Hydrous Molybdenum Oxide Coating of Zinc Metal Anode via the Facile Electrodeposition Strategy and Its Performance Improvement Mechanisms for Aqueous Zinc−Ion Batteries
Abstract
:1. Introduction
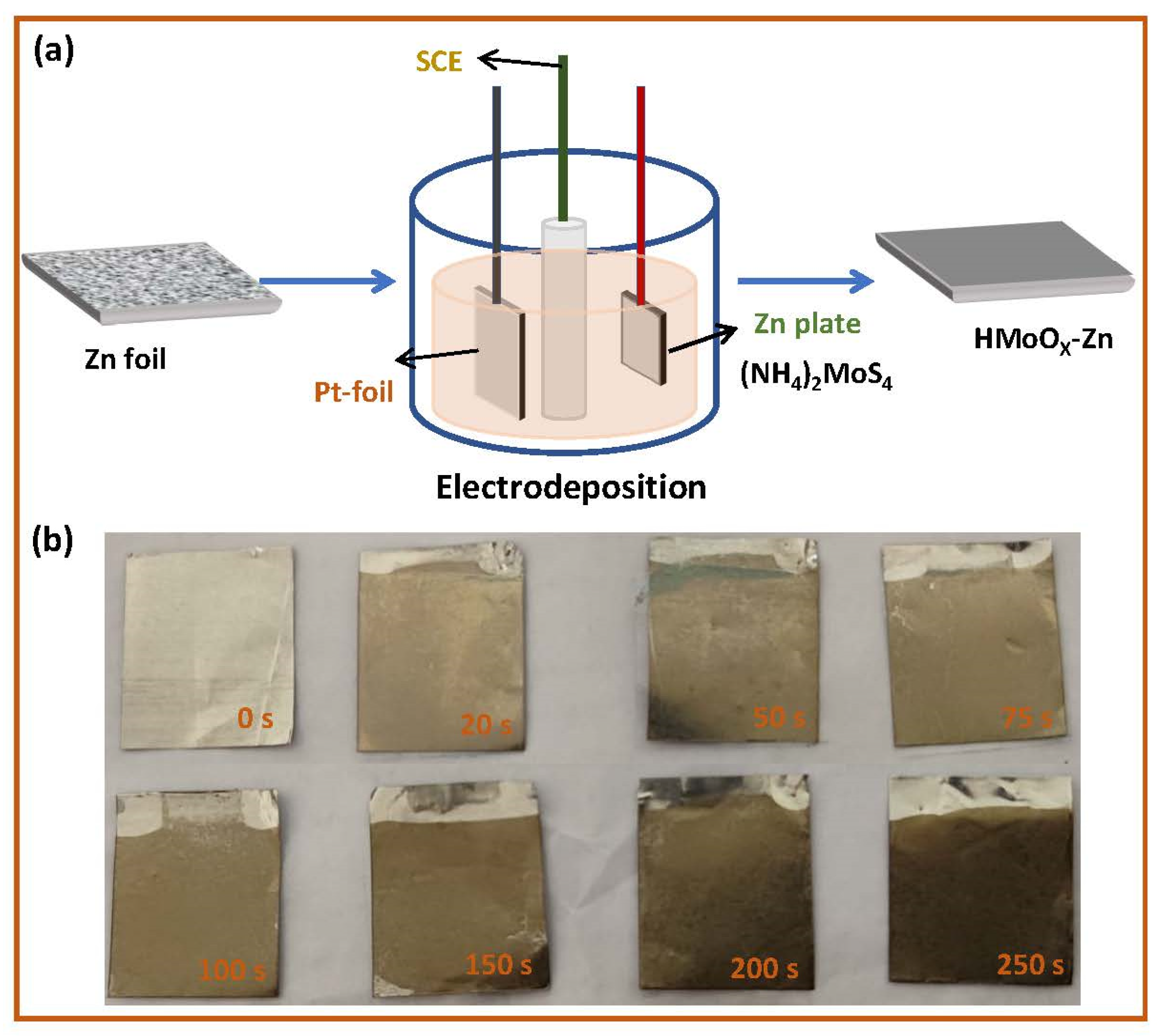
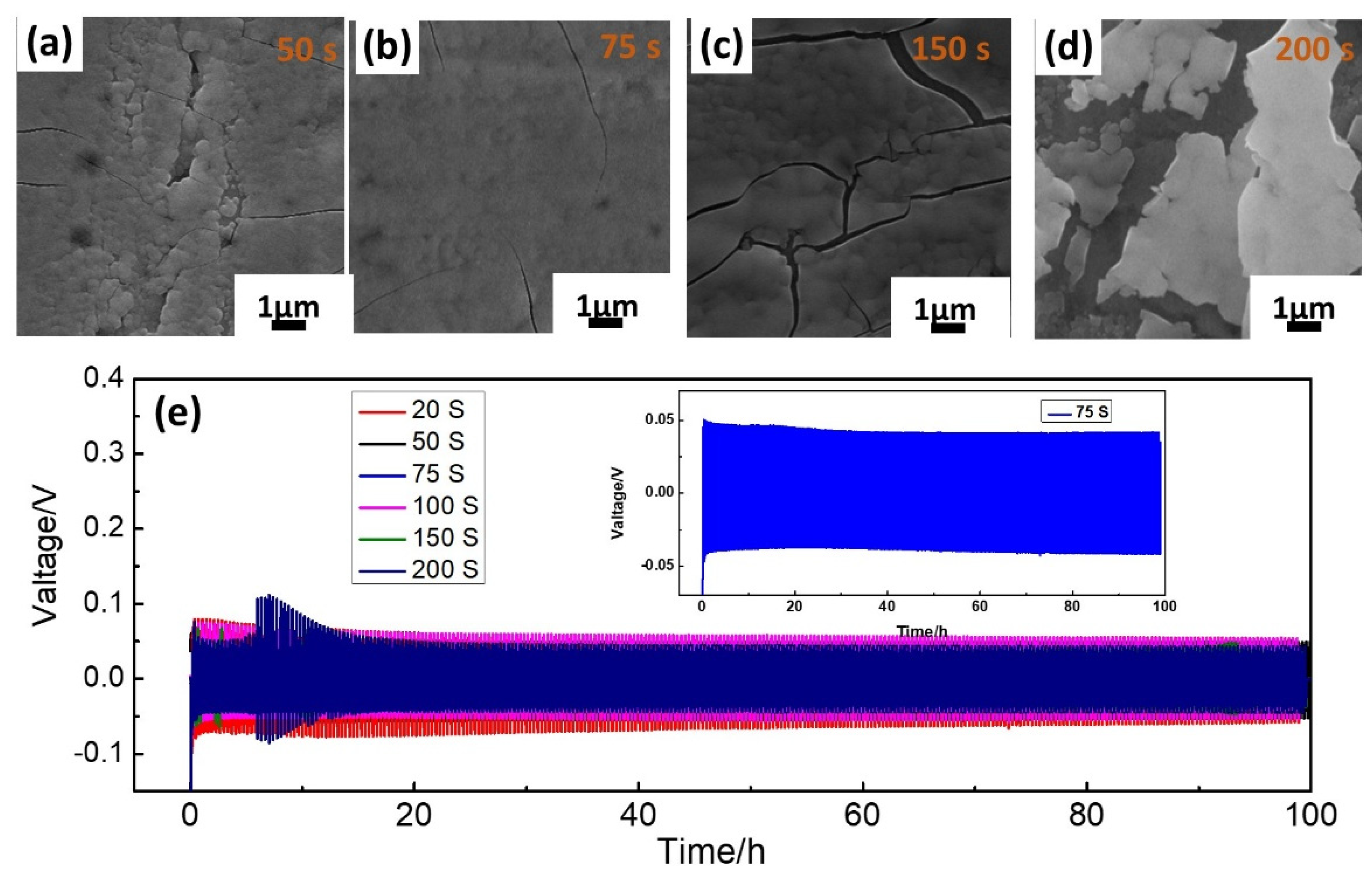
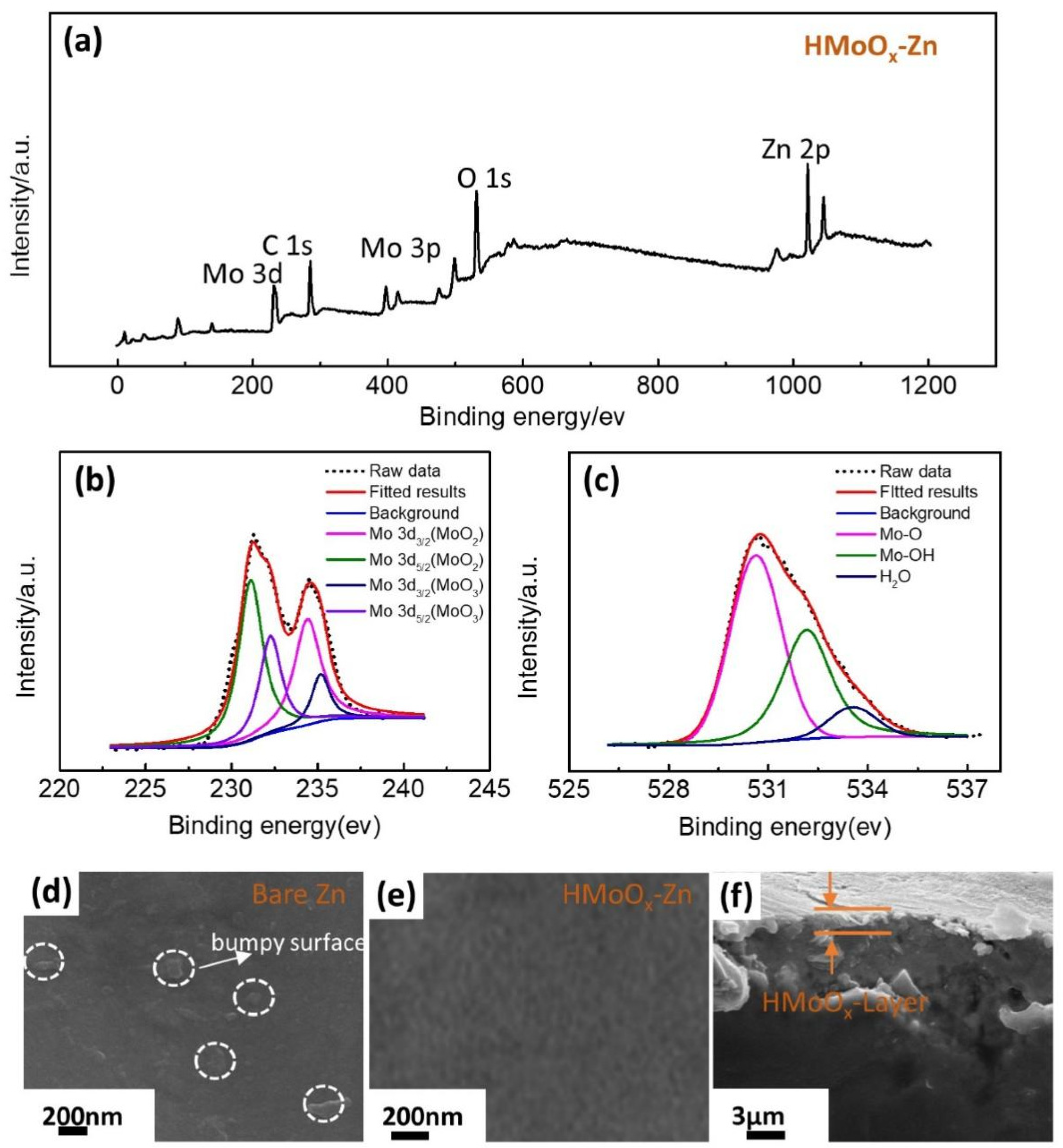
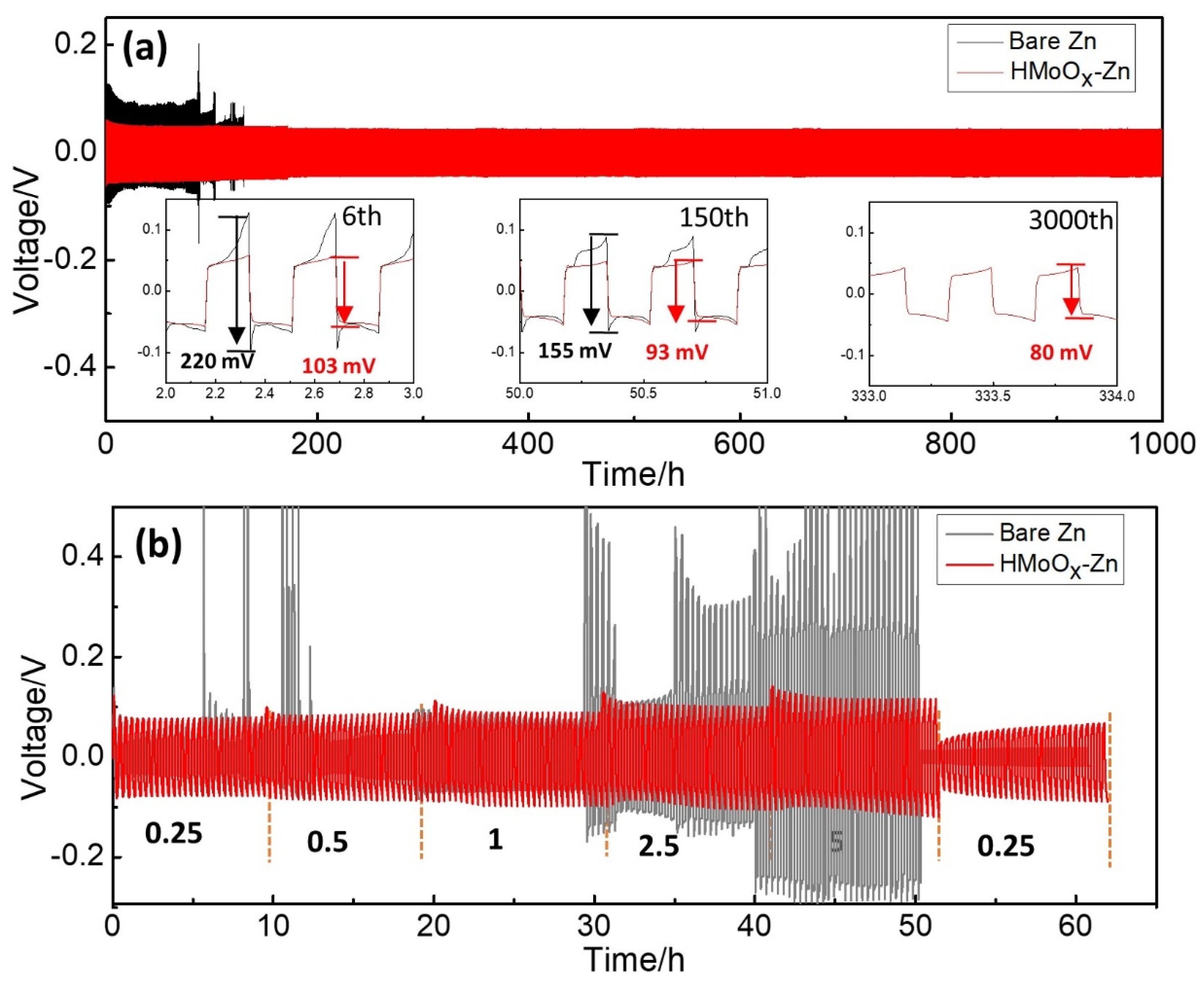
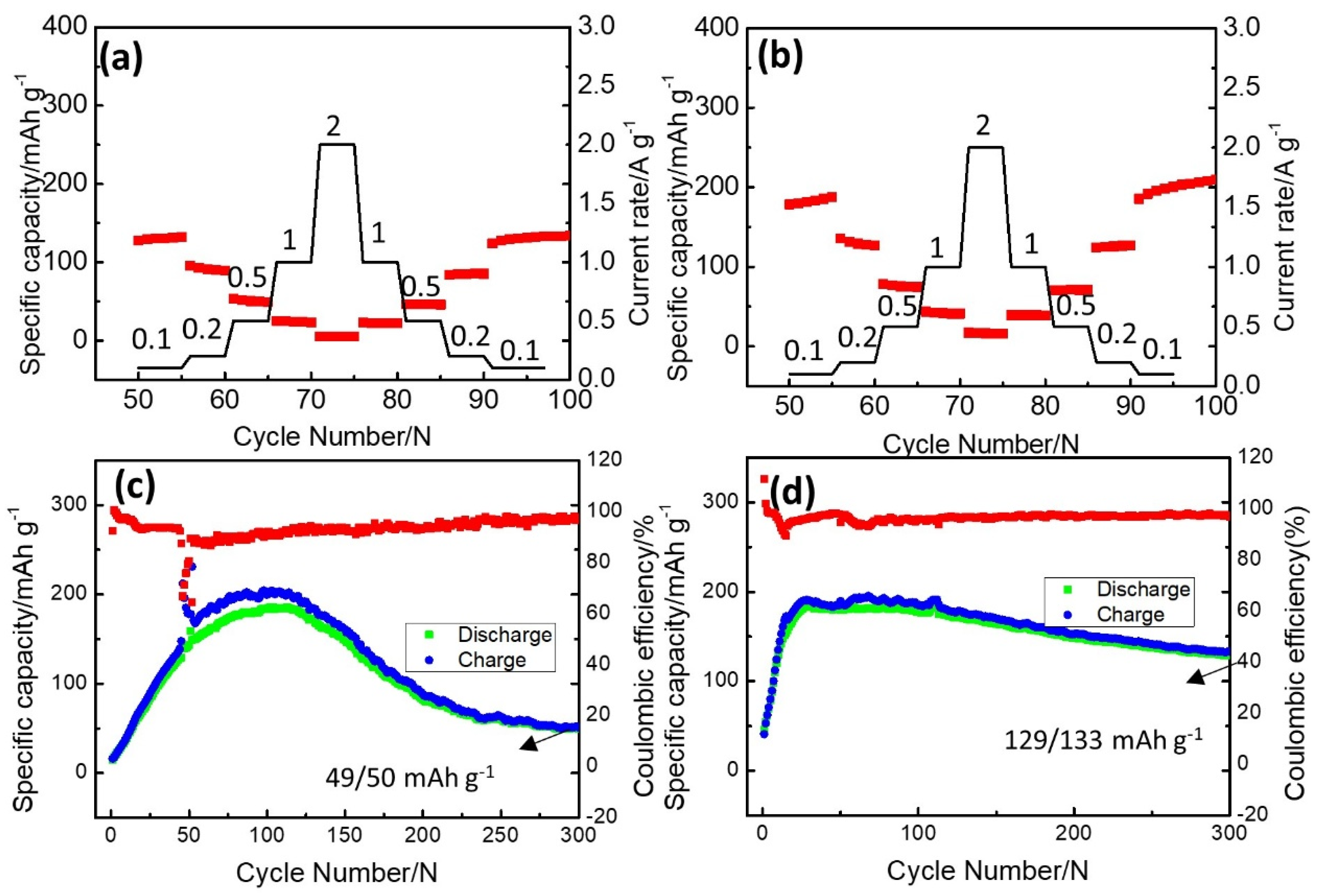
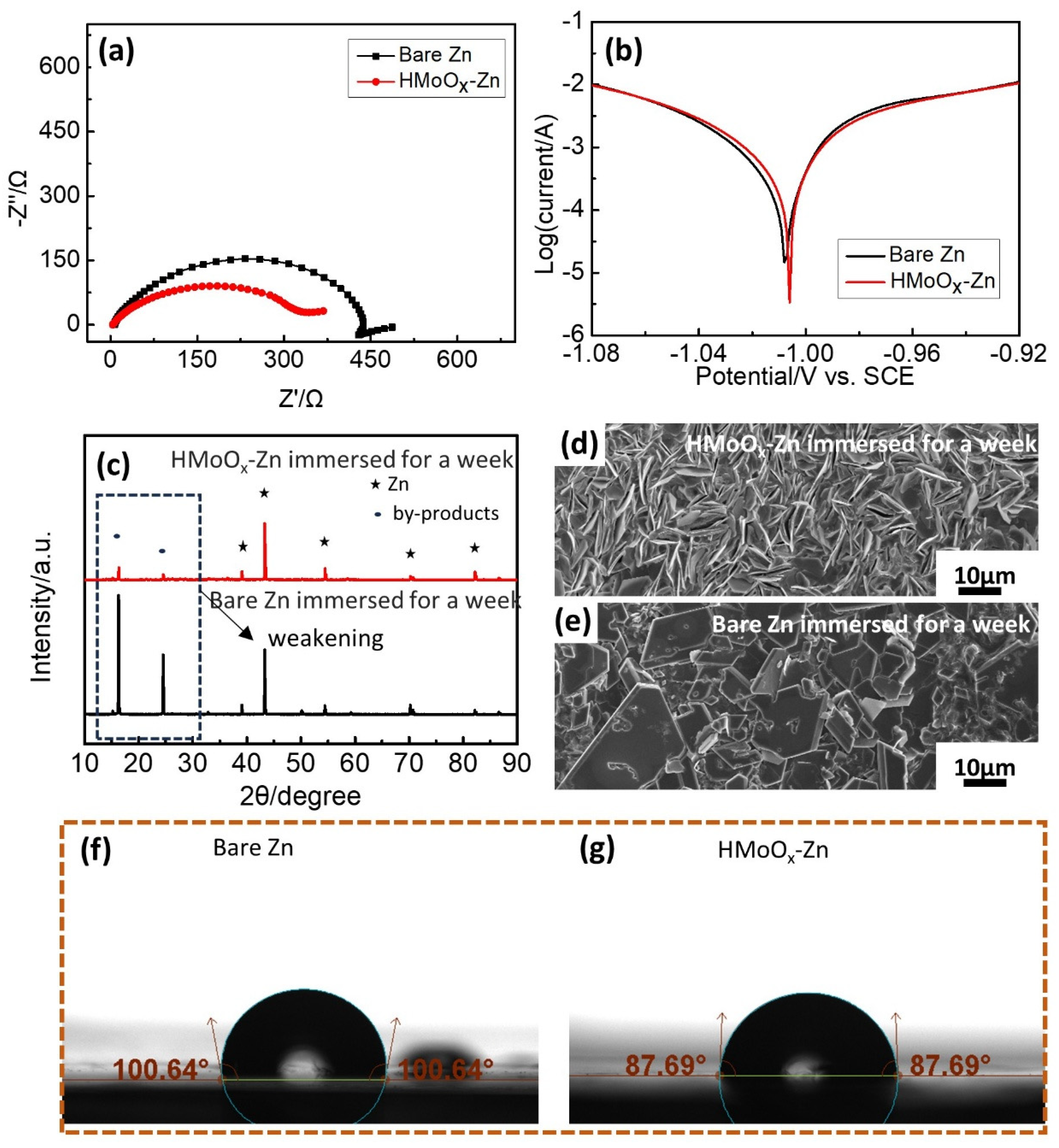
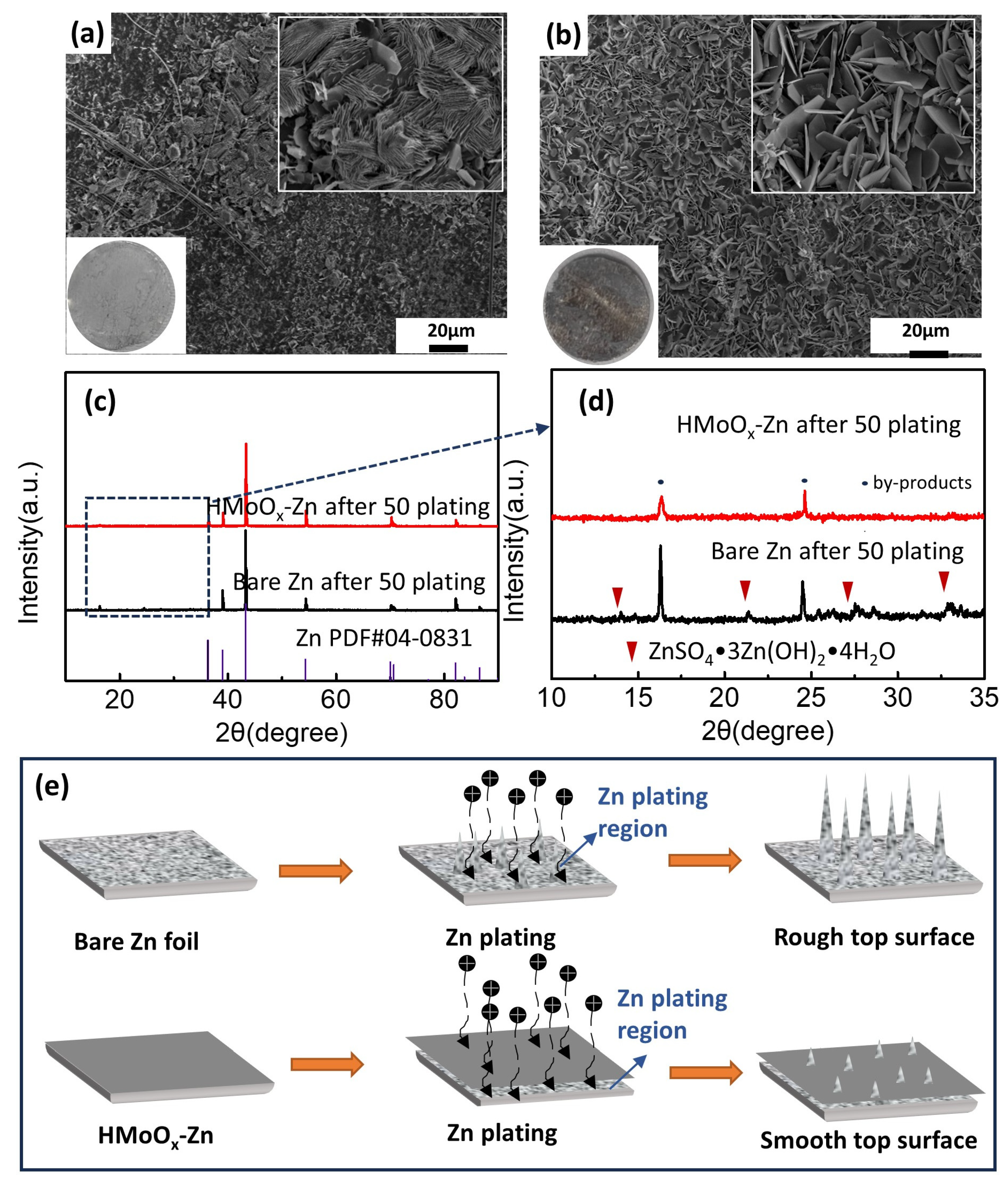
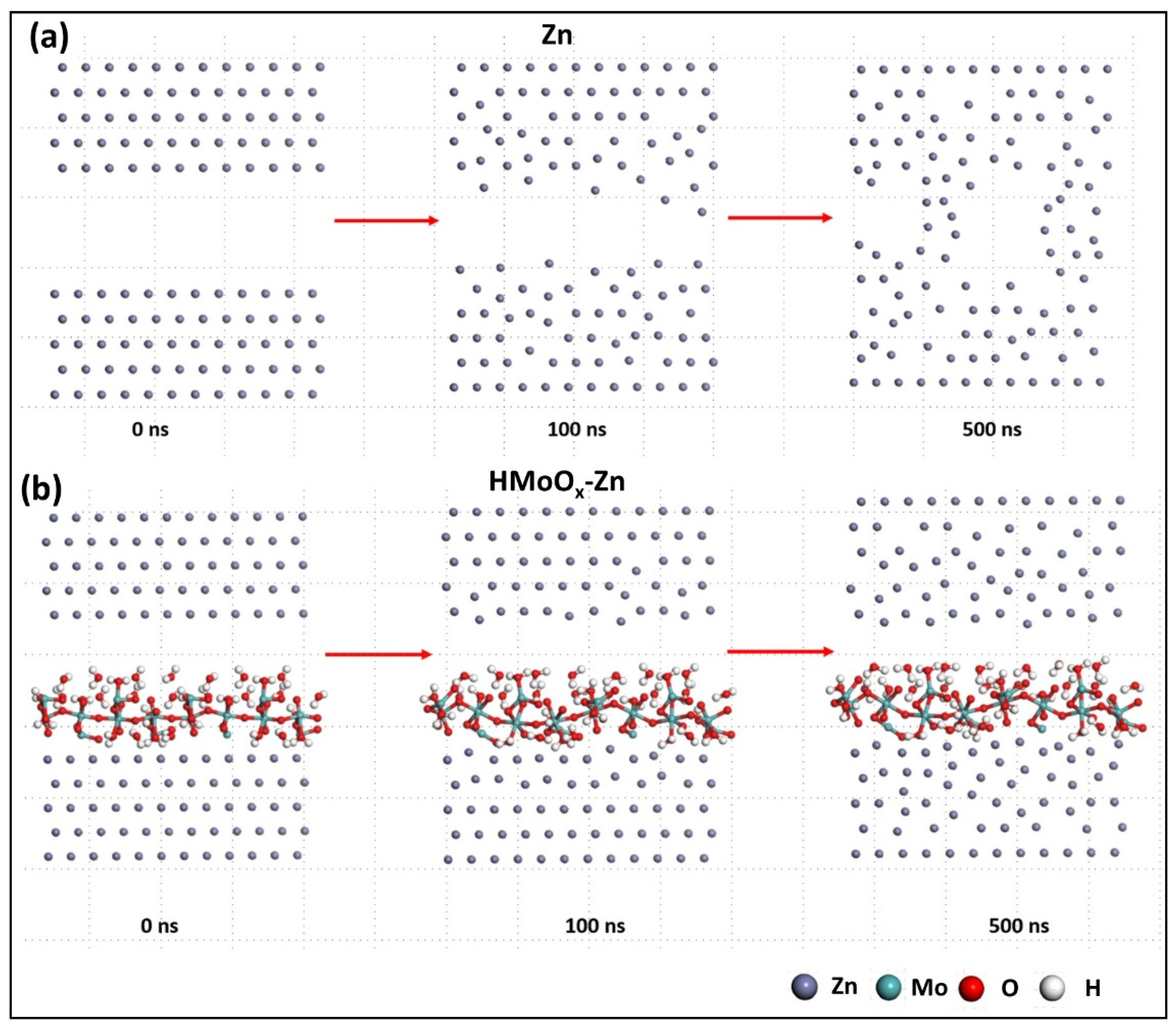
2. Results and Discussion
3. Materials and Methods
3.1. Materials’ Synthesis
3.2. Materials’ Characterizations
3.3. Electrochemical Measurements
3.4. Theoretical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, Z.; Fan, K.; Liu, T.; Xu, Z.; Chen, G.; Zhang, H.; Li, H.; Guo, X.; Zhang, X.; Zhu, Y. Mitigating lattice distortion of high−voltage LiCoO2 via core−shell structure induced by cationic heterogeneous Co−Doping for lithium−ion batteries. Nano−Micro Lett. 2024, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, T.; Ding, Y.; Sun, X.; Huang, J.; Qiao, J.; Peng, S. Controlled nucleation and growth for the dendrite−free zinc anode in aqueous zinc−ion battery. J. Alloys Compd. 2024, 970, 172584. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Li, J.; Li, Y.; Wang, L.; Xu, H.; Han, W. Flexible TiVCTx MXene film for high−performance magnesium−ion storage device. J. Colloid Interface Sci. 2024, 657, 550–558. [Google Scholar] [CrossRef]
- Mathiyalagan, K.; Shin, D.; Lee, Y.-C. Difficulties, strategies, and recent research and development of layered sodium transition metal oxide cathode materials for high−energy sodium−ion batteries. J. Energy Chem. 2023, 90, 40–57. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, H.; Lu, Y.; Zhang, W.; Li, Z. The synergistic effect of Lewis acidic etching V4C3 (MXene)@ CuSe2/CoSe2 as an advanced cathode material for aluminum batteries. J. Mater. Sci. Technol. 2024, 177, 205–213. [Google Scholar] [CrossRef]
