chlorella for Catalytic Depolymerization of Lignin into Monophenols. ACS Omega 2022, 7, 13134–13143. [Google Scholar] [CrossRef]
- Zhu, J.J.; Chen, L.H.; Gleisner, R.; Zhu, J.Y. Co-production of bioethanol and furfural from poplar wood via low temperature (≤90 °C) acid hydrotropic fractionation (AHF). Fuel 2019, 254, 115572. [Google Scholar] [CrossRef]
- Ma, Q.L.; Zhu, J.J.; Gleisner, R.; Yang, R.D.; Zhu, J.Y. Valorization of Wheat Straw Using a Recyclable Hydrotrope at Low Temperatures (≤90 °C). ACS Sustain. Chem. Eng. 2018, 6, 14480–14489. [Google Scholar] [CrossRef]
- Cai, C.; Hirth, K.; Gleisner, R.; Lou, H.M.; Qiu, X.Q.; Zhu, J.Y. Maleic acid as a dicarboxylic acid hydrotrope for sustainable fractionation of wood at atmospheric pressure and ≤100 °C: Mode and utility of lignin esterification. Green Chem. 2020, 22, 1605–1617. [Google Scholar] [CrossRef]
- Su, C.; Hirth, K.; Liu, Z.L.; Cao, Y.F.; Zhu, J.Y. Maleic acid hydrotropic fractionation of wheat straw to facilitate value-added multi-product biorefinery at atmospheric pressure. Glob. Chang. Biol. Bioenergy 2021, 13, 1407–1424. [Google Scholar] [CrossRef]
- Ma, R.S.; Xu, Y.; Zhang, X. Catalytic Oxidation of Biorefinery Lignin to Value-added Chemicals to Support Sustainable Biofuel Production. Chemsuschem 2015, 8, 24–51. [Google Scholar] [CrossRef]
- Mamleeva, N.A.; Autlov, S.A.; Fionov, A.V.; Bazarnova, N.G.; Lunin, V.V. The oxidative destruction of lignin in the ozonation of wood. Russ. J. Phys. Chem. A 2009, 83, 745–751. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, S.; Wang, W.; Linhardt, R.J.; Ragauskas, A.J. Preparation of Highly Reactive Lignin by Ozone Oxidation: Application as Surfactants with Antioxidant and Anti-UV Properties. ACS Sustain. Chem. Eng. 2020, 8, 22–28. [Google Scholar] [CrossRef]
- Khadre, M.A.; Yousef, A.E.; Kim, J.G. Microbiological aspects of ozone applications in food: A review. J. Food Sci. 2001, 66, 1242–1252. [Google Scholar] [CrossRef]
- Seta, F.T.; An, X.Y.; Liu, L.Q.; Zhang, H.; Yang, J.; Zhang, W.; Nie, S.X.; Yao, S.Q.; Cao, H.B.; Xu, Q.L.; et al. Preparation and characterization of high yield cellulose nanocrystals (CNC) derived from ball mill pretreatment and maleic acid hydrolysis. Carbohydr. Polym. 2020, 234, 115942. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.K.; Bhardwaj, N.K.; Ghatak, H.R. Developments in Ozone-Based Bleaching of Pulps. Ozone-Sci. Eng. 2020, 42, 194–210. [Google Scholar] [CrossRef]
- Garcia, J.C.; Lopez, F.; Perez, A.; Pelach, M.A.; Mutje, P.; Colodette, J.L. Initiating ECF bleaching sequences of eucalyptus kraft pulps with Z/D and Z/E stages. Holzforschung 2010, 64, 1–6. [Google Scholar] [CrossRef]
- Travaini, R.; Martín-Juarez, J.; Lorenzo-Hernando, A.; Bolado-Rodríguez, S. Ozonolysis: An advantageous pretreatment for lignocellulosic biomass revisited. Bioresour. Technol. 2016, 199, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Zhao, L.H.; Ren, J.L.; He, B.H. Structural Changes of Alkali Lignin under Ozone Treatment and Effect of Ozone-Oxidized Alkali Lignin on Cellulose Digestibility. Processes 2022, 10, 559. [Google Scholar] [CrossRef]
- Liu, Z.L.; Meng, L.K.; Chen, J.Q.; Cao, Y.F.; Wang, Z.G.; Ren, H. The utilization of soybean straw III: Isolation and characterization of lignin from soybean straw. Biomass Bioenergy 2016, 94, 12–20. [Google Scholar] [CrossRef]
