Preparation of MAZ-Type Zeolite with High Silica
Abstract
:1. Introduction
2. Results and Discussion
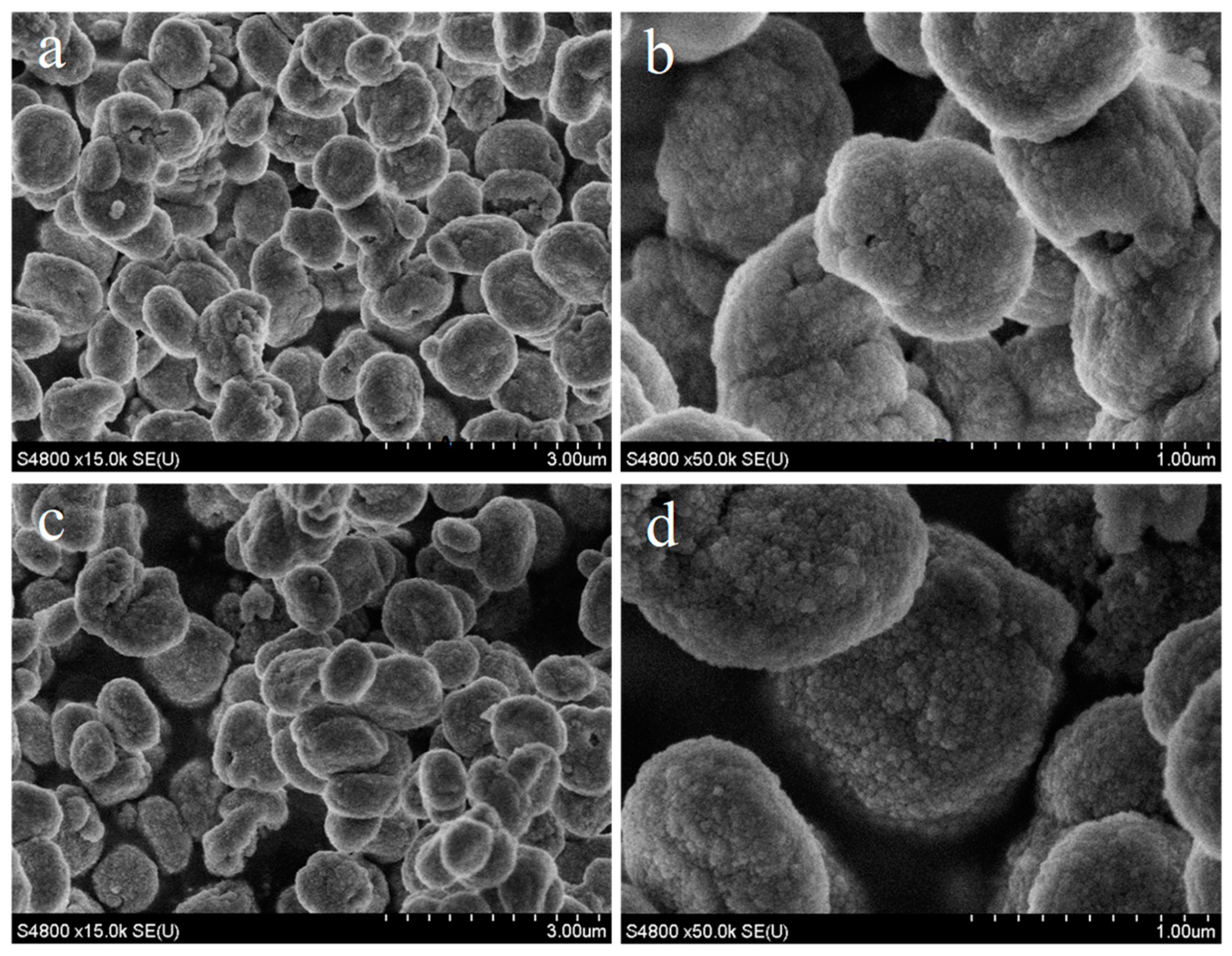
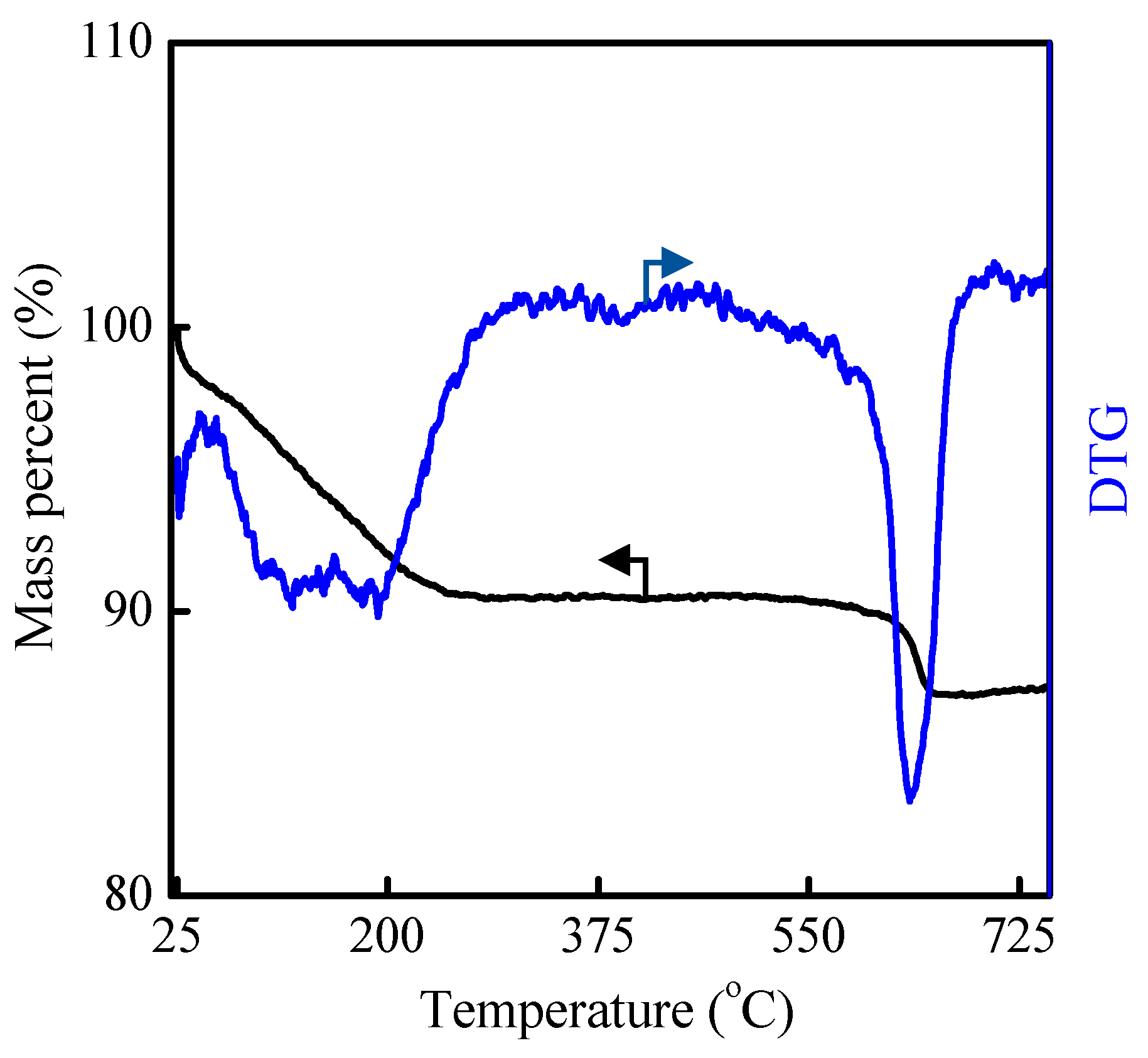
2.1. Hydrothermal Synthesis of MAZ Zeolite
2.2. Preparation of MAZ Zeolite with High Silica by Post-Treated Route
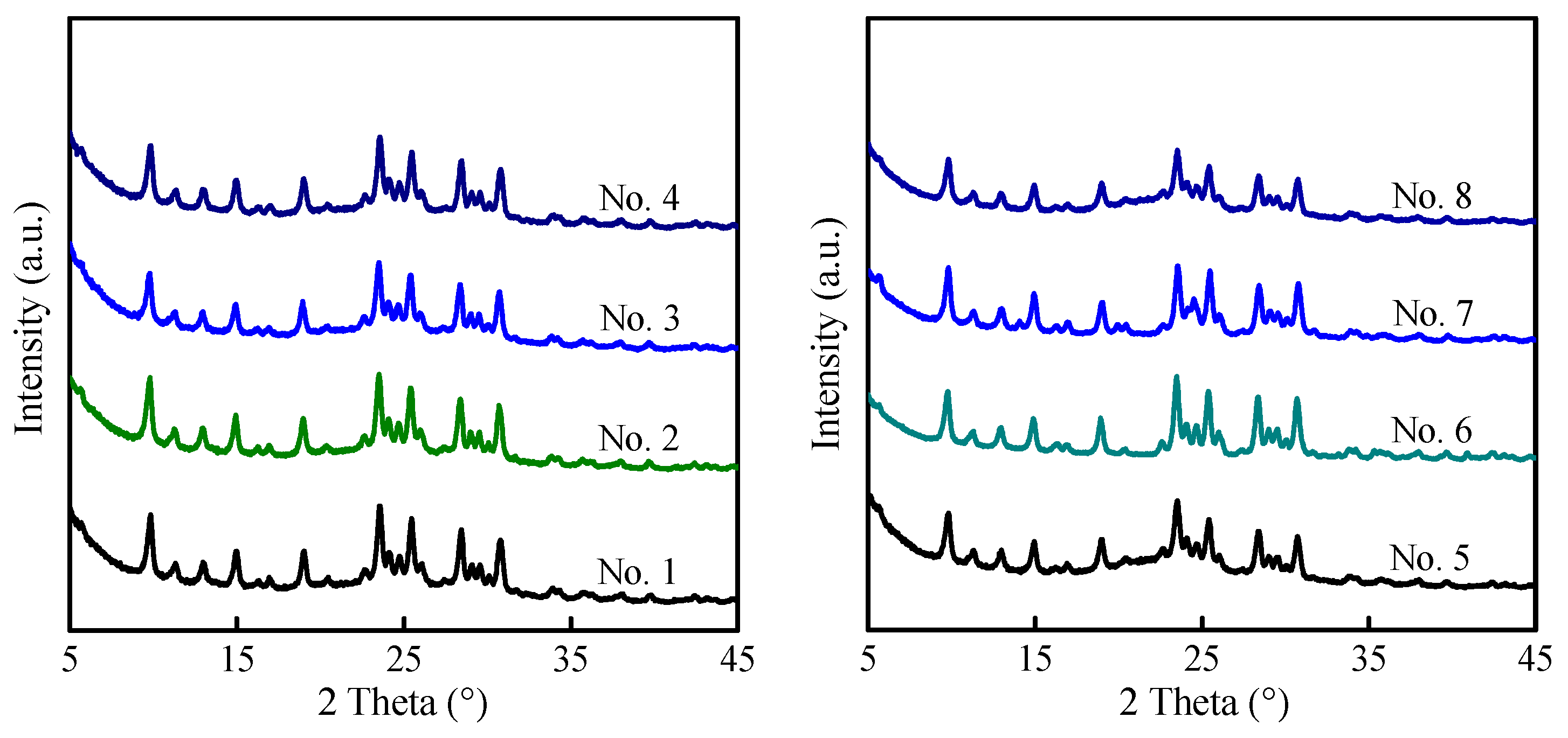
2.3. Preparation of MAZ Zeolite with High Silica by Interzeolite Transformation
3. Materials and Methods
3.1. Materials
3.2. Hydrothermal Synthesis of MAZ Zeolite
3.3. Post-Treated Dealumination of H-MAZ Zeolite
3.3.1. Acetic Acid Treatment followed by Hydrochloric Acid Treatment in Ethanol Solution
3.3.2. Steaming Treatment
3.4. Interzeolite Transformation of FAU to MAZ Zeolite
3.5. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, P.; Shen, B.; Gao, J. Synthesis of MAZ/ZSM-5 Composite Zeolite and Its Catalytic Performance in FCC Gasoline Aromatization. Catal. Commun. 2007, 8, 1161–1166. [Google Scholar] [CrossRef]
- Lei, C.; Dong, Z.; Martínez, C.; Martínez-Triguero, J.; Chen, W.; Wu, Q.; Meng, X.; Parvulescu, A.; De Baerdemaeker, T.; Müller, U.; et al. A Cationic Oligomer as An Organic Template for Direct Synthesis of Aluminosilicate ITH Zeolite. Angew. Chem. Int. Ed. 2020, 59, 15649–15655. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Moliner, M.; Corma, A. Building Zeolites from Precrystallized Units: Nanoscale Architecture. Angew. Chem. Int. Ed. 2018, 57, 15330–15353. [Google Scholar] [CrossRef] [PubMed]