- Gu, H.; Yang, X.; Chen, S.; Zhang, W.; Yang, H.Y.; Li, Z. Oxygen vacancies boosted proton intercalation kinetics for aqueous aluminum–manganese Batteries. Nano Lett. 2023, 23, 11842–11849. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.M.; Santos, É.A.; Martins, P.R.; Silva, C.G.; Zanin, H. Emerging medium− and high−entropy materials as catalysts for lithium−sulfur batteries. Energy Storage Mater. 2023, 63, 102999. [Google Scholar] [CrossRef]
- Li, X.; Ye, P.; Dou, A.; Jiang, Z.; Naveed, A.; Zhou, Y.; Su, M.; Zhang, P.; Liu, Y. Nanoporous Nb2O5 coatings enabled long−life and deeply rechargeable zinc anodes for aqueous zinc−ion batteries. J. Energy Storage 2024, 76, 109874. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Du, C.; Zhou, X.; Xiong, H.; Wang, G.; Lu, X. Achieving stable Zn metal anode via a hydrophobic and Zn2+−conductive amorphous carbon interface. J. Colloid Interface Sci. 2024, 657, 644–652. [Google Scholar] [CrossRef]
- Zhao, J.; Ying, Y.; Wang, G.; Hu, K.; Di Yuan, Y.; Ye, H.; Liu, Z.; Lee, J.Y.; Zhao, D. Covalent organic framework film protected zinc anode for highly stable rechargeable aqueous zinc−ion batteries. Energy Storage Mater. 2022, 48, 82–89. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Hu, Y.; Hu, K.; Lin, X.; Liu, X.; Reddy, K.M.; Xie, G.; Qiu, H.J. Highly strengthened and toughened Zn−Li−Mn alloys as long−cycling life and dendrite-free Zn anode for aqueous zinc−ion batteries. Small 2022, 18, 2200787. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hu, A.; Yan, Z.; Chen, J.; He, M.; Zhou, B.; Pan, Y.; Fan, Y.; Gao, D.; Long, J. Enhancing Zn2+ diffusion for dendrite−free zinc anodes via a robust zincophilic clay mineral coating. Chem. Eng. J. 2024, 479, 147410. [Google Scholar] [CrossRef]
- Xu, P.; Wang, C.; Zhao, B.; Zhou, Y.; Cheng, H. An interfacial coating with high corrosion resistance based on halloysite nanotubes for anode protection of zinc−ion batteries. J. Colloid Interface Sci. 2021, 602, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Hu, C.; Zhang, Q.; Wu, T.; Xie, C.; Wang, H.; Tang, Y.; Ji, X.; Wang, H. Bulk−phase reconstruction enables robust Zinc metal anodes for aqueous Zinc−Ion batteries. Angew. Chem. 2023, 135, e202308017. [Google Scholar] [CrossRef]
- Wang, S.-B.; Ran, Q.; Yao, R.-Q.; Shi, H.; Wen, Z.; Zhao, M.; Lang, X.-Y.; Jiang, Q. Lamella−nanostructured eutectic zinc–aluminum alloys as reversible and dendrite−free anodes for aqueous rechargeable batteries. Nat. Commun. 2020, 11, 1634. [Google Scholar] [CrossRef]
- Zhu, C.; Li, P.; Xu, G.; Cheng, H.; Gao, G. Recent progress and challenges of Zn anode modification materials in aqueous Zn−ion batteries. Coord. Chem. Rev. 2023, 485, 215142. [Google Scholar] [CrossRef]
- Zhou, J.; Xie, M.; Wu, F.; Mei, Y.; Hao, Y.; Li, L.; Chen, R. Encapsulation of metallic Zn in a hybrid MXene/Graphene aerogel as a stable Zn anode for foldable Zn−Ion batteries. Adv. Mater. 2022, 34, 2106897. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wang, X.; Zhou, S.; Zou, X.; Sun, H.; Liu, D.; Zhu, G. A specific free−volume network as synergistic zinc–ion–conductor interface towards stable zinc anode. Energy Storage Mater. 2022, 53, 909–916. [Google Scholar] [CrossRef]
- Kang, L.; Cui, M.; Jiang, F.; Gao, Y.; Luo, H.; Liu, J.; Liang, W.; Zhi, C. Nanoporous CaCO3 coatings enabled uniform Zn stripping/plating for long−life zinc rechargeable aqueous batteries. Adv. Energy Mater. 2018, 8, 1801090. [Google Scholar] [CrossRef]
- Tao, F.; Liu, Y.; Ren, X.; Wang, J.; Zhou, Y.; Miao, Y.; Ren, F.; Wei, S.; Ma, J. Different surface modification methods and coating materials of zinc metal anode. J. Energy Chem. 2022, 66, 397–412. [Google Scholar] [CrossRef]
- Naveed, A.; Yang, H.; Yang, J.; Nuli, Y.; Wang, J. Highly reversible and rechargeable safe Zn batteries based on a triethyl phosphate electrolyte. Angew. Chem. Int. Ed. 2019, 58, 2760–2764. [Google Scholar] [CrossRef] [PubMed]
- Naveed, A.; Yang, H.; Shao, Y.; Yang, J.; Yanna, N.; Liu, J.; Shi, S.; Zhang, L.; Ye, A.; He, B. A highly reversible Zn anode with intrinsically safe organic electrolyte for long−cycle−life batteries. Adv. Mater. 2019, 31, 1900668. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Cheng, Z.; Zhang, H.; Li, J.; Liu, D.; Liao, Y.; Meng, J.; Shen, Y.; Huang, Y. Monosodium glutamate, an effective electrolyte additive to enhance cycling performance of Zn anode in aqueous battery. Nano Energy 2022, 98, 107220. [Google Scholar] [CrossRef]
- Shen, F.; Du, H.; Qin, H.; Wei, Z.; Kuang, W.; Hu, N.; Lv, W.; Yi, Z.; Huang, D.; Chen, Z. Mediating triple ions migration behavior via a fluorinated separator interface toward highly reversible aqueous Zn batteries. Small 2024, 20, 2305119. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Li, X.; Li, N.; Xie, K.; Wang, J.-G.; Liu, X.; Wei, B. Graphene−boosted, high−performance aqueous Zn−ion battery. ACS Appl. Mater. Interfaces 2018, 10, 25446–25453. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Zhou, W.; Huang, A.; Chen, M.; Chen, J.; Tian, Q.; Xu, J. Modifying the Zn anode with carbon black coating and nanofibrillated cellulose binder: A strategy to realize dendrite−free Zn−MnO2 batteries. J. Colloid Interface Sci. 2020, 577, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; He, Q.; Li, Z.; Li, Q.; Zhang, Y.; Meng, J.; Liu, X.; Li, S.; Wu, B.; Chen, L. A novel dendrite−free Mn2+/Zn2+ hybrid battery with 2.3 V voltage window and 11000−cycle lifespan. Adv. Energy Mater. 2019, 9, 1901469. [Google Scholar] [CrossRef]