- Delmas, G.H.; Benjelloun-Mlayah, B.; Le Bigot, Y.; Delmas, M. Functionality of Wheat Straw Lignin Extracted in Organic Acid Media. J. Appl. Polym. Sci. 2011, 121, 491–501. [Google Scholar] [CrossRef]
- Mohammadpour, R.; Sadeghi, G.M.M. Potential use of black liquor as lignin source for synthesis of polyurethane foam. J. Polym. Res. 2020, 27, 362. [Google Scholar] [CrossRef]
- Shukry, N.; Fadel, S.M.; Agblevor, F.A.; Ei-Kalyoubi, S.F. Some physical properties of acetosolv lignins from bagasse. J. Appl. Polym. Sci. 2008, 109, 434–444. [Google Scholar] [CrossRef]
- Faravelli, T.; Frassoldati, A.; Migliavacca, G.; Ranzi, E. Detailed kinetic modeling of the thermal degradation of lignins. Biomass Bioenergy 2010, 34, 290–301. [Google Scholar] [CrossRef]
- Yang, J.Y.; Yu, Q.F.; Li, M.F. Freeze-thaw assisted maleic acid pretreatment of eucalyptus to prepare cellulose nanocrystals and degraded lignin. Bioresour. Technol. 2023, 384, 129365. [Google Scholar] [CrossRef] [PubMed]
- Figueirêdo, M.B.; Heeres, H.J.; Deuss, P.J. Ozone mediated depolymerization and solvolysis of technical lignins under ambient conditions in ethanol. Sustain. Energy Fuels 2020, 4, 265–276. [Google Scholar] [CrossRef]
- Wang, R.; Chen, C.L.; Gratzl, J.S. Ozonation of pine kraft lignin in alkaline solution. Part 1: Ozonation, characterization of kraft lignin and its ozonated preparations. Holzforschung 2004, 58, 622–630. [Google Scholar] [CrossRef]
- Xiao, M.Z.; Chen, W.J.; Hong, S.; Pang, B.; Cao, X.F.; Wang, Y.Y.; Yuan, T.Q.; Sun, R.C. Structural characterization of lignin in heartwood, sapwood, and bark of eucalyptus. Int. J. Biol. Macromol. 2019, 138, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.L.; Zhao, C.; Shao, Q.J.; Hassan, M. Structural Characterization of Corn Stover Lignin after Hydrogen Peroxide Presoaking Prior to Ammonia Fiber Expansion Pretreatment. Energy Fuels 2018, 32, 6022–6030. [Google Scholar] [CrossRef]
- Xie, D.; Gan, T.; Su, C.; Han, Y.; Liu, Z.L.; Cao, Y.F. Structural characterization and antioxidant activity of water-soluble lignin-carbohydrate complexes (LCCs) isolated from wheat straw. Int. J. Biol. Macromol. 2020, 161, 315–324. [Google Scholar] [CrossRef]
- Khongchamnan, P.; Wanmolee, W.; Laosiripojana, N.; Champreda, V.; Suriyachai, N.; Kreetachat, T.; Sakulthaew, C.; Chokejaroenrat, C.; Imman, S. Solvothermal-Based Lignin Fractionation From Corn Stover: Process Optimization and Product Characteristics. Front. Chem. 2021, 9, 697237. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Jiang, B.; Cao, T.Y.; Gu, F.; Wu, W.J.; Jin, Y.C. Comparison of the Structural Characteristics of Cellulolytic Enzyme Lignin Preparations Isolated from Wheat Straw Stem and Leaf. ACS Sustain. Chem. Eng. 2017, 5, 342–349. [Google Scholar] [CrossRef]
- Giummarella, N.; Lawoko, M. Structural Basis for the Formation and Regulation of Lignin-Xylan Bonds in Birch. ACS Sustain. Chem. Eng. 2016, 4, 5319–5326. [Google Scholar] [CrossRef]
- Ibanez, A.B.; Bauer, S. Downscaled method using glass microfiber filters for the determination of Klason lignin and structural carbohydrates. Biomass Bioenergy 2014, 68, 75–81. [Google Scholar] [CrossRef]
- Li, X.K.; Zhao, X.; Zhu, H.W.; Wang, X.; Zhou, J.H. Research of Inhibiting Lignin Condensation by Ethylene Glycol During DES Pretreatment Process. Trans. China Pulp Pap. 2022, 37, 39–46. (In Chinese) [Google Scholar]
