Evolution of Aroma Profiles in Vitis vinifera L. Marselan and Merlot from Grapes to Wines and Difference between Varieties
Abstract
1. Introduction
2. Results
2.1. Aroma Profile of the Ripe Berries of Two Varieties of Grapes
2.1.1. Free-Form Aroma Compounds in Two Varieties
| Aroma Components | Retention Index | Standards | Quantitative Methods | Concentrations (μg/kg) | |
|---|---|---|---|---|---|
| Marselan | Merlot | ||||
| C6/C9 compounds | |||||
| 1-Hexanal | 1090.2 | Hexanal | A.Q. | 6271.59 ± 821.86 ** | 3793.55 ± 302.11 |
| 3-Hexenal | 1142.1 | Hexanal | R.Q. | 439.67 ± 51.74 ** | 191.92 ± 35.84 |
| (E)-2-Hexenal | 1227.4 | (E)-2-Hexenal | A.Q. | 8507.44 ± 929.72 ** | 4190.59 ± 316.91 |
| 1-Hexen-3-ol | 1247.3 | (Z)-3-Hexenol | R.Q. | 21.85 ± 3.12 | 13.12 ± 8.58 |
| 1-Hexanol | 1349.2 | 1-Hexanol | A.Q. | 392.91 ± 22.53 * | 138.11 ± 21.36 |
| (Z)-3-Hexen-1-ol | 1381.7 | (Z)-3-Hexenol | A.Q. | 592.3 ± 49.64 ** | 9.28 ± 1.54 |
| 1-Nonanal | 1397.9 | Nonanal | A.Q. | 2.64 ± 0.39 | 2.01 ± 0.46 |
| (E)-2-Hexen-1-ol | 1402.4 | (E)-2-Hexenol | A.Q. | 1031.45 ± 288.38 ** | 298.84 ± 42.68 |
| (E,E)-2,4-Hexadienal | 1410.5 | (E,Z)-2,6-Nonadienal | R.Q. | 53.54 ± 6.01 ** | 30.33 ± 2.61 |
| (E)-2-Nonenal | 1543.1 | (E)-2-Nonenal | A.Q. | 0.30 ± 0.05 | 0.29 ± 0.01 |
| (E,Z)-2,6-Nonadienal | 1594.8 | (E,Z)-2,6-Nonadienal | A.Q. | 1.17 ± 0.23 | 1.08 ± 0.15 |
| 1-Nonanol | 1659.3 | 2-Nonanol | R.Q. | 0.25 ± 0.03 | 0.22 ± 0.05 |
| Straight-chain aliphatics | |||||
| 1-Octanal | 1005 | Octanal | A.Q. | 0.37 ± 0.1 | 0.26 ± 0.14 |
| 4-Hexen-1-ol acetate | 1321 | (Z)-3-Hexenyl acetate | R.Q. | 3.29 ± 0.35 | nd |
| (Z)-2-Penten-1-ol | 1317.1 | (Z)-2-Hexen-1-ol | R.Q. | 10.67 ± 0.59 * | 6.77 ± 1.79 |
| (Z)-2-Heptenal | 1190.6 | (Z)-2-Heptenal | A.Q. | 0.75 ± 0.19* | 0.35 ± 0.07 |
| (E)-2-Octenal | 1436.0 | (E)-2-Heptenal | R.Q. | 489,747.86 ± 36,722.12 | 411,913.44 ± 60,763.77 |
| 1-Octen-3-ol | 1446.8 | 1-Octen-3-ol | A.Q. | 0.75 ± 0.07 | 0.65 ± 0.47 |
| (E,E)-2,4-Heptadienal | 1501.0 | (E,Z)-2,6-Nonadienal | R.Q. | 2.05 ± 0.06 ** | 1.34 ± 0.18 |
| 1-Decanal | 1504.1 | Decanal | A.Q. | 0.45 ± 0.03 | 0.33 ± 0.1 |
| 1-Octanol | 1555.4 | 1-Octanol | A.Q. | 0.40 ± 0.04 | 0.27 ± 0.14 |
| (E)-2-Decenoic acid | 2271 | Decanoic acid | R.Q. | 0.34 ± 0.08 | 0.25 ± 0.02 |
| Aromatic compounds | |||||
| p-Cymene | 1278.1 | p-Cymene | A.Q. | 3.63 ± 0.17 ** | 0.61 ± 0.12 |