- Hao, J.; Li, B.; Li, X.; Zeng, X.; Zhang, S.; Yang, F.; Liu, S.; Li, D.; Wu, C.; Guo, Z. An in−depth study of Zn metal surface chemistry for advanced aqueous Zn−ion batteries. Adv. Mater. 2020, 32, 2003021. [Google Scholar] [CrossRef]
- He, H.; Tong, H.; Song, X.; Song, X.; Liu, J. Highly stable Zn metal anodes enabled by atomic layer deposited Al2O3 coating for aqueous zinc−ion batteries. J. Mater. Chem. A 2020, 8, 7836–7846. [Google Scholar] [CrossRef]
- Liang, P.; Yi, J.; Liu, X.; Wu, K.; Wang, Z.; Cui, J.; Liu, Y.; Wang, Y.; Xia, Y.; Zhang, J. Highly reversible Zn anode enabled by controllable formation of nucleation sites for Zn−based batteries. Adv. Funct. Mater. 2020, 30, 1908528. [Google Scholar] [CrossRef]
- Hieu, L.T.; So, S.; Kim, I.T.; Hur, J. Zn anode with flexible β−PVDF coating for aqueous Zn−ion batteries with long cycle life. Chem. Eng. J. 2021, 411, 128584. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, J.; Hu, Z.; Li, J.; Li, J.; Zhang, Y.; Wang, C.; Cui, G. Long−life and deeply rechargeable aqueous Zn anodes enabled by a multifunctional brightener−inspired interphase. Energy Environ. Sci. 2019, 12, 1938–1949. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, C.; Pan, J.; Tian, F.; Zeng, S.; Yang, J.; Qian, Y. Polypyrrole−controlled plating/stripping for advanced zinc metal anodes. Mater. Today Energy 2020, 17, 100443. [Google Scholar] [CrossRef]
- Pu, X.; Jiang, B.; Wang, X.; Liu, W.; Dong, L.; Kang, F.; Xu, C. High−performance aqueous zinc−ion batteries realized by MOF materials. Nano−Micro Lett. 2020, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chang, Z.; Qiao, Y.; Deng, H.; Mu, X.; He, P.; Zhou, H. Constructing a super−saturated electrolyte front surface for stable rechargeable aqueous zinc batteries. Angew. Chem. 2020, 132, 9463–9467. [Google Scholar] [CrossRef]
