A Close View of the Production of Bioactive Fungal Metabolites Mediated by Chromatin Modifiers
Abstract
:1. Introduction
2. The Production of Secondary Metabolites by Fungi
3. Epigenetic-Induced Production of Secondary Metabolites by Fungi
4. Protocols and Methodologies for Experiments with Small Molecules Elicitors
| Fungi Species [Conditions] | Modulator * (Concentration) | Metabolites Detected/Isolated (Bold Numbers Refer to Figure 3) | Ref. |
|---|---|---|---|
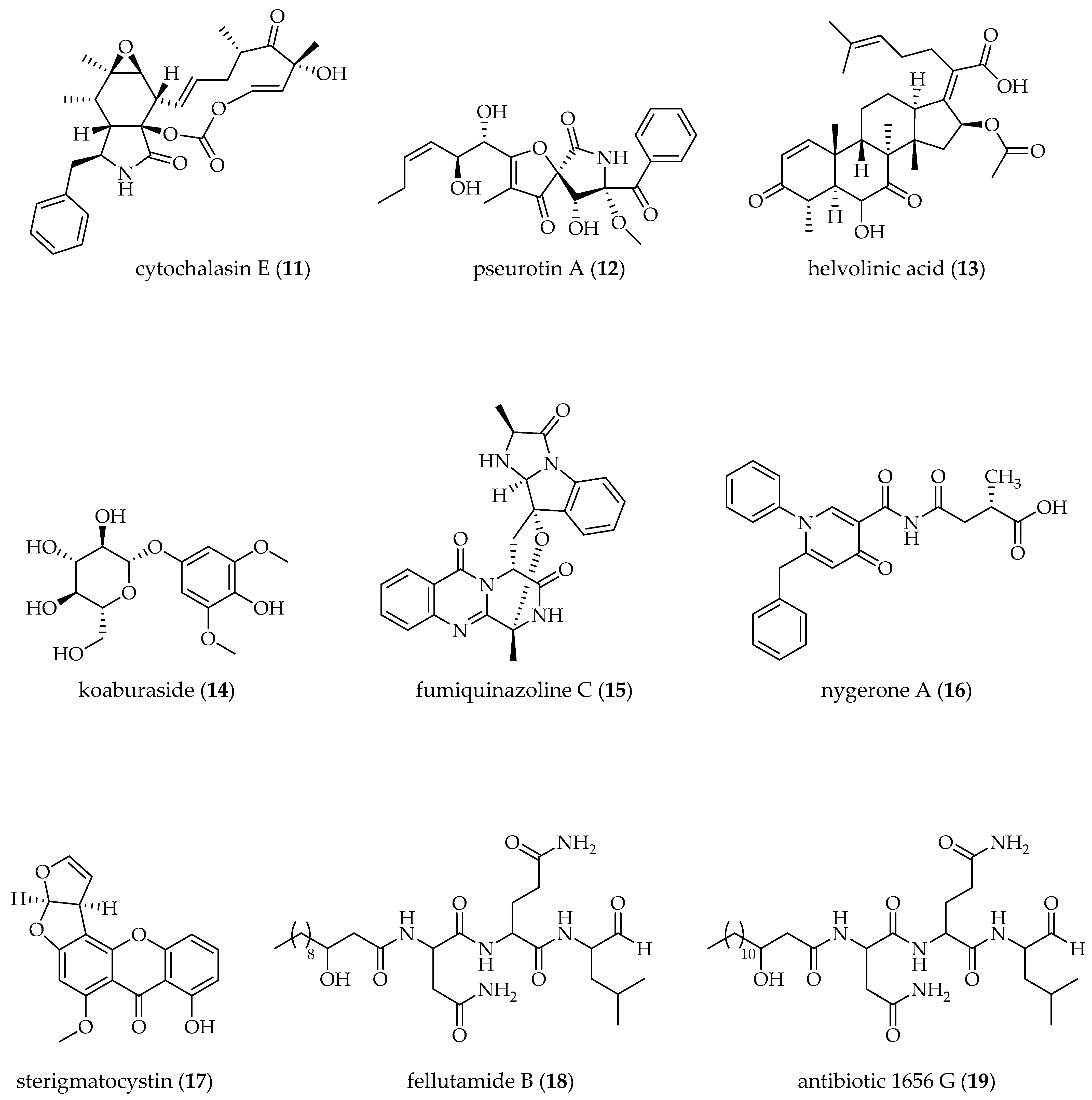
| A. clavatus [25 °C, 150 rpm, 72 h] | 5-AZA A (5 µM); BUT B (5 µM); TCA B (5 µM) | cytochalasin E (11) (↑) pseurotin A (12) (↑) | [58] |
| AGMA + 5-AZA (5 µM each) | pseurotin A (12) (↑) | ||
| VPA B (5 µM) | cytochalasin E (11) (↑) pseurotin A (12) (↑) | ||
| A. fumigatus [28 °C, static, 30 days] | 5-AZA + SBHA B (500 µM each) | helvolinic acid (13) (↨) koaburaside (14) (↨) | [61] |
| A. fumigatus GA-L7 [28 °C, 100 rpm, 8 days] | VPA (500 µM) | fumiquinazoline C (15) (↑) | [69] |
| A. niger ATCC1015 [25 °C, static, 2 weeks] | SAHA (10 µM) | nygerone A (16) (↑) | [63] |
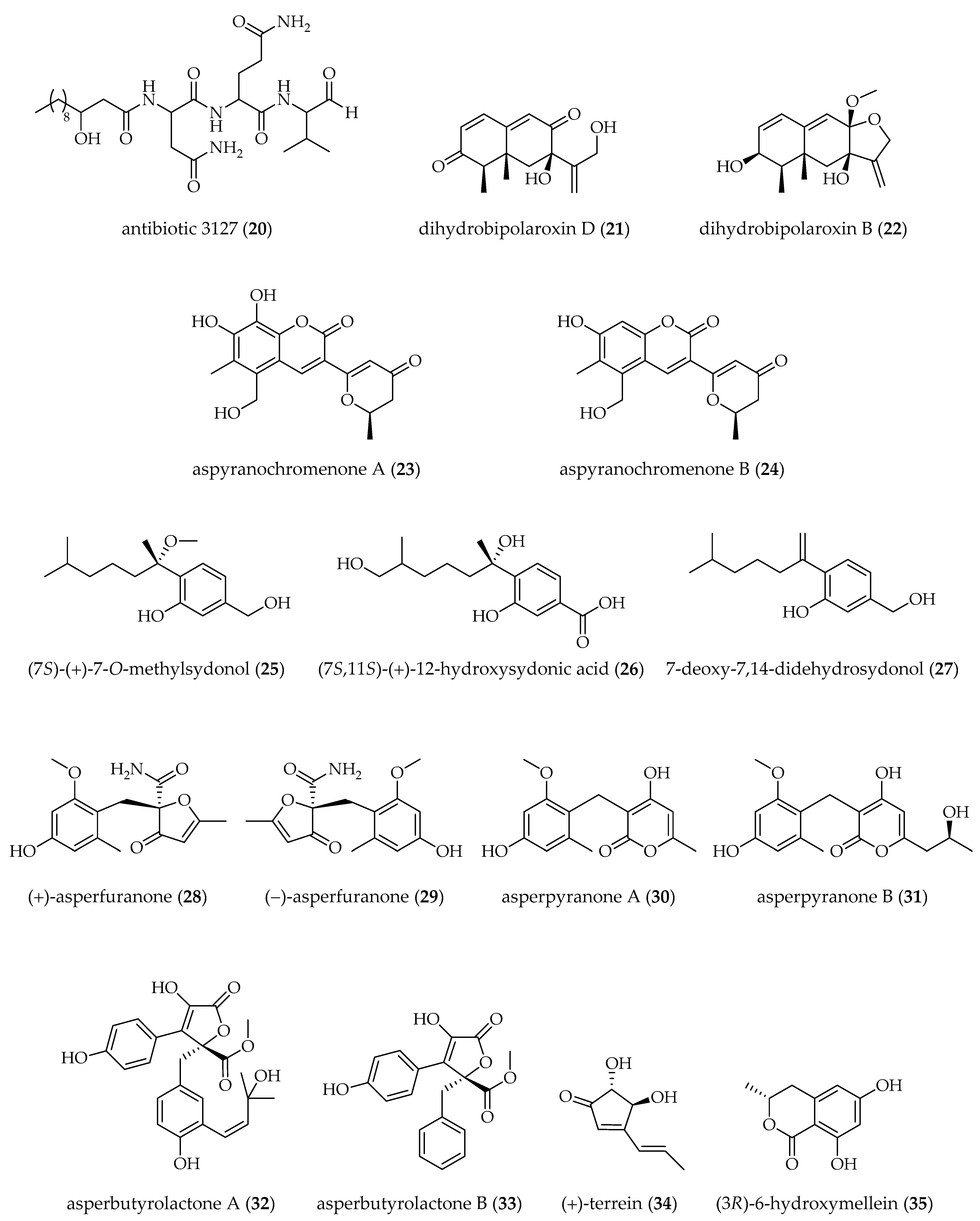
| A. nidulans [37 °C, 250 rpm, 96 h] | SAHA (100 µM) | sterigmatocystin (17) (↓) fellutamide B (18) (↑) antibiotic 1656 G (19) (↑) antibiotic 3127 (20) (↑) | [66] |
| Aspergillus sp. SCSIOW2 [28 °C, static, 15 days] | 5-AZA + SBHA (1 mM each) | dihydrobipolaroxin D (21) † dihydrobipolaroxin B (22) † | [70] |
| Aspergillus sp. AST0006 [28 °C, 160 rpm, 8 days] | SAHA (250–500 µM) | aspyranochromenone A (23) † aspyranochromenone B (24) † | [62] |
| A. sydowii [25 °C, 150 rpm, 10 days] | 5-AZA (100 µM) | (7S)-(+)-7-O-methylsydonol (25) † (7S,11S)-(+)-12-hydroxysydonic acid (26) † 7-deoxy-7,14-didehydrosydonol (27) † | [60] |
| A. terreus RA2905 [28 °C, 150 rpm, 7 days] | SAHA (100 µM) | (+) and (−)-asperfuranone (28, 29) asperpyranone A (30) asperpyranone B (31) | [67] |
| A. terreus GZU-31-1 [rt, static, 30 days] | 5-AZA (50 µM) | asperbutyrolactone A (32) † asperbutyrolactone B (33) † | [59] |
| A. terreus PF26 [28 °C, 180 rpm, 24 days] | SAHA (500 µM) | (+)-terrein (34) (↑) (3R)-6-hydroxymellein (35) (↑) | [68] |
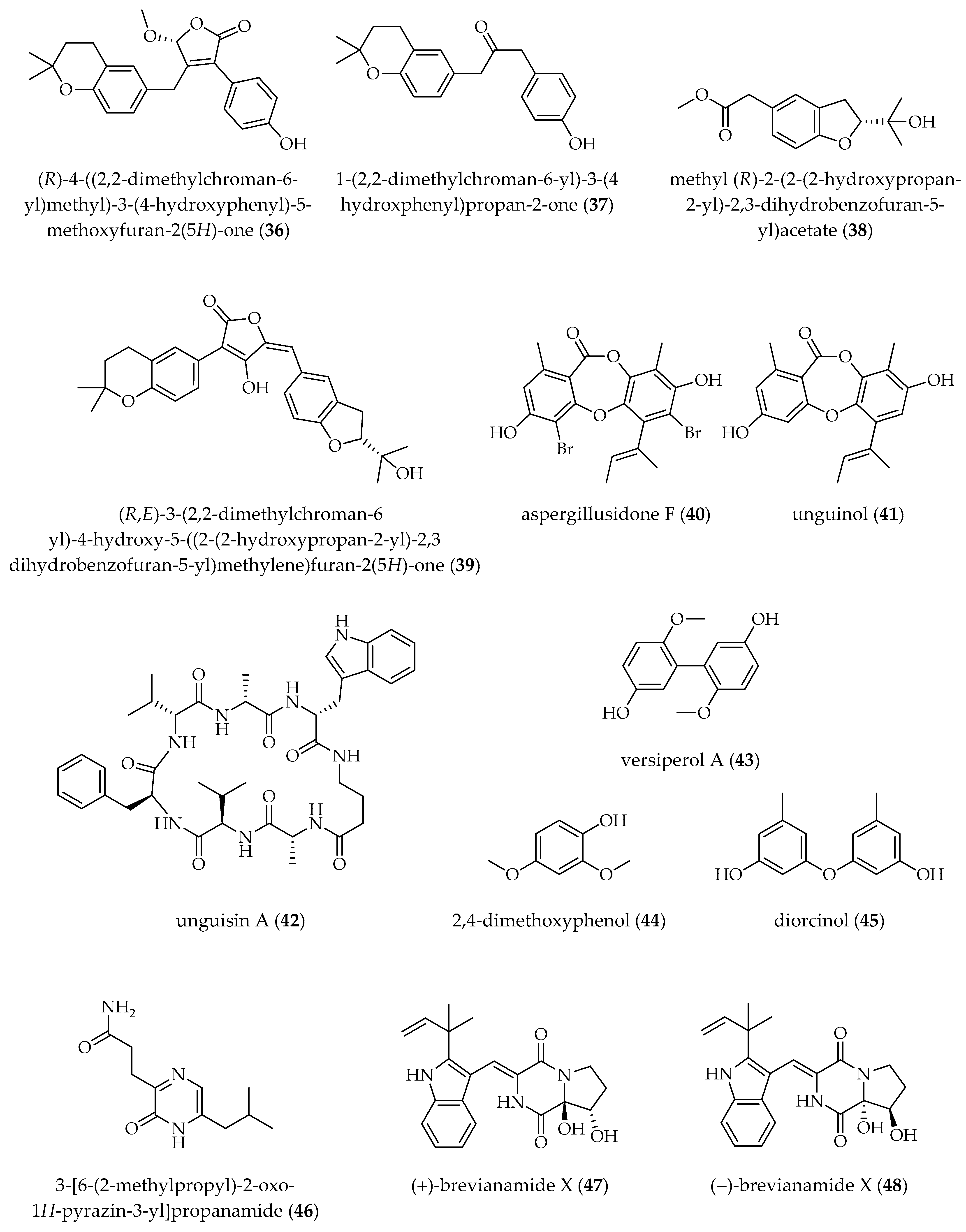
| A. terreus OUCMDZ-2739 [25 °C, static, 30 days] | TSA B (10 µM) | (R)-4-((2,2-dimethylchroman-6-yl)methyl)-3-(4-hydroxyphenyl)-5-methoxyfuran-2(5H)-one (36) 1-(2,2-dimethylchroman-6-yl)-3-(4-hydroxyphenyl)propan-2-one (37) methyl (R)-2-(2-(2-hydroxypropan-2-yl)-2,3-dihydrobenzofuran-5-yl) acetate (38) (R,E)-3-(2,2-dimethylchroman-6-yl)-4-hydroxy-5-((2-(2-hydroxypropan-2-yl)-2,3-dihydrobenzofuran-5-yl)methylene)furan-2(5H)-one (39) | [71] |
| A. unguis DLEP2008001 [28 °C, 150 rpm, 20 days] | PRO A (1 µM) | aspergillusidone F (40) (↨) unguinol (41) (↨) unguisin A (42) (↨) | [72] |
| A. versicolor MCCC 3A00080 [25 °C, 150 rpm, 15 days] | SAHA (20 mg/L) | versiperol A (43) † 2,4-dimethoxyphenol (44) diorcinol (45) | [73] |
| A. versicolor OUCMDZ-2738 [25 °C, static, 30 days] | SAHA (10 µM) | 3-[6-(2-methylpropyl)-2-oxo-1H-pyrazin-3-yl]propanamide (46) † (+)- and (−)-brevianamide X (47, 48) (±)-brevianamide R (49) (±)-brevianamide Q (50) diorcinol C (51) diorcinol E (52) diorcinol (45) methyl diorcinol-4-carboxylate (53) | [64] |
| A. versicolor XS-20090066 [rt, 30 days] | SAHA + 5-AZA (100 µM each) | kipukasin K † (54) (↨) kipukasin L † (55) (↨) aspergillusene E † (56) (↨) | [74] |
