Effect of Cap Management Frequency on the Phenolic, Chromatic, and Sensory Composition of Cabernet Sauvignon Wines from the Central Coast of California over Two Vintages
Abstract
1. Introduction
2. Results and Discussion
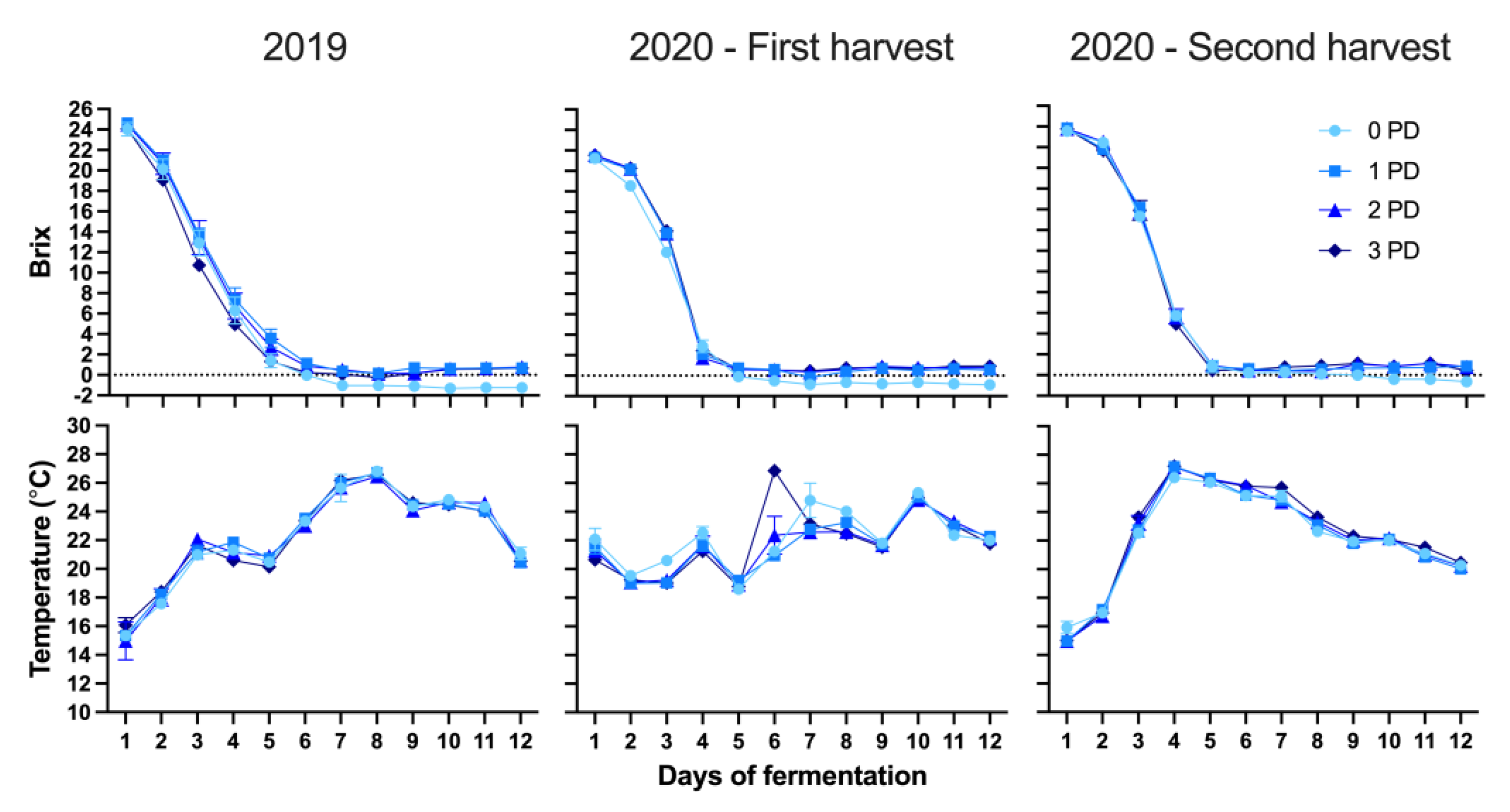
2.1. Basic Fruit and Wine Chemistry
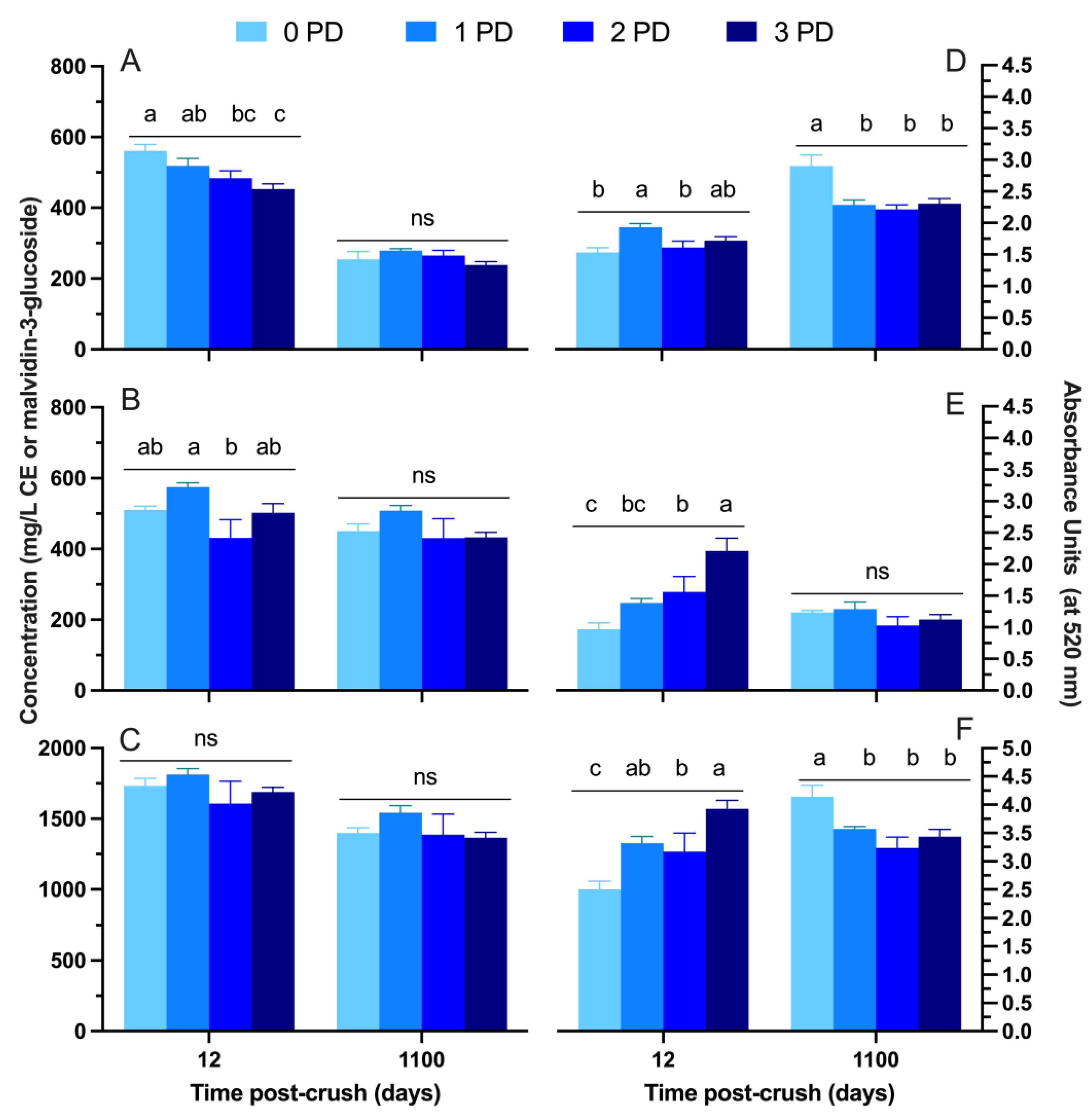
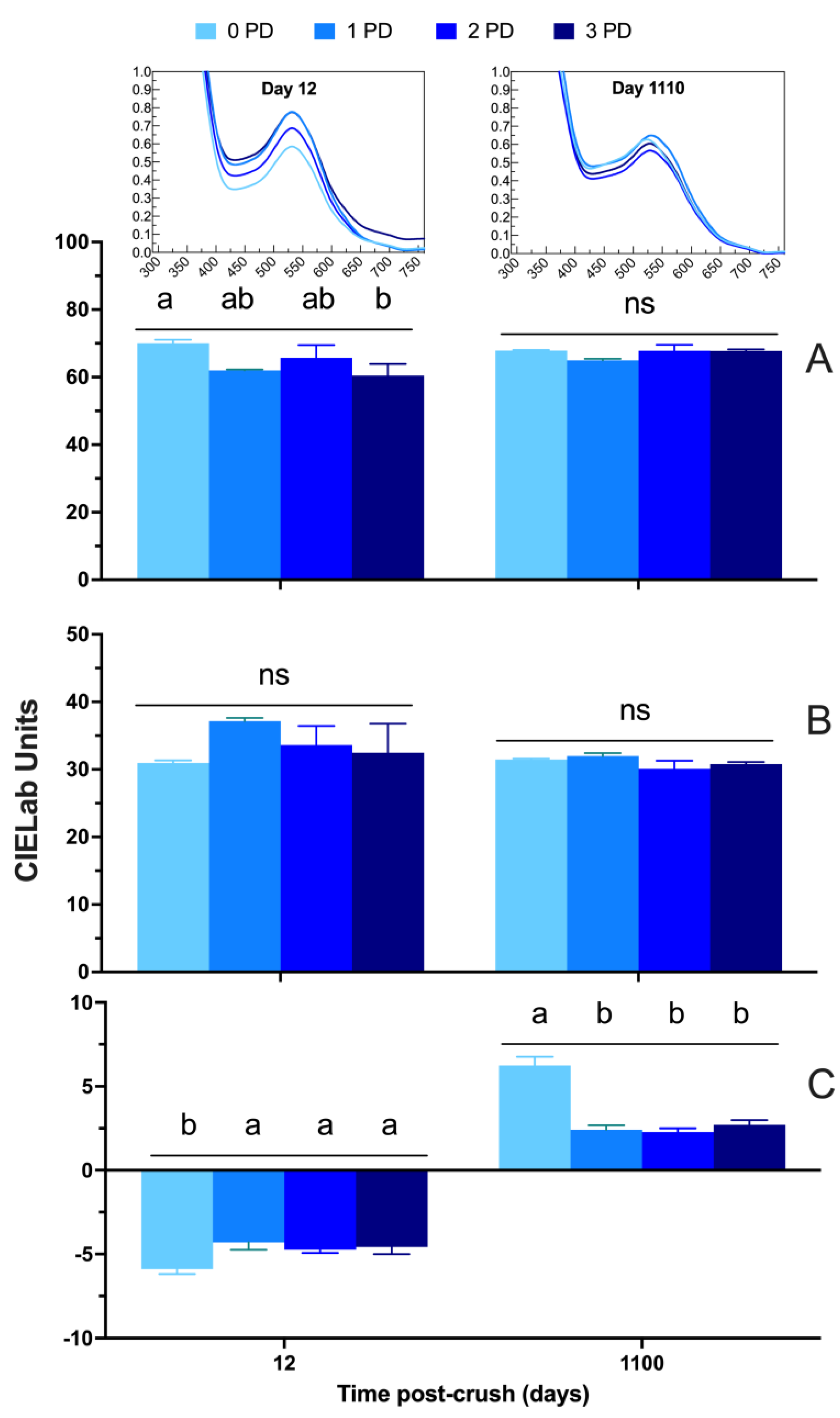
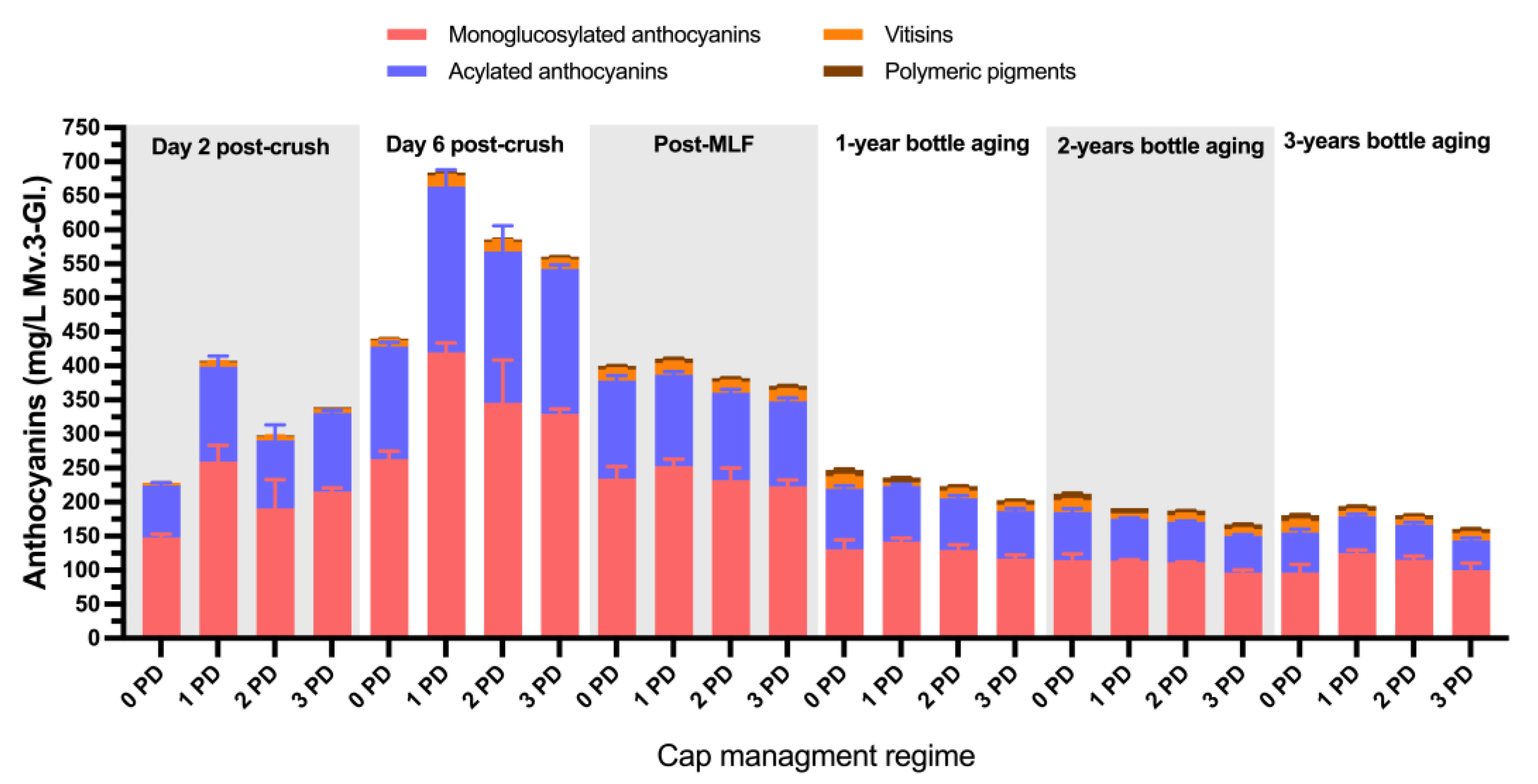
2.2. Phenolic and Chromatic Composition of the Wines
2.2.1. 2019 Vintage
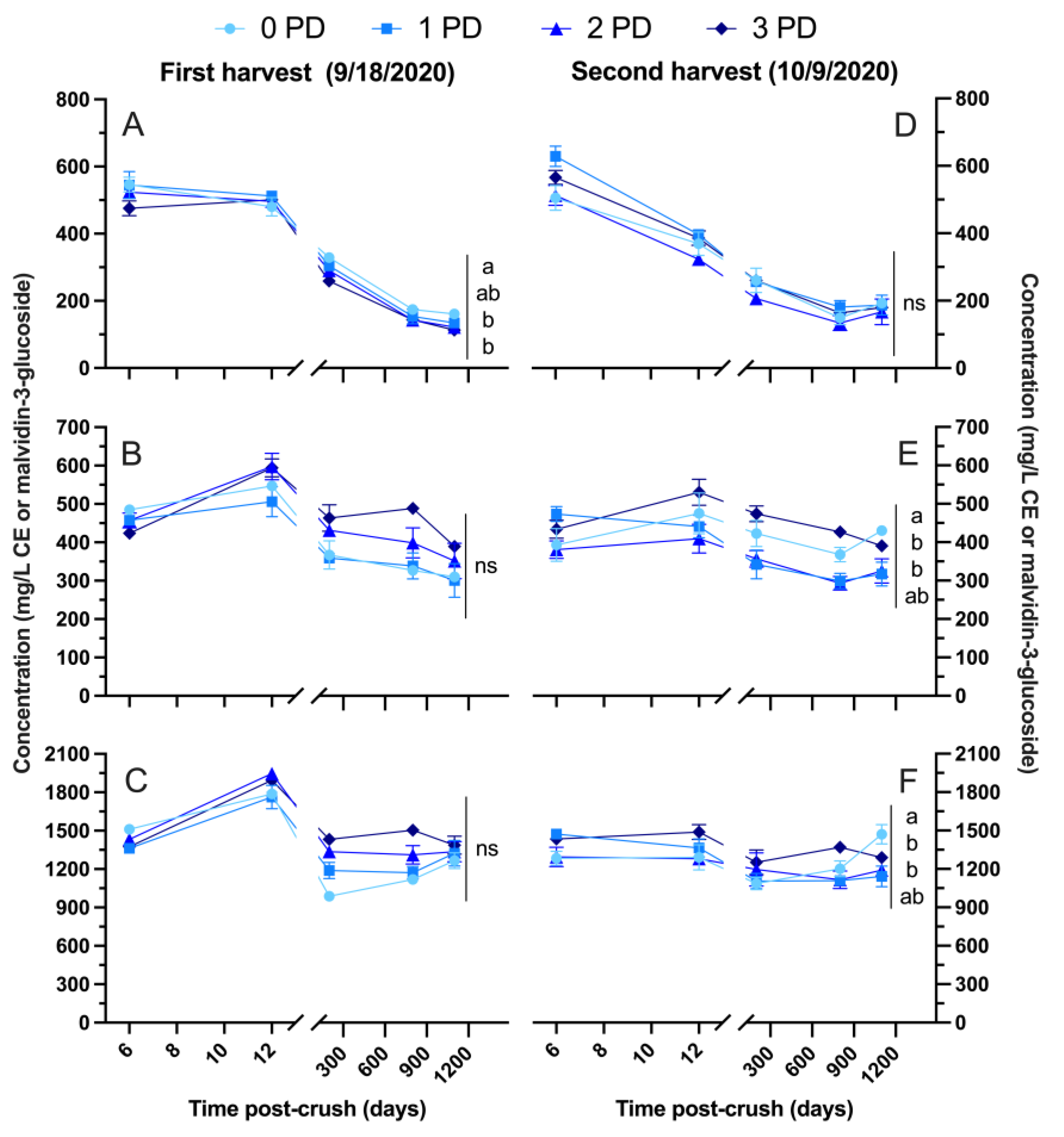
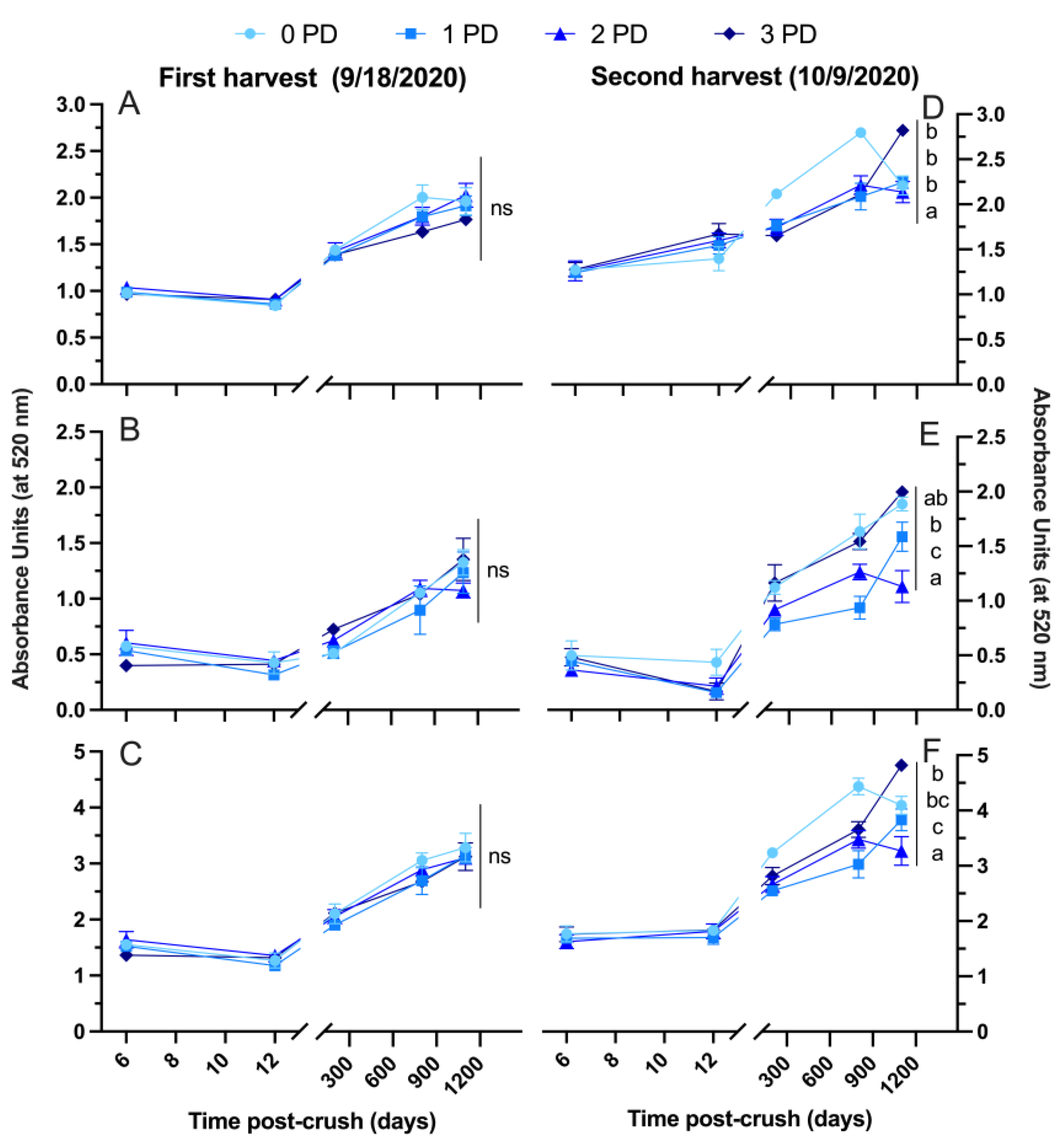
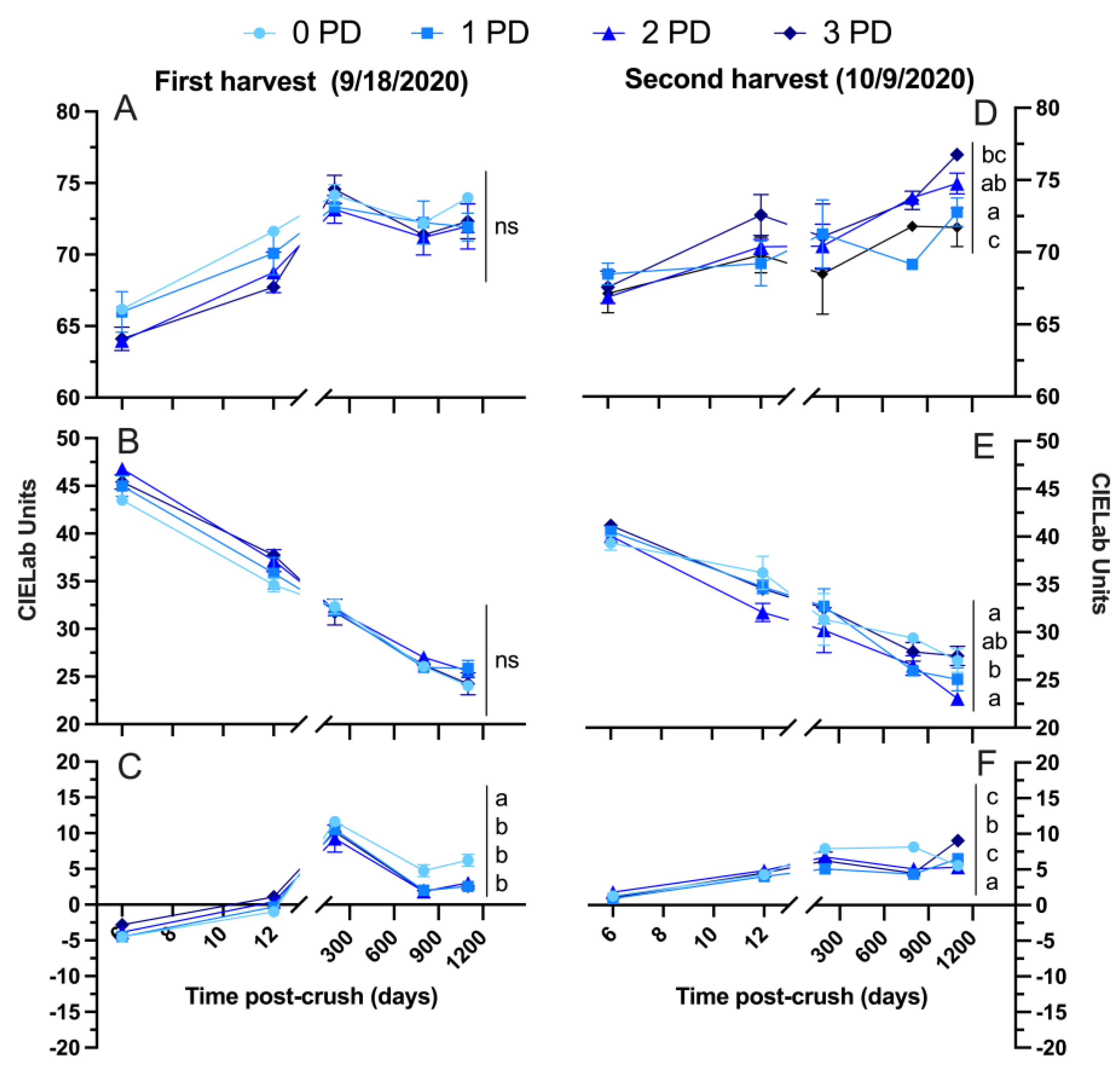
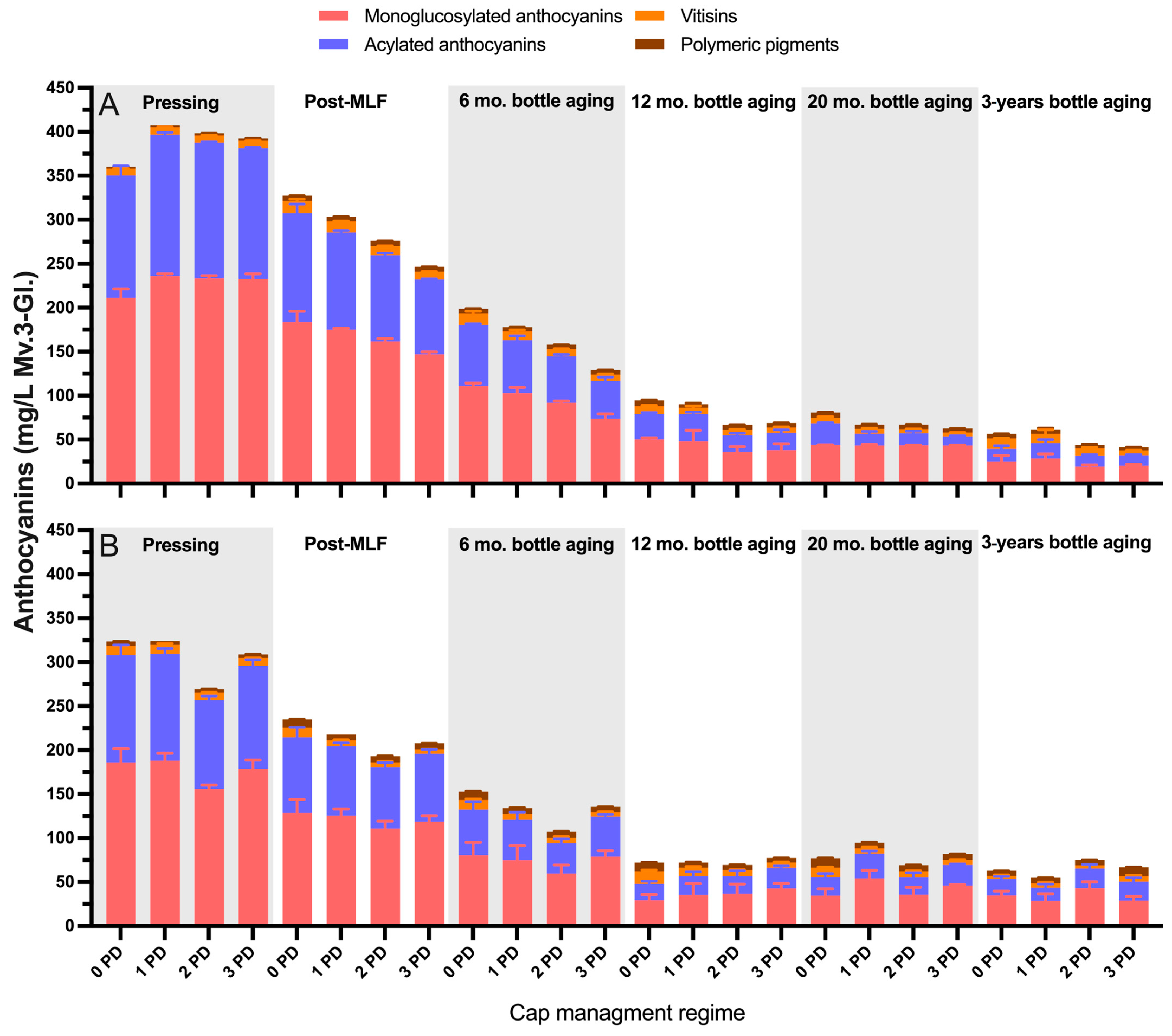
2.2.2. 2020 Vintage
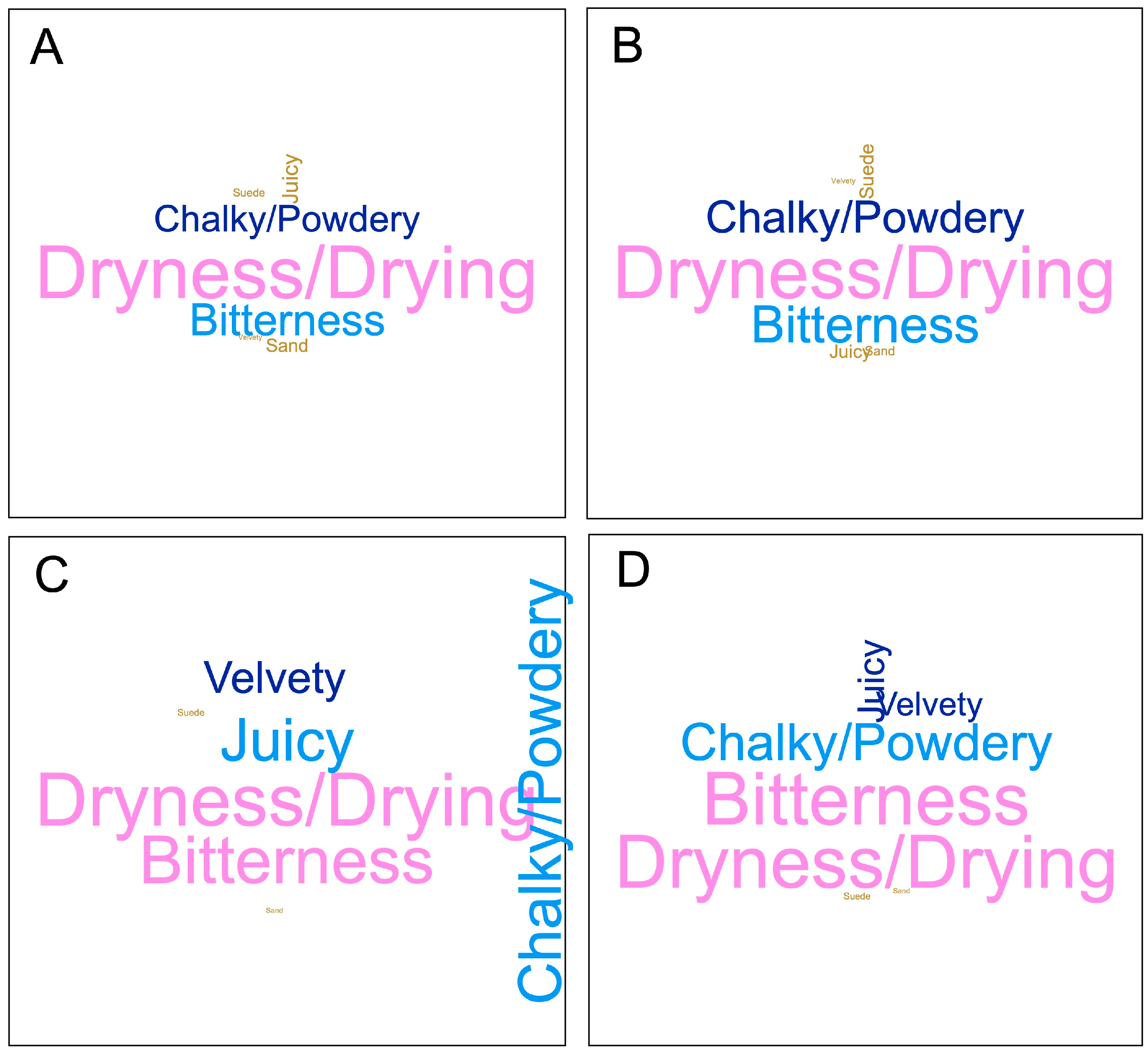
2.2.3. Sensory Analysis
3. Materials and Methods
3.1. Grapes and Vineyard Site
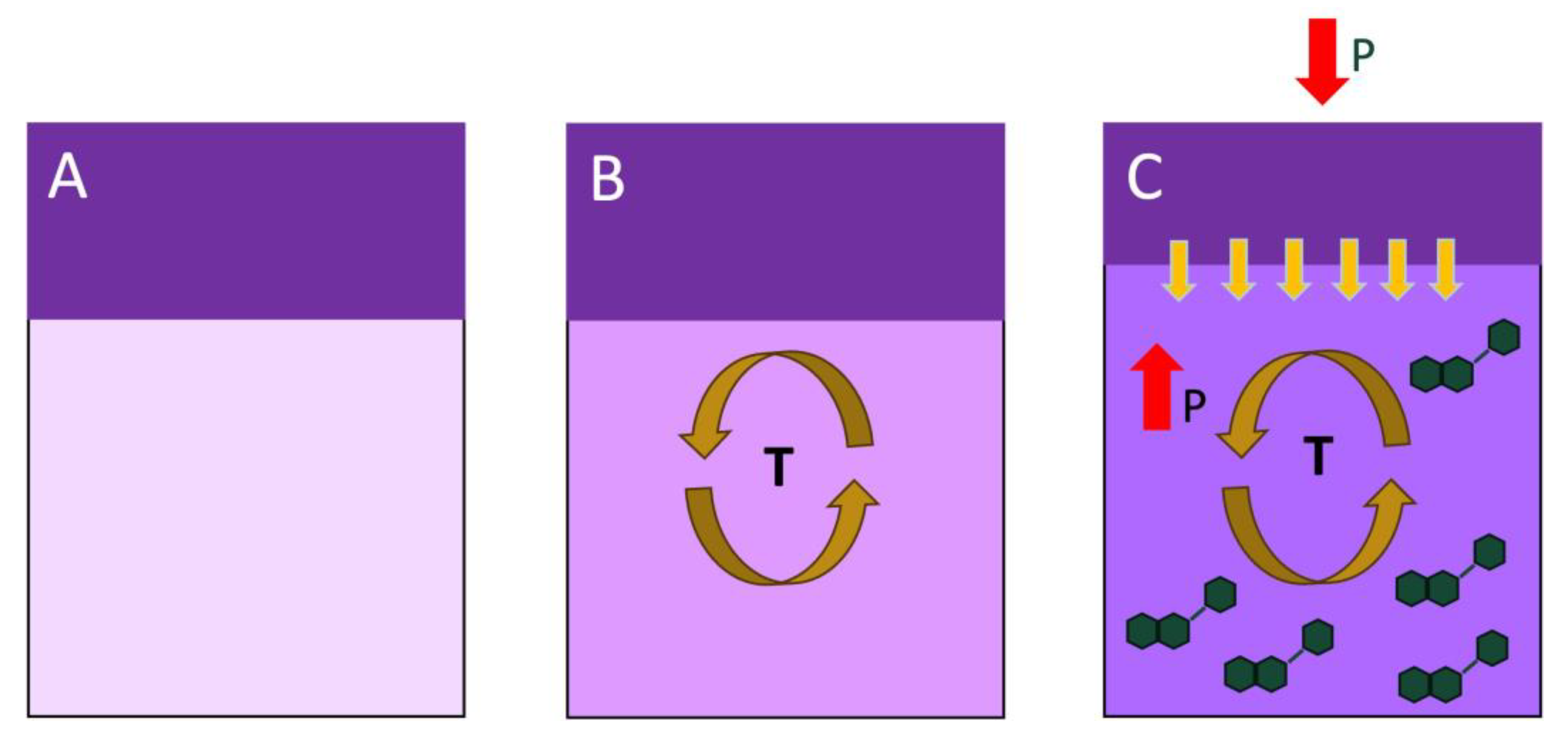
3.2. Winemaking and Experimental Design
3.3. Wine Basic Chemical Composition
3.4. Wine Spectrophotometric Analysis
3.5. Wine Analysis by HPLC-Diode Array Detector-MS
3.6. Sensory Analysis
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Setford, P.C.; Jeffery, D.W.; Grbin, P.R.; Muhlack, R.A. Factors Affecting Extraction and Evolution of Phenolic Compounds During Red Wine Maceration and the Role of Process Modelling. Trends Food Sci. Technol. 2017, 69, 106–117. [Google Scholar] [CrossRef]