| Benzaldehyde | 1535.2 | Benzaldehyde | A.Q. | 24.31 ± 5.27 * | 7.12 ± 5.92 |
| Phenylacetaldehyde | 1655.8 | Phenylacetaldehyde | A.Q. | 120.39 ± 22.98 * | 43.17 ± 19.87 |
| Acetophenone | 1664.4 | Phenylacetaldehyde | R.Q. | 7.45 ± 1.02 | 7.13 ± 1.22 |
| Methyl salicylate | 1793.3 | Methyl salicylate | A.Q. | 0.99 ± 0.11 | 1.20 ± 0.04 * |
| Phenethyl alcohol | 1921.3 | Phenethyl alcohol | A.Q. | 56.33 ± 1.64 ** | 11.59 ± 3.45 |
| Phenol | 2015.8 | Phenol | A.Q. | 7.18 ± 0.62 | 6.08 ± 0.47 |
| Branched-chain aliphatics | |||||
| 2-Ethylhexanol | 1486.6 | 2-Ethylhexanol | A.Q. | 0.87 ± 0.15 | 0.77 ± 0.15 |
| Terpenes | |||||
| α-Terpinene | 1702.2 | Terpinolene | R.Q. | 4.25 ± 0.24 ** | nd |
| γ-Terpinene | 1238.5 | Terpinolene | R.Q. | 3.48 ± 0.31 ** | nd |
| Terpinolene | 1289.9 | Terpinolene | A.Q. | 2.72 ± 0.09 ** | nd |
| α-Terpineol | 1702.3 | α-Terpineol | A.Q. | 1.94 ± 0.37 ** | 0.30 ± 0.1 |
| Terpinen-4-ol | 1608.2 | α-Terpineol | R.Q. | 1.07 ± 0.12 ** | 0.29 ± 0.09 |
| Linalool | 1546.1 | Linalool | A.Q. | 4.10 ± 0.49 * | 0.36 ± 0.07 |
| L-Menthol | 1643.1 | α-Terpineol | R.Q. | 1.68 ± 0.18 | 1.65 ± 0.03 |
| Norisoprenoids | |||||
| (E)-β-Damascenone | 1835.3 | β-Damascenone | A.Q. | 32.07 ± 8.64 * | 10.40 ± 1.97 |
| α-Ionene | 1541.7 | β-Ionone | R.Q. | 1.39 ± 0.13 ** | nd |
2.1.2. Glycosidically Bound Aroma Compounds in Two Varieties
| Aroma Components | Retention Index | Standards | Quantitative Methods | Concentrations (μg/kg) | |
|---|---|---|---|---|---|
| Marselan | Merlot | ||||
| C6/C9 compounds | |||||
| 1-Hexanol | 1349.2 | 1-Hexanol | A.Q. | 63.7 ± 6.01 ** | 40.07 ± 5.15 |
| (Z)-3-Hexen-1-ol | 1381.7 | (Z)-3-Hexenol | A.Q. | 38.76 ± 4.49 ** | 11.08 ± 0.88 |
| (E)-2-Hexen-1-ol | 1402.4 | (E)-2-Hexenol | A.Q. | 44.27 ± 6.54 | 92.76 ± 3.52 ** |
| 2-Nonanol | 1084.2 | 2-Nonanol | A.Q. | 0.27 ± 0.02 | 0.28 ± 0.03 |
| 1-Nonanol | 1659.3 | 2-Nonanol | R.Q. | 0.32 ± 0.07 | 0.32 ± 0.13 |
| (Z)-3-Nonen-1-ol | 1685.6 | 2-Nonanol | R.Q. | 0.17 ± 0.03 | 0.20 ± 0.01 |
| Straight-chain aliphatics | |||||
| 2-Heptanol | 1315 | 2-Heptanol | A.Q. | 0.66 ± 0.09 ** | 0.24 ± 0.02 |
| 2-Octanol | 1550.6 | 1-Octanol | R.Q. | 0.26 ± 0.02 | 0.28 ± 0.03 |
| 1-Octen-3-ol | 1446.8 | 1-Octen-3-ol | A.Q. | 0.57 ± 0.09 | 1.24 ± 0.41 |
| 1-Heptanol | 1451.5 | 1-Heptanol | A.Q. | 0.68 ± 0.07 | 0.64 ± 0.2 |
| 1-Decanol | 1970.2 | 1-Decanol | A.Q. | 0.18 ± 0.05 | 0.19 ± 0.06 |
| 1-Octanol | 1555.4 | 1-Octanol | A.Q. | 0.58 ± 0.06 | 0.62 ± 0.22 |
| Branched-chain aliphatics | |||||
| Isoamyl alcohol | 1199.8 | 3-Methylpentanol | R.Q. | 7.91 ± 1.42 | 12.71 ± 5.75 |
| 2-Ethylhexanol | 1486.6 | 2-Ethylhexanol | A.Q. | 0.52 ± 0.19 | 0.57 ± 0.11 |
| Aromatic compounds | |||||
| Methyl salicylate | 1793.3 | Methyl salicylate | A.Q. | 1.85 ± 0.29 | 2.92 ± 1.43 |
| Benzyl alcohol | 1885.7 | Benzyl alcohol | A.Q. | 235.87 ± 28.17 | 512.25 ± 10.05 ** |
| Phenethyl alcohol | 1921.3 | Phenethyl alcohol | A.Q. | 181.72 ± 25.1 * | 106.52 ± 21.13 |
| Phenol | 2015.8 | Phenol | A.Q. | 4.50 ± 0.3 | 4.99 ± 0.37 |
| Terpenes | |||||
| Linalool | 1546.1 | Linalool | A.Q. | 1.59 ± 0.19 * | 0.76 ± 0.35 |
| α-Terpineol | 1702.3 | α-Terpineol | A.Q. | 1.42 ± 0.16 ** | 0.60 ± 0.11 |
| Norisoprenoids | |||||
| Vitispirane A | 1539.3 | β-Damascenone | R.Q. | 14.93 ± 1.58 ** | 8.15 ± 0.09 |
| Vitispirane B | 1542 | β-Damascenone | R.Q. | 13.82 ± 1.37 ** | 7.93 ± 0.39 |
| (E)-β-Damascenone | 1835.3 | β-Damascenone | A.Q. | 20.98 ± 1.6 | 19.11 ± 0.53 |
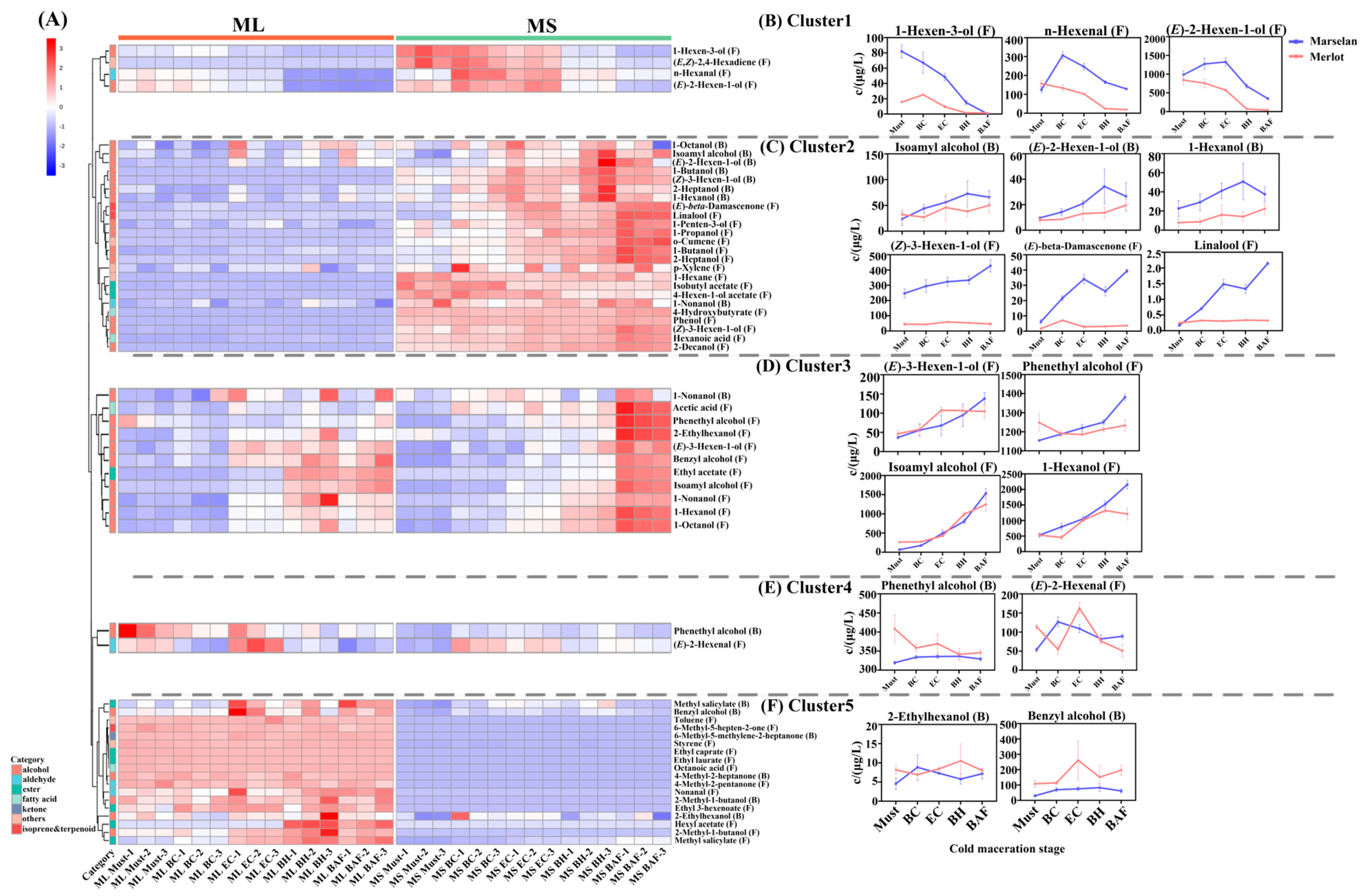
2.2. Evolution of Aroma Compounds during Cold Maceration Stage
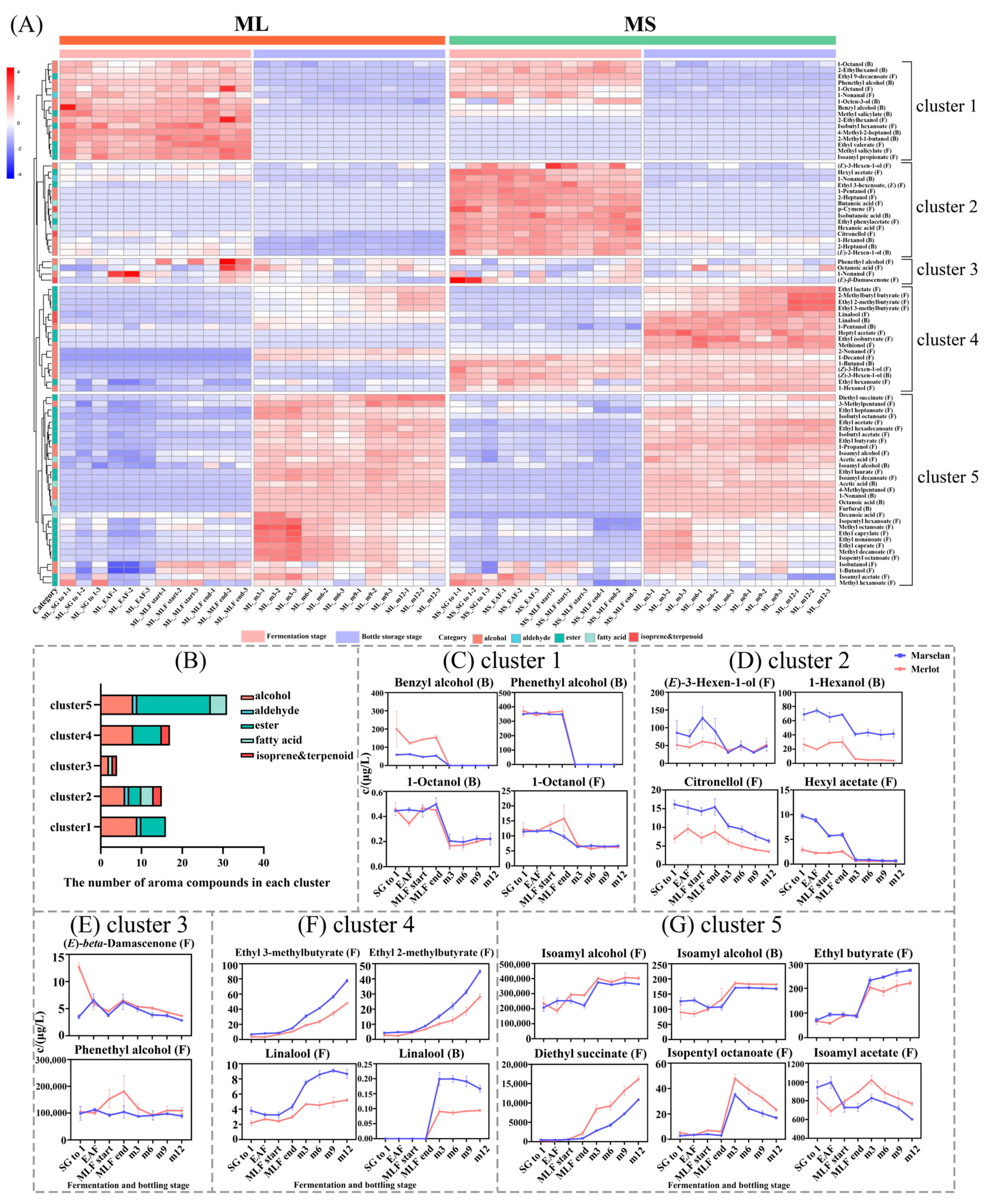
2.3. Evolution of Aroma Compounds during Fermentation and Bottle Storage
2.4. Aroma Characteristics of ‘Merlot’ and ‘Marselan’ Wines Based on Odor Activity Value
3. Discussion
4. Materials and Methods
4.1. Winemaking and Sampling
4.1.1. Collection of Grape Berries Samples
4.1.2. Winemaking and Sampling
4.2. Determination of Free and Bound Aroma Compounds in Grape Berries
4.2.1. Extraction of Aroma Compounds in Berries
4.2.2. Determination of Aroma Compounds in Grape
4.2.3. Identification and Quantification of Grape Aroma Compounds
4.3. Determination of Aroma Compounds in the Free and Bound States of Wine
4.3.1. Extraction of Wine Aroma Compounds
4.3.2. Determination of Wine Aroma Compounds
4.3.3. Determination of Free and Bound Aroma Compounds in Wine
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Before cold maceration |
| EC | After cold maceration |
| BH | Before heating |
| BAF | Before alcoholic fermentation |
| SG to 1 | Specific gravity down to 1.00 |
| EAF | The end of alcoholic fermentation |
| MLF start | The beginning of malolactic fermentation |
| MLF end | The end of malolactic fermentation |
| 3m | The bottle storage stages of 3 months |
| 6m | The bottle storage stages of 6 months |
| 9m | The bottle storage stages of 9 months |
| 12m | The bottle storage stages of 12 months |
| MS | Marselan |
| ML | Merlot |
| OVA | The odor activity value |
| VIP | Variance importance |
References
- Fariña, L.; Villar, V.; Ares, G.; Carrau, F.; Dellacassa, E.; Boido, E. Volatile composition and aroma profile of Uruguayan Tannat wines. Food Res. Int. 2015, 69, 244–255. [Google Scholar] [CrossRef]
- Azzi-Achkouty, S.; Estephan, N.; Ouaini, N.; Rutledge, D.N. Headspace solid-phase microextraction for wine volatile analysis. Crit. Rev. Food Sci. Nutr. 2017, 57, 2009–2020. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Massonnet, M.; Cantu, D. The genetic basis of grape and wine aroma. Hortic. Res. 2019, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Villamor, R.R.; Ross, C.F. Wine matrix compounds affect perception of wine aromas. Annu. Rev. Food Sci. Technol. 2013, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Selli, S.; Cabaroglu, T.; Canbas, A.; Erten, H.; Nurgel, C.; Lepoutre, J.P.; Gunata, Z. Volatile composition of red wine from cv. Kalecik Karasι grown in central Anatolia. Food Chem. 2004, 85, 207–213. [Google Scholar] [CrossRef]
- He, Y.; Wang, X.Y.; Li, P.H.; Lv, Y.C.; Nan, H.L.; Wen, L.K.; Wang, Z.T. Research progress of wine aroma components: A critical review. Food Chem. 2023, 402, 134491. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.V.; Block, D.E. A review of wine fermentation process modeling. J. Food Eng. 2020, 273, 109783. [Google Scholar] [CrossRef]
- De Castilhos, M.B.M.; Del Bianchi, V.L.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Sensory descriptive and comprehensive GC–MS as suitable tools to characterize the effects of alternative winemaking procedures on wine aroma. Part I: BRS Carmem and BRS Violeta. Food Chem. 2019, 272, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Pripis-Nicolau, L.; de Revel, G.; Bertrand, A.; Lonvaud-Funel, A. Methionine catabolism and production of volatile sulphur compounds by Œnococcus œni. J. Appl. Microbiol. 2004, 96, 1176–1184. [Google Scholar] [CrossRef]
- Liu, S.X.; Lou, Y.; Li, Y.X.; Zhao, Y.; Laaksonen, O.; Li, P.; Zhang, J.J.; Battino, M.; Yang, B.R.; Gu, Q. Aroma characteristics of volatile compounds brought by variations in microbes in winemaking. Food Chem. 2023, 420, 136075. [Google Scholar] [CrossRef]
- Alessandrini, M.; Gaiotti, F.; Belfiore, N.; Matarese, F.; D’Onofrio, C.; Tomasi, D. Influence of vineyard altitude on Glera grape ripening (Vitis vinifera L.): Effects on aroma evolution and wine sensory profile. J. Sci. Food Agric. 2007, 97, 2695–2705. [Google Scholar] [CrossRef] [PubMed]
- María, P.J.; Carolina, M.G.; Ángeles, P.B.M. Understanding human salivary esterase activity and its variation under wine consumption conditions. RSC Adv. 2020, 10, 24352–24361. [Google Scholar] [CrossRef]
- Tao, Y.S.; Li, N. Research progress on aroma compounds in wine. J. Food Sci. Technol. 2023, 41, 28–40, (In Chinese with English abstract). [Google Scholar]
- Casassa, L.F.; Bolcato, E.A.; Sari, S.E.; Sari, S.E. Effects of maceration length after prefermentative cold soak: Detailed chromatic, phenolic and sensory composition of cabernet sauvignon, malbec and merlot wines. J. Food Compos. Anal. 2021, 104, 104168. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; du Toit, W. Cold maceration application in red wine production and its effects on phenolic compounds: A review. LWT-Food Sci. Technol. 2018, 95, 200–208. [Google Scholar] [CrossRef]
- Álvarez, I.; Aleixandre, J.L.; García, M.J.; Lizama, V. Impact of prefermentative maceration on the phenolic and volatile compounds in Monastrell red wines. Anal. Chim. Acta 2006, 563, 109–115. [Google Scholar] [CrossRef]
- Naviglio, D.; Formato, A.; Scaglione, G.; Montesano, D.; Pellegrino, A.; Villecco, F.; Gallo, M. Study of the Grape Cryo-Maceration Process at Different Temperatures. Foods 2018, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, S.; Charpentier, C.; Feuillat, M.; Voilley, A. Influence of yeast walls on the behavior of aroma compounds in a model wine. Am. J. Enol. Vitic. 1994, 45, 29–33. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Z.; Xia, H.; Zhang, Q.; Zhang, J. Typical aroma of merlot dry red wine from eastern foothill of Helen mountain in Ningxia, China. Molecules 2023, 28, 5682. [Google Scholar] [CrossRef]
- Lyu, J.; Ma, Y.; Xu, Y.; Nie, Y.; Tang, K. Characterization of the key aroma compounds in Marselan wine by gas chromatography-olfactometry, ouantitative measurements, aroma recombination, and omission tests. Molecules 2019, 24, 2978. [Google Scholar] [CrossRef]
- Zhang, H.L.; Xia, N.Y.; Yao, X.C.; Duan, C.Q.; Pan, Q.H. Effects of phenolic evolution on color characteristics of single-cultivar Vitis vinifera L. Marselan and Merlot wines during vinification and aging. Foods 2024, 13, 494. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Pérez, A.; Vila-López, R.; Fernández-Fernández, J.I.; Martínez-Cutillas, A.; Gil-Muñoz, R. Influence of cold pre-fermentation treatments on the major volatile compounds of three wine varieties. Food Chem. 2013, 139, 770–776. [Google Scholar] [CrossRef]
- Alti-Palacios, L.; Martínez, J.; Teixeira, J.A.C.; Câmara, J.S.; Perestrelo, R. Influence of cold pre-fermentation maceration on the volatilomic pattern and aroma of white wines. Foods 2023, 12, 1135. [Google Scholar] [CrossRef] [PubMed]
- Malherbe, S.; Tredoux, A.G.J.; Nieuwoudt, H.H.; du Toit, M. Comparative metabolic profiling to investigate the contribution of O. oeni MLF starter cultures to red wine composition. J. Ind. Microbiol. Biotechnol. 2012, 39, 477–494. [Google Scholar] [CrossRef]
- Antalick, G.; Perello, M.C.; de Revel, G. Characterization of fruity aroma modifications in red wines during malolactic fermentation. J. Agric. Food Chem. 2012, 60, 12371–12383. [Google Scholar] [CrossRef] [PubMed]
- Dziadas, M.; Jeleń, H.H. Comparison of enzymatic and acid hydrolysis of bound flavor compounds in model system and grapes. Food Chem. 2016, 190, 412–418. [Google Scholar] [CrossRef]
- Yang, Y.; Frank, S.; Wei, X.B.; Wang, X.J.; Li, Y.K.; Steinhaus, M.; Tao, Y.S. Molecular rearrangement of four typical grape free terpenes in the wine environment. J. Agric. Food Chem. 2023, 71, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xing, R.R.; Li, Z.; Yang, D.M.; Pan, Q.H. Evolution of volatile compounds, aroma attributes, and sensory perception in bottle-aged red wines and their correlation. Eur. Food Res. Technol. 2016, 242, 1937–1948. [Google Scholar] [CrossRef]
- Lee, D.H.; Kang, B.S.; Park, H.J. Effect of oxygen on volatile and sensory characteristics of cabernet sauvignon during secondary shelf life. J. Agric. Food Chem. 2011, 59, 11657–11666. [Google Scholar] [CrossRef]
- Ugliano, M. Oxygen contribution to wine aroma evolution during bottle aging. J. Agric. Food Chem. 2013, 61, 6125–6136. [Google Scholar] [CrossRef]
- Dimkou, E.; Ugliano, M.; Dieval, J.B.; Vidal, S.; Aagaard, O.; Raunut, D.; Jung, R. Impact of Headspace oxygen and closure on sulfur dioxide, color, and hydrogen sulfide levels in a Riesling wine. Am. J. Enol. Vitic. 2011, 62, 261–269. [Google Scholar] [CrossRef]
- Ruiz-Moreno, M.J.; Muñoz-Redondo, J.M.; Cuevas, F.J.; Marrufo-Curtido, A.; León, J.M.; Ramírez, P.; Moreno-Rojas, J.M. The influence of pre-fermentative maceration and ageing factors on ester profile and marker determination of Pedro Ximenez sparkling wines. Food Chem. 2017, 230, 697–704. [Google Scholar] [CrossRef]
- Díaz-Maroto, M.C.; Schneider, R.; Baumes, R. Formation pathways of ethyl esters of branched short-chain fatty acids during wine aging. J. Agric. Food Chem. 2005, 53, 3503–3509. [Google Scholar] [CrossRef] [PubMed]
- Pittari, E.; Moio, L.; Piombino, P. Interactions between polyphenols and volatile compounds in wine: A literature review on physicochemical and sensory insights. Appl. Sci. 2021, 11, 1157. [Google Scholar] [CrossRef]
- Rodríguez-Bencomo, J.J.; Muñoz-González, C.; Andújar-Ortiz, I.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Pozo-Bayón, M.Á. Assessment of the effect of the non-volatile wine matrix on the volatility of typical wine aroma compounds by headspace solid phase microextraction/gas chromatography analysis. J. Sci. Food Agric. 2011, 91, 2484–2494. [Google Scholar] [CrossRef]
- Ling, M.Q.; Qi, M.Y.; Li, S.Y.; Shi, Y.; Pan, Q.H.; Cheng, C.F.; Yang, W.M.; Duan, C.Q. The influence of polyphenol supplementation on ester formation during red wine alcoholic fermentation. Food Chem. 2022, 377, 131961. [Google Scholar] [CrossRef]
- Yao, X.C.; Zhang, H.L.; Ma, X.R.; Xia, N.Y.; Duan, C.Q.; Yang, W.M.; Pan, Q.H. Leaching and evolution of anthocyanins and aroma compounds during Cabernet Sauvignon wine fermentation with whole-process skin-seed contact. Food Chem. 2023, 436, 137727. [Google Scholar] [CrossRef] [PubMed]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to prefermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Franco, M.; Peinado, R.A.; Medina, M.; Moreno, J. Off-vine grape drying effect on volatile compounds and aromatic series in must from Pedro Ximenez grape variety. J. Agric. Food Chem. 2004, 52, 3905–3910. [Google Scholar] [CrossRef] [PubMed]
- Culleré, L.; Cacho, J.; Ferreira, V. An Assessment of the Role Played by Some Oxidation-Related Aldehydes in Wine Aroma. J. Agric. Food Chem. 2007, 55, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.S.; Zhang, L. Intensity prediction of typical aroma characters of cabernet sauvignon wine in Changli County (China). LWT-Food Sci. Technol. 2010, 43, 1550–1556. [Google Scholar] [CrossRef]
- Buttery, R.G.; Seifert, R.M.; Guadagni, D.G.; Ling, L.C. Characterization of some volatile constituents of bell peppers. J. Agric. Food Chem. 1969, 17, 1322. [Google Scholar] [CrossRef] [PubMed]
- Sinuco, D.C.; Steinhaus, M.; Osorio, C.; Schieberle, P. Quantitation of the odour-active compounds in Andes berry (Rubus glaucus Benth) fruit using the molecular sensory approach. Eur. Food Res. Technol. 2013, 236, 373–378. [Google Scholar] [CrossRef]
- Peng, C.T.; Wen, Y.; Tao, Y.S.; Lan, Y.Y. Modulating the formation of meili wine aroma by prefermentative freezing process. J. Agric. Food Chem. 2013, 61, 1542–1553. [Google Scholar] [CrossRef] [PubMed]
- Guth, H. Identification of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3022–3026. [Google Scholar] [CrossRef]
- Moyano, L.; Zea, L.; Moreno, J.; Medina, M. Analytical study of aromatic series in Sherry wines subjected to biological aging. J. Agric. Food Chem. 2002, 50, 7356–7361. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, Y.-L.; Xia, N.-Y.; Wang, Y.-C.; Zhang, H.-L.; Yang, W.-M.; Duan, C.-Q.; Pan, Q.-H. Evolution of Aroma Profiles in Vitis vinifera L. Marselan and Merlot from Grapes to Wines and Difference between Varieties. Molecules 2024, 29, 3250. https://doi.org/10.3390/molecules29143250
Ge Y-L, Xia N-Y, Wang Y-C, Zhang H-L, Yang W-M, Duan C-Q, Pan Q-H. Evolution of Aroma Profiles in Vitis vinifera L. Marselan and Merlot from Grapes to Wines and Difference between Varieties. Molecules. 2024; 29(14):3250. https://doi.org/10.3390/molecules29143250
Chicago/Turabian StyleGe, Yi-Lin, Nong-Yu Xia, Ya-Chen Wang, Hua-Lin Zhang, Wei-Ming Yang, Chang-Qing Duan, and Qiu-Hong Pan. 2024. "Evolution of Aroma Profiles in Vitis vinifera L. Marselan and Merlot from Grapes to Wines and Difference between Varieties" Molecules 29, no. 14: 3250. https://doi.org/10.3390/molecules29143250
APA StyleGe, Y.-L., Xia, N.-Y., Wang, Y.-C., Zhang, H.-L., Yang, W.-M., Duan, C.-Q., & Pan, Q.-H. (2024). Evolution of Aroma Profiles in Vitis vinifera L. Marselan and Merlot from Grapes to Wines and Difference between Varieties. Molecules, 29(14), 3250. https://doi.org/10.3390/molecules29143250