- Liu, M.; Yang, L.; Liu, H.; Amine, A.; Zhao, Q.; Song, Y.; Yang, J.; Wang, K.; Pan, F. Artificial solid−electrolyte interface facilitating dendrite−free zinc metal anodes via nanowetting effect. ACS Appl. Mater. Interfaces 2019, 11, 32046–32051. [Google Scholar] [CrossRef]
- Jin, S.; Chen, Z.; Bai, S.; Zhang, Y. Highly reversible Zn anode enabled by porous BaSO4 coating with wide band gap and high dielectric constant. J. Power Sources 2024, 591, 233894. [Google Scholar] [CrossRef]
- Lin, Y.; Ta, L.; Meng, J.; Song, Y.; Liu, X.-X. Electrodepositing amorphous molybdenum oxides for aqueous NH4+ storage. Chem. Commun. 2023, 59, 1481–1484. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yang, L.; Lu, X.; Mao, J.; Zhang, Y.; Wu, Y.; Tang, Y. Synthesis, characterization and lithium−storage performance of MoO2/carbon hybrid nanowires. J. Mater. Chem. 2010, 20, 2807–2812. [Google Scholar] [CrossRef]
- Shi, J.; Hou, Y.; Liu, Z.; Zheng, Y.; Wen, L.; Su, J.; Li, L.; Liu, N.; Zhang, Z.; Gao, Y. The high−performance MoO3−x/MXene cathodes for zinc−ion batteries based on oxygen vacancies and electrolyte engineering. Nano Energy 2022, 91, 106651. [Google Scholar] [CrossRef]
- Li, B.; Xue, J.; Lv, X.; Zhang, R.; Ma, K.; Wu, X.; Dai, L.; Wang, L.; He, Z. A facile coating strategy for high stability aqueous zinc ion batteries: Porous rutile nano−TiO2 coating on zinc anode. Surf. Coat. Technol. 2021, 421, 127367. [Google Scholar] [CrossRef]
- Noori, Y.J.; Thomas, S.; Ramadan, S.; Smith, D.E.; Greenacre, V.K.; Abdelazim, N.; Han, Y.; Beanland, R.; Hector, A.L.; Klein, N.; et al. Large−area electrodeposition of few−Layer MoS2 on graphene for 2D material heterostructures. ACS Appl. Mater. Interfaces 2020, 12, 49786–49794. [Google Scholar] [CrossRef]
- Bhoyate, S.; Mhin, S.; Jeon, J.-e.; Park, K.; Kim, J.; Choi, W. Stable and high−energy−density Zn−ion rechargeable batteries based on a MoS2−coated Zn anode. ACS Appl. Mater. Interfaces 2020, 12, 27249–27257. [Google Scholar] [CrossRef]
- Cao, H.-Z.; Tong, C.-J.; Zhang, H.-B.; Zheng, G.-Q. Mechanism of MoO2 electrodeposition from ammonium molybdate solution. Trans. Nonferrous Met. Soc. China 2019, 29, 1744–1752. [Google Scholar] [CrossRef]
- Ravichandran, K.; Dineshbabu, N.; Arun, T.; Manivasaham, A.; Sindhuja, E. Synergistic effects of Mo and F doping on the quality factor of ZnO thin films prepared by a fully automated home−made nebulizer spray technique. Appl. Surf. Sci. 2017, 392, 624–633. [Google Scholar] [CrossRef]
- Li, Y.; Guan, Q.; Cheng, J.; Wang, B. Amorphous H0.82MoO3.26 cathodes based long cyclelife fiber−shaped Zn−ion battery for wearable sensors. Energy Storage Mater. 2022, 49, 227–235. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, B.; Xu, J.; Jia, G.; Chen, S.; Rawat, R.S.; Fan, H.J. Rapid synthesis of cobalt nitride nanowires: Highly efficient and low-cost catalysts for oxygen evolution. Angew. Chem. 2016, 128, 8812–8816. [Google Scholar] [CrossRef]
- Cao, K.; Liu, H.; Li, Y.; Wang, Y.; Jiao, L. Encapsulating sulfur in δ−MnO2 at room temperature for Li−S battery cathode. Energy Storage Mater. 2017, 9, 78–84. [Google Scholar] [CrossRef]
- Wang, M.; Cao, C.; Su, F.; Wang, Y.; Liu, L.; Wang, W.; Zhang, W.; Shen, H.; Zhang, J. Optimized cyclic durability of α−MnO2 nanosheets for zinc ion storage through synergistic effect of lithium ions pre−embedding and electrolyte additives. Electrochim. Acta 2022, 403, 139699. [Google Scholar] [CrossRef]
- Ye, P.; Li, X.; He, K.; Dou, A.; Wang, X.; Naveed, A.; Zhou, Y.; Su, M.; Zhang, P.; Liu, Y. A semi−interpenetrating network polymer coating for dendrite−free Zn anodes. J. Power Sources 2023, 558, 232622. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, C.; Yu, Y.; Yan, M.; Wei, Q.; He, P.; Dong, Y.; Zhang, Z.; Wang, X.; Mai, L. Ultrathin surface coating enables stabilized zinc metal anode. Adv. Mater. Interfaces 2018, 5, 1800848. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.; Gu, C.; Zhang, X.; Okhawilai, M.; Wang, S.; Han, J.; Qin, J.; Huang, Y. Modulating Zn deposition via ceramic−cellulose separator with interfacial polarization effect for durable zinc anode. Nano Energy 2021, 89, 106322. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab−initio total energy calculations for metals and semiconductors using a plane−wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented−wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA−type density functional constructed with a long−range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Shi, Y.; Bian, W.; Wu, H.; Chen, Y.; Zhou, C.; Chen, X.; Zhang, W.; Shen, H. Hydrous Molybdenum Oxide Coating of Zinc Metal Anode via the Facile Electrodeposition Strategy and Its Performance Improvement Mechanisms for Aqueous Zinc−Ion Batteries. Molecules 2024, 29, 3229. https://doi.org/10.3390/molecules29133229
Yuan J, Shi Y, Bian W, Wu H, Chen Y, Zhou C, Chen X, Zhang W, Shen H. Hydrous Molybdenum Oxide Coating of Zinc Metal Anode via the Facile Electrodeposition Strategy and Its Performance Improvement Mechanisms for Aqueous Zinc−Ion Batteries. Molecules. 2024; 29(13):3229. https://doi.org/10.3390/molecules29133229
Chicago/Turabian StyleYuan, Jianwei, Yutao Shi, Weibai Bian, Huaren Wu, Yingjun Chen, Chengcheng Zhou, Xiaohui Chen, Wei Zhang, and Hailin Shen. 2024. "Hydrous Molybdenum Oxide Coating of Zinc Metal Anode via the Facile Electrodeposition Strategy and Its Performance Improvement Mechanisms for Aqueous Zinc−Ion Batteries" Molecules 29, no. 13: 3229. https://doi.org/10.3390/molecules29133229