| A. wentii na-3 [25 °C, static, 30 days] | SAHA (20 µM) | aspewentin A † (57) aspewentin B † (58) aspewentin C (59) | [65] |
5. Methodological and Analytical Approaches
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, C.; Ha, Y.; Liu, X.; Wang, N.; Lian, X.Y.; Zhang, Z. Isolation and Structure Elucidation of New Metabolites from the Mariana-Trench-Associated Fungus Aspergillus sp. SY2601. Molecules 2024, 29, 459. [Google Scholar] [CrossRef] [PubMed]
- Mbaoji, F.N.; Nweze, J.A.; Yang, L.; Huang, Y.; Huang, S.; Onwuka, A.M.; Peter, I.E.; Mbaoji, C.C.; Jiang, M.; Zhang, Y.; et al. Novel Marine Secondary Metabolites Worthy of Development as Anticancer Agents: A Review. Molecules 2021, 26, 5769. [Google Scholar] [CrossRef] [PubMed]
- Santiago, K.A.A.; Edrada-Ebel, R.; Cheow, Y.L.; Ting, A.S.Y. Biodiscovery of potential antibacterial diagnostic metabolites from the endolichenic fungus Xylaria venustula using LC–MS-based metabolomics. Biology 2021, 10, 191. [Google Scholar] [CrossRef]
- Zhang, X.; Song, C.; Bai, Y.; Hu, J.; Pan, H. Cytotoxic and antimicrobial activities of secondary metabolites iso-lated from the deep-sea-derived Actinoalloteichus cyanogriseus 12A22. 3 Biotech 2021, 11, 283. [Google Scholar] [CrossRef]
- Hawksworth, D.L. The fungal dimension of biodiversity: Magnitude, significance, and conservation. Mycol. Res. 1991, 95, 641–655. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Lücking, R. Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol. Spectr. 2017, 5, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Hussain, M.; Zhang, W.; Stadler, M.; Liu, X.; Xiang, M. Current insights into fungal species diversity and perspective on naming the environmental DNA sequences of fungi. Mycology 2019, 10, 127–140. [Google Scholar] [CrossRef]
- Hyde, K.D.; Jeewon, R.; Chen, Y.J.; Bhunjun, C.S.; Calabon, M.S.; Jiang, H.B.; Lin, C.G.; Norphanphoun, C.; Sysouphanthong, P.; Pem, D.; et al. The numbers of fungi: Is the descriptive curve flattening? Fungal Divers. 2020, 103, 219–271. [Google Scholar] [CrossRef]
- Niskanen, T.; Lücking, R.; Dahlberg, A.; Gaya, E.; Suz, L.M.; Mikryukov, V.; Liimatainen, K.; Druzhinina, I.; Westrip, J.R.S.; Mueller, M.; et al. Pushing the Frontiers of Biodvesity Research: Unveiling the Global Diversity, Distribution, and Conservation of Fungi. Annu. Rev. Environ. Resour. 2023, 48, 149–176. [Google Scholar] [CrossRef]
- Varrella, S.; Barone, G.; Tangherlini, M.; Rastelli, E.; Dell’Anno, A.; Corinaldesi, C. Diversity, Ecological Role and Biotechnological Potential of Antarctic Marine Fungi. J. Fungi 2021, 7, 391. [Google Scholar] [CrossRef]
- Yurchenko, A.N.; Girich, E.V.; Yurchenko, E.A. Metabolites of Marine Sediment-Derived Fungi: Actual Trends of Biological Activity Studies. Mar. Drugs 2021, 19, 88. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, V.N.; Carvalho, C.R.; Martins, L.B.M.; Barreto, D.L.; da Silva, B.F.; Queiroz, S.C.N.; Tamang, P.; Bajsa-Hirschel, J.; Cantrell, C.L.; Duke, S.O.; et al. Bioactive Me-tabolites Produced by Fungi Present in Antarctic, Arctic, and Alpine Ecosystems. In Fungi Bioactive Metabolites: Integration of Pharmaceutical Applications, 1st ed.; Deshmukh, S.K., Takahashi, J.A., Saxena, S., Eds.; Springer Nature: Singapore, 2024; Volume 1, pp. 537–563. [Google Scholar] [CrossRef]
