Synergistic Effect of Doxorubicin and Blue Light Irradiation on the Antitumor Treatment of HepG2 Cells in Liver Cancer
Abstract
:1. Introduction
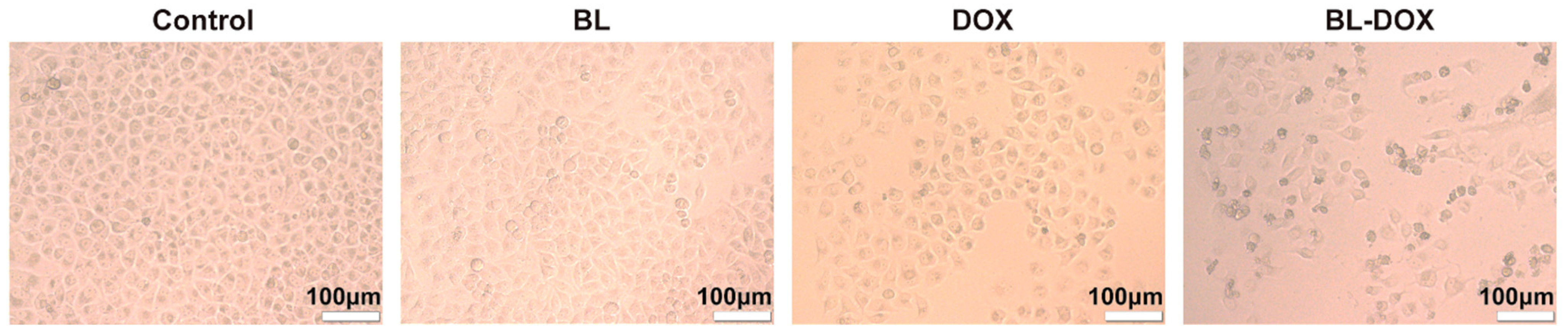
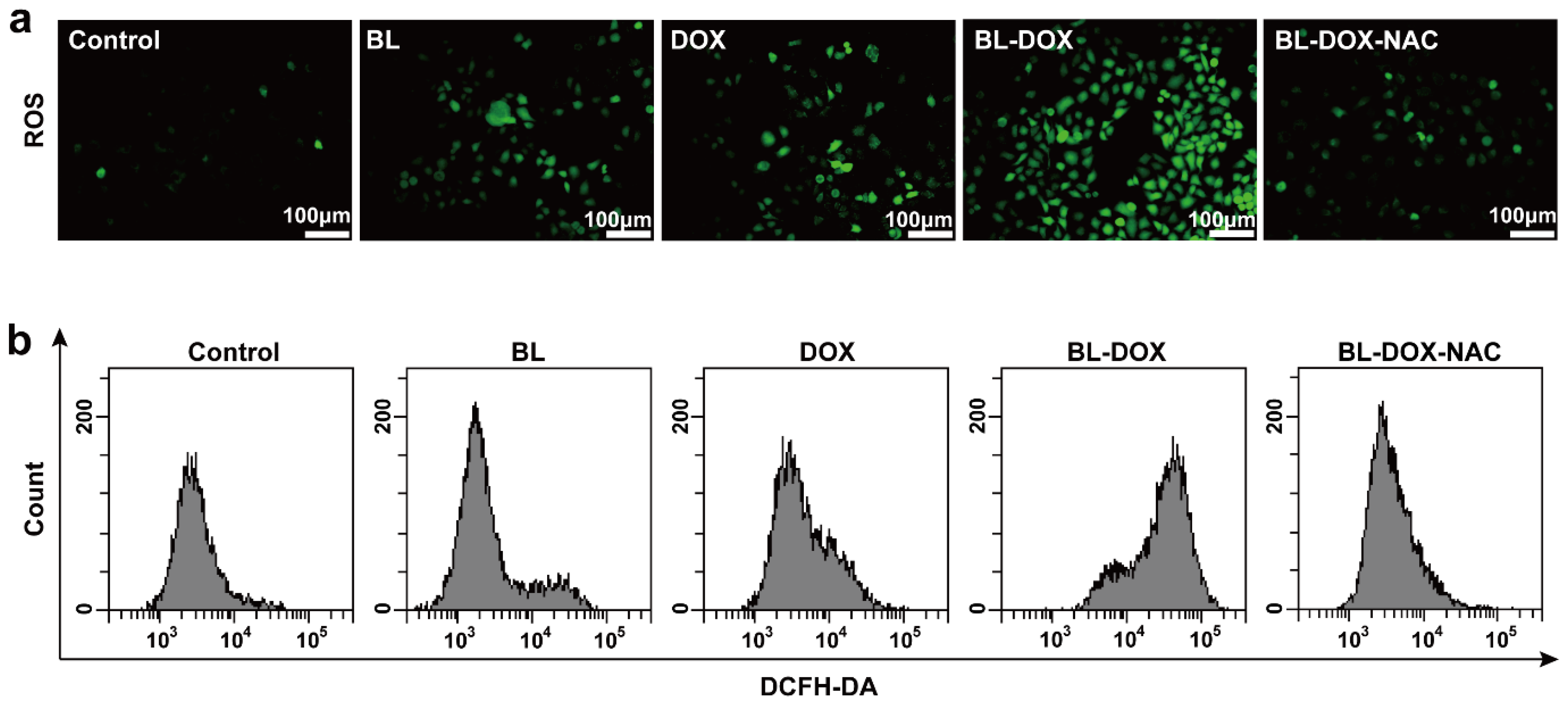
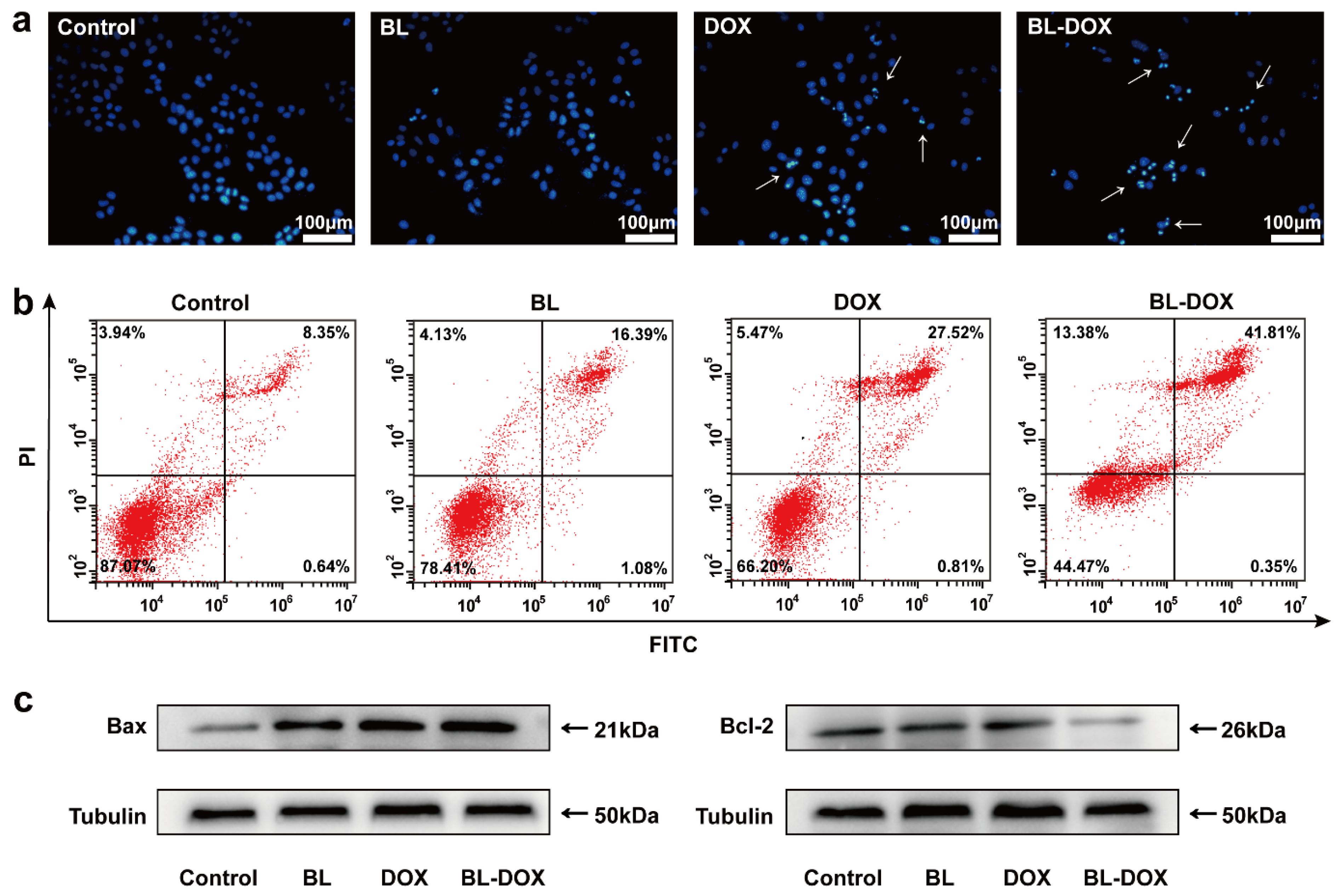
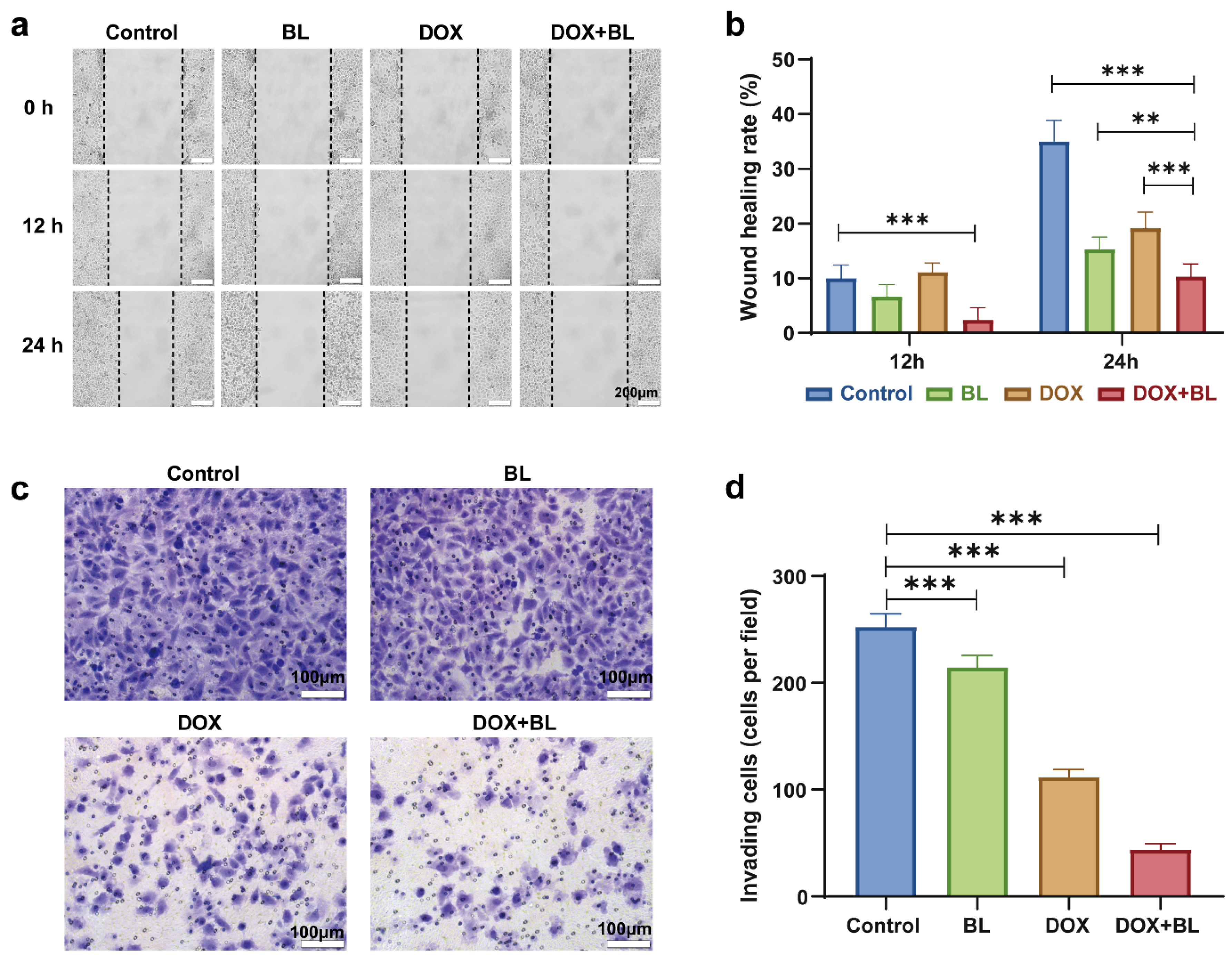
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Cell Culture
3.3. BL Irradiation System for Cell Treatments
3.4. Growth Viability Assay
3.5. ROS Examination
3.6. Apoptosis Analysis
3.7. Intracellular TNF-α Assay
3.8. Detection of Superoxide Dismutase (SOD) Activity
3.9. Malondialdehyde (MDA) Examination
3.10. Hoechst 33258 Staining
3.11. Cell Migration Assay and Cell Invasion Assay
3.12. Western Blot
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lafaro, K.J.; Demirjian, A.N.; Pawlik, T.M. Epidemiology of Hepatocellular Carcinoma. Surg. Oncol. Clin. N. Am. 2015, 24, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Martinelli, E.; Comm, E.G. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann. Oncol. 2021, 32, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.-Y.; Yeh, M.-L.; Yu, M.-L. An EASL position paper for systemic treatment of hepatocellular carcinoma: Go forward courageously. J. Hepatol. 2022, 76, 478–480. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Kawamura, Y.; Hasegawa, K.; Tateishi, R.; Kariyama, K.; Shiina, S.; Toyoda, H.; Imai, Y.; Hiraoka, A.; Ikeda, M.; et al. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer 2021, 10, 181–223. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.R.; Cao, M.M.; Lei, L.; Yang, F.; Li, H.; Yan, X.X.; He, S.Y.; Zhang, S.L.; Teng, Y.; Xia, C.F.; et al. Burden of liver cancer: From epidemiology to prevention. Chin. J. Cancer Res. 2022, 34, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Nicoletto, R.E.; Ofner, C.M., III. Cytotoxic mechanisms of doxorubicin at clinically relevant concentrations in breast cancer cells. Cancer Chemother. Pharm. 