- Yilmaztekin, M.; Kocabey, N.; Hayaloglu, A.A. Effect of Maceration Time on Free and Bound Volatiles of Red Wines from Cv. Karaoğlan (Vitis vinifera L.) Grapes Grown in Arapgir, Turkey. J. Food Sci. 2015, 80, C556–C563. [Google Scholar] [CrossRef] [PubMed]
- Casassa, L.F.; Beaver, C.W.; Mireles, M.S.; Harbertson, J.F. Effect of Extended Maceration and Ethanol Concentration on the Extraction and Evolution of Phenolics, Colour Components and Sensory Attributes of Merlot Wines. Aust. J. Grape Wine Res. 2013, 19, 25–39. [Google Scholar] [CrossRef]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and Their Variation in Red Wines I. Monomeric Anthocyanins and Their Color Expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef] [PubMed]
- Medina-Plaza, C.; Beaver, J.W.; Lerno, L.; Dokoozlian, N.; Ponangi, R.; Blair, T.; Block, D.E.; Oberholster, A. Impact of Temperature, Ethanol and Cell Wall Material Composition on Cell Wall-Anthocyanin Interactions. Molecules 2019, 24, 3350. [Google Scholar] [CrossRef] [PubMed]
- Lerno, L.; Reichwage, M.; Ponangi, R.; Hearne, L.; Block, D.E.; Oberholster, A. Effects of Cap and Overall Fermentation Temperature on Phenolic Extraction in Cabernet Sauvignon Fermentations. Am. J. Enol. Vitic. 2015, 66, 444–453. [Google Scholar] [CrossRef]
- Miller, K.V.; Noguera, R.; Beaver, J.; Medina-Plaza, C.; Oberholster, A.; Block, D.E. A Mechanistic Model for the Extraction of Phenolics from Grapes During Red Wine Fermentation. Molecules 2019, 24, 1275. [Google Scholar] [CrossRef] [PubMed]
- Frost, S.C.; Blackman, J.W.; Hjelmeland, A.K.; Ebeler, S.E.; Heymann, H. Extended Maceration and Cap Management Impacts on the Phenolic, Volatile, and Sensory Profiles of Merlot Wine. Am. J. Enol. Vitic. 2018, 69, 360–370. [Google Scholar] [CrossRef]
- Morata, A.; González, C.; Tesfaye, W.; Loira, I.; Suárez-Lepe, J.A. Chapter 3—Maceration and Fermentation: New Technologies to Increase Extraction. In Red Wine Technology; Morata, A., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 35–49. [Google Scholar]
- Bosso, A.; Panero, L.; Petrozziello, M.; Follis, R.; Motta, S.; Guaita, M. Influence of Submerged-Cap Vinification on Polyphenolic Composition and Volatile Compounds of Barbera Wines. Am. J. Enol. Vitic. 2011, 62, 503–511. [Google Scholar] [CrossRef]
- Chittenden, R.; Annand, M.; King, P.; Russell, G. The Effect of Half Plunging and No Plunging as Alternative Winemaking Techniques on Phenolic Extraction and Pigment Composition of Wine. S. Afr. J. Enol. Vitic. 2015, 36, 134–145. [Google Scholar] [CrossRef][Green Version]
- Casassa, L.F.; Kuster, S.; Perlette, D.J.; Bargetto, K.L. Chemical and Chromatic Composition of Wines Produced with Reduced Cap Management at Industrial Scale on Two Clones of Pinot Noir from California. J. Food Comp. Anal. 2024, 125, 105728. [Google Scholar] [CrossRef]
- Durner, D. 12—Improvement and Stabilization of Red Wine Color. In Handbook on Natural Pigments in Food and Beverages; Carle, R., Schweiggert, R.M., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 239–264. [Google Scholar]
- Adams, D.O.; Harbertson, J.F.; Picciotto, E.A. Fractionation of Red Wine Polymeric Pigments by Protein Precipitation and Bisulfite Bleaching. In Red Wine Color; Acs Symposium Series; American Chemical Society: Washington, DC, USA, 2004; Volume 886, pp. 275–288. [Google Scholar]
- Uysal, R.S.; Issa-Issa, H.; Sendra, E.; Carbonell-Barrachina, Á.A. Changes in Anthocyanin Pigments, Trans-Resveratrol, and Colorimetric Characteristics of Fondillón Wine and Other “Monastrell” Wines During the Aging Period. Eur. Food Res. Technol. 2023, 249, 1821–1831. [Google Scholar] [CrossRef]
- Allegro, G.; Pastore, C.; Valentini, G.; Muzzi, E.; Filippetti, I. Influence of Berry Ripeness on Accumulation, Composition and Extractability of Skin and Seed Flavonoids in cv. Sangiovese (Vitis vinifera L.). J. Sci. Food Agric. 2016, 96, 4553–4559. [Google Scholar] [CrossRef] [PubMed]
- Watrelot, A.A.; Heymann, H.; Waterhouse, A.L. Red Wine Dryness Perception Related to Physicochemistry. J. Agric. Food Chem. 2019, 68, 2964–2972. [Google Scholar] [CrossRef] [PubMed]
- Peleg, H.; Gacon, K.; Schlich, P.; Noble, A.C. Bitterness and Astringency of Flavan-3-ol Monomers, Dimers and Trimers. J. Sci. Food Agric. 1999, 79, 1123–1128. [Google Scholar] [CrossRef]
- Iland, P.; Bruer, N.; Edwards, G.; Caloghiris, S.; Wilkes, E. Chemical Analysis of Grapes and Wine Techniques and Concepts, 2nd ed.; Patrick Iland Wine Promotions Pty Ltd: Adelaide, Australia, 2012; p. 118. [Google Scholar]
- Harbertson, J.F.; Picciotto, E.A.; Adams, D.O. Measurement of Polymeric Pigments in Grape Berry Extracts and Wines Using a Protein Precipitation Assay Combined with Bisulfite Bleaching. Am. J. Enol. Vitic. 2003, 54, 301–306. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Kennedy, J.A.; Adams, D.O. Tannin in Skins and Seeds of Cabernet Sauvignon, Syrah, and Pinot Noir Berries During Ripening. Am. J. Enol. Vitic. 2002, 53, 54–59. [Google Scholar] [CrossRef]
- Pérez-Caballero, V.; Ayala, F.; Echávarri, J.F.; Negueruela, A.I. Proposal for a New Standard Oiv Method for Determination of Chromatic Characteristics of Wine. Am. J. Enol. Vitic. 2003, 54, 59–62. [Google Scholar] [CrossRef]
- Downey, M.O.; Rochfort, S. Simultaneous Separation by Reversed-Phase High-Performance Liquid Chromatography and Mass Spectral Identification of Anthocyanins and Flavonols in Shiraz Grape Skin. J. Chromatogr. A 2008, 1201, 43–47. [Google Scholar] [CrossRef] [PubMed]
| Vintage | Harvest Date | Brix | pH | Titratable Acidity (g/L Tartaric Acid) | Malic Acid (g/L) | Yeast Available Nitrogen (mg/L) | Potassium (mg/L) |
|---|---|---|---|---|---|---|---|
| 2019 | 10/15/2019 | 25.3 ± 0.05 | 3.63 ± 0.01 | 6.70 ± 0.05 | 1.06 ± 0.12 | 182 ± 13 | 2020 ± 100 |
| 2020 | 9/18/2020 | 20.9 ± 0.07 | 3.55 ± 0.02 | 5.32 ± 0.11 | 0.91 ± 0.14 | 194 ± 25 | 2230 ± 90 |
| 10/9/2020 | 24.7 ± 0.09 | 3.76 ± 0.02 | 4.20 ± 0.02 | 0.46 ± 0.03 | 177 ± 18 | 2405 ± 35 |
| Harvest | Winemaking Treatment | Ripeness Level | Alcohol % (v/v) | pH | Titratable Acidity (g/L) | Malic Acid (g/L) | Lactic Acid (g/L) | Acetic Acid (g/L) | Glucose + Fructose (g/L) |
|---|---|---|---|---|---|---|---|---|---|
| 2019 | 0 PD | Ripe | 14.22 ± 0.01 a | 3.94 ± 0.00 a | 5.12 ± 0.04 a | 0.05 ± 0.00 a | 1.39 ± 0.02 a | 0.34 ± 0.02 b | 0.33 ± 0.02 a |
| 1 PD | Ripe | 14.11 ± 0.01 a | 3.99 ± 0.01 a | 5.37 ± 0.11 a | 0.03 ± 0.03 b | 1.37 ± 0.00 a | 0.43 ± 0.04 a | 0.31 ± 0.05 a | |
| 2 PD | Ripe | 13.91 ± 0.01 a | 3.93 ± 0.00 a | 5.26 ± 0.03 a | 0.04 ± 0.00 ab | 1.40 ± 0.02 a | 0.42 ± 0.03 a | 0.27 ± 0.03 a | |
| 3 PD | Ripe | 13.86 ± 0.01 a | 3.95 ± 0.00 a | 5.14 ± 0.04 a | 0.00 ± 0.00 c | 1.38 ± 0.01 a | 0.44 ± 0.02 a | 0.31 ± 0.00 a | |
| p-value | 0.135 | 0.063 | 0.127 | 0.001 b | 0.830 | 0.004 | 0.373 | ||
| 2020 | 0 PD | Unripe | 11.73 ± 0.04 a | 3.95 ± 0.02 a | 4.82 ± 0.16 a | 0.05 ± 0.00 a | 1.21 ± 0.21 a | 0.45 ± 0.01 a | 0.23 ± 0.06 a |
| 1 PD | Unripe | 11.89 ± 0.02 a | 3.94 ± 0.01 a | 4.74 ± 0.35 a | 0.04 ± 0.01 a | 1.21 ± 0.17 a | 0.38 ± 0.02 a | 0.03 ± 0.06 a | |
| 2 PD | Unripe | 11.82 ± 0.02 a | 3.94 ± 0.01 a | 4.82 ± 0.17 a | 0.03 ± 0.04 a | 1.16 ± 0.07 a | 0.41 ± 0.02 a | 0.03 ± 0.02 a | |
| 3 PD | Unripe | 11.74 ± 0.04 a | 3.94 ± 0.04 a | 4.84 ± 0.22 a | 0.02 ± 0.00 b | 1.19 ± 0.11 a | 0.41 ± 0.04 a | 0.03 ± 0.01 a | |
| p-value | 0.168 | 0.752 | 0.239 | 0.012 | 0.424 | 0.279 | 0.219 | ||
| 0 PD | Ripe | 12.55 ± 0.03 a | 3.89 ± 0.06 a | 4.49 ± 0.22 a | 0.04 ± 0.02 a | 0.63 ± 0.11 a | 0.37 ± 0.04 a | 0.11 ± 0.03 a | |
| 1 PD | Ripe | 12.60 ± 0.02 a | 3.73 ± 0.04 a | 5.77 ± 0.12 a | 0.01 ± 0.02 a | 1.23 ± 0.12 a | 0.52 ± 0.04 a | 0.07 ± 0.01 a | |
| 2 PD | Ripe | 12.56 ± 0.03 a | 3.77 ± 0.06 a | 5.12 ± 0.32 a | 0.01 ± 0.02 a | 1.04 ± 0.02 a | 0.46 ± 0.02 a | 0.08 ± 0.01 a | |
| 3 PD | Ripe | 12.40 ± 0.02 a | 3.77 ± 0.04 a | 5.13 ± 0.22 a | 0.00 ± 0.00 a | 0.91 ± 0.13 a | 0.38 ± 0.04 a | 0.07 ± 0.01 a | |
| p-value | 0.330 | 0.562 | 0.523 | 0.280 | 0.638 | 0.138 | 0.919 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casassa, L.F.; LoMonaco, I.; Velasco, M.; Papageorgas, D.D. Effect of Cap Management Frequency on the Phenolic, Chromatic, and Sensory Composition of Cabernet Sauvignon Wines from the Central Coast of California over Two Vintages. Molecules 2024, 29, 2509. https://doi.org/10.3390/molecules29112509
Casassa LF, LoMonaco I, Velasco M, Papageorgas DD. Effect of Cap Management Frequency on the Phenolic, Chromatic, and Sensory Composition of Cabernet Sauvignon Wines from the Central Coast of California over Two Vintages. Molecules. 2024; 29(11):2509. https://doi.org/10.3390/molecules29112509
Chicago/Turabian StyleCasassa, L. Federico, Isabelle LoMonaco, Marcel Velasco, and Dimos D. Papageorgas. 2024. "Effect of Cap Management Frequency on the Phenolic, Chromatic, and Sensory Composition of Cabernet Sauvignon Wines from the Central Coast of California over Two Vintages" Molecules 29, no. 11: 2509. https://doi.org/10.3390/molecules29112509
APA StyleCasassa, L. F., LoMonaco, I., Velasco, M., & Papageorgas, D. D. (2024). Effect of Cap Management Frequency on the Phenolic, Chromatic, and Sensory Composition of Cabernet Sauvignon Wines from the Central Coast of California over Two Vintages. Molecules, 29(11), 2509. https://doi.org/10.3390/molecules29112509