- Orfali, R.; Aboseada, M.A.; Abdel-Wahab, N.M.; Hassan, H.M.; Perveen, S.; Ameen, F.; Alturki, E.; Abdelmohsen, U.R. Recent updates on the bioactive compounds of the marine-derived genus Aspergillus. RSC Adv. 2021, 11, 17116–17150. [Google Scholar] [CrossRef] [PubMed]
- Gozari, M.; Alborz, M.; El-Seedi, H.R.; Jassbi, A.R. Chemistry, biosynthesis and biological activity of terpenoids and meroterpenoids in bacteria and fungi isolated from different marine habitats. Eur. J. Med. Chem. 2021, 210, 112957. [Google Scholar] [CrossRef]
- Zou, J.X.; Song, Y.P.; Ji, N.Y. Deoxytrichodermaerin, a harziane lactone from the marine algicolous fungus Trichoderma longibrachiatum A-WH-20-2. Nat. Prod. Res. 2021, 35, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, D.; Weston, M.; Vannette, R.L. Bees just wanna have fungi: A review of bee associations with non-pathogenic fungi. FEMS Microbiol. Ecol. 2023, 99, fiad077. [Google Scholar] [CrossRef]
- Mongkolsamrit, S.; Sandargo, B.; Ebada, S.S.; Noisripoom, W.; Jaiyen, S.; Luangsa-Ard, J.J.; Stadler, M. Bhushaniella gen. nov. (Cordycipitaceae) on spider eggs sac: A new genus from Thailand and its bioactive secondary metabolites. Mycol. Prog. 2023, 22, 64. [Google Scholar] [CrossRef]
- Ortega, H.E.; Torres-Mendoza, D.; Caballero, E.Z.; Cubilla-Rios, L. Structurally uncommon secondary metabolites derived from endophytic fungi. J. Fungi 2021, 7, 570. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, X.; Al Anbari, W.H.; Zhou, Q.; Zhou, P.; Zhang, M.; Zeng, F.; Chen, C.; Tong, Q.; Wang, J.; et al. Cysteine Residue Containing Merocytochalasans and 17,18-seco-Aspochalasins from Aspergillus micronesiensis. J. Nat. Prod. 2019, 82, 2653–2658. [Google Scholar] [CrossRef]
- Gubiani, J.R.; Oliveira, M.C.; Neponuceno, R.A.; Camargo, M.J.; Garcez, W.S.; Biz, A.R.; Soares, M.A.; Araujo, A.R.; Bolzani, V.d.S.; Lisboa, H.C.F.; et al. Cytotoxic prenylated indole alkaloid produced by the endophytic fungus Aspergillus terreus P63. Phytochem. Lett. 2019, 32, 162–167. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, Z.; Liu, L.; Chen, S. The chemistry and biology of fungal meroterpenoids (2009–2019). Org. Biomol. Chem. 2021, 19, 1644–1704. [Google Scholar] [CrossRef]
- Stroe, M.C.; Gao, J.; Pitz, M.; Fischer, R. Complexity of fungal polyketide biosynthesis and function. Mol. Microbiol. 2024, 121, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.-L.; Wei, W.-J.; Li, H.-Y.; Wang, L.-D.; Dong, S.-H.; Gao, K. Phomotide A, a novel polyketide, from the endophytic fungus Phomopsis sp. CFS42. Tetrahedron Lett. 2020, 61, 151468. [Google Scholar] [CrossRef]
- Bashir, F.; Sultana, K.; Khalid, M.; Rabia, H. Kojic Acid: A Comprehensive Review. Asian J. Allied Health Sci. (AJAHS) 2021, 6, 13–21. [Google Scholar] [CrossRef]
- Chen, S.; Cai, R.; Liu, Z.; Cui, H.; She, Z. Secondary metabolites from mangrove-associated fungi: Source, chemistry and bioactivities. Nat. Prod. Rep. 2022, 39, 560–595. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Murtaza, G.; Ditta, A. Nutritional, Medicinal, and Cosmetic Value of Bioactive Compounds in Button Mushroom (Agaricus bisporus): A Review. Appl. Sci. 2021, 11, 5943. [Google Scholar] [CrossRef]
- Pimenta, L.P.; Gomes, D.C.; Cardoso, P.G.; Takahashi, J.A. Recent Findings in Azaphilone Pigments. J. Fungi 2021, 7, 541. [Google Scholar] [CrossRef] [PubMed]