- Su, C.; Hirth, K.; Liu, Z.L.; Cao, Y.F.; Zhu, J.Y. Acid hydrotropic fractionation of switchgrass at atmospheric pressure using maleic acid in comparison with p-TsOH: Advantages of lignin esterification. Ind. Crop. Prod. 2021, 159, 113017. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.D.; Yoo, C.G.; Yang, X.H.; Lin, X.L.; Ralph, J.; Pan, X.J. An uncondensed lignin depolymerized in the solid state and isolated from lignocellulosic biomass: A mechanistic study. Green Chem. 2018, 20, 4224–4235. [Google Scholar] [CrossRef]
- Yue, P.P.; Hu, Y.J.; Fu, G.Q.; Sun, C.X.; Li, M.F.; Peng, F.; Sun, R.C. Structural Differences between the Lignin-Carbohydrate Complexes (LCCs) from 2-and 24-Month-Old Bamboo (Neosinocalamus affinis). Int. J. Mol. Sci. 2018, 19, 1. [Google Scholar] [CrossRef] [PubMed]



| Item | Temp. c (°C) | O3 Holding Time (min) | Ground Wheat Straw | Ball-Milled Wheat Straw | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MA d | MA/O3 | O3 e | O3/H2SO4 f | MA | MA/O3 | O3 | O3/H2SO4 | |||
| Yield (%) | 60 °C | 0 | 78.40 ± 0.3 | N/A | 63.66 ± 0.5 | N/A | ||||
| 9 | N/A g | 81.20 ± 0.4 | 92.33 ± 0.5 | 94.33 ± 0.3 | N/A | 63.05 ± 0.3 | 82.67 ± 0.4 | 70.33 ± 0.6 | ||
| Lignin removal ratio b (%) | 0 | 18.80 ± 0.2 | N/A | 28.46 ± 0.8 | N/A | |||||
| 9 | N/A | 23.32 ± 0.1 | 4.77 ± 0.7 | 14.32 ± 0.3 | N/A | 38.07 ± 0.2 | 15.78 ± 0.7 | 28.25 ± 0.7 | ||
| Dextran removal ratio b (%) | 0 | 13.77 ± 0.3 | N/A | 22.28 ± 0.3 | N/A | |||||
| 9 | N/A | 21.53 ± 0.3 | 5.15 ± 0.6 | 10.54 ± 0.2 | N/A | 31.44 ± 0.1 | 14.95 ± 0.6 | 25.98 ± 0.9 | ||
| Xylan removal ratio b (%) | 0 | 13.76 ± 0.4 | N/A | 55.16 ± 0.4 | N/A | |||||
| 9 | N/A | 25.35 ± 0.2 | 10.15 ± 0.4 | 17.53 ± 0.7 | N/A | 71.98 ± 0.1 | 23.37 ± 0.3 | 59.34 ± 0.5 | ||
| Name of Sample | MWL | MA | O3 | MA/O3 | O3/H2SO4 |
|---|---|---|---|---|---|
| Mw | 14,678 | 8105 | 9730 | 7758 | 7990 |
| Mn | 4258 | 3563 | 1832 | 1954 | 1802 |
| PI | 3.45 | 2.27 | 5.31 | 3.97 | 4.43 |
| Characteristics | MWL | MA | O3 | O3/H2SO4 | MA/O3 |
|---|---|---|---|---|---|
| Lignin interunit linkages | |||||
| β-O-4 | 50 | 52 | 0 | 0 | 0 |
| β-β | 1 | 10 | 0 | 0 | 0 |
| β-5 | 2 | 7 | 0 | 0 | 0 |
| Condensed degree a | 6 | 10 | 0 | 0 | 0 |
| Lignin aromatic units | |||||
| G | 51 | 53 | 62 | 69 | 100 |
| S | 45 | 43 | 38 | 31 | 0 |
| H | 4 | 4 | 0 | 0 | 0 |
| S/G | 0.87 | 0.81 | 0.61 | 0.45 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Liu, Z.; Zhou, Z.; Li, R.; Li, L.; Cao, Y. The Synergetic Reduction of the Condensation Degree of Dissolved Lignin (DL) during the Refining Process of Wheat Straw Biomass Based on the MA/O3 System. Molecules 2024, 29, 3228. https://doi.org/10.3390/molecules29133228
Chen X, Liu Z, Zhou Z, Li R, Li L, Cao Y. The Synergetic Reduction of the Condensation Degree of Dissolved Lignin (DL) during the Refining Process of Wheat Straw Biomass Based on the MA/O3 System. Molecules. 2024; 29(13):3228. https://doi.org/10.3390/molecules29133228
Chicago/Turabian StyleChen, Xiuguang, Zhulan Liu, Zhenyu Zhou, Renai Li, Lizi Li, and Yunfeng Cao. 2024. "The Synergetic Reduction of the Condensation Degree of Dissolved Lignin (DL) during the Refining Process of Wheat Straw Biomass Based on the MA/O3 System" Molecules 29, no. 13: 3228. https://doi.org/10.3390/molecules29133228
APA StyleChen, X., Liu, Z., Zhou, Z., Li, R., Li, L., & Cao, Y. (2024). The Synergetic Reduction of the Condensation Degree of Dissolved Lignin (DL) during the Refining Process of Wheat Straw Biomass Based on the MA/O3 System. Molecules, 29(13), 3228. https://doi.org/10.3390/molecules29133228