- Toulhoat, H.; Fomena, M.L.; de Bruin, T. Computational Study of the Effect of Confinement within Microporous Structures on the Activity and Selectivity of Metallocene Catalysts for Ethylene Oligomerization. J. Am. Chem. Soc. 2011, 133, 2481–2491. [Google Scholar] [CrossRef] [PubMed]
- Shigeishi, R.A.; Chiche, B.H.; Fajula, F. CO Adsorption on Superacid Sites on Dealuminated Mazzite. Microporous Mesoporous Mater. 2001, 43, 211–226. [Google Scholar] [CrossRef]
- Lv, T.; Wang, Y.; Zhang, S.; Feng, Z.; Zheng, J.; Yang, J.; Liu, X.; Liu, X.; Meng, C. Solid-State Transformation of TMA-Magadiite into Zeolite Omega and Detailed Insights into the Crystallization Process. Dalton Trans. 2019, 48, 16974–16985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, L.; Yang, Z.; Han, S.; Zhu, Q.; Wang, L.; Liu, C.; Meng, X.; Xiao, F. Design of Fast Crystallization of Nanosized Zeolite Omega Crystals at Higher Temperatures. Chin. J. Catal. 2019, 40, 1093–1099. [Google Scholar] [CrossRef]
- Ogawa, A.; Iyoki, K.; Kamimura, Y.; Elangovan, S.P.; Itabashi, K.; Okubo, T. Seed-Directed, Rapid Synthesis of MAZ-Type Zeolites without Using Organic Structure-Directing Agent. Microporous Mesoporous Mater. 2014, 186, 21–28. [Google Scholar] [CrossRef]
- Mekki, A.; Mokhtar, A.; Hachemaoui, M.; Beldjilali, M.; Meliani, M.F.; Zahmani, H.H.; Hacini, S.; Boukoussa, B. Fe and Ni Nanoparticles-Loaded Zeolites as Effective Catalysts for Catalytic Reduction of Organic Pollutants. Microporous Mesoporous Mater. 2021, 310, 110597. [Google Scholar] [CrossRef]
- Honda, K.; Yashiki, A.; Sadakane, M.; Sano, T. Hydrothermal Conversion of FAU and ∗BEA-Type Zeolites into MAZ-Type Zeolites in the Presence of Non-Calcined Seed Crystals. Microporous Mesoporous Mater. 2014, 196, 254–260. [Google Scholar] [CrossRef]
- Wouters, B.H.; Chen, T.; Goossens, A.M.; Martens, J.A.; Grobet, P.J. Determination of the AlT1/AlT2 Ratio in MAZ Zeolites Using Line Shapes of MQ MAS NMR. J. Phys. Chem. B 1999, 103, 8093–8096. [Google Scholar] [CrossRef]
- Zanjanchi, M.A.; Mohabbati, E. Spectroscopic Studies of the Zeolite Materials: Interaction of Extra-Framework Aluminum with Acetylacetone and Hydroxyl Groups. J. Mol. Struct. 2003, 645, 171–176. [Google Scholar] [CrossRef]
- McQueen, D.; Chiche, B.H.; Fajula, F.; Auroux, A.; Guimon, C.; Fitoussi, F.; Schulz, P. A Multitechnique Characterization of the Acidity of Dealuminated Mazzite. J. Catal. 1996, 161, 587–596. [Google Scholar] [CrossRef]
- Brouwer, D.H.; Brouwer, C.C.; Mesa, S.; Semelhago, C.A.; Steckley, E.E.; Sun, M.P.Y.; Mikolajewski, J.G.; Baerlocher, C. Solid-State 29Si NMR Spectra of Pure Silica Zeolites for the International Zeolite Association Database of Zeolite Structures. Microporous Mesoporous Mater. 2020, 297, 110000. [Google Scholar] [CrossRef]
- Chatelard, C.; Dodin, M.; Martinez-Franco, R.; Tuel, A. Di- and Trioxacyclohexane as Structure Directing Molecules in the Synthesis of Zeolites Omega and ECR-1. Microporous Mesoporous Mater. 2021, 38, 111015. [Google Scholar] [CrossRef]
- Gackowski, M.; Kuterasiński, L.; Podobiński, J.; Korzeniowska, A.; Sulikowski, B.; Datka, J. Hierarchical Zeolite Mazzite: Physicochemical Properties and α-Pinene Isomerization. Appl. Catal. A Gen. 2019, 578, 53–62. [Google Scholar] [CrossRef]
- Zhang, S.L.; Wang, Y.; Lv, T.M.; Feng, Z.; Liu, X.; Meng, C. Hydrothermal Conversion of Zeolite Omega from Magadiite with Assistance of Seed Crystals. Mater. Today Chem. 2021, 20, 100440. [Google Scholar] [CrossRef]
- Bouchiba, N.; Toumi, N.; Bengueddach, A. Preparation of a New Micro-Mesoporous Omega Zeolite by Hydrothermal Route: Effect of Crystallization Time. Silicon 2022, 14, 5085–5090. [Google Scholar] [CrossRef]
- Allain, J.F.; Magnoux, P.; Schulz, P.; Guisnet, M. Hydroisomerization of n-Hexane over Platinum Mazzite and Platinum Mordenite Catalysts—Kinetics and Mechanism. Appl. Catal. A Gen. 1997, 152, 221–235. [Google Scholar] [CrossRef]
- Gackowski, M.; Tarach, K.; Kuterasiński, L.; Podobiński, J.; Jarczewski, S.; Kuśtrowski, P.; Datka, J. Hierarchical Zeolites Y Obtained by Desilication: Porosity, Acidity and Catalytic Properties. Microporous Mesoporous Mater. 2018, 263, 282–288. [Google Scholar] [CrossRef]