- Poorniammal, R.; Prabhu, S.; Dufossé, L.; Kannan, J. Safety Evaluation of Fungal Pigments for Food Applications. J. Fungi 2021, 7, 692. [Google Scholar] [CrossRef] [PubMed]
- Collemare, J.; Seidl, M.F. Chromatin-dependent regulation of secondary metabolite biosynthesis in fungi: Is the picture complete? FEMS Microbiol. Rev. 2019, 43, 591–607. [Google Scholar] [CrossRef]
- Garello, M.; Piombo, E.; Buonsenso, F.; Prencipe, S.; Valente, S.; Meloni, G.R.; Marcet-Houben, M.; Gabaldón, T.; Spadaro, D. Several secondary metabolite gene clusters in the genomes of ten Penicillium spp. raise the risk of multiple mycotoxin occurrence in chestnuts. Food Microbiol. 2024, 122, 104532. [Google Scholar] [CrossRef]
- Hüttel, W. Echinocandins: Structural diversity, biosynthesis, and development of antimycotics. Appl. Microbiol. Biotechnol. 2021, 105, 55–66. [Google Scholar] [CrossRef]
- Cox, R.J.; Skellam, E.; Williams, K. Biosynthesis of Fungal Polyketides. In Physiology and Genetics: Selected Basic and Applied Aspects—The Mycota Series, 2nd ed.; Anke, T., Schüffler, A., Eds.; Springer: Cham, Switzerland, 2018; Volume 15, pp. 385–412. [Google Scholar] [CrossRef]
- Pavesi, C.; Flon, V.; Mann, S.; Leleu, S.; Prado, S.; Franck, X. Biosynthesis of azaphilones: A review. Nat. Prod. Rep. 2021, 38, 1058–1071. [Google Scholar] [CrossRef] [PubMed]
- Caceres, I.; Al Khoury, A.; El Khoury, R.; Lorber, S.; Oswald, I.P.; El Khoury, A.; Atoui, A.; Puel, O.; Bailly, J.D. Aflatoxin Biosynthesis and Genetic Regulation: A Review. Toxins 2020, 12, 150. [Google Scholar] [CrossRef] [PubMed]
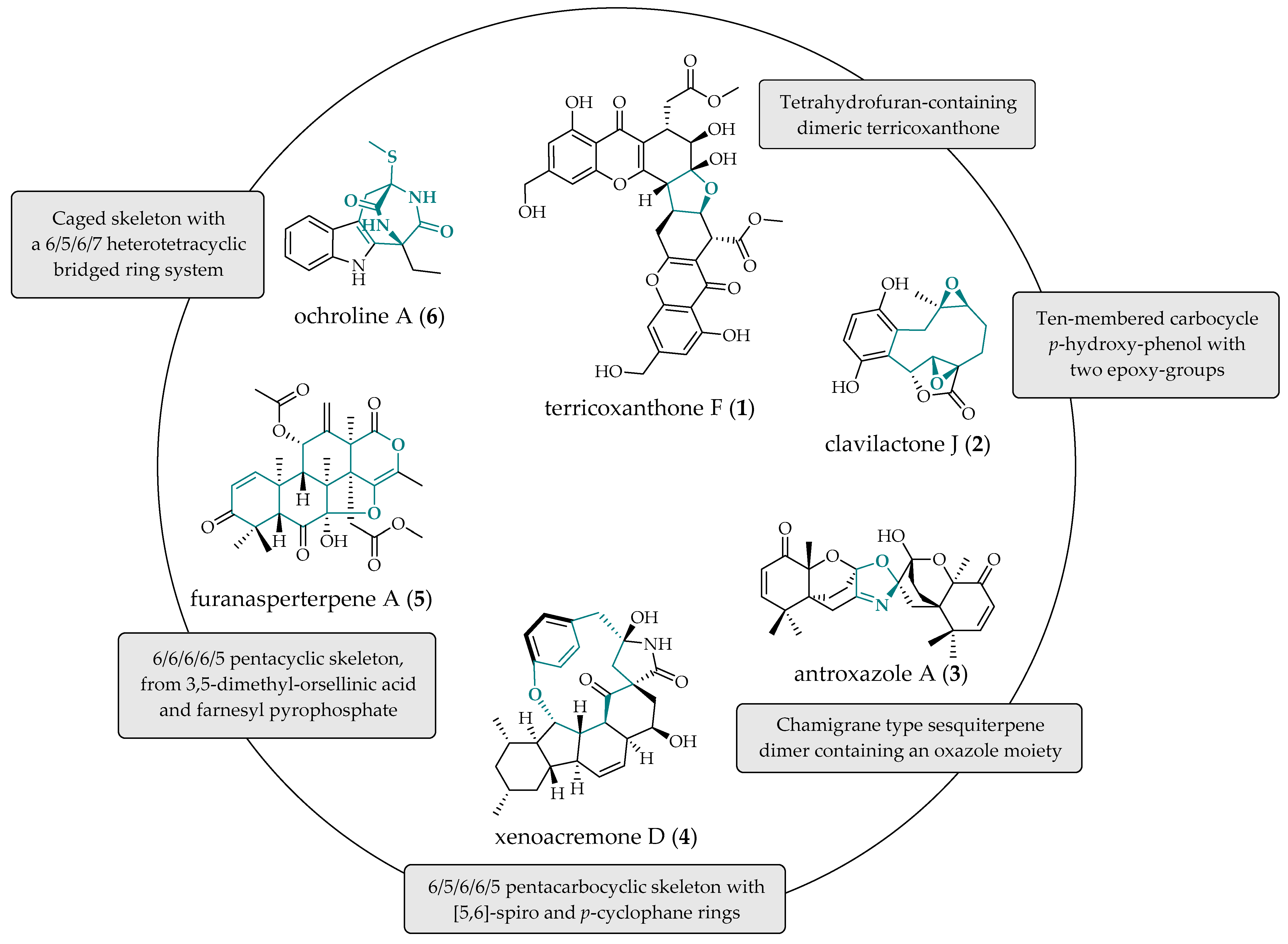
- Chen, H.W.; Wu, X.Y.; Zhao, Z.Y.; Huang, Z.Q.; Lei, X.S.; Yang, G.X.; Li, J.; Xiong, J.; Hu, J.F. Terricoxanthones A–E, unprecedented dihydropyran-containing dimeric xanthones from the endophytic fungus Neurospora terricola HDF-Br-2 associated with the vulnerable conifer Pseudotsuga Gaussenii. Phytochemistry 2024, 219, 113963. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Li, Q.; Chen, M.; Wu, H.; Yang, J.; Sun, Z.; Xu, X.; Ma, G. Novel geranylhydroquinone derived meroterpenoids from the fungus Clitocybe clavipes and their cytotoxic activity. Fitoterapia 2022, 161, 105251. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yu, W.W.; Isaka, M.; Cox, R.J.; Liu, J.K.; Feng, T. Antroxazole A, an oxazole-containing chamigrane dimer from the fungus Antrodiella albocinnamomea with immunosuppressive activity. Org. Biomol. Chem. 2022, 20, 7278–7283. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, L.; Wang, A.; Li, L.; Zhao, S.; Wang, Y.; Sun, Y. Xenoacremones D–H, Bioactive Tyrosine-decahydrofluorene Analogues from the Plant-Derived Fungus Xenoacremonium sinensis. Mar. Drugs 2022, 20, 375. [Google Scholar] [CrossRef]
- Chen, B.; Shi, Z.; Wang, Y.; Chen, M.; Yang, C.; Cui, H.; Su, T.; Kwan, H.Y. Discovery of a novel anti-obesity meroterpenoid agent targeted subcutaneous adipose tissue. Phytomedicine 2022, 106, 154396. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Qin, S.; Xu, L.; Sang, Z.; Chen, C.; Tan, J.; Huang, Y.; Li, M.; Zou, Z. Ochrolines A-C, three new indole diketopiperazines from cultures of endophytic fungi Bionectria ochroleuca SLJB-2. Fitoterapia 2024, 173, 105809. [Google Scholar] [CrossRef]
- Takahashi, J.A.; Marques, J.G.S.; Ferreira, M.R.; Santos, T.P.; Rosário, G.O.C. Recent Advances in Pharmaceutically Important Compounds from Endophytic Fungi. In Fungi Bioactive Metabolites: Integration of Pharmaceutical Applications, 1st ed.; Deshmukh, S.K., Takahashi, J.A., Saxena, S., Eds.; Springer: Singapore, 2024; Volume 1, pp. 3–28. [Google Scholar] [CrossRef]
- Bode, H.B.; Bethe, B.; Höfs, R.; Zeeck, A. Big Effects from Small Changes: Possible Ways to Explore Nature’s Chemical Diversity. Chem. Bio. Chem. 2002, 3, 619–627. [Google Scholar] [CrossRef]
- Achimón, F.; Krapacher, C.R.; Jacquat, A.G.; Pizzolitto, R.P.; Zygadlo, J.A. Carbon sources to enhance the biosynthesis of useful secondary metabolites in Fusarium verticillioides submerged cultures. World J. Microbiol. Biotechnol. 2021, 37, 78. [Google Scholar] [CrossRef]
- de Oliveira, G.P.; Barreto, D.L.C.; Ramalho Silva, M.; Augusti, R.; Evódio Marriel, I.; Gomes de Paula Lana, U.; Takahashi, J.A. Biotic stress caused by in vitro co-inoculation enhances the expression of acetylcholinesterase inhibitors by fungi. Nat. Prod. Res. 2022, 36, 4266–4270. [Google Scholar] [CrossRef]
- Ogbe, A.A.; Finnie, J.F.; Van Staden, J. The role of endophytes in secondary metabolites accumulation in medicinal plants under abiotic stress. S. Afr. J. Bot. 2020, 134, 126–134. [Google Scholar] [CrossRef]
- Jia, X.; Song, J.; Wu, Y.; Feng, S.; Sun, Z.; Hu, Y.; Yu, M.; Han, R.; Zeng, B. Strategies for the Enhancement of Secondary Me-tabolite Production via Biosynthesis Gene Cluster Regulation in Aspergillus oryzae. J. Fungi 2024, 10, 312. [Google Scholar] [CrossRef]
- Staropoli, A.; Iacomino, G.; De Cicco, P.; Woo, S.L.; Di Costanzo, L.; Vinale, F. Induced secondary metabolites of the beneficial fungus Trichoderma harzianum M10 through OSMAC approach. Chem. Biol. Technol. Agric. 2023, 10, 28. [Google Scholar] [CrossRef]
- Yu, H.B.; Ning, Z.; Hu, B.; Zhu, Y.P.; Lu, X.L.; He, Y.; Jiao, B.-H.; Liu, X.Y. Cytosporin Derivatives from Arctic-Derived Fungus Eutypella sp. D-1 via the OSMAC Approach. Mar. Drugs 2023, 21, 382. [Google Scholar] [CrossRef]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Chung, Y.M.; Wu, Y.C.; Hunyadi, A.; Wang, C.C.; Chang, F.R. Natural products development under epigenetic modulation in fungi. Phytochem. Rev. 2020, 19, 1323–1340. [Google Scholar] [CrossRef]
- Kramer, H.M.; Cook, D.E.; Seidl, M.F.; Thomma, B.P. Epigenetic regulation of nuclear processes in fungal plant pathogens. PLoS Pathog. 2023, 19, e1011525. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Hou, X.; Fu, J.; Zhang, J.; Wang, J.; Zhao, Z.; Xu, D.; Lai, D.; Zhou, L. Recent Advances in Search of Bioactive Secondary Metabolites from Fungi Triggered by Chemical Epigenetic Modifiers. J. Fungi 2023, 9, 172. [Google Scholar] [CrossRef]
- Cimermancic, P.; Medema, M.H.; Claesen, J.; Kurita, K.; Brown, L.C.W.; Mavrommatis, K.; Pati, A.; Godfrey, P.A.; Koehrsen, M.; Clardy, J.; et al. Insights into secondary metabolism from a global analysis of prokaryotic biosynthetic gene clusters. Cell 2024, 158, 412–421. [Google Scholar] [CrossRef]
- Chen, J.-J.; Han, M.-Y.; Gong, T.; Qiao, Y.-M.; Yang, J.-L.; Zhu, P. Epigenetic modification enhances ergot alkaloid pro-duction of Claviceps purpurea. Biotechnol. Lett. 2019, 41, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Galagan, J.E.; Calvo, S.E.; Cuomo, C.; Ma, L.J.; Wortman, J.R.; Batzoglou, S.; Lee, S.-I.; Baştürkmen, M.; Spevak, C.C.; Clutterbuck, J.; et al. Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature 2005, 438, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Harshitha, K.; Nair, A.R.; Pillai, P.P. Overview of bioactive metabolite(s) produced by endophytes and future perspectives on epigenetic modification/regulation of cryptic biosynthetic pathways. Phytochem. Lett. 2023, 53, 116–131. [Google Scholar] [CrossRef]
- Hur, J.Y.; Jeong, E.; Kim, Y.C.; Lee, S.R. Strategies for Natural Product Discovery by Unlocking Cryptic Biosynthetic Gene Clusters in Fungi. Separations 2023, 10, 333. [Google Scholar] [CrossRef]
- Zutz, C.; Gacek, A.; Sulyok, M.; Wagner, M.; Strauss, J.; Rychli, K. Small Chemical Chromatin Effectors Alter Secondary Metabolite Production in Aspergillus clavatus. Toxins 2013, 5, 1723–1741. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, X.; Tang, Y.; Liu, Y.; Zhao, Z.; Cui, H. New butanolide derivatives from the marine derived fungus Aspergillus terreus GZU-31-1 by chemical epigenetic manipulation. Nat. Prod. Res. 2022, 38, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Wei, C.; Chuang, D.; El-Shazly, M.; Hsieh, C.; Asai, T.; Oshima, Y.; Hsieh, T.; Hwang, T.; Wu, Y.; et al. An epigenetic modifier enhances the production of anti-diabetic and anti-inflammatory sesquiterpenoids from Aspergillus sydowii. Bioorganic Med. Chem. 2013, 21, 3866–3872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, J.; Wei, C.; Deng, X.; Xu, J. Chemical epigenetic modifiers enhance the production of immunosuppressants from the endophytic fungus Aspergillus fumigatus isolated from Cynodon dactylon. Nat. Prod. Res. 2022, 36, 4481–4485. [Google Scholar] [CrossRef] [PubMed]
- De Amorim, M.R.; Wijeratne, E.M.K.; Zhou, S.; Arnold, A.E.; Batista, A.N.L.; Batista, J.M.; dos Santos, L.C.; Gunatilaka, A.A.L. An epigenetic modifier induces production of 3-(4-oxopyrano)-chromen-2-ones in Aspergillus sp. AST0006, an endophytic fungus of Astragalus lentiginosus. Tetrahedron 2020, 76, 131525. [Google Scholar] [CrossRef]
- Henrikson, J.C.; Hoover, A.R.; Joyner, P.M.; Cichewicz, R.H. A chemical epigenetics approach for engineering the in situ biosynthesis of a cryptic natural product from Aspergillus niger. Org. Biomol. Chem. 2009, 7, 435–438. [Google Scholar] [CrossRef]
- Liu, W.; Wang, L.; Wang, B.; Xu, Y.; Zhu, G.; Lan, M.; Zhu, W.; Sun, K. Diketopiperazine and Diphenylether Derivatives from Marine Algae-Derived Aspergillus versicolor OUCMDZ-2738 by Epigenetic Activation. Mar. Drugs 2019, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Liang, X.; Liu, X.; Ji, N. Aspewentins A–C, Norditerpenes from a Cryptic Pathway in an Algicolous Strain of Aspergillus wentii. J. Nat. Prod. 2014, 77, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Albright, J.C.; Henke, M.T.; Soukup, A.A.; McClure, R.A.; Thomson, R.J.; Keller, N.P.; Kelleher, N.L. Large-Scale Metabolomics Reveals a Complex Response of Aspergillus nidulans to Epigenetic Perturbation. ACS Chem. Biol. 2015, 10, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shi, X.; Zhang, Y.; Shao, C.; Fu, X.; Li, X.; Yao, G.; Wang, C. Benzyl Furanones and Pyrones from the Marine-Derived Fungus Aspergillus terreus Induced by Chemical Epigenetic Modification. Molecules 2020, 25, 3927. [Google Scholar] [CrossRef]
- Xiao, L.; Yin, Y.; Sun, W.; Zhang, F.; Li, Z. Enhanced production of (+)-terrein by Aspergillus terreus strain PF26 with epigenetic modifier suberoylanilide hydroxamic acid. Process Biochem. 2013, 48, 1635–1639. [Google Scholar] [CrossRef]
