Abstract
Liver disease is a global health problem that affects the well-being of tens of thousands of people. Dihydroquercetin (DHQ) is a flavonoid compound derived from various plants. Furthermore, DHQ has shown excellent activity in the prevention and treatment of liver injury, such as the inhibition of hepatocellular carcinoma cell proliferation after administration, the normalization of oxidative indices (like SOD, GSH) in this tissue, and the down-regulation of pro-inflammatory molecules (such as IL-6 and TNF-α). DHQ also exerts its therapeutic effects by affecting molecular pathways such as NF-κB and Nrf2. This paper discusses the latest research progress of DHQ in the treatment of various liver diseases (including viral liver injury, drug liver injury, alcoholic liver injury, non-alcoholic liver injury, fatty liver injury, and immune liver injury). It explores how to optimize the application of DHQ to improve its effectiveness in treating liver diseases, which is valuable for preparing potential therapeutic drugs for human liver diseases in conjunction with DHQ.
1. Introduction
The liver is an indispensable organ for the maintenance of life activities of the organism and is the leading site for anabolism, catabolism, and energy metabolism [1]. Liver injury is a condition in which hepatocyte damage to the liver occurs due to various causes, which in turn affects normal liver function. Globally, about 2 million people die from liver disease every year (data provided by the Institute for Health Metrics and Evaluation, University of Washington and Department of Surgery, University of Texas Southwestern, 2014) [2,3]. Liver injury occurs due to several factors, the most common of which are infectious, including hepatitis A, B, C, D, and E [4,5]. In addition, other factors such as drugs, alcohol, fat, and autoimmune can trigger liver injury. Liver injury can be categorized into viral liver injury, drug liver injury, alcoholic liver injury, fatty liver injury, and autoimmune liver injury. Liver injury, if not controlled in a timely and reasonable manner, can lead to the occurrence of serious diseases such as cirrhosis and even liver cancer [6,7]. Therefore, it is crucial to fully understand liver diseases and find safe and effective treatment methods to prevent their occurrence and development.
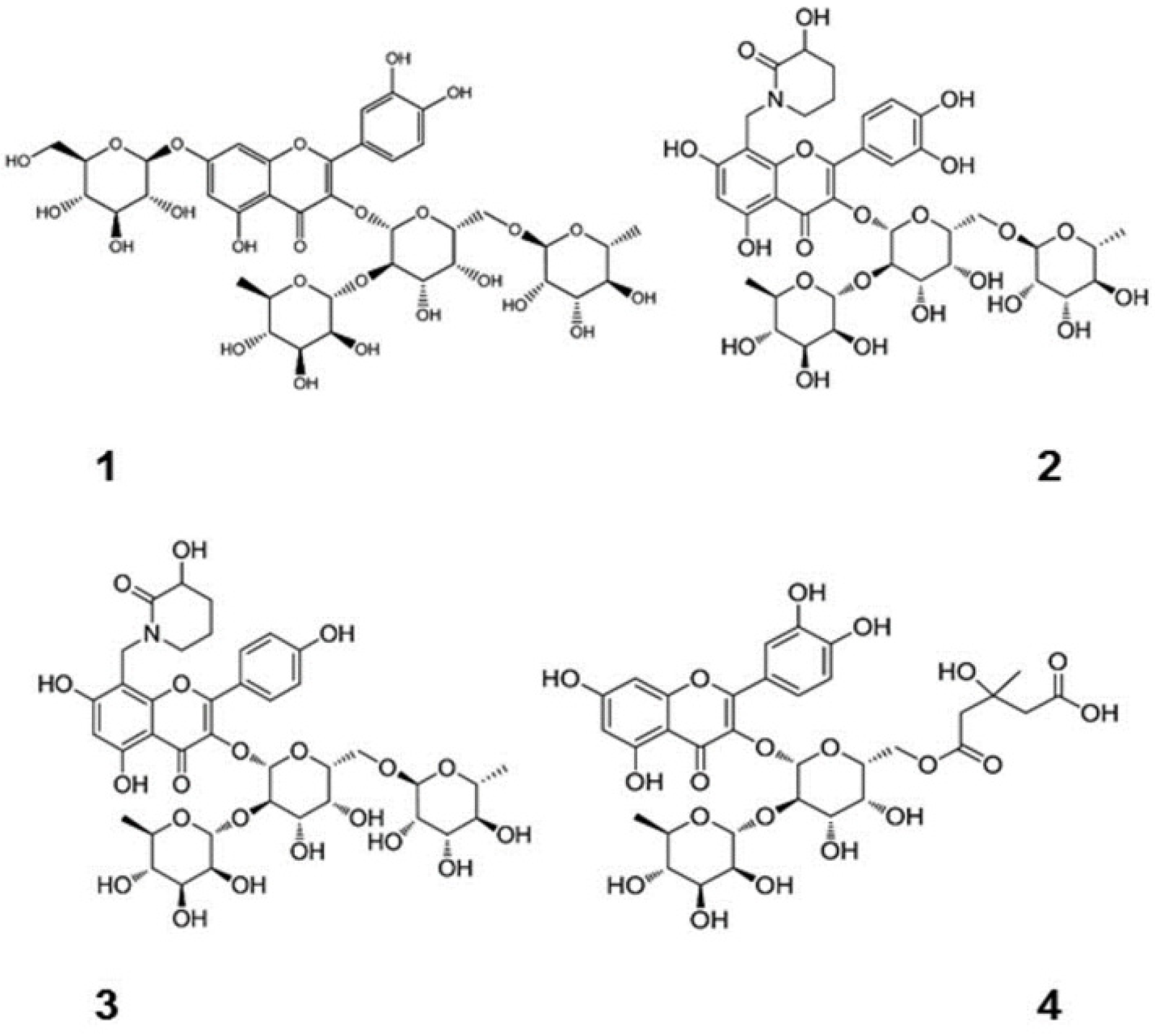
In recent years, natural medicinal plants have shown promising potential in protecting against liver injury, with flavonoids standing out as a particularly noteworthy area of research [8,9,10]. Pardede et al. [11] demonstrated the hepatoprotective effect of the active compound rutin on Tert-butyl Hydroperoxide (tBHP)-induced Hep G2. Kondeva et al. [12] found that flavonoids extracted from many species of the genus Astragalus effectively inhibited the release of lactate dehydrogenase (LDH) and reduced the production of malondialdehyde (MDA), thereby preventing liver injury. The effects of flavonoid structures on hepatotoxicity were analyzed using a QSAR model with LDH activity and MDA production as indicators. The analysis results showed that the presence of aromatic hydroxyl groups in flavonoids increased the number of non-hydrogen bonded aromatic carbon atoms attached to them. The negative regression coefficients in the model reveal that the higher the number of such aromatic carbon atoms, the lower the toxicity of the compounds to the liver. At the same time, the lipophilicity of flavonoids increases with the decrease in the number of hexose units. The positive regression coefficients in the model confirm the idea that the more lipophilic a compound is, the more toxic it is to the liver. In summary, flavonoids may have this hepatoprotective property due to the variety of functional groups contained in their glycosidic structure or glycosyl portion, and these different functional groups confer specific bioactivities (Figure 1). Dihydroquercetin (DHQ), as a natural and highly active flavonoid, is more widely distributed in plants, including tiger balm, mulberry, grape, elderberry, mistletoe bark, and wood bean root. Nevertheless, it is mainly derived from the roots of larch in alpine regions [13]. Like other flavonoids, DHQ possesses a wide range of bioactivities. It can be used to treat many causes of liver disease as well as anti-inflammatory [14,15], antiviral [16], antidiabetic [17], and other factors. Therefore, it is widely used in medicine and other fields and has a large potential for development.
Figure 1.
Chemical structures of flavonoids containing different sugar groups and DHQ [12]. (These figures were reprinted with permission).
In this review, scientific databases such as X-MOL, PubMed, Web of Science, and Google Scholar were searched from 1990 to the present to find out the online scientific literature on the preventive and curative effects of DHQ on liver injury and the improvement of DHQ bioavailability. The keywords searched were dihydroquercetin, taxifolin, liver injury, and biological availability. Based on this review, this paper summarizes the mechanism of action of DHQ in liver injury and the research progress in recent years in terms of five aspects: pharmacological liver injury, alcoholic liver injury, non-alcoholic liver injury, fatty liver injury, and immune liver injury. We also reviewed the technical means to improve the biological availability of DHQ to provide theoretical support for the broad application of DHQ in the future.
2. Mechanism of Action of DHQ in Protection against Liver Injury
2.1. Ameliorative Effects on Drug-Induced Liver Injury
Drug-Induced Liver Injury (DILI) is a condition in which liver function is impaired during drug use due to the drug itself, its metabolites, or an individual’s abnormal sensitivity and decreased tolerance to the drug, also known as drug-induced liver disease [18]. This injury can present with acute or chronic hepatitis symptoms, and liver function usually resolves spontaneously after the discontinuation of the drug in mild cases. Regardless, in severe cases, DILI can be life-threatening and requires urgent treatment [19]. It is noteworthy that DILI does not discriminate between populations and may occur in people with otherwise healthy livers or affect people with severe disease. DILI is complex and difficult to predict. Therefore, monitoring changes in liver function during drug therapy and recognizing and managing drug-related liver injury promptly are essential for patient safety.
2.1.1. Ameliorative Effects of Acetaminophen (APAP)-Induced Liver Injury
Acetaminophen (APAP) has been one of the widely used antipyretic and analgesic drugs worldwide since 1950 [20,21]. The drug is generally considered safe at the recommended dose (no more than 4 g per day) [22]. However, if this dosage limit is exceeded, an overdose can cause severe liver damage and may even progress to acute liver failure (ALF), a potential risk that should not be ignored [23,24,25]. Research has indicated that the metabolic processes of drugs and their effects on diverse intracellular signaling pathways play a central role in the mechanism of drug-induced hepatotoxicity, especially perturbations involving mitochondrial function, which are of particular importance [23]. When widespread necrosis occurs in the liver, the ensuing sterile inflammatory response is a crucial component of the body’s attempt to restore and repair damaged tissue. However, this inflammatory response acts as a double-edged sword: on the one hand, it is an integral part of tissue repair; on the other hand, if it is not regulated correctly, it may exacerbate the pre-existing damage and result in a more complex pathological state [26]. This is a controversial area. Despite this, a more profound comprehension of these inflammatory pathways is expected to facilitate the discovery of new therapeutic targets, which are especially important during the transition from the injury phase to the regeneration phase.
Chen et al. [27] first investigated the protective effect of DHQ against APAP-induced liver injury in a mouse model. Mice were injected intraperitoneally with a certain amount of APAP to establish the model. One hour later, they were treated with different concentrations (0, 20, 40 mg/kg) of DHQ. The high dose of DHQ was able to effectively inhibit the mRNA expression of the TLR4 gene and down-regulate the mRNA levels of pro-inflammatory factors TNF-α and IL-6. It significantly restored the reduction of antioxidant enzyme activities induced by APAP at both the mRNA and protein levels. The expression of Bcl-2 and pro-caspase-3 was increased. In contrast, the expression of Bax was suppressed, suggesting its positive effects in attenuating oxidative stress and inflammatory responses and inhibiting hepatocyte apoptosis, thus effectively reducing APAP-induced liver injury.
Hu et al. [28] investigated the effect of DHQ on APAP-induced hepatotoxicity in mice. They found that 80 mg/kg of DHQ alone effectively inhibited the expression of CYP2E1 in the liver tissue of mice. In addition, DHQ reversed the APAP-induced decrease in L-02 cell viability and reduced the accumulation of intracellular ROS levels. Glutamate cysteine ligase (GCL), a key rate-limiting enzyme for synthesizing glutathione, is essential for enhancing the body’s resistance to oxidative stress. GCLC and GCLM are the catalytic and regulatory subunits of GCL, respectively [29,30,31]. Following DHQ treatment, the antioxidant function was achieved by upregulating the expression of GCLC, thereby preventing APAP-induced liver injury.
Zai et al. [32] investigated the protective effect of DHQ against APAP-induced hepatocyte injury with different concentrations (12.5–100 μM). It was discovered that DHQ attenuated APAP-induced cell proliferation inhibition and lactate dehydrogenase (LDH) release in a dose-dependent manner and was able to block APAP-induced cell necrosis and extracellular signal-regulated kinase-c-Jun-N-terminal kinase (ERK-JNK) stress responses. In addition, DHQ improved cellular ROS levels in a dose-dependent manner. Mitochondrial dysfunction is a crucial factor in generating high levels of reactive oxygen species (ROS), and an initial increase in ROS will further exacerbate mitochondrial damage [33]. The results showed that APAP treatment resulted in the loss of mitochondrial membrane potential (MMP), followed by the reversal of ROS accumulation and mitochondrial dysfunction. DHQ also initiated the Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) cascade phosphorylation process, thereby regulating the expression of anti-apoptotic Bcl-2 family proteins. In addition, DHQ induces autophagy, mediating its protective effect on hepatocytes. Notably, this protective effect can be reversed by intervening with chloroquine (CQ) and inhibiting the autophagic process.
2.1.2. Ameliorative Effects against Carbon Tetrachloride (CCl4)-Induced Liver Injury
During CCl4-induced hepatic fibrosis, free radicals and cytokines released from damaged hepatocytes activate HSCs and initiate fibrogenesis [34]. The characteristics of CCl4-induced histological and biochemical changes reflect the pattern of human liver fibrosis and cirrhotic disease. In addition, some of the mechanisms of CCl4 induction have been demonstrated [35,36].
Liu et al. [37] treated CCl4-induced acute liver injury in mice with different concentrations (20, 40, and 80 mg/kg) of DHQ, and the present study demonstrated that DHQ inhibited hepatic stellate cell (HSC) activation and extracellular matrix production by regulating the PI3K/AKT/mTOR and TGF-β1/Smads pathways. Additionally, DHQ was found to attenuate CCl4-induced oxidative stress and apoptosis, suggesting its potential as an effective hepatoprotective agent.
2.1.3. Ameliorative Effects against Other Drug-Induced Liver Injury
Cyclophosphamide (CP), as a potent anticancer drug and immunosuppressant, has demonstrated significant clinical efficacy [38,39,40]. Meanwhile, it is associated with numerous adverse effects, especially causing hepatic impairment [41]. Althunibat et al. [42] investigated the potential protective role of DHQ by a mouse model of CP-induced hepatotoxicity. NF-κB p65 activation and pro-inflammatory cytokines are key factors in CP-induced liver injury. It was shown that DHQ treatment reduced NF-κB p65 protein expression and significantly lowered the levels of TNF-α, IL-1β and IL-6 while effectively inhibiting the process of apoptosis through the up-regulation of the expression of the anti-apoptotic protein Bcl-2 and the reduction in the levels of the pro-apoptotic proteins Bax and caspase-3. Together, these findings support the anti-inflammatory and protective effects of DHQ in attenuating CP-induced liver injury.
As a pesticide ingredient of natural origin, rotenone has been recognized as an environmental pollutant. It has been reported in the literature to cause organ function damage, exhibiting potentially toxic effects [43,44,45]. After using rotenone to cause liver injury, Akinmoladun et al. [46] found that liver function indices after treatment with DHQ (0.25, 0.5, and 1 mg/kg) showed significant improvement, including bilirubin level, γ-glutamyltransferase, alkaline phosphatase, alanine aminotransferase and aspartate aminotransferase activities, and total protein concentration. The levels/activities of hepatic oxidative stress indicators showed that DHQ could effectively attenuate the hepatic injury induced by rotenone and exert its protective effects by enhancing the antioxidant mechanism and reducing the level of oxidative stress.
Pazopanib, a tyrosine kinase inhibitor, is commonly used for the treatment of metastatic renal cell carcinoma and advanced soft tissue sarcoma, and it induces varying degrees of hepatotoxicity [41,47,48]. AkAgunduz et al. [49] explored the effect of DHQ (50 mg/kg) on pazopanib-induced hepatotoxicity. It not only attenuated pazopanib-induced liver injury at the histopathological level but also showed positive improvement in biochemical indexes and effectively inhibited the further development of oxidative hepatotoxicity. These discoveries emphasize the value of DHQ as a potential adjuvant therapy in attenuating the hepatotoxicity of the chemotherapeutic drug pazopanib and provide a theoretical basis for further preclinical and clinical studies.
Cisplatin, a widely used platinum-based chemotherapeutic agent, has been used in the treatment of a wide range of cancer types, including gastric and ovarian cancers [50,51,52]. Although it has demonstrated significant efficacy against these malignant tumors and the efficacy is usually enhanced with the elevated drug dose, its non-selective mechanism of action also poses a severe challenge to patients [53,54]. With increasing therapeutic doses, cisplatin inevitably produces toxic effects on several organs and physiological systems, such as hepatotoxicity and nephrotoxicity, limiting its clinical application [55,56]. Kurt et al. [57] investigated the effect of DHQ on cisplatin-induced oxidative liver injury in rats using biochemical methods. After treatment with DHQ (50 mg/kg), the reduction in serum alanine aminotransferase (AST) and alanine aminotransferase (ALT) levels and the normalization of other biochemical indices were observed. The intervention of DHQ effectively reduced the degree of oxidative stress and thus exerted a protective effect on the liver.
2.2. Ameliorative Effects on Alcoholic Liver Injury
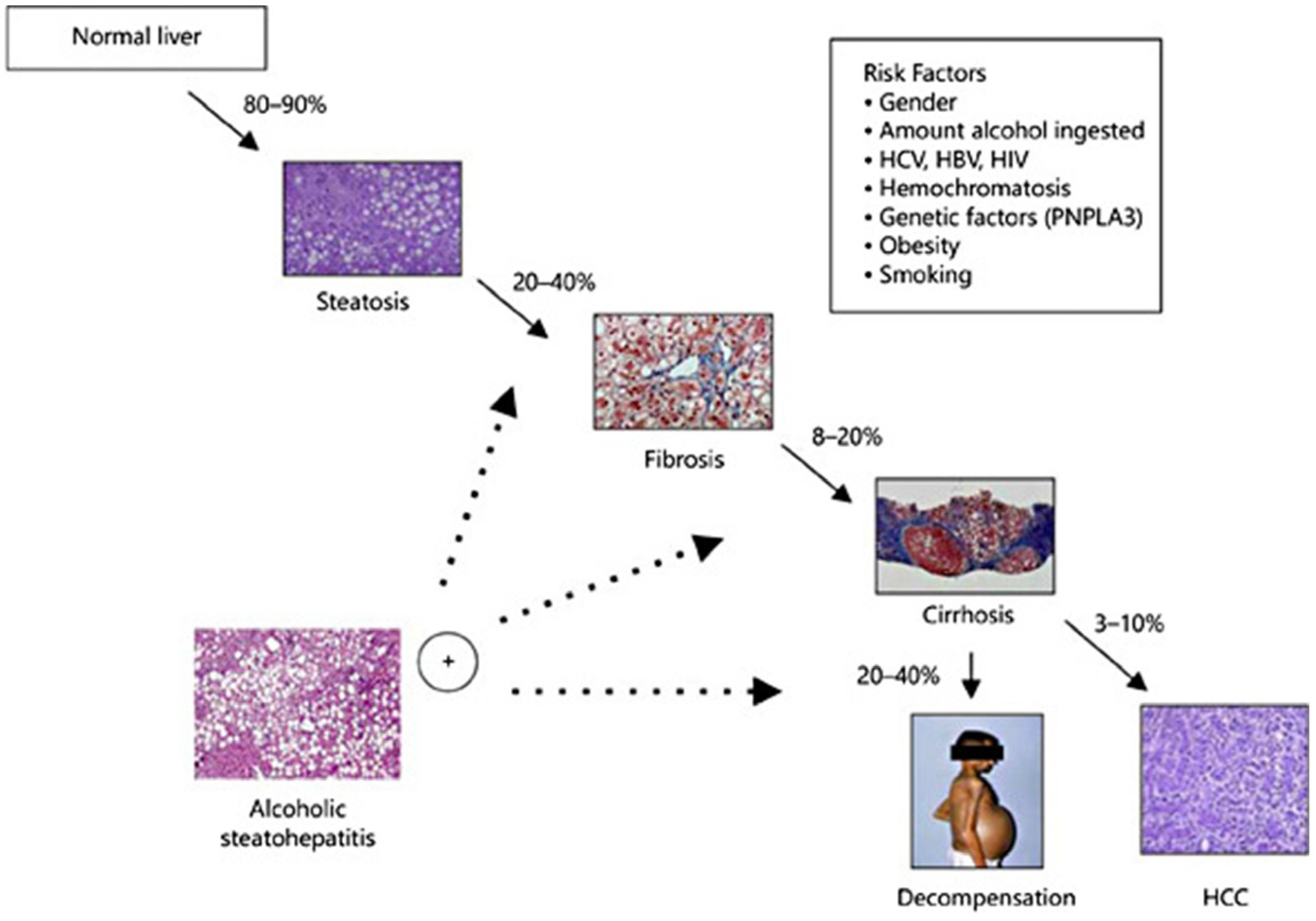
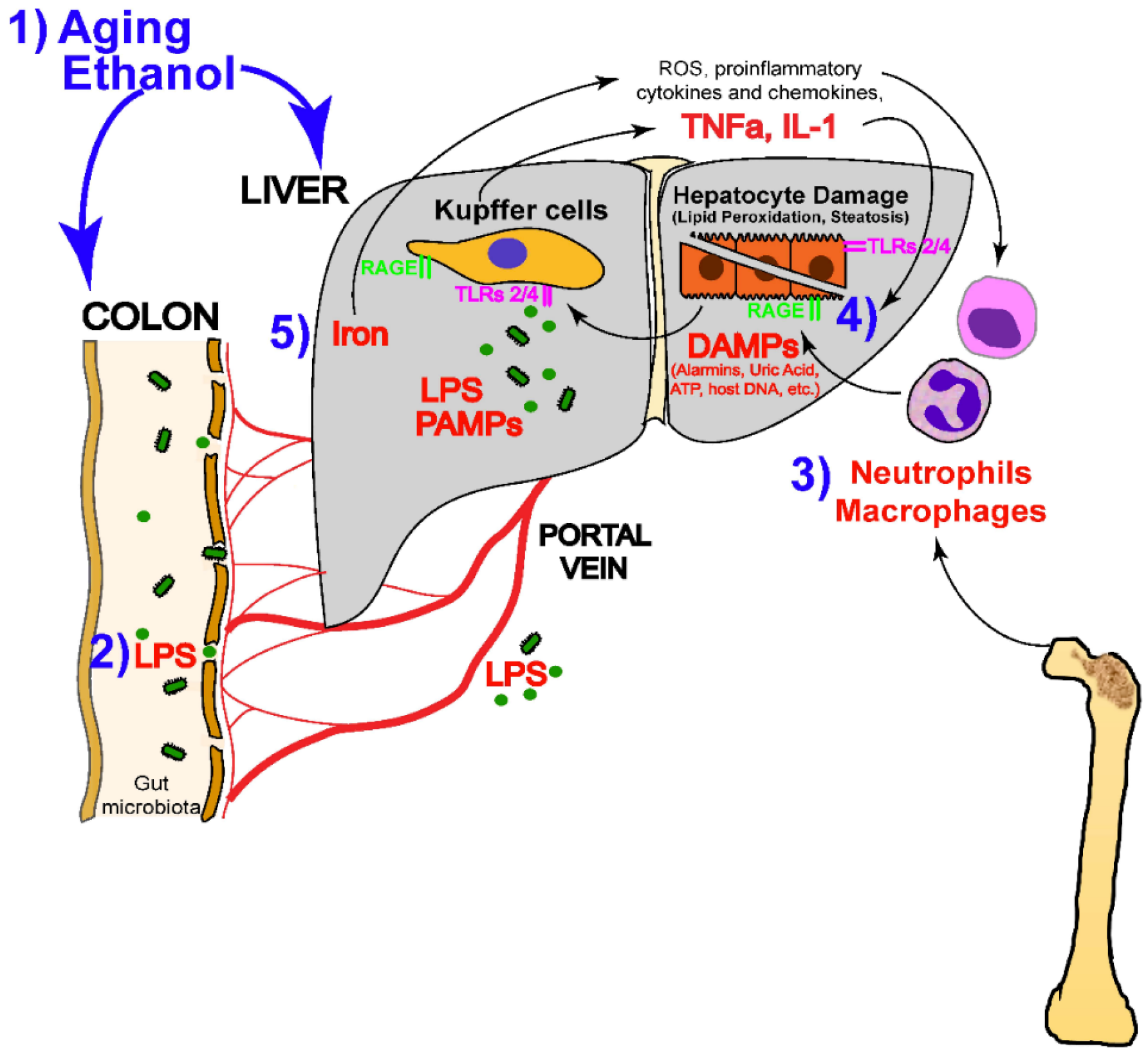
Excessive alcohol consumption has varying chances of leading to the development of different kinds of alcohol-related liver disease (ALD), including a range of diseases such as asymptomatic hepatic steatosis, hepatic fibrosis, and cirrhosis (Figure 2) [58,59,60,61,62]. Alcohol metabolite toxicity reduces liver compensatory capacity, interferes with homeostasis, and makes the liver vulnerable [63]. Critical aspects in the development of ALD include (1) aging and gender differences; (2) aging and gender differences; (3) infiltration of neutrophils and bone marrow-derived macrophages; (4) alcohol metabolism and fatty acid synthesis; and (5) iron deposition and ROS production (Figure 3).
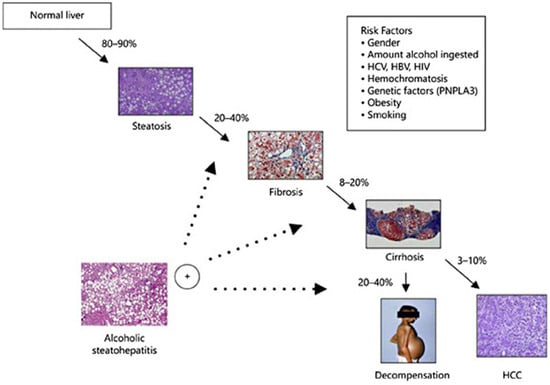
Figure 2.
Progression of ALD and susceptible risk factors for accelerated progression [64]. (These figures were reprinted with permission).
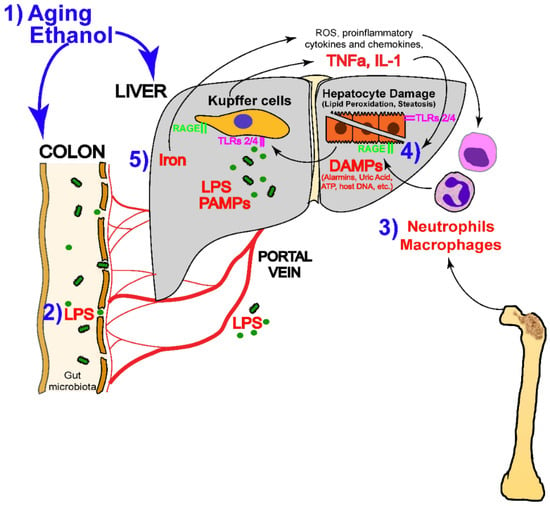
Figure 3.
Alcohol-mediated liver injury and the mechanisms that drive it (LPS, lipopolysaccharide; DAMP, disease-associated molecular patterns; ATP, adenosine triphosphate; TNFα, tumor necrosis factor-α; IL-1, interleukin-1; ROS, reactive oxygen species; PAMPs, pathogen-associated molecular patterns) [65]. (These figures were reprinted with permission).
In order to explore the pathogenesis of ALD, interventional therapy, and to understand the effects of alcohol metabolism on the microenvironment of liver tissues, Ding et al. [8] explored the potential protective effects of different concentrations of DHQ (20, 40, and 80 mg/kg) against acute alcoholic liver injury in mice. The experimental results showed that the treatment of DHQ decreased alanine aminotransferase (ALT) and increased aspartate aminotransferase (AST) while contributing to the increase in superoxide dismutase (SOD), glutathione (GSH) and malondialdehyde (MDA). Histopathologic examination showed that alcohol-induced hepatocellular injury and inflammatory invasion were reduced after treatment with DHQ. These findings support the role of DHQ as a hepatoprotective agent, which contributes to the amelioration of alcoholic liver injury through its antioxidant, anti-inflammatory, and direct protective effects on hepatocytes. Meanwhile, Western blot analysis and real-time fluorescence quantitative PCR (rt-PCR) results showed that DHQ was able to reduce the level of tumor necrosis factor-α (TNF-α), block the activation pathway of nuclear factor-κB (NF-κB) in the liver, and effectively reverse the alcohol-induced. The process of alcohol-induced apoptosis was effectively reversed by adjusting the expression of PI3K/Akt signaling pathway and its downstream apoptosis-related factors.
2.3. Ameliorative Effects on Fatty Liver Injury
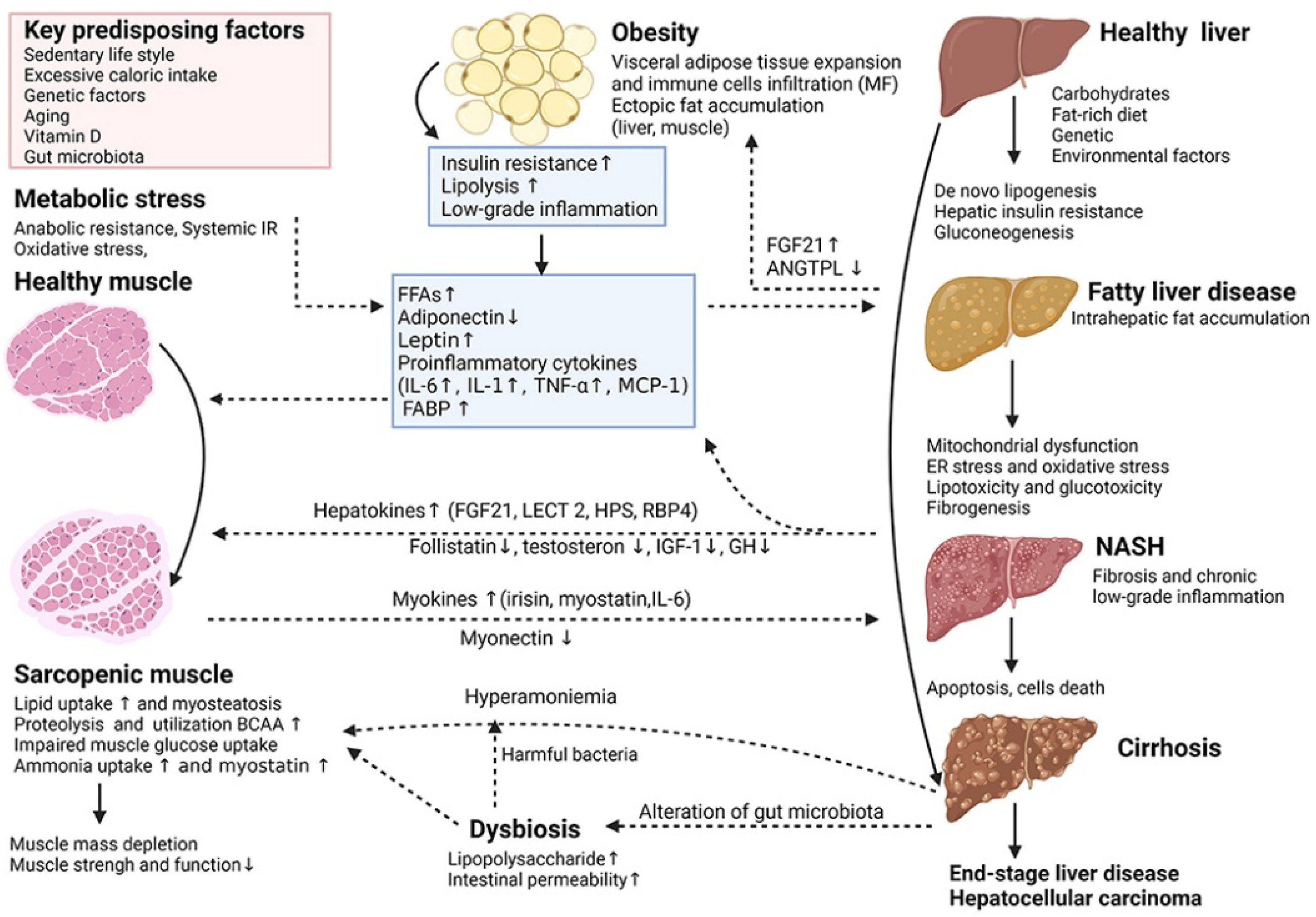
Non-alcoholic fatty liver disease (NAFLD) is one of the most prevalent types of chronic liver disease worldwide, affecting approximately one-quarter of the general population, with a particular predilection for patients with obesity and type 2 diabetes mellitus (T2DM) [66,67,68]. The scope of the disease is broad, encompassing both non-alcoholic simple fatty liver disease (NAFL) and the more severe non-alcoholic steatohepatitis (NASH). Notably, patients with NASH have a significantly higher risk of developing advanced liver fibrosis, cirrhosis, and even hepatocellular carcinoma (HCC) [69,70,71]. The pathomechanism of NASH is complex (Figure 4), involving the interplay of metabolic stress and inflammatory response. Given this, therapeutic strategies for NASH need to focus on stopping the progression of the condition to hepatocellular carcinoma, and it is therefore critical to assess whether therapies are effective in blocking this process.
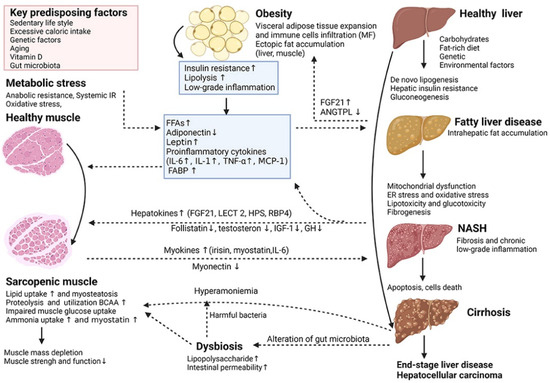
Figure 4.
Key cellular and molecular mechanisms underlying the complex interactions between adipose tissue, sarcopenia and non-alcoholic fatty liver disease (NAFLD) [72]. (These figures were reprinted with permission).
Lee et al. [73] explored the effect of DHQ on free fatty acid (FFA)-induced insulin resistance in the liver. FFA treatment inhibited cellular glucose uptake, whereas DHQ at concentrations of 25 and 50 µM was able to reverse this effect and promote glucose uptake. In addition, DHQ upregulated the expression levels of key proteins involved in insulin signaling—p-PI3K, p-IRS1, p-AKT, p-AMPK and p-ACC—in FFA-treated hepatocytes. It was also noted that FFA treatment led to a rise in miR-195 expression, but DHQ treatment significantly reduced it dose-dependently. The modulation of miR-195 levels by the transfection of miR-195 mimics and inhibitors, respectively, further confirmed that DHQ enhanced the expression of p-IRS1, p-PI3K, p-AMPK, p-AKT, and p-ACC through this pathway, thus revealing the mechanism by which DHQ alleviates FFA-induced liver damage by modulating miR-195 expression. These findings provide new perspectives for understanding the molecular mechanisms of DHQ in ameliorating fatty liver-associated insulin resistance.
Inoue et al. [74] DHQ at different concentrations (0.05 and 3%) used in the treatment of a NASH mouse model significantly prevented the development of hepatic steatosis, chronic inflammation and liver fibrosis. The mechanism of action includes a direct effect on hepatocytes, i.e., the inhibition of lipid accumulation [75]. FGF21 is a potent inducer of thermogenic genes in brown adipose tissue [76]. mRNA expression of FGF21 was increased in the liver and brown adipose tissue by DHQ treatment and significantly increased brown adipocyte-specific genes, and mature brown adipocyte secreted factors (FGF21 and IL-6) of mRNA expression. These results suggest that DHQ acts through a dual mechanism: directly regulating brown adipocyte function while promoting FGF21 expression in the liver. When exploring the effects of DHQ on hepatic steatosis in depth, the researchers found that the up-regulated expression of genes associated with lipogenesis (SREBP1c, FAS, SCDc1, and ACC and inflammatory genes (TNFα, Il-1β, and EMR1 (F4/80)) was significantly suppressed in the high-dose DQH-treated group. The histological examination further revealed the presence of small, dysplastic nodules characterized by atypical hepatocellular hyperplasia with enlarged nuclei and deepened pigmentation in non-tumorigenic NASH liver samples. DHQ treatment significantly reduced the mRNA expression of genes associated with inflammation and fibrosis in liver tumor lesions without affecting CD206, a representative marker of tumor-associated macrophages. In summary, the study by Inoue et al. not only provides strong evidence for the therapeutic effects of DHQ in successive stages of NASH but also provides insight into the novel mechanisms of DHQ action.
2.4. Ameliorative Effects against Autoimmune Hepatitis
Autoimmune hepatitis is a severe health challenge affecting a large number of patients worldwide. The cases of the disease increased abruptly in 2014 (Table 1), and disease progression is often accompanied by a poor prognosis [77]. In this context, DHQ, through its potent antioxidant properties and anti-inflammatory effects, can significantly alleviate the symptoms of acute or severe (fulminant) hepatitis, offering new hope for intervening in this type of autoimmune liver disease [78]. Zhao et al. [79] found that DHQ at a dose of 30 mg/kg significantly ameliorated cutin A (Con A)-induced liver injury in mice. Specifically, DHQ was able to significantly enhance the survival rate of mice while effectively reducing the serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), indicators of liver function. Given that oxidative stress and pro-inflammatory mediators released by macrophages play a vital role in the development of immune-mediated hepatitis [80], DHQ demonstrated the ability to significantly inhibit interferon-gamma (IFN-γ) and tumor necrosis factor-α (TNF-α) mRNA expression and their secretion. This mechanism of action involves the enhancement of nuclear factor E2-related factor 2 (Nrf2) expression, which is not only increased in the cytoplasm but also shifted to the nucleus, thereby significantly elevating heme oxygenase-1 (HO-1) expression in a time- and dose-dependent manner. The protective effect of DHQ on Con A-induced hepatic injury may be due to the activation of Nrf2/1. The protective mechanism of DHQ against Con A-induced liver injury may lie in the activation of the Nrf2/HO-1 pathway to remove oxidative stress and increase HO-1 activity, as well as the modulation of MAPK signaling in macrophages to inhibit the release of inflammatory mediators, thereby effectively alleviating immune-mediated liver injury. Together, these findings emphasize the effectiveness and mechanism of DHQ as a potential therapeutic tool in response to specific models of liver injury.
Chen et al. [81] identified that the immunomodulatory effects of DHQ on DHQ (5 mg/kg) effectively ameliorated Con A-mediated immune, hepatic injury by reducing the expression of pro-inflammatory mediators and inhibiting the infiltration of CD4+/CD8+ T cells in the liver. In addition, DHQ may also provide HepG2 cells with protective effects against TNF-α/ActD-induced apoptosis by modulating the caspase pathway and NF-κB signaling pathway, thus demonstrating its dual benefits in immunomodulation and cytoprotection.

Table 1.
Studies on the incidence and prevalence of autoimmune hepatitis [82]. (The data are reprinted with permission).
Table 1.
Studies on the incidence and prevalence of autoimmune hepatitis [82]. (The data are reprinted with permission).
| Incidence/100,000 | Rrevalence/100,000 | Ref. | Year | Cases |
|---|---|---|---|---|
| 0.8 | —— | [83] | 1997 | 496 |
| 3.0 | —— | [84] | 2007 | 200 |
| 0.85 | 10.7 | [85] | 2008 | 473 |
| 1.68 | 23.9 | [78] | 2014 | 1721 |
| 1.1 | 18.3 | [86] | 2014 | 1313 |
| 2.0 | 24.5 | [87] | 2010 | 138 |
| 0.67 | 11.0 | [88] | 2013 | 100 |
| 1.37 | 11.61 | [89] | 2004 | 13 |
| —— | 42.9 | [90] | 2002 | 77 |
3. Improvement of DHQ Bioavailability
Drug bioavailability is an essential aspect of pharmacology that affects the effectiveness of drug therapy [91]. Although a large number of studies have amply demonstrated the great potential of DHQ for clinical applications, in fact, systematic studies on the stability of natural products are quite limited [92]. Previous studies have pointed out that DHQ undergoes polymerization when electrolyzed in a neutral environment (pH 7.0) [93], which undoubtedly poses a challenge to the stable use of the drug. In addition, despite its high water solubility, the oral bioavailability of DHQ is unsatisfactory, being only about 36%, especially in the form of lipid-based drug delivery [94,95]. Of more significant concern, DHQ may also be broken down by microbial communities in the gut [96], further affecting its bioavailability. Given all these limitations, finding effective methods to enhance the bioavailability of DHQ has become a key aspect in driving the development of novel drug delivery systems. This requires an in-depth investigation of the chemical and biological properties of DHQ, but also innovative drug formulation techniques to ensure that the compound is absorbed and utilized more stably and efficiently after entry into the body, thus fully exploiting its potential therapeutic benefits (Figure 5).
The development of innovative materials is leading a revolution in medicine, significantly advancing the progress of drug manufacturing [97]. Xiong and other scholars [98] have exhaustively elaborated in their study a cutting-edge strategy that utilizes metal-organic frameworks (MOFs) cross-linked with cyclodextrins to significantly enhance the efficiency of chemotherapeutic drug-targeted delivery and uptake to localized lesions. This technological breakthrough opens up new possibilities for precision medicine. In another study, Song et al. [99] introduced a novel class of organic mineral matrix materials, such as liposomal dispersions prepared using natural clay. These novel materials not only ensured the stability of the formulation but also broadened the solubility range of the drug, providing an innovative solution for the delivery of difficult-to-solve drugs. Ding et al. [100] explored the hepatoprotective mechanism of DHQ liposomes by in vivo experiments after preparing DHQ liposomes using a thin-film dispersion method. The results showed that DHQ liposomes (50 mg/kg) significantly inhibited LPS/D-galactose-induced acute hepatic injury in mice through antioxidant effects. A review article by V. Ambrogi [101], on the other hand, looked at the functional expansion of traditional materials from an entirely new perspective. Taking calcium carbonate as an example, this classic material, which has been traditionally used as pH-responsive fillers, is now being utilized and revitalized through the exploration of the application of different crystal forms. Calcium carbonate is being developed as a novel carrier matrix for drug molecules [102], and utilizing its unique physical and chemical properties, these matrices can effectively enhance the bioavailability of drugs, bringing revolutionary thinking to drug design and delivery technologies. These recent advances in material science undoubtedly provide strong support for improving drug therapeutic efficacy, reducing side effects and developing novel drug release systems.
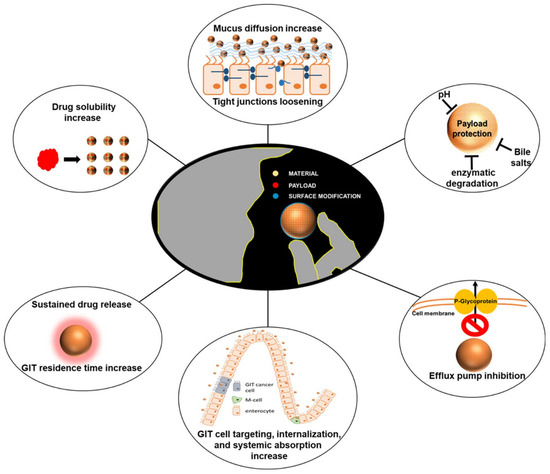
Figure 5.
Characterization of nanodelivery systems for improving the bioavailability of oral therapeutic drugs [103]. (These figures were reprinted with permission).
Figure 5.
Characterization of nanodelivery systems for improving the bioavailability of oral therapeutic drugs [103]. (These figures were reprinted with permission).
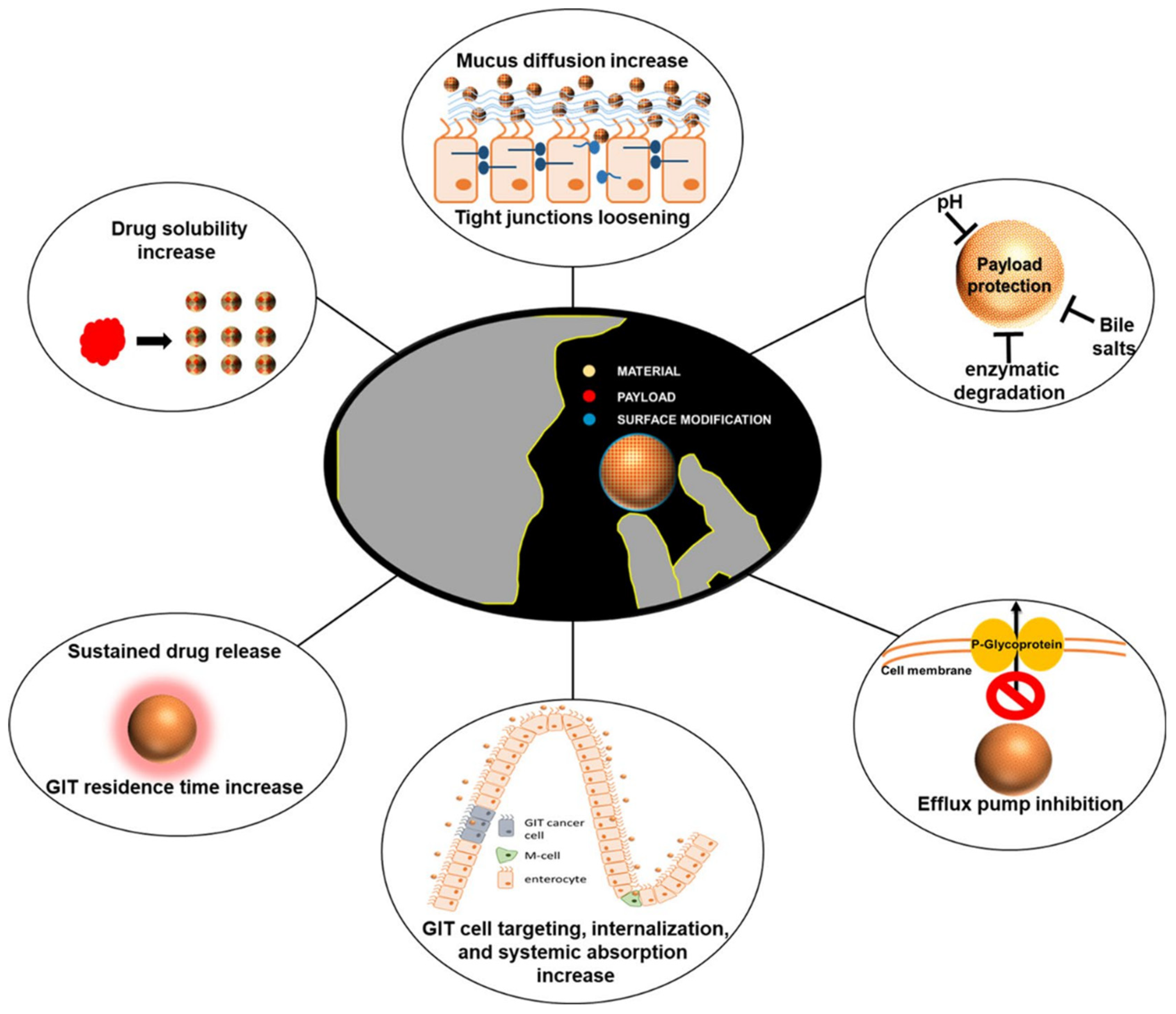
4. Prospects and Outlook
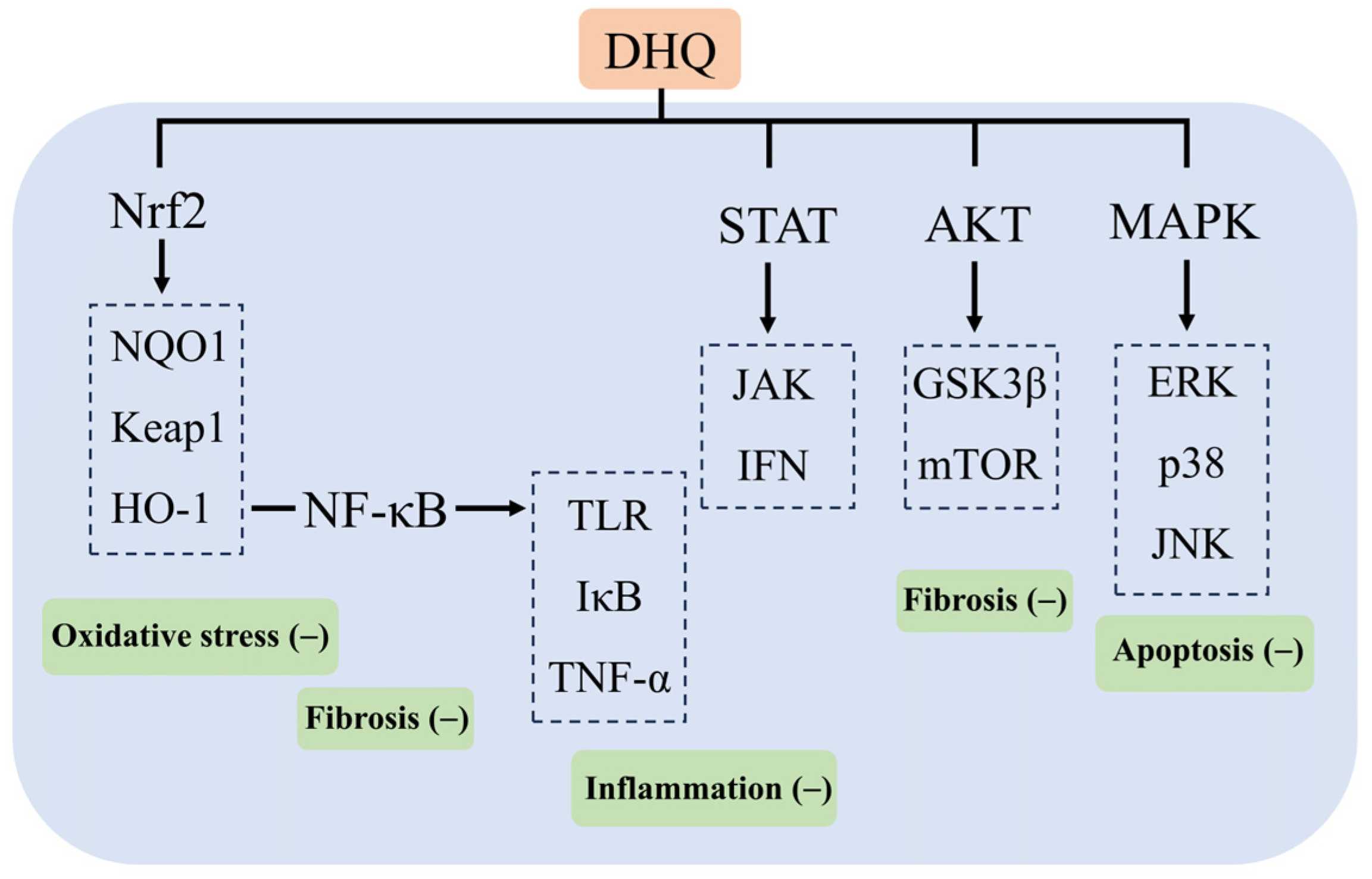
In Russia, DHQ is added to more than 250 products, of which 142 are food supplements and 40 are food products, and no adverse reactions have been reported during the 15–20 years of use and sale of their products [104]. DHQ has been included in the Russian Pharmacopoeia for hepatoprotection, the treatment of diabetes, atherosclerosis, and so on. Some patients have been taking 600 mg per day for two weeks, with no adverse reactions. Meanwhile, on 13 December 2016, a panel of experts from the European Union Food Safety Authority EFSA reviewed the composition of the DHQ extract provided by Ametis JSC. It stated that it was adequately characterized and had no safety concerns, and no genotoxicity was found. A 90-day subchronic rat study according to OECD standards showed no adverse effects at the highest dose (1500 mg/kg body weight) [99,105]. Thus, the aim of this paper is to review the mechanism of action of the natural flavonoid DHQ in the prevention and treatment of various experimental liver injuries and its recent findings. As a crucial organ in the human body, the liver undertakes the core functions of synthesis, metabolism and detoxification. Therefore, the effective prevention and treatment of liver injury have been a hot issue in the field of medical research. The review shows that DHQ is particularly effective in combating chemical, pathological, immune, and pharmacological liver injuries and that DHQ exerts its effects in protecting the liver from injury through a variety of mechanisms, including the attenuation of oxidative stress and inflammation, the modulation of lipid metabolism, antiviral activity, and immunomodulation. These mechanisms of action involve multiple key signaling pathways and their targets, such as TLR and IκB in the NF-κB signaling pathway; ERK, p38, and JNK in the MAPK pathway; GSK3β and mTOR in the Akt pathway; HO-1, NQO1, and Keap1 in the Nrf2 pathway; and JAK and IFN in the STAT pathway, which demonstrates the multidimensional protective effects of DHQ at the molecular level (Figure 6). Although DHQ has demonstrated a wide range of potential applications in experimental studies for the prevention and treatment of liver injury, its low oral bioavailability has become a significant bottleneck limiting its clinical application. For this reason, several strategies to enhance the bioavailability of DHQ are also discussed in the paper, aiming to provide theoretical and technical support for the subsequent DHQ-based clinical trial studies and to promote the conversion of pharmaceutical preparations containing DHQ as the main active ingredient for clinical treatment. In the future, by profoundly exploring the potential clinical value of DHQ in different types of liver injury, as well as further clarifying the specific targets of its action, we can not only lay a solid foundation for the clinical application of DHQ but also provide valuable insights and directions for the development of novel therapeutic drugs for liver diseases, which is expected to make breakthroughs in the field of preventing and treating liver diseases.
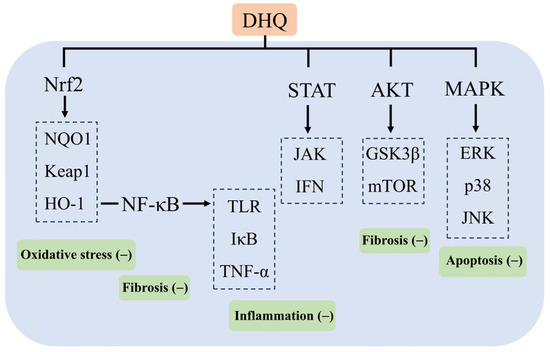
Figure 6.
Mechanism of action of DHQ in ameliorating liver injury.
Author Contributions
Conceptualization, H.W.; formal analysis, X.B. and Z.C.; investigation, T.Z. and X.L.; Visualization, Q.D. and J.Y.; writing—original draft preparation, H.W.; writing—review and editing, C.D. and W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Jilin College of Agricultural Science and Technology Projects (20230049).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the findings are available from the corresponding authors upon reasonable request.
Acknowledgments
The author would like to thank the Jilin Jianwei Natural Biotechnology Co., Ltd.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ding, L.; Liu, Y.; Kang, M.; Wei, X.; Geng, C.; Liu, W.; Han, L.; Yuan, F.; Wang, P.; Wang, B.; et al. UPLC-QTOF/MS Metabolomics and Biochemical Assays Reveal Changes in Hepatic Nutrition and Energy Metabolism during Sexual Maturation in Female Rainbow Trout (Oncorhynchus mykiss). Biology 2022, 11, 1679. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Mokdad, A.A.; Lopez, A.D.; Shahraz, S.; Lozano, R.; Mokdad, A.H.; Stanaway, J.; Murray, C.J.; Naghavi, M. Liver cirrhosis mortality in 187 countries between 1980 and 2010: A systematic analysis. BMC Med. 2014, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Huang, Z.; Wang, S.; Zhao, Z.; Yi, P.; Chen, Y.; Xiao, M.; Quan, J.; Hu, X. The Hepatic Nerves Regulated Inflammatory Effect in the Process of Liver Injury: Is Nerve the Key Treating Target for Liver Inflammation? Inflammation 2023, 46, 1602–1611. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Frias, F.; Rando-Segura, A.; Quer, J. Solved the enigma of pediatric severe acute hepatitis of unknown origin? Front. Cell Infect. Microbiol. 2023, 13, 1175996. [Google Scholar] [CrossRef]
- Nan, Y.; Su, H.; Lian, X.; Wu, J.; Liu, S.; Chen, P.; Liu, S. Pathogenesis of Liver Fibrosis and Its TCM Therapeutic Perspectives. Evid.-Based Complement. Altern. Med. 2022, 2022, 5325431. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Lei, J.; Li, P.; Wang, Y.; Wang, J.; Song, T.; Zhu, B.; Jia, J.; Miao, J.; Cui, H. Hedan tablet ameliorated non-alcoholic steatohepatitis by moderating NF-kappaB and lipid metabolism-related pathways via regulating hepatic metabolites. J. Cell Mol. Med. 2024, 28, e18194. [Google Scholar] [CrossRef]
- Ding, C.; Zhao, Y.; Chen, X.; Zheng, Y.; Liu, W.; Liu, X. Taxifolin, a novel food, attenuates acute alcohol-induced liver injury in mice through regulating the NF-κB-mediated inflammation and PI3K/Akt signalling pathways. Pharm. Biol. 2021, 59, 868–879. [Google Scholar] [CrossRef]
- Kim, M.; Jee, S.-C.; Sung, J.-S. Hepatoprotective Effects of Flavonoids against Benzo[a]Pyrene-Induced Oxidative Liver Damage along Its Metabolic Pathways. Antioxidants 2024, 13, 180. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Kim, S.H.; Chang, S.N.; Lee, J.-H.; Hwang, B.S.; Woo, J.-T.; Kang, S.C.; Lee, J.; Park, J.G. Efficacy of Polymethoxylated Flavonoids from Citrus depressa Extract on Alcohol-induced Liver Injury in Mice. Biotechnol. Bioprocess. Eng. 2019, 24, 907–914. [Google Scholar] [CrossRef]
- Pardede, A.; Adfa, M.; Kusnanda, A.J.; Ninomiya, M.; Koketsu, M. Flavonoid rutinosides from Cinnamomum parthenoxylon leaves and their hepatoprotective and antioxidant activity. Med. Chem. Res. 2017, 26, 2074–2079. [Google Scholar] [CrossRef]
- Kondeva-Burdina, M.; Doytchinova, I.; Krasteva, I.; Ionkova, I.; Manov, V. Hepato-, neuroprotective effects and QSAR studies on flavoalkaloids and flavonoids from Astragalus monspessulanus. Biotechnol. Biotechnol. Equip. 2019, 33, 1434–1443. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, M.; Cui, G.; Yang, X.; Gu, H.; Yang, L. Optimization of arabinogalactan and taxifolin extraction process from Dahurian larch (Larix gmelinii) and evaluation of the effects on activities of -amylase, -glycosidase, and pancreatic lipase in vitro. J. Food Biochem. 2018, 42. [Google Scholar] [CrossRef]
- Cai, C.; Liu, C.; Zhao, L.; Liu, H.; Li, W.; Guan, H.; Zhao, L.; Xiao, J. Effects of Taxifolin on Osteoclastogenesis in vitro and in vivo. Front. Pharmacol. 2018, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Rysenga, C.E.; May-Zhang, L.; Zahavi, M.; Knight, J.S.; Ali, R.A. Taxifolin inhibits NETosis through activation of Nrf2 and provides protective effects in models of lupus and antiphospholipid syndrome. Rheumatology 2023, 63, 2006–2015. [Google Scholar] [CrossRef] [PubMed]
- Zivkovic, L.; Bajić, V.; Topalović, D.; Bruić, M.; Spremo-Potparević, B. Antigenotoxic Effects of Biochaga and Dihydroquercetin (Taxifolin) on H2O2-Induced DNA Damage in Human Whole Blood Cells. Oxidative Med. Cell. Longev. 2019, 2019, 5039372. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ma, Y.; Xu, Y. Taxifolin Shows Anticataractogenesis and Attenuates Diabetic Retinopathy in STZ-Diabetic Rats via Suppression of Aldose Reductase, Oxidative Stress, and MAPK Signaling Pathway. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 599–608. [Google Scholar] [CrossRef]
- Copple, I.M.; Park, B.K.; Goldring, C.E. Gene Signatures Reduce the Stress of Preclinical Drug Hepatotoxicity Screening. Hepatology 2021, 74, 513–515. [Google Scholar] [CrossRef]
- Weiler-Normann, C.; Schramm, C. Drug induced liver injury and its relationship to autoimmune hepatitis. J. Hepatol. 2011, 55, 747–749. [Google Scholar] [CrossRef]
- Li, J.; Tang, X.; Wen, X.; Ren, X.; Zhang, H.; Du, Y.; Lu, J. Mitochondrial Glrx2 Knockout Augments Acetaminophen-Induced Hepatotoxicity in Mice. Antioxidants 2022, 11, 1643. [Google Scholar] [CrossRef]
- Gong, L.; Liao, L.; Dai, X.; Xue, X.; Peng, C.; Li, Y. The dual role of immune response in acetaminophen hepatotoxicity: Implication for immune pharmacological targets. Toxicol. Lett. 2021, 351, 37–52. [Google Scholar] [CrossRef]
- Shiffman, S.; Battista, D.R.; Kelly, J.P.; Malone, M.K.; Weinstein, R.B.; Kaufman, D.W. Exceeding the maximum daily dose of acetaminophen with use of different single-ingredient OTC formulations. J. Am. Pharm. Assoc. 2018, 58, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H. How relevant are neutrophils for acetaminophen hepatotoxicity? Hepatology 2006, 43, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Dore, J.P.; Butterworth, J.F. Acetaminophen dosing in the era of enhanced recovery after surgery. Paediatr. Anaesth. 2019, 29, 5. [Google Scholar] [CrossRef]
- Hersh, E.V.; Pinto, A.; Moore, P.A. Adverse drug interactions involving common prescription and over-the-counter analgesic agents. Clin. Ther. 2007, 29, 2477–2497. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Kubes, P. Innate immune cells orchestrate the repair of sterile injury in the liver and beyond. Eur. J. Immunol. 2019, 49, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, J.; Hu, Z.; Zhang, Q.; Li, X.; Huang, D. Protective effects of dihydroquercetin on an APAP-induced acute liver injury mouse model. Int. J. Clin. Exp. Pathol. 2017, 10, 10223–10232. [Google Scholar] [PubMed]
- Hu, C.; Ye, J.; Zhao, L.; Li, X.; Wang, Y.; Liu, X.; Pan, L.; You, L.; Chen, L.; Jia, Y.; et al. 5,7,3′,4′-flavan-on-ol (taxifolin) protects against acetaminophen-induced liver injury by regulating the glutathione pathway. Life Sci. 2019, 236, 116939. [Google Scholar] [CrossRef]
- Zhang, J.; Hosoya, T.; Maruyama, A.; Nishikawa, K.; Maher, J.M.; Ohta, T.; Motohashi, H.; Fukamizu, A.; Shibahara, S.; Itoh, K.; et al. Nrf2 Neh5 domain is differentially utilized in the transactivation of cytoprotective genes. Biochem. J. 2007, 404, 459–466. [Google Scholar] [CrossRef]
- Huang, J.; Jia, Y.; Li, Q.; Son, K.; Hamilton, C.; Burris, W.R.; Bridges, P.J.; Stromberg, A.J.; Matthews, J.C. Glutathione content and expression of proteins involved with glutathione metabolism differs in longissimus dorsi, subcutaneous adipose, and liver tissues of finished vs. growing beef steers. J. Anim. Sci. 2018, 96, 5152–5165. [Google Scholar] [CrossRef]
- Hasan, M.N.; Akond, Z.; Alam, J.; Begum, A.A.; Rahman, M.; Mollah, N.H. Toxic Dose prediction of Chemical Compounds to Biomarkers using an ANOVA based Gene Expression Analysis. Bioinformation 2018, 14, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Zai, W.; Chen, W.; Luan, J.; Fan, J.; Zhang, X.; Wu, Z.; Ding, T.; Ju, D.; Liu, H. Dihydroquercetin ameliorated acetaminophen-induced hepatic cytotoxicity via activating JAK2/STAT3 pathway and autophagy. Appl. Microbiol. Biotechnol. 2018, 102, 1443–1453. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wu, F.; Lin, S.; Pan, X.; Jin, L.; Lu, T.; Shi, L.; Wang, Y.; Xu, A.; Li, X. Adiponectin protects against acetaminophen-induced mitochondrial dysfunction and acute liver injury by promoting autophagy in mice. J. Hepatol. 2014, 61, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Perez, T.R. Is cirrhosis of the liver experimentally produced by CCl4 and adequate model of human cirrhosis? Hepatology 1983, 3, 112–120. [Google Scholar]
- Weber, L.W.D.; Boll, M.; Stampfl, A. Hepatotoxicity and mechanism of action of haloalkanes: Carbon tetrachloride as a toxicological model. Crit. Rev. Toxicol. 2003, 33, 105–136. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, W.; Ding, C.; Zhao, Y.; Chen, X.; Ling, D.; Zheng, Y.; Cheng, Z. Taxifolin, Extracted from Waste Larix olgensis Roots, Attenuates CCl(4)-Induced Liver Fibrosis by Regulating the PI3K/AKT/mTOR and TGF-beta1/Smads Signaling Pathways. Drug. Des. Devel. Ther. 2021, 15, 871–887. [Google Scholar] [CrossRef] [PubMed]
- Helsby, N.; Yong, M.; van Kan, M.; de Zoysa, J.; Burns, K. The importance of both CYP2C19 and CYP2B6 germline variations in cyclophosphamide pharmacokinetics and clinical outcomes. Br. J. Clin. Pharmacol. 2019, 85, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Ahlmann, M.; Hempel, G. The effect of cyclophosphamide on the immune system: Implications for clinical cancer therapy. Cancer Chemother. Pharmacol. 2016, 78, 661–671. [Google Scholar] [CrossRef]
- Liao, S.; Wei, C.; Wei, G.; Liang, H.; Peng, F.; Zhao, L.; Li, Z.; Liu, C.; Zhou, Q. Cyclophosphamide activates ferroptosis-induced dysfunction of Leydig cells via SMAD2 pathwaydagger. Biol. Reprod. 2024, 110, 1012–1024. [Google Scholar] [CrossRef]
- Akinmoladun, A.C.; Oladejo, C.O.; Josiah, S.S.; Famusiwa, C.D.; Ojo, O.B.; Olaleye, M.T. Catechin, quercetin and taxifolin improve redox and biochemical imbalances in rotenone-induced hepatocellular dysfunction: Relevance for therapy in pesticide-induced liver toxicity? Pathophysiology 2018, 25, 365–371. [Google Scholar] [CrossRef]
- Patwa, J.; Khan, S.; Jena, G. Nicotinamide attenuates cyclophosphamide-induced hepatotoxicity in SD rats by reducing oxidative stress and apoptosis. J. Biochem. Mol. Toxicol. 2020, 34, e22558. [Google Scholar] [CrossRef]
- Althunibat, O.Y.; Abukhalil, M.H.; Jghef, M.M.; Alfwuaires, M.A.; Algefare, A.I.; Alsuwayt, B.; Alazragi, R.; Abourehab, M.A.S.; Almuqati, A.F.; Karimulla, S.; et al. Hepatoprotective effect of taxifolin on cyclophosphamide-induced oxidative stress, inflammation, and apoptosis in mice: Involvement of Nrf2/HO-1 signaling. Biomol. Biomed. 2023, 23, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, X.; Feng, Y.; Nie, X.; Liu, Q.; Du, X.; Wu, Y.; Liu, T.; Zhu, X. Rotenone, an environmental toxin, causes abnormal methylation of the mouse brain organoid's genome and ferroptosis. Int. J. Med. Sci. 2022, 19, 1184–1197. [Google Scholar] [CrossRef] [PubMed]
- Azimullah, S.; Meeran, M.F.N.; Ayoob, K.; Arunachalam, S.; Ojha, S.; Beiram, R. Tannic Acid Mitigates Rotenone-Induced Dopaminergic Neurodegeneration by Inhibiting Inflammation, Oxidative Stress, Apoptosis, and Glutamate Toxicity in Rats. Int. J. