Facile Synthesis of Novel Magnetic Janus Graphene Oxide for Efficient and Recyclable Demulsification of Crude Oil-in-Water Emulsion
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of MJGO
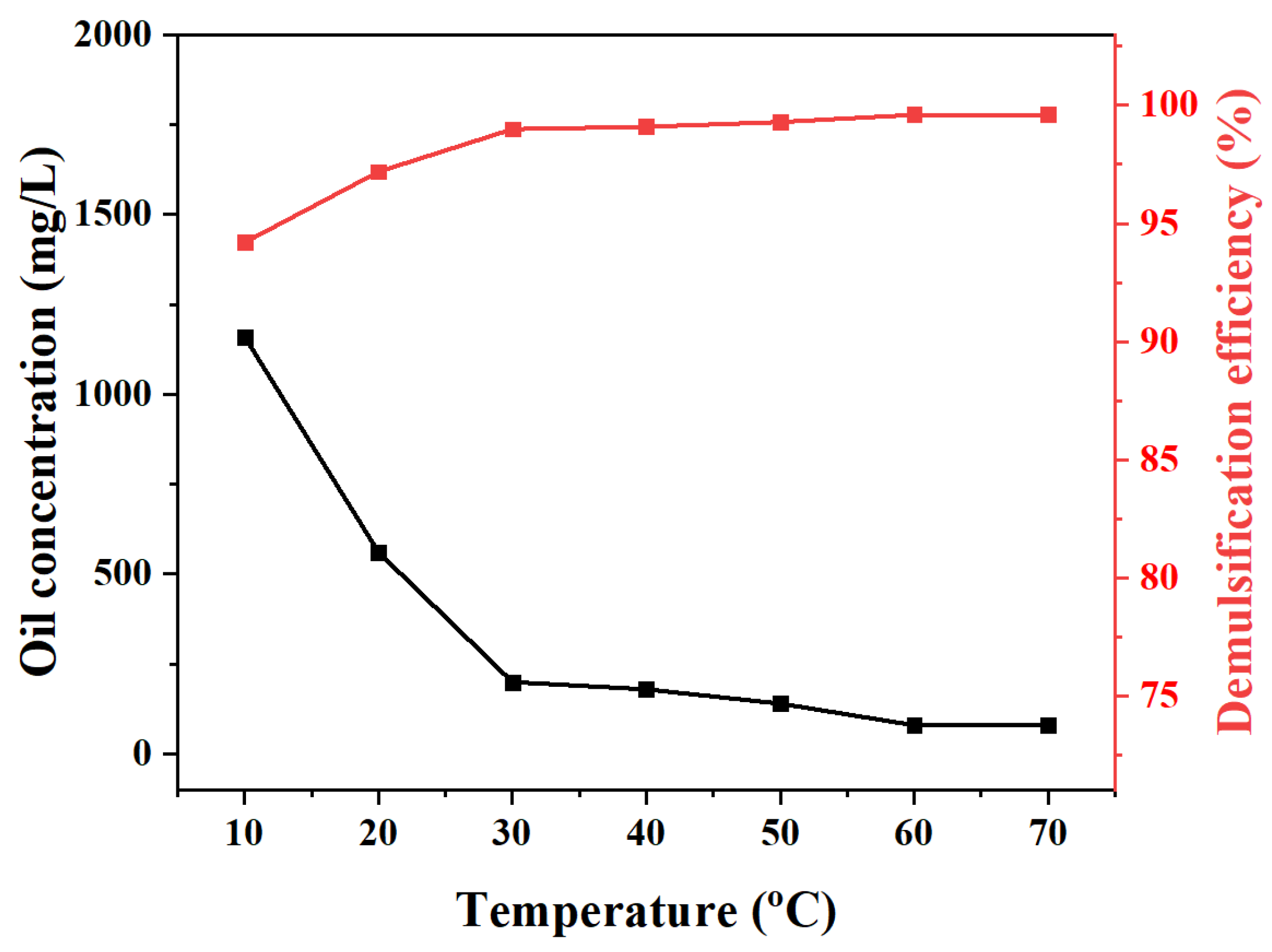
2.2. Demulsification Efficiency Tests of MJGO
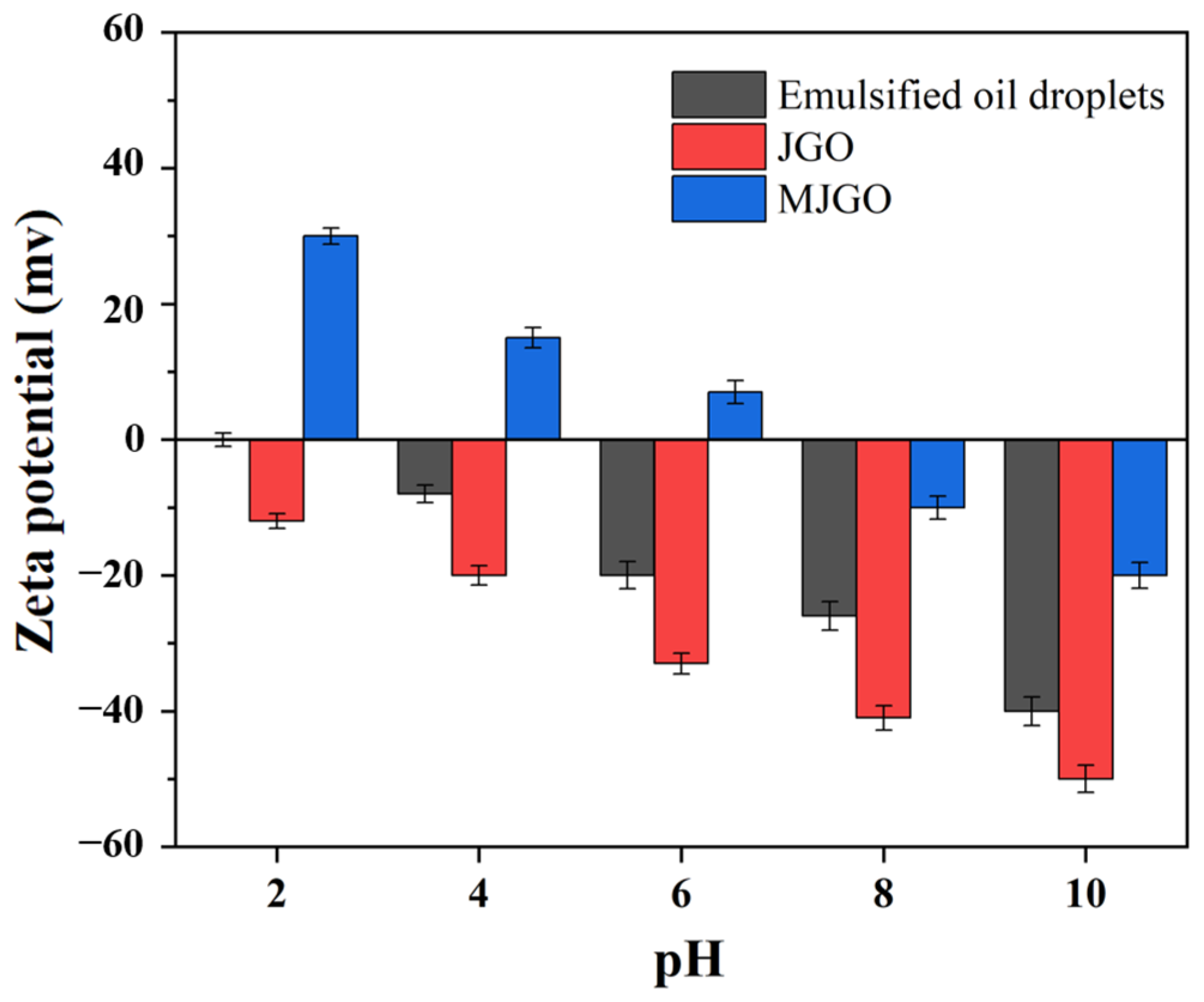
2.3. Demulsification Mechanism of MJGO
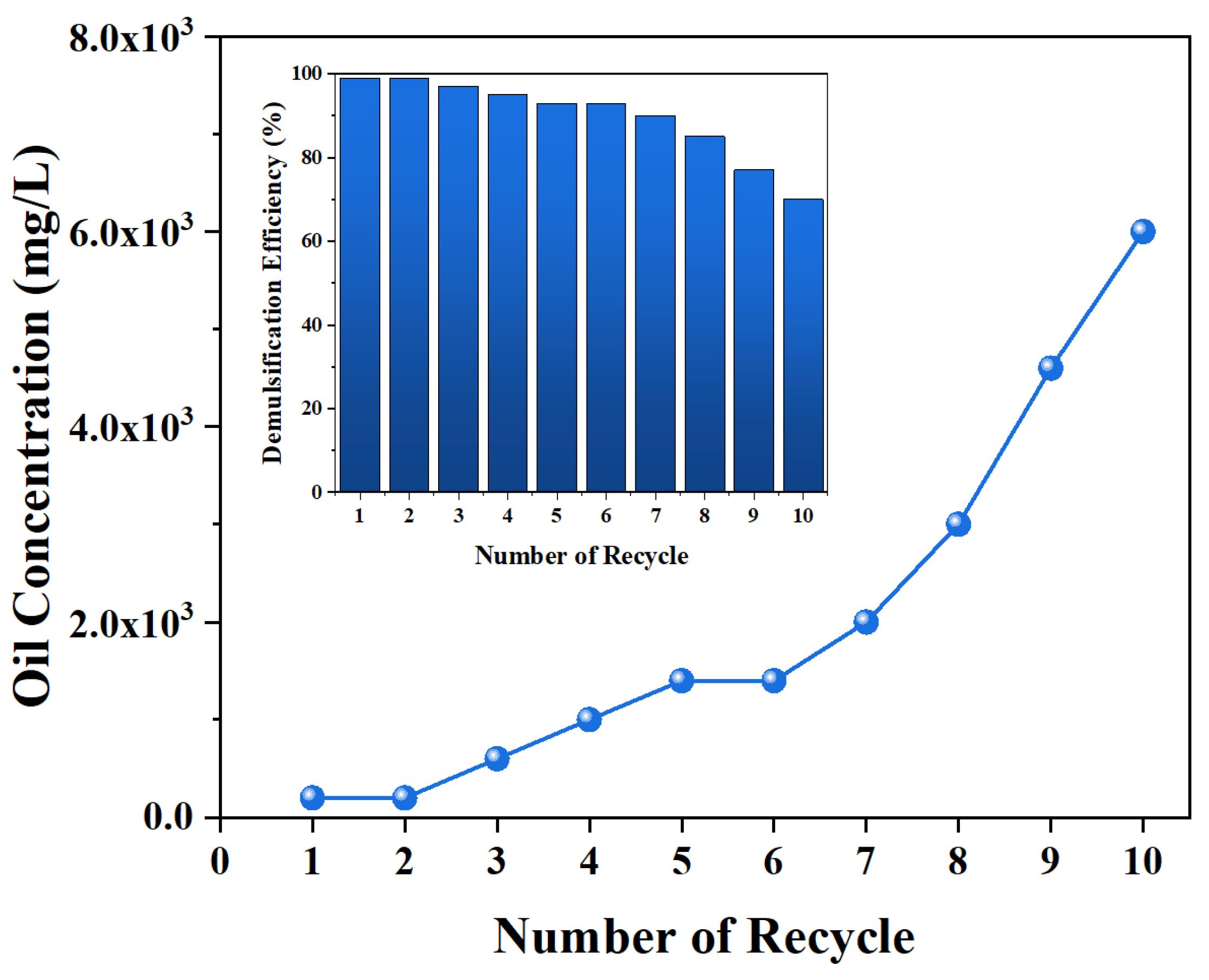
2.4. Recycling Tests of MJGO
3. Materials and Methods
3.1. Materials
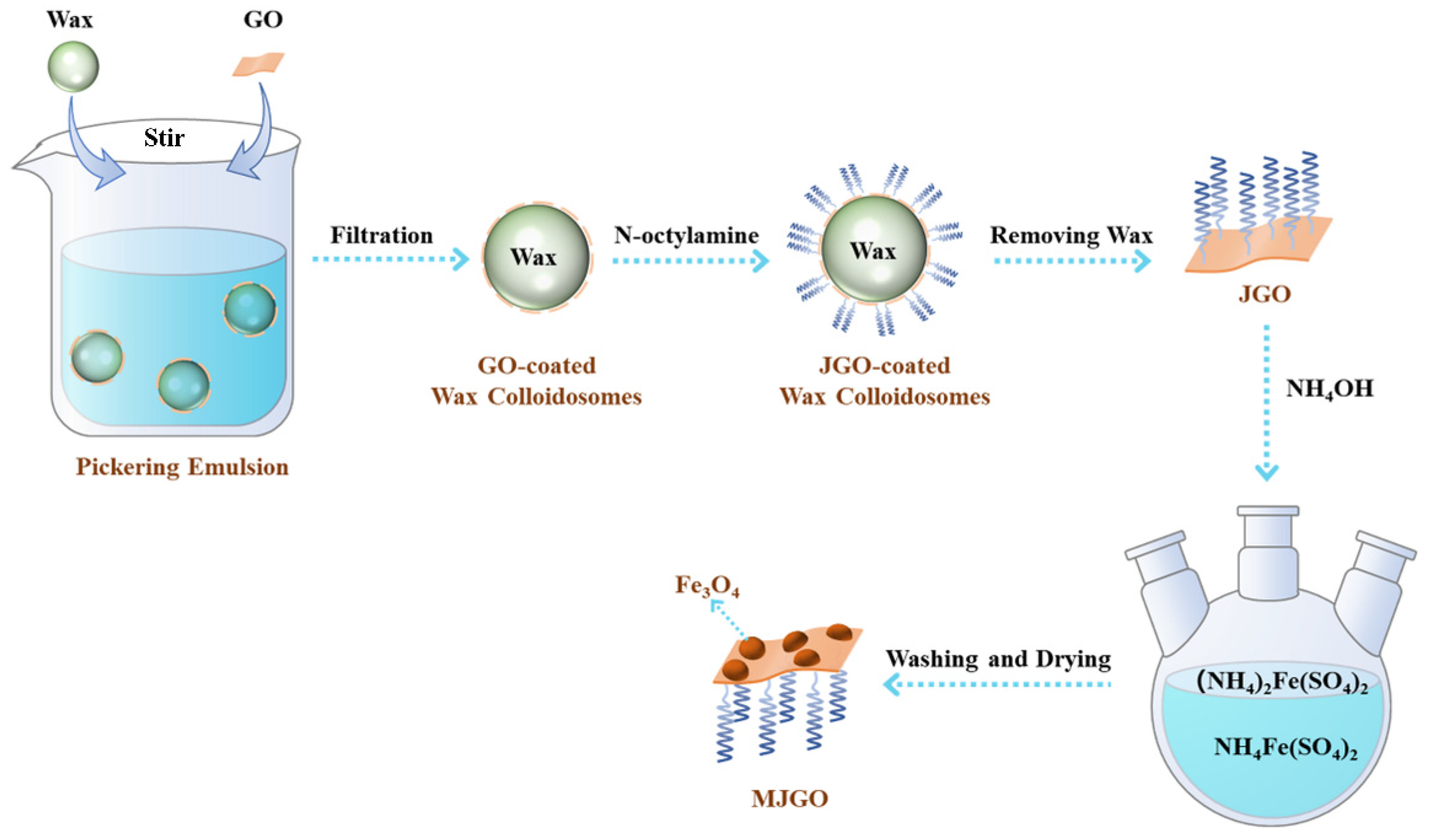
3.2. Preparation of MJGO
3.3. Characterization
3.4. Preparation of Crude Oil-in-Water Emulsion
3.5. Emulsion Separation
3.6. Recycle Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adetunji, A.I.; Olaniran, A.O. Treatment of industrial oily wastewater by advanced technologies: A review. Appl. Water Sci. 2021, 11, 98. [Google Scholar] [CrossRef]
- de Medeiros, A.D.M.; da Silva, J.C.G., Jr.; de Amorim, J.D.P.; Durval, I.J.B.; Costa, A.F.D.; Sarubbo, L.A. Oily Wastewater Treatment: Methods, Challenges, and Trends. Processes 2022, 10, 743. [Google Scholar] [CrossRef]
- Binazadeh, M. Cellulose derivative polymers for demulsification of lagoon wastewater-in-crude oil systems: An efficient water reuse strategy. J. Petrol. Sci. Eng. 2022, 211, 110120. [Google Scholar] [CrossRef]
- Chen, L.H.; Ye, F.; Liu, H.J.; Jiang, X.B.; Zhao, Q.M.; Ai, G.S.; Shen, L.W.; Feng, X.N.; Yang, Y.; Mi, Y.Z. Demulsification of oily wastewater using a nano carbon black modified with polyethyleneimine. Chemosphere 2022, 295, 133857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kuang, J.Z.; Li, B.; Mi, Y.Z.; Yang, Y.; Feng, X.N. The demulsification of oily wastewater by a hyperbranched polymer grafted SiO2. J. Dispers. Sci. Technol. 2023, 44, 651–659. [Google Scholar] [CrossRef]
- Alao, K.T.; Alara, O.R.; Abdurahman, N.H. Trending approaches on demulsification of crude oil in the petroleum industry. Appl. Petrochem. Res. 2021, 11, 281–293. [Google Scholar] [CrossRef]
- Wang, Y.B.; Du, C.C.; Yan, Z.F.; Duan, W.H.; Deng, J.; Luo, G.S. Rapid demulsification and phase separation in a miniaturized centrifugal demulsification device. Chem. Eng. J. 2022, 446, 137276. [Google Scholar] [CrossRef]
- Ye, F.; Shen, L.W.; Liu, S.; Liu, H.Y.; Zhang, X.Y.; Zhang, Z.J.; Yang, Y.; Feng, X.N.; Tang, Y.Q.; Xiang, D.; et al. Demulsification of amphiphilic gemini ionic liquids and its demulsification mechanism. Chemosphere 2022, 309, 136650. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.; Naderifar, A.; Rahidi, A.M.; Alaei, M. Recent advances in oil/water separation using nanomaterial-based filtration methods for crude oil processing-a review. J. Petrol. Sci. Eng. 2022, 215, 110617. [Google Scholar] [CrossRef]
- Mu, Y.; Liu, J.; Liu, J.; Ren, S.L. Preparation and demulsification performance of modified attapulgite nanoparticle demulsifier. Fuel 2022, 313, 123038. [Google Scholar] [CrossRef]
- Xie, H.S.; Zhao, W.Y.; Zhang, X.H.; Wang, Z.L. Demulsification of Bacteria-Stabilized Pickering Emulsions Using Modified Silica Nanoparticles. ACS Appl. Mater. Interfaces 2022, 14, 24102–24112. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Raza, A.; Imran, M.; Ul-Hamid, A.; New, A.S.; Ali, S. Hydrothermal Synthesis of Silver Decorated Reduced Graphene Oxide (rGO) Nanoflakes with Effective Photocatalytic Activity for Wastewater Treatment. Nanoscale Res. Lett. 2020, 15, 95. [Google Scholar] [CrossRef] [PubMed]
- Mittal, H.; Al Alili, A.; Morajkar, P.P.; Alhassan, S.M. GO crosslinked hydrogel nanocomposites of chitosan/carboxymethyl cellulose—A versatile adsorbent for the treatment of dyes contaminated wastewater. Int. J. Biol. Macromol. 2021, 167, 1248–1261. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Vasseghian, Y.; Khataee, A.; Harati, M.; Arfaeinia, H. Ultrasound-assisted synthesis of FeTiO3/GO nanocomposite for photocatalytic degradation of phenol under visible light irradiation. Sep. Purif. Technol. 2021, 261, 118274. [Google Scholar] [CrossRef]
- Velusamy, S.; Roy, A.; Sundaram, S.; Mallick, T.K. A Review on Heavy Metal Ions and Containing Dyes Removal Through Graphene Oxide-Based Adsorption Strategies for Textile Wastewater Treatment. Chem. Rec. 2021, 21, 1570–1610. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, X.C.; Jia, W.H.; Li, Z.Y.; Zhao, Y.P.; Ren, S.L. Demulsification of Crude Oil-in-Water Emulsions Driven by Graphene Oxide Nanosheets. Energy Fuels 2015, 29, 4644–4653. [Google Scholar] [CrossRef]
- Luo, D.; Wang, F.; Vu, B.V.; Chen, J.F.; Bao, J.M.; Cai, D.; Willson, R.C.; Ren, Z.F. Synthesis of graphene-based amphiphilic Janus nanosheets via manipulation of hydrogen bonding. Carbon 2018, 126, 105–110. [Google Scholar] [CrossRef]
- Huang, P.; Jia, H.; Wang, T.Y.; Xu, Y.B.; Zhang, L.Y.; Wei, X.; Jia, H.D.; Wen, S.J.; Lv, K.H.; Liu, D.X. Effects of Modification Degrees on the Colloidal Stability of Amphiphilic Janus Graphene Oxide in Aqueous Solution with and without Electrolytes. Langmuir 2021, 37, 10061–10070. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Huang, P.; Han, Y.G.; Wang, Q.X.; Wei, X.; Huang, W.J.; Dai, J.J.; Song, J.Y.; Yan, H.; Liu, D.X. Synergistic effects of Janus graphene oxide and surfactants on the heavy oil/water interfacial tension and their application to enhance heavy oil recovery. J. Mol. Liq. 2020, 314, 113791. [Google Scholar] [CrossRef]
- Lv, K.H.; Huang, P.; Zhou, Z.S.; Wei, X.; Luo, Q.; Huang, Z.M.; Yan, H.; Jia, H. Study of Janus Amphiphilic Graphene Oxide as a High-Performance Shale Inhibitor and Its Inhibition Mechanism. Front. Chem. 2020, 8, 201. [Google Scholar] [CrossRef]
- Alazab, A.A.; Saleh, T.A. Magnetic hydrophobic cellulose-modified polyurethane filter for efficient oil-water separation in a complex water environment. J. Water Process Eng. 2022, 50, 103125. [Google Scholar] [CrossRef]
- Huang, X.G.; Wei, J.W.; Zhang, Y.K.; Qian, B.B.; Jia, Q.; Liu, J.; Zhao, X.J.; Shao, G.F. Ultralight Magnetic and Dielectric Aerogels Achieved by Metal-Organic Framework Initiated Gelation of Graphene Oxide for Enhanced Microwave Absorption. Nano-Micro Lett. 2022, 14, 107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Peng, S.S.; Liu, Z.Y.; Li, Y.; Huang, J.; Li, J.; Wan, H.R.; Zhou, S.; Gao, Z.J.; Chen, T. Ag NPs decorated on the magnetic Fe3O4@PDA as efficient catalyst for organic pollutants removal and as effective antimicrobial agent for microbial inhibition. J. Alloys Compd. 2022, 928, 167257. [Google Scholar] [CrossRef]
- Xu, H.Y.; Jia, W.H.; Ren, S.L.; Wang, J.Q. Novel and recyclable demulsifier of expanded perlite grafted by magnetic nanoparticles for oil separation from emulsified oil wastewaters. Chem. Eng. J. 2018, 337, 10–18. [Google Scholar] [CrossRef]
- Chen, Y.N.; Lin, X.; Liu, N.; Cao, Y.Z.; Lu, F.; Xu, L.X.; Feng, L. Magnetically Recoverable Efficient Demulsifier for Water-in-Oil Emulsions. Chemphyschem 2015, 16, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Oketani, R.; Terada, T.; Leproux, P.; Morono, Y.; Kano, H. Label-Free Identification of Spore-Forming Bacteria Using Ultrabroadband Multiplex Coherent Anti-Stokes Raman Scattering Microspectroscopy. J. Phys. Chem. B 2023, 127, 1940–1946. [Google Scholar] [CrossRef] [PubMed]
- Jaberifard, F.; Arsalani, N.; Ghorbani, M.; Mostafavi, H. Incorporating halloysite nanotube/carvedilol nanohybrids into gelatin microsphere as a novel oral pH-sensitive drug delivery system. Colloids Surf. A 2022, 637, 128122. [Google Scholar] [CrossRef]
- Zuo, S.Y.; Li, D.Y.; Guan, Z.Y.; Yang, F.; Song, J.Q.; Xu, H.M.; Xia, D.S.; Li, H.X.; Li, X.H. A directional Built-in electric field mediates the electron transfer synergy mechanism of the Radical/Nonradical pathway in FeOCl-CuO. Chem. Eng. J. 2022, 430, 133004. [Google Scholar] [CrossRef]
- Nerantzaki, M.; Michel, A.; Petit, L.; Garnier, M.; Ménager, C.; Griffete, N. Biotinylated magnetic molecularly imprinted polymer nanoparticles for cancer cell targeting and controlled drug delivery. Chem. Commun. 2022, 58, 5642–5645. [Google Scholar] [CrossRef]
- Mirzaee, M.; Bahramian, B.; Gholampour, P.; Teymouri, S.; Khorsand, T. Preparation and characterization of Fe3O4@Boehmite core-shell nanoparticles to support molybdenum or vanadium complexes for catalytic epoxidation of alkenes. Appl. Organomet. Chem. 2019, 33, e4792. [Google Scholar] [CrossRef]
- Keyikoglu, R.; Karatas, O.; Khataee, A.; Kobya, M.; Can, O.T.; Soltani, R.D.C.; Isleyen, M. Peroxydisulfate activation by in-situ synthesized Fe3O4 nanoparticles for degradation of atrazine: Performance and mechanism. Sep. Purif. Technol. 2020, 247, 116925. [Google Scholar] [CrossRef]
- Angeloni, L.; Passeri, D.; Corsetti, S.; Peddis, D.; Mantovani, D.; Rossi, M. Single nanoparticles magnetization curves by controlled tip magnetization magnetic force microscopy. Nanoscale 2017, 9, 18000–18011. [Google Scholar] [CrossRef] [PubMed]
- Stan, C.; Cristescu, C.P.; Balasoiu, M.; Perov, N.; Duginov, V.N.; Mamedov, T.N.; Fetisov, L. Investigations of a Fe3O4-Ferrofluid at different temperatures by means of magnetic measurements. Univ. Politeh. Buchar. Sci. Bull. Ser. A 2011, 73, 117–124. [Google Scholar]
- Xu, Y.B.; Wang, Y.F.; Wang, T.Y.; Zhang, L.Y.; Xu, M.M.; Jia, H. Demulsification of Heavy Oil-in-Water Emulsion by a Novel Janus Graphene Oxide Nanosheet: Experiments and Molecular Dynamic Simulations. Molecules 2022, 27, 2191. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Naghmash, A.A. Evaluation of the separation efficiency of synthetic polymeric demulsifier for crude oil. Egypt. J. Chem. 2022, 65, 817–822. [Google Scholar] [CrossRef]
- Zaman, H.; Ali, N.; Shah, A.U.A.; Gao, X.Y.; Zhang, S.Z.; Hong, K.; Bilal, M. Effect of pH and salinity on stability and dynamic properties of magnetic composite amphiphilic demulsifier molecules at the oil-water interface. J. Mol. Liq. 2019, 290, 111186. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, G.; Zhu, L.J.; Deng, W.S.; Wang, C.T.; Ren, T.H.; Zhu, B.K.; Zeng, Z.X. Desert beetle-like microstructures bridged by magnetic Fe3O4 grains for enhancing oil-in-water emulsion separation performance and solar-assisted recyclability of graphene oxide. Chem. Eng. J. 2022, 427, 130904. [Google Scholar] [CrossRef]
- Azizi, N.; Bashipour, F. Demulsification of water-in-oil emulsions applying Fe3O4 magnetic nanoparticles for demulsifier modification: Experimental optimization via response surface methodology. J. Petrol. Sci. Eng. 2022, 216, 110806. [Google Scholar] [CrossRef]
- Aleem, W.; Mellon, N. Model for the prediction of separation profile of oil-in-water emulsion. J. Dispers. Sci. Technol. 2018, 39, 8–17. [Google Scholar] [CrossRef]
- Arab, D.; Kantzas, A.; Bryant, S.L. Nanoparticle stabilized oil in water emulsions: A critical review. J. Petrol. Sci. Eng. 2018, 163, 217–242. [Google Scholar] [CrossRef]
- Ge, B.; Han, L.; Gao, B.; Zhang, T.X.; Li, X.L.; Zhu, X.T.; Pu, X.P.; Li, W.Z. A mesoporous SiO2/TiO2 composite used for various emulsions separation. Sep. Sci. Technol. 2019, 54, 962–969. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, X.W.; Wang, Y.L.; Qi, Y.F.; Zhang, Y.N.; Luo, J.L.; Cui, P.; Jiang, W. A review on oil/water emulsion separation membrane material. J. Environ. Chem. Eng. 2022, 10, 107257. [Google Scholar] [CrossRef]
- Zuo, J.H.; Chen, J.H.; Wen, X.F.; Xu, S.P.; Pi, P.H. Advanced Materials for Separation of Oil/Water Emulsion. Prog. Chem. 2019, 31, 1440–1458. [Google Scholar] [CrossRef]
- Gyergyek, S.; Lisjak, D.; Bekovic, M.; Grilc, M.; Likozar, B.; Necemer, M.; Makovec, D. Magnetic Heating of Nanoparticles Applied in the Synthesis of a Magnetically Recyclable Hydrogenation Nanocatalyst. Nanomaterials 2020, 10, 1142. [Google Scholar] [CrossRef] [PubMed]
- Song, X.L.; Li, L.; Geng, Z.R.; Zhou, L.; Ji, L.J. Effective and selective adsorption of As(III) via imprinted magnetic Fe3O4/HTCC composite nanoparticles. J. Environ. Chem. Eng. 2017, 5, 16–25. [Google Scholar] [CrossRef]
- Zhang, S.X.; Zhang, Y.Y.; Liu, J.S.; Xu, Q.; Xiao, H.Q.; Wang, X.Y.; Xu, H.; Zhou, J. Thiol modified Fe3O4@SiO2 as a robust, high effective, and recycling magnetic sorbent for mercury removal. Chem. Eng. J. 2013, 226, 30–38. [Google Scholar] [CrossRef]
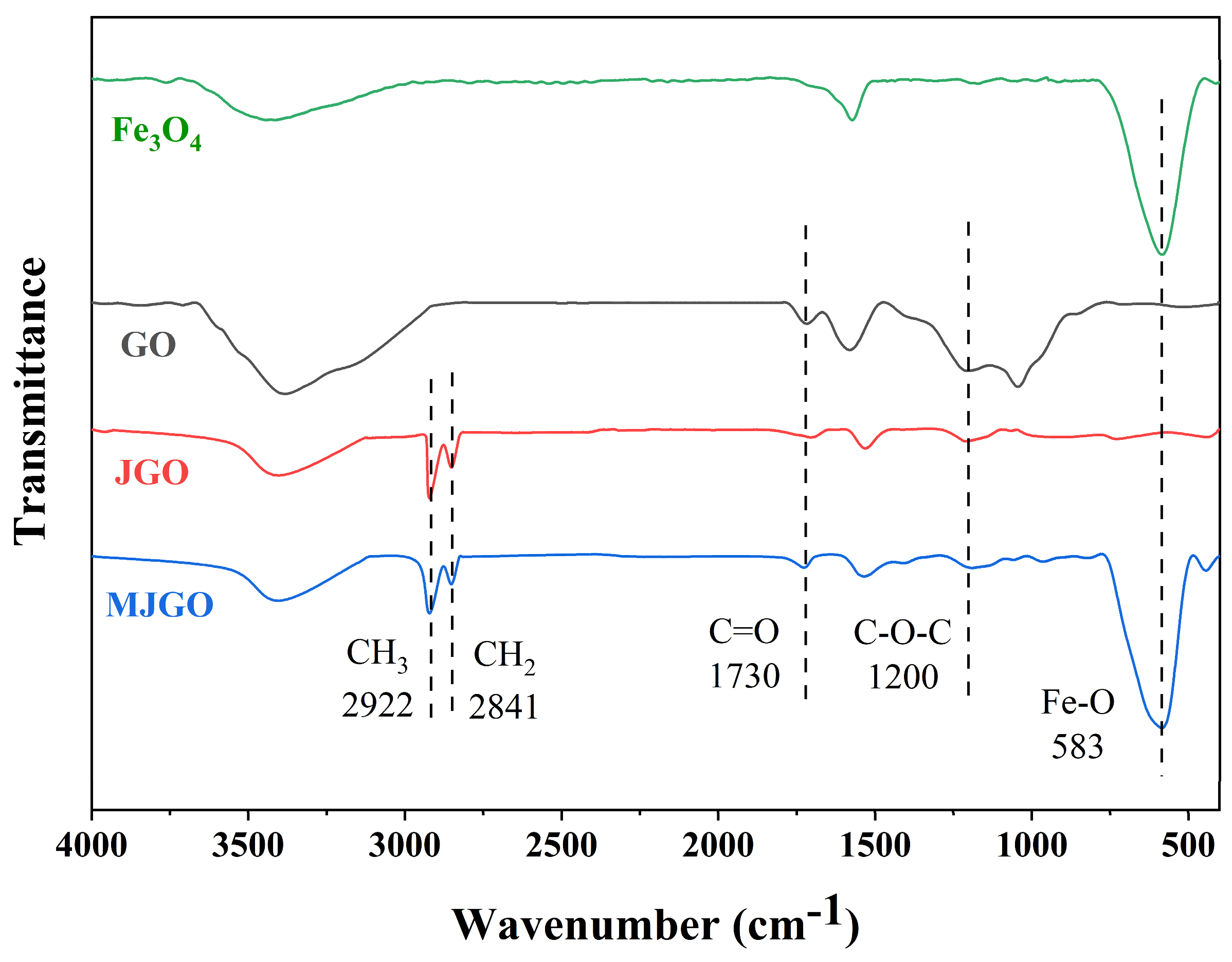
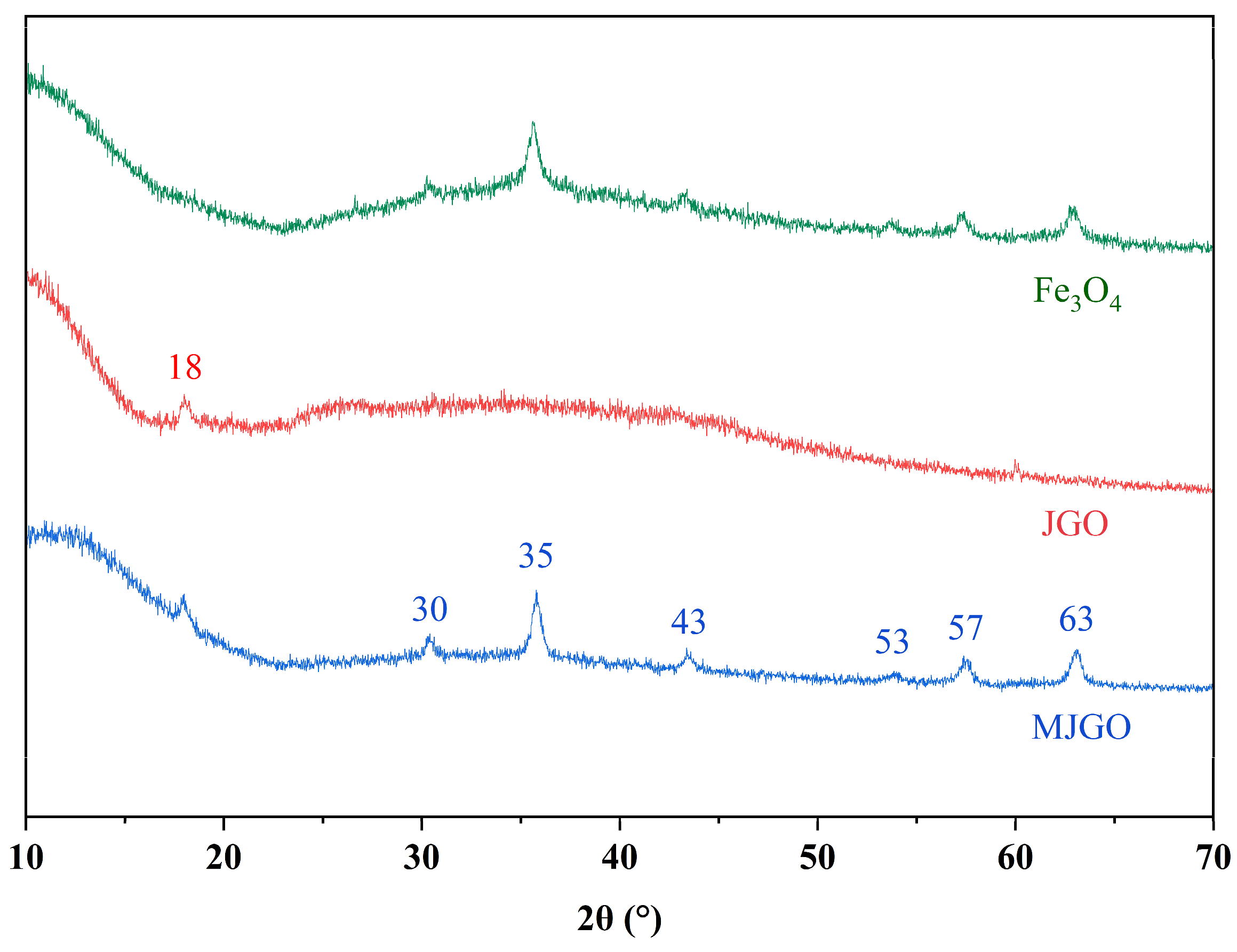
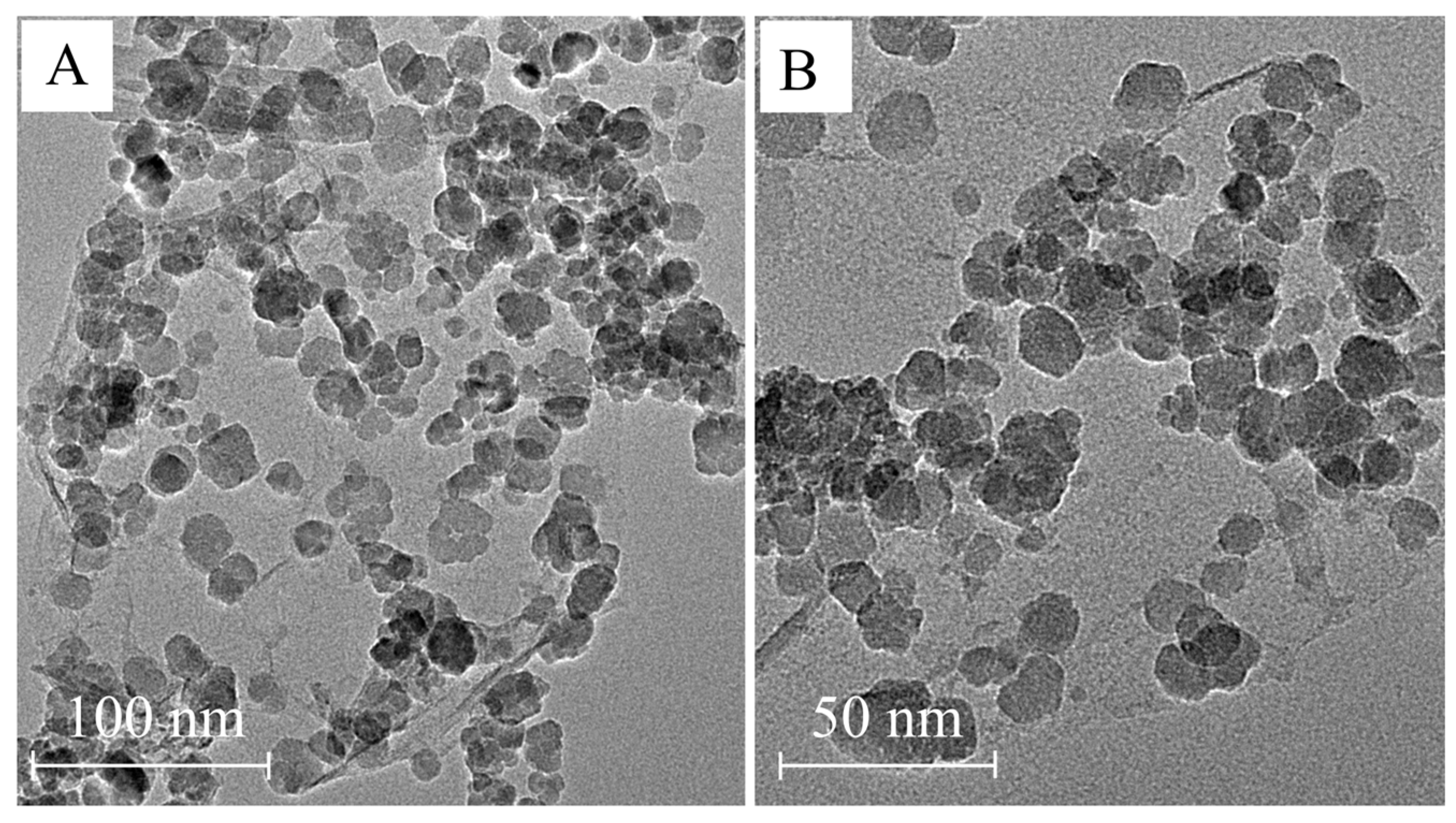
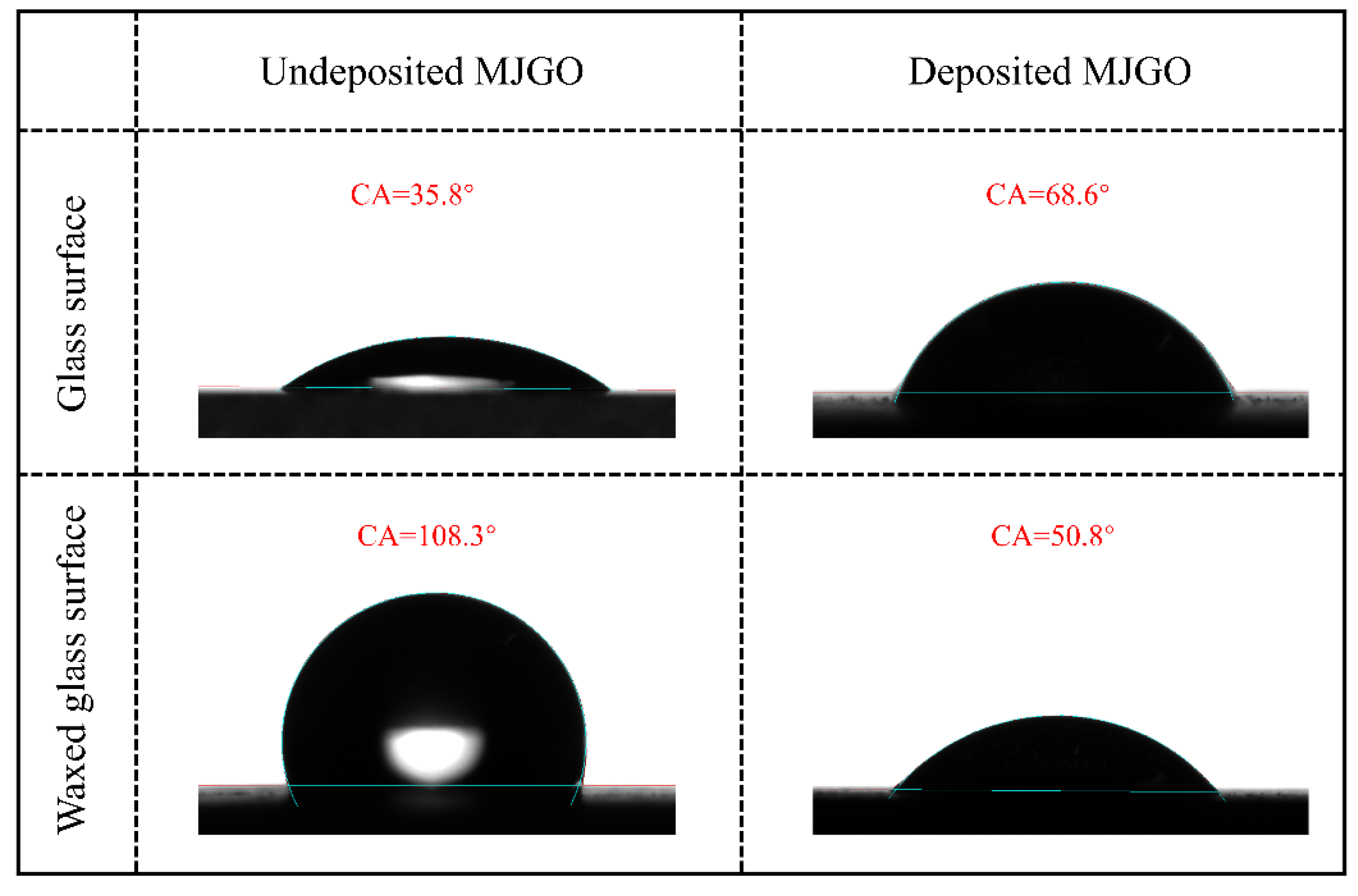
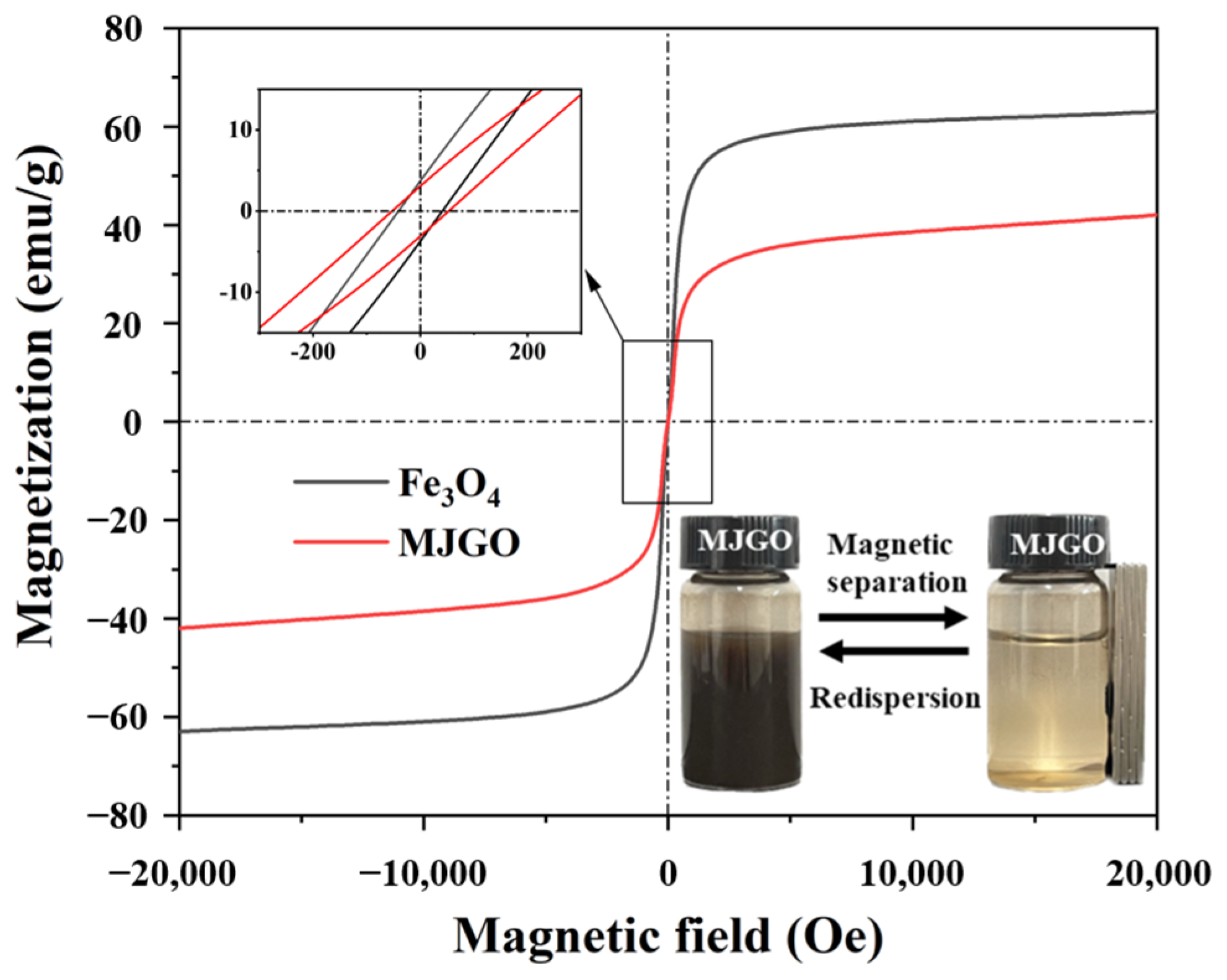

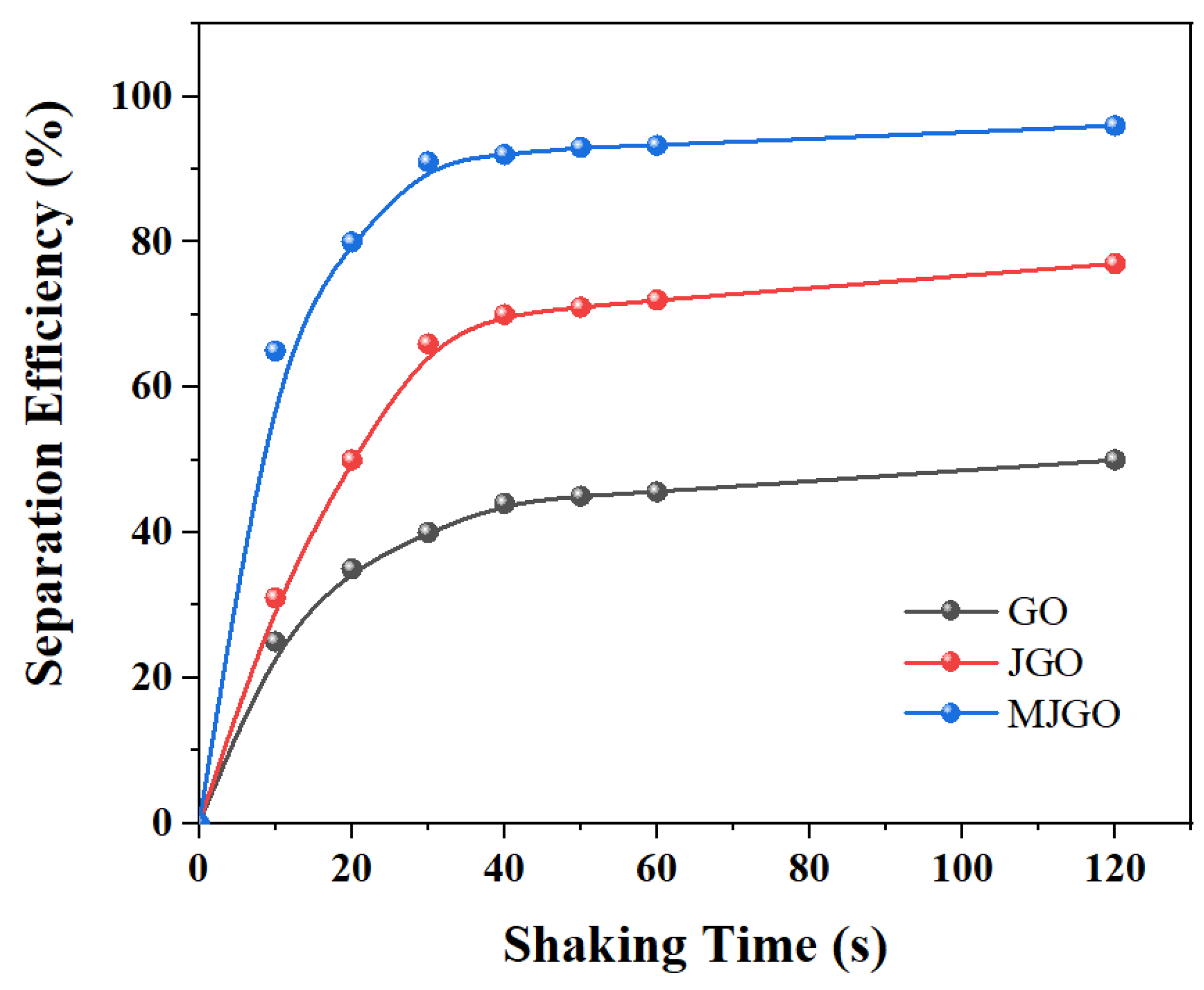
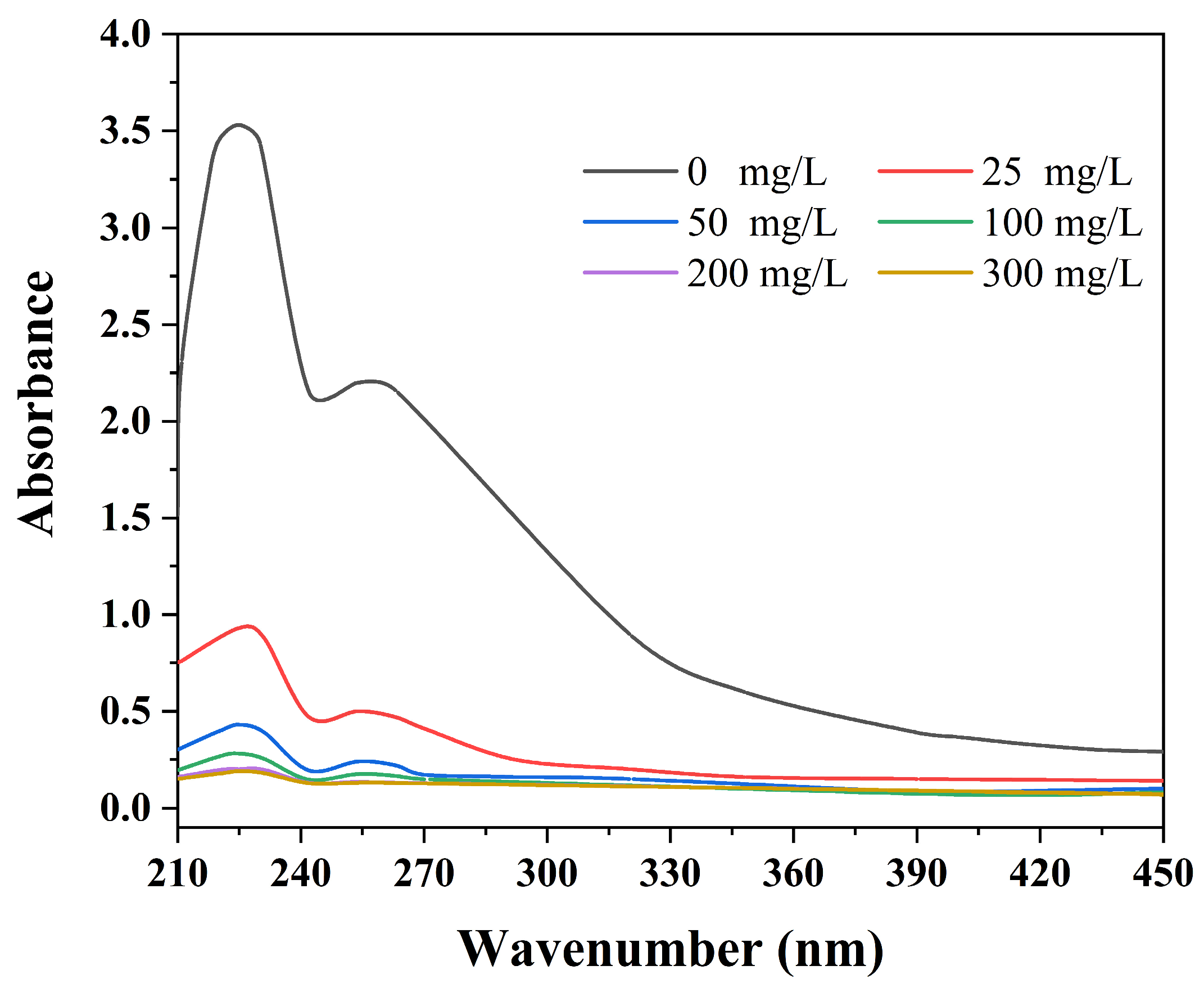
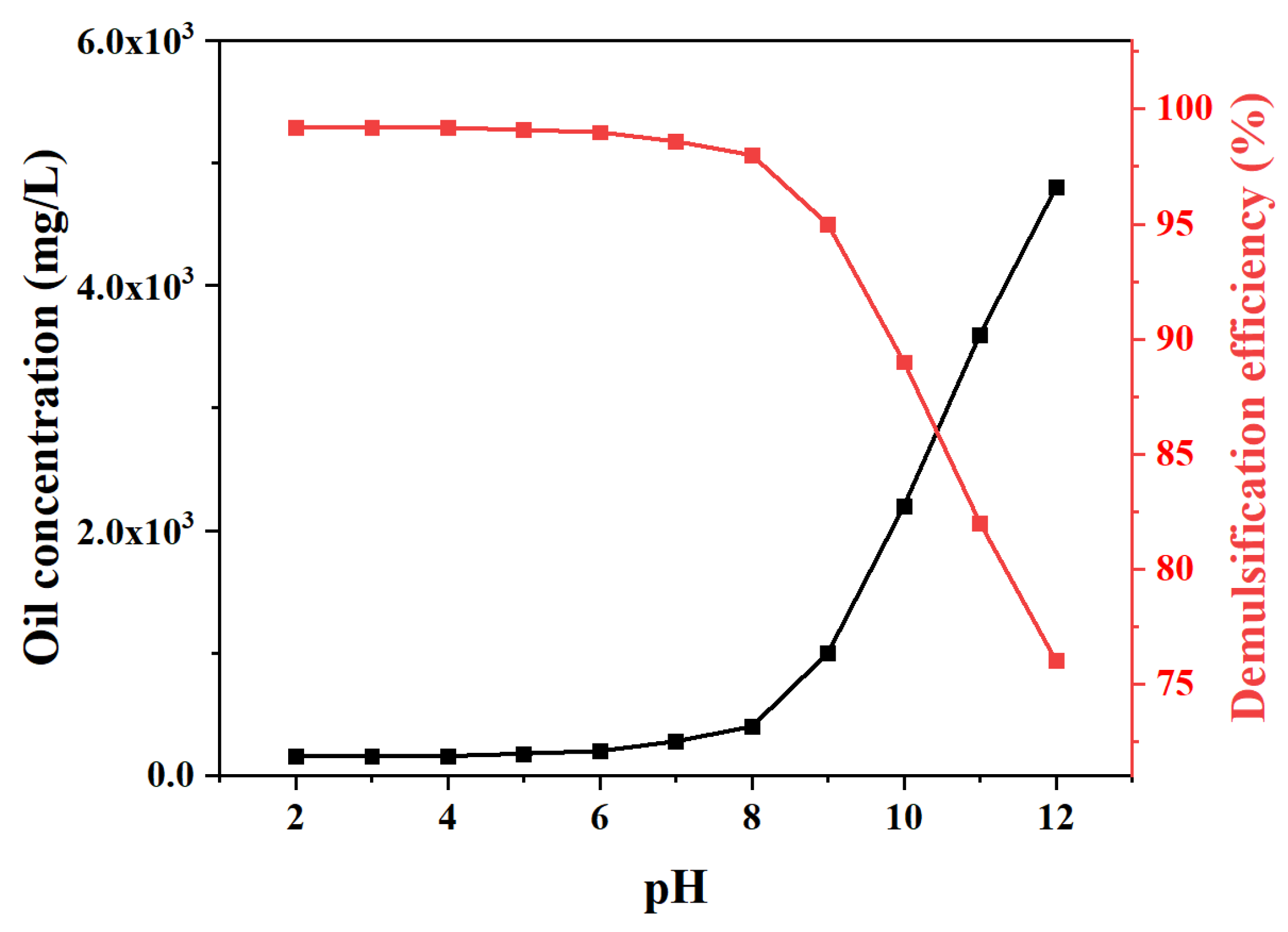
| Sample | 2θ | Crystal Plane (h k l) | FWHM (°) | Crystal Size, D (nm) | d-Spacing (Å) |
|---|---|---|---|---|---|
| MJGO | 30 | (2 2 0) | 0.32 | 26 | 0.29 |
| 35 | (3 1 1) | 0.31 | 27 | 0.25 | |
| 43 | (4 0 0) | 0.39 | 22 | 0.21 | |
| 53 | (4 2 2) | 0.60 | 15 | 0.17 | |
| 57 | (5 1 1) | 0.49 | 18 | 0.16 | |
| 63 | (5 3 3) | 0.47 | 20 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Cheng, L.; Wang, Y.; Jia, H. Facile Synthesis of Novel Magnetic Janus Graphene Oxide for Efficient and Recyclable Demulsification of Crude Oil-in-Water Emulsion. Molecules 2024, 29, 3307. https://doi.org/10.3390/molecules29143307
Xu Y, Cheng L, Wang Y, Jia H. Facile Synthesis of Novel Magnetic Janus Graphene Oxide for Efficient and Recyclable Demulsification of Crude Oil-in-Water Emulsion. Molecules. 2024; 29(14):3307. https://doi.org/10.3390/molecules29143307
Chicago/Turabian StyleXu, Yingbiao, Li Cheng, Yefei Wang, and Han Jia. 2024. "Facile Synthesis of Novel Magnetic Janus Graphene Oxide for Efficient and Recyclable Demulsification of Crude Oil-in-Water Emulsion" Molecules 29, no. 14: 3307. https://doi.org/10.3390/molecules29143307
APA StyleXu, Y., Cheng, L., Wang, Y., & Jia, H. (2024). Facile Synthesis of Novel Magnetic Janus Graphene Oxide for Efficient and Recyclable Demulsification of Crude Oil-in-Water Emulsion. Molecules, 29(14), 3307. https://doi.org/10.3390/molecules29143307