- Magotra, A.; Kumar, M.; Kushwaha, M.; Awasthi, P.; Raina, C.; Gupta, A.P.; Shah, B.A.; Gandhi, S.G.; Chaubey, A. Epigenetic modifier induced enhancement of fumiquinazoline C production in Aspergillus fumigatus (GA-L7): An endophytic fungus from Grewia asiatica L. AMB Expr. 2017, 7, 43. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.; Tang, J.; Li, X. Eremophilane Sesquiterpenes from a Deep Marine-Derived Fungus, Aspergillus sp. SCSIOW2, Cultivated in the Presence of Epigenetic Modifying Agents. Molecules 2016, 21, 473. [Google Scholar] [CrossRef]
- Sun, K.; Zhu, G.; Hao, J.; Wang, Y.; Zhu, W. Chemical-epigenetic method to enhance the chemodiversity of the marine algicolous fungus, Aspergillus terreus OUCMDZ-2739. Tetrahedron 2018, 74, 83–87. [Google Scholar] [CrossRef]
- Yang, W.; Bao, H.; Liu, Y.; Nie, Y.; Yang, J.; Hong, P.; Zhang, Y. Depsidone Derivatives and a Cyclopeptide Produced by Marine Fungus Aspergillus unguis under Chemical Induction and by Its Plasma Induced Mutant. Molecules 2018, 23, 2245. [Google Scholar] [CrossRef]
- Zhu, J.X.; Ding, L.; Shan He, S. Discovery of a new biphenyl derivative by epigenetic manipulation of marine-derived fungus Aspergillus versicolor. Nat. Prod. Res. 2019, 33, 1191–1195. [Google Scholar] [CrossRef]
- Wu, J.; Yao, G.; Shi, X.; Rehman, S.U.; Xu, Y.; Fu, X.; Zhang, X.; Liu, Y.; Wang, C. Epigenetic Agents Trigger the Production of Bioactive Nucleoside Derivatives and Bisabolane Sesquiterpenes from the Marine-Derived Fungus Aspergillus versicolor. Front. Microbiol. 2020, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Borin, G.P.; Oliveira, J.V.D.C. Assessing the intracellular primary metabolic profile of Trichoderma reesei and Aspergillus niger grown on different carbon sources. Front. Fungal Biol. 2022, 3, 998361. [Google Scholar] [CrossRef] [PubMed]
- Bills, G.F.; Platas, G.; Fillola, A.; Jiménez, M.R.; Collado, J.; Vicente, F.; Martín, J.; González, A.; Bur-Zimmermann, J.; Tormo, J.R.; et al. Enhancement of antibiotic and secondary metabolite detection from filamentous fungi by growth on nutritional arrays. J. Appl. Microbiol. 2008, 104, 1644–1658. [Google Scholar] [CrossRef]
- Smedsgaard, J. Micro-scale extraction procedure for standardized screening of fungal metabolite production in cultures. J. Chromatogr. A 1997, 760, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Aldholmi, M.; Wilkinson, B.; Ganesan, A. Epigenetic modulation of secondary metabolite profiles in Aspergillus calidoustus and Aspergillus westerdijkiae through histone deacetylase (HDAC) inhibition by vorinostat. J. Antibiot. 2020, 73, 410–413. [Google Scholar] [CrossRef]
- Ying, Y.M.; Li, L.; Yu, H.F.; Xu, Y.L.; Huang, L.; Mao, W.; Tong, C.P.; Zhang, Z.D.; Zhan, Z.J.; Zhang, Y. Induced production of a new polyketide in Penicillium sp. HS-11 by chemical epigenetic manipulation. Nat. Prod. Res. 2021, 35, 3446–3451. [Google Scholar] [CrossRef]
- Jordan, A.; Stoy, P.; Sneddon, H.F. Chlorinated Solvents: Their Advantages, Disadvantages, and Alternatives in Organic and Medicinal Chemistry. Chem. Rev. 2020, 121, 1582–1622. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.U.; Wang, Y.; Soh, L.S.; Yong, W.F. Are green solvents truly green? Integrating life cycle assessment and tech-no-economic analysis for sustainable membrane fabrication. Green Chem. 2023, 25, 4501–4512. [Google Scholar] [CrossRef]
- Wang, X.; Serrano, R.; González-Menéndez, V.; Mackenzie, T.A.; Ramos, M.C.; Frisvad, J.C.; Larsen, T.O. A Molecular Networking Based Discovery of Diketopiperazine Heterodimers and Aspergillicins from Aspergillus caelatus. J. Nat. Prod. 2022, 85, 25–33. [Google Scholar] [CrossRef]
- Christiansen, J.V.; Larsen, T.O.; Frisvad, J.C. Production of Fungal Quinones: Problems and Prospects. Biomolecules 2022, 12, 1041. [Google Scholar] [CrossRef]
- Wang, X.; Subko, K.; Kildgaard, S.; Frisvad, J.C.; Larsen, T.O. Mass Spectrometry-Based Network Analysis Reveals New Insights into the Chemodiversity of 28 Species in Aspergillus section Flavi. Front. Fungal Biol. 2021, 2, 719420. [Google Scholar] [CrossRef] [PubMed]
- Charria-Girón, E.; Sauer, C.; García, D.; Ebada, S.S.; Marin-Felix, Y. Neochetracin: An Unusual Chetracin-Type Epithiodiketopiperazine Derivative Produced by the Fungus Amesia Atrobrunnea. ACS Omega 2024, 9, 24009–24014. [Google Scholar] [CrossRef] [PubMed]
- Wennrich, J.P.; Ebada, S.S.; Sepanian, E.; Holzenkamp, C.; Khalid, S.J.; Schrey, H.; Maier, W.; Mándi, A.; Kurtán, T.; Ashrafi, S.; et al. Omnipo-lyphilins A and B: Chlorinated cyclotetrapeptides and naphtho-α-pyranones from the plant nematode-derived fungus Polyphilus sieberi. J. Agric. Food Chem. 2024, 72, 6998–7009. [Google Scholar] [CrossRef]
- Wubshet, S.G.; Nyberg, N.T.; Tejesvi, M.V.; Pirttilä, A.M.; Kajula, M.; Mattila, S.; Staerk, D. Targeting high-performance liquid chromatography–high-resolution mass spectrometry–solid-phase extraction–nuclear magnetic resonance analysis with high-resolution radical scavenging profiles—Bioactive secondary metabolites from the endophytic fungus Penicillium namyslowskii. J. Chromatogr. A 2013, 1302, 34–39. [Google Scholar] [CrossRef]
- Bao, B.; Wang, Z.; Thushara, D.; Liyanage, A.; Gunawardena, S.; Yang, Z.; Zhao, S. Recent advances in micro-fluidics-based chromatography—A mini review. Separations 2021, 8, 3. [Google Scholar] [CrossRef]
- Kawai, T.; Matsumori, N.; Otsuka, K. Recent advances in microscale separation techniques for lipidome analysis. Analyst 2021, 146, 7418–7430. [Google Scholar] [CrossRef]
- Danne-Rasche, N.; Rubenzucker, S.; Ahrends, R. Uncovering the complexity of the yeast lipidome by means of nLC/NSI-MS/MS. Anal. Chim. Acta 2020, 1140, 199–209. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, J.A.; de Queiroz, L.L.; Vidal, D.M. A Close View of the Production of Bioactive Fungal Metabolites Mediated by Chromatin Modifiers. Molecules 2024, 29, 3536. https://doi.org/10.3390/molecules29153536
Takahashi JA, de Queiroz LL, Vidal DM. A Close View of the Production of Bioactive Fungal Metabolites Mediated by Chromatin Modifiers. Molecules. 2024; 29(15):3536. https://doi.org/10.3390/molecules29153536
Chicago/Turabian StyleTakahashi, Jacqueline Aparecida, Laura Lima de Queiroz, and Diogo Montes Vidal. 2024. "A Close View of the Production of Bioactive Fungal Metabolites Mediated by Chromatin Modifiers" Molecules 29, no. 15: 3536. https://doi.org/10.3390/molecules29153536
APA StyleTakahashi, J. A., de Queiroz, L. L., & Vidal, D. M. (2024). A Close View of the Production of Bioactive Fungal Metabolites Mediated by Chromatin Modifiers. Molecules, 29(15), 3536. https://doi.org/10.3390/molecules29153536