- Rejmak, P.; Datka, J.; Broclawik, E. Identity of Two Types of Strong Brønsted Acid Sites in Mazzite Revealed by CO Probe: IR Study and Periodic DFT Modeling. Int. J. Quantum Chem. 2019, 119, e25873. [Google Scholar] [CrossRef]
- Guisnet, M.; Ayrault, P.; Datka, J. Acid Properties of Mazzite Zeolites Studied by IR Spectroscopy. Microporous Mesoporous Mater. 1998, 20, 283–291. [Google Scholar] [CrossRef]
- Goossens, A.M.; Feijen, E.J.P.; Verhoeven, G.; Wouters, B.H.; Grobet, P.J.; Jacobs, P.A.; Martens, J.A. Crystallization of MAZ-Type Zeolites Using Tetramethylammonium, Sodium and n-Hexane Derivatives as Structure- and Composition-Directing Agents. Microporous Mesoporous Mater. 2000, 35, 555–572. [Google Scholar] [CrossRef]
- Xu, H.; Dong, P.; Liu, L.; Wang, J.G.; Deng, F.; Dong, J.X. Synthesis and Characterization of Zeolite Mazzite Analogue in Na2O–Al2O3–SiO2–Piperazine–H2O. J. Porous Mater. 2007, 14, 97–101. [Google Scholar] [CrossRef]
- Mekki, A.; Boukoussa, B. Structural, Textural and Toluene Adsorption Properties of Microporous–Mesoporous Zeolite Omega Synthesized by Different Methods. J. Mater. Sci. 2019, 54, 8096–8107. [Google Scholar] [CrossRef]
- Knorpp, A.J.; Newton, M.A.; Mizuno, S.C.M.; Zhu, J.; Mebrate, H.; Pinar, A.B.; van Bokhoven, J.A. Comparative Performance of Cu-Zeolites in the Isothermal Conversion of Methane to Methanol. Chem. Commun. 2019, 55, 11794–11797. [Google Scholar] [CrossRef] [PubMed]
- Knorpp, A.J.; Pinar, A.B.; Newton, M.; Sushkevich, V.; van Bokhoven, J.A. Copper-Exchanged Omega (MAZ) Zeolite: Copper-Concentration Dependent Active Sites and Its Unprecedented Methane to Methanol Conversion. ChemCatChem 2018, 10, 5593–5596. [Google Scholar] [CrossRef]
- Hakiki, A.; Boukoussa, B.; Kibou, Z.; Terrab, I.; Ghomari, K.; Choukchou-Braham, N.; Hamacha, R.; Bengueddach, A.; Azzouz, A. Correlation of Hydrophilic Character and Surface Basicity of Exchanged Omega-Catalyzed MCR Process. Thermochim. Acta 2018, 662, 108–115. [Google Scholar] [CrossRef]
- Zhang, S.; Lv, T.; Feng, Z.; Liu, X.; Wang, Y.; Meng, C. Solid-State and Organic Template-Free Synthesis of Zeolite Omega by Conversion of Magadiite in the Presence of Seed Crystals and Investigation of Conversion Mechanism. Ind. Eng. Chem. Res. 2020, 59, 19574–19583. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, W.; Wang, L.; Song, W.; Hu, Y. Low-Cost and Environmental-Friendly Route for Synthesizing Nano-Rod Aluminosilicate MAZ Zeolite. Molecules 2022, 27, 7930. [Google Scholar] [CrossRef]
- Martucci, A.; Alberti, A.; Guzman-Castillo, M.L.; Renzo, F.D.; Fajula, F. Crystal Structure of Zeolite Omega, the Synthetic Counterpart of the Natural Zeolite Mazzite. Microporous Mesoporous Mater. 2003, 63, 33–42. [Google Scholar] [CrossRef]
- Dutartre, R.; de Ménorval, L.C.; Renzo, F.D.; McQueen, D.; Fajula, F.; Schulz, P. Mesopore Formation during Steam Dealumination of Zeolites: Influence of Initial Aluminum Content and Crystal Size. Microporous Mater. 1996, 6, 311–320. [Google Scholar] [CrossRef]
- Yue, C.; Xie, W.; Liu, Y.; Wu, H.; Li, X.; Wu, P. Hydrothermal Synthesis of MWW-Type Analogues Using Linear-Type Quaternary Alkylammonium Hydroxides as Structure-Directing Agents. Microporous Mesoporous Mater. 2011, 142, 347–353. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, H.; Jiang, J.; Guan, Y.; Wu, P. Sn-Beta Zeolite Hydrothermally Synthesized via Interzeolite Transformation as Efficient Lewis Acid Catalyst. J. Catal. 2017, 352, 1–12. [Google Scholar] [CrossRef]
- Kumar, A.; Nguyen, A.H.; Okumu, R.; Shepherd, T.D.; Molinero, V. Could Mesophases Play a Role in the Nucleation and Polymorph Selection of Zeolites. J. Am. Chem. Soc. 2018, 140, 16071–16086. [Google Scholar] [CrossRef]
- Jain, R.; Mallette, A.J.; Rimer, J.D. Controlling Nucleation Pathways in Zeolite Crystallization: Seeding Conceptual Methodologies for Advanced Materials Design. J. Am. Chem. Soc. 2021, 143, 21446–21460. [Google Scholar] [CrossRef]


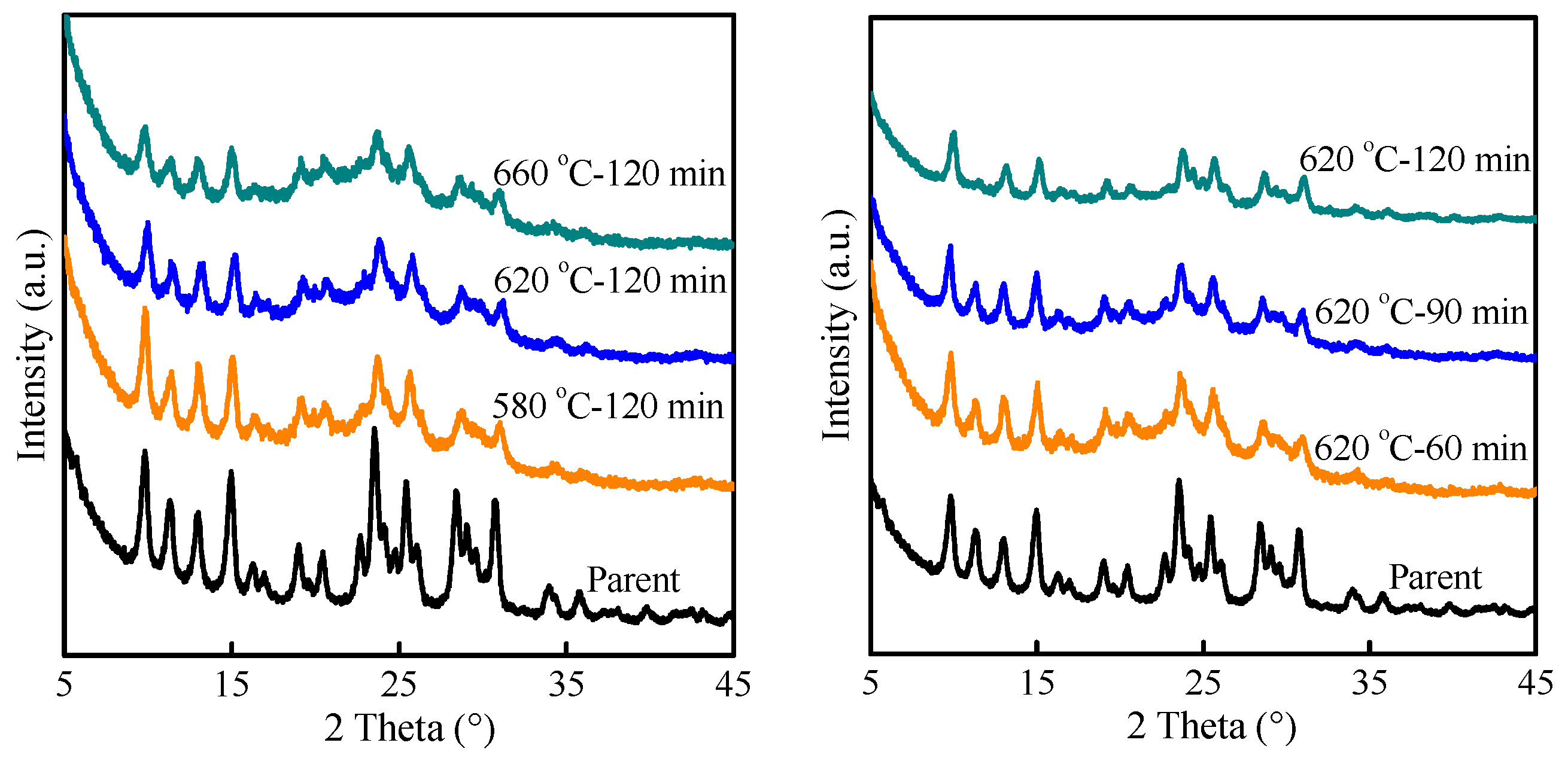
| No. | Steaming Treatment | HNO3 Acid Leaching | Bulk Si/Al a | Crystallinity (%) | |||
|---|---|---|---|---|---|---|---|
| T (°C) | Time (h) | Concentration (M) | Time (h) | T (°C) | |||
| 1 | 580 | 2 | 1 | 2 | 80 | 10.4 | 85.7 |
| 2 | 620 | 2 | 1 | 2 | 80 | 11.8 | 77.9 |
| 3 | 660 | 2 | 1 | 2 | 80 | 15.7 | 64.5 |
| 4 | 620 | 1 | 1 | 2 | 80 | 8.3 | 86.6 |
| 5 | 620 | 1.5 | 1 | 2 | 80 | 9.7 | 83.0 |
| 6 | 620 | 2 | 0.5 | 2 | 80 | 7.2 | 81.1 |
| 7 | 620 | 2 | 2 | 2 | 80 | 16.9 | 74.4 |
| No. | Synthesis Conditions | Product | ||||
|---|---|---|---|---|---|---|
| Si/Al Ratio of Starting FAU | OH Source | Seed (wt%) | Phase | Yield (%) | Bulk Si/Al b | |
| 1 | 6 | LiOH | 0.33 | MAZ | 78 | 4.8 |
| 2 | 19 | LiOH | 0.25 | MAZ | 39 | 6.4 |
| 3 | 19 | LiOH | 0.33 | MAZ | 34 | 6.7 |
| 4 | 19 | NaOH | 0.33 | MAZ | 22 | 4.2 |
| 5 | 44 | LiOH | 0.33 | MAZ | 15 | 6.3 |
| 6 | 44 | NaOH | 0.25 | MAZ | 11 | 3.4 |
| 7 | 44 | NaOH | 0.33 | MAZ | 12 | 3.6 |
| 8 | 168 | LiOH | 0.33 | MAZ | 8 | 7.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bo, S.; Yang, K.; Lü, H.; Zhu, Z. Preparation of MAZ-Type Zeolite with High Silica. Molecules 2024, 29, 3315. https://doi.org/10.3390/molecules29143315
Bo S, Yang K, Lü H, Zhu Z. Preparation of MAZ-Type Zeolite with High Silica. Molecules. 2024; 29(14):3315. https://doi.org/10.3390/molecules29143315
Chicago/Turabian StyleBo, Songcheng, Kaixuan Yang, Hongying Lü, and Zhiguo Zhu. 2024. "Preparation of MAZ-Type Zeolite with High Silica" Molecules 29, no. 14: 3315. https://doi.org/10.3390/molecules29143315