2022, 89, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ren, W.; Zhong, T.; Zhang, S.; Huang, D.; Guo, Y.; Yao, X.; Wang, C.; Zhang, W.Q.; Zhang, X.; et al. Tumor-specific pH-responsive peptide-modified pH-sensitive liposomes containing doxorubicin for enhancing glioma targeting and anti-tumor activity. J. Control. Release 2016, 222, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H.; Van der Speeten, K.; Stuart, O.A.; Chang, D. Impact of surgical and clinical factors on the pharmacology of intraperitoneal doxorubicin in 145 patients with peritoneal carcinomatosis. Eur. J. Surg. Oncol. 2011, 37, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Al-Shammari, A.M.; Rameez, H.; Al-Taee, M.F. Newcastle disease virus, rituximab, and doxorubicin combination as anti-hematological malignancy therapy. Oncol. Virother. 2016, 5, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Wei, H.; Lai, X.; Ge, Y.; Xu, S.; Meng, J.; Wen, T.; Liu, J.; Zhang, W.; Wang, J.; et al. Co-delivery of homoharringtonine and doxorubicin boosts therapeutic efficacy of refractory acute myeloid leukemia. J. Control. Release 2020, 327, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Poon, R.T.P.; Borys, N. Lyso-thermosensitive liposomal doxorubicin: A novel approach to enhance efficacy of thermal ablation of liver cancer. Expert Opin. Pharmacother. 2009, 10, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Pham, C.V.; Wang, T.; Al Shamaileh, H.; Chowdhury, R.; Patel, S.; Li, Y.; Kong, L.; Hou, Y.; Zhu, Y.; et al. Inhibition of Autophagy Promotes the Elimination of Liver Cancer Stem Cells by CD133 Aptamer-Targeted Delivery of Doxorubicin. Biomolecules 2022, 12, 1623. [Google Scholar] [CrossRef] [PubMed]
- Cote, B.; Carlson, L.J.; Rao, D.A.; Alani, A.W.G. Combinatorial resveratrol and quercetin polymeric micelles mitigate doxorubicin induced cardiotoxicity in vitro and in vivo. J. Control. Release 2015, 213, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Synowiec, E.; Hoser, G.; Bialkowska-Warzecha, J.; Pawlowska, E.; Skorski, T.; Blasiak, J. Doxorubicin Differentially Induces Apoptosis, Expression of Mitochondrial Apoptosis-Related Genes, and Mitochondrial Potential in BCR-ABL1-Expressing Cells Sensitive and Resistant to Imatinib. Biomed. Res. Int. 2015, 2015, 673512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, J.; Yan, M. Targeted hepatocellular carcinoma therapy: Transferrin modified, self-assembled polymeric nanomedicine for co-delivery of cisplatin and doxorubicin. Drug Dev. Ind. Pharm. 2016, 42, 1590–1599. [Google Scholar] [CrossRef] [PubMed]
- Pieniazek, A.; Czepas, J.; Piasecka-Zelga, J.; Gwozdzinski, K.; Koceva-Chyla, A. Oxidative stress induced in rat liver by anticancer drugs doxorubicin, paclitaxel and docetaxel. Adv. Med. Sci. 2013, 58, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Oshikata, A.; Matsushita, T.; Ueoka, R. Enhancement of drug efflux activity via MDR1 protein by spheroid culture of human hepatic cancer cells. J. Biosci. Bioeng. 2011, 111, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Greco, G.; Ulfo, L.; Turrini, E.; Marconi, A.; Costantini, P.E.; Marforio, T.D.; Mattioli, E.J.; Di Giosia, M.; Danielli, A.; Fimognari, C.; et al. Light-Enhanced Cytotoxicity of Doxorubicin by Photoactivation. Cells 2023, 12, 392. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.F.; Alves, J.R.; da Fonseca, A.d.S.; Mencalha, A.L. Low power blue LED exposure increases effects of doxorubicin on MDA-MB-231 breast cancer cells. Photodiagn. Photodyn. Ther. 2018, 24, 250–255. [Google Scholar] [CrossRef]
- Leanse, L.G.; dos Anjos, C.; Mushtaq, S.; Dai, T.H. Antimicrobial blue light: A ‘Magic Bullet’ for the 21st century and beyond? Adv. Drug Deliv. Rev. 2022, 180, 114057. [Google Scholar] [CrossRef]
- Yang, J.L.; Fu, Q.Q.; Jiang, H.; Li, Y.H.; Liu, M.Q. Progress of phototherapy for osteosarcoma and application prospect of blue light photobiomodulation therapy. Front. Oncol. 2022, 12, 1022973. [Google Scholar] [CrossRef]
- Takeuchi, M.; Nishisho, T.; Toki, S.; Kawaguchi, S.; Tamaki, S.; Oya, T.; Uto, Y.; Katagiri, T.; Sairyo, K. Blue light induces apoptosis and autophagy by promoting ROS-mediated mitochondrial dysfunction in synovial sarcoma. Cancer Med. 2023, 12, 9668–9683. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.J.; Liu, Y.G.; Yuan, Q.X.; Liu, J.S.; Liu, Y.; Li, H.D.; Wang, D. Blue light-induced apoptosis of human promyelocytic leukemia cells via the mitochondrial-mediated signaling pathway. Oncol. Lett. 2018, 15, 6291–6296. [Google Scholar] [CrossRef]
- Zhuang, J.J.; Liu, J.S.; Liu, Y.G.; Li, H.D.; Wang, D.; Teng, L.S. Enhanced proliferation inhibition of HL60 cells treated by synergistic all-trans retinoic acid/blue light/nanodiamonds. RSC Adv. 2017, 7, 38895–38901. [Google Scholar] [CrossRef]
- Yan, G.; Zhang, L.; Feng, C.; Gong, R.; Idiiatullina, E.; Huang, Q.; He, M.; Guo, S.; Yang, F.; Li, Y.; et al. Blue light emitting diodes irradiation causes cell death in colorectal cancer by inducing ROS production and DNA damage. Int. J. Biochem. Cell Biol. 2018, 103, 81–88. [Google Scholar] [CrossRef]
- Li, C.; Zhu, G.; Cui, Z.; Zhang, J.; Zhang, S.; Wei, Y. The strong inhibitory effect of combining anti-cancer drugs AT406 and rocaglamide with blue LED irradiation on colorectal cancer cells. Photodiagn. Photodyn. Ther. 2020, 30, 101797. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Gong, R.; Zheng, Q.; Yang, G.; He, M.; Lei, H.; Li, X.; Zhang, L.; Xu, Z.; Liu, S.; et al. Synergistic anti-tumor effects of arsenic trioxide and blue LED irradiation on human osteosarcoma. Int. J. Biol. Sci. 2019, 15, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Shakibaie, M.; Vaezjalali, M.; Rafii-Tabar, H.; Sasanpour, P. Synergistic effect of phototherapy and chemotherapy on bladder cancer cells. J. Photochem. Photobiol. B 2019, 193, 148–154. [Google Scholar] [CrossRef]
- Teng, Y.; Li, Z.G.; Liu, J.S.; Teng, L.S.; Li, H.D. Proliferation inhibition and apoptosis of liver cancer cells treated by blue light irradiation. Med. Oncol. 2023, 40, 227. [Google Scholar] [CrossRef]
- Seow, T.K.; Liang, R.; Leow, C.K.; Chung, M.C.M. Hepatocellular carcinoma: From bedside to proteomics. Proteomics 2001, 1, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, B.M.; Banik, B.K.; Borah, P.; Jain, A. Reactive Oxygen Species (ROS): Key Components in Cancer Therapies. Anti-Cancer Agents 2022, 22, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Slimen, I.B.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben Mrad, M.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperther. 2014, 30, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Skulachev, V.P. Why are mitochondria involved in apoptosis? Permeability transition pores and apoptosis as selective mechanisms to eliminate superoxide-producing mitochondria and cell. FEBS Lett. 1996, 397, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Li, J.; Zhou, Y.; Yang, S.; Chen, W.; Li, F.; You, B.; Liu, Y.; Zhang, X. Targeted Delivery of Doxorubicin via CD147-Mediated ROS/pH Dual-Sensitive Nanomicelles for the Efficient Therapy of Hepatocellular Carcinoma. AAPS J. 2018, 20, 34. [Google Scholar] [CrossRef] [PubMed]
- Edlich, F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem. Biophys. Res. Commun. 2018, 500, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.J.; Han, L.H.; Cong, R.S.; Liang, J. Caspase family proteases and apoptosis. Acta Biochim. Biophys. Sin. 2005, 37, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Kawamura, N.; Kumagai, J.; Fukuda, J.; Tanaka, T. Tumor necrosis factor regulation of apoptosis in mouse preimplantation embryos and its antagonism by transforming growth factor alpha/phosphatidylionsitol 3-kinase signaling system. Biol. Reprod. 2007, 76, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Sidoti-de Fraisse, C.; Rincheval, V.; Risler, Y.; Mignotte, B.; Vayssiere, J.L. TNF-alpha activates at least two apoptotic signaling cascades. Oncogene 1998, 17, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Munoz, M.F.; Argueelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J. Critical methods in Free Radical Biology & Medicine. Free Radic. Biol. Med. 2009, 47 (Suppl. S2), S207. [Google Scholar] [CrossRef]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.N.; Bhowmick, N.A. Role of EMT in Metastasis and Therapy Resistance. J. Clin. Med. 2016, 5, 17. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, Y.; Li, Z.; Liu, J.; Teng, L.; Li, H. Synergistic Effect of Doxorubicin and Blue Light Irradiation on the Antitumor Treatment of HepG2 Cells in Liver Cancer. Molecules 2024, 29, 3360. https://doi.org/10.3390/molecules29143360
Teng Y, Li Z, Liu J, Teng L, Li H. Synergistic Effect of Doxorubicin and Blue Light Irradiation on the Antitumor Treatment of HepG2 Cells in Liver Cancer. Molecules. 2024; 29(14):3360. https://doi.org/10.3390/molecules29143360
Chicago/Turabian StyleTeng, Yun, Zhige Li, Junsong Liu, Lesheng Teng, and Hongdong Li. 2024. "Synergistic Effect of Doxorubicin and Blue Light Irradiation on the Antitumor Treatment of HepG2 Cells in Liver Cancer" Molecules 29, no. 14: 3360. https://doi.org/10.3390/molecules29143360




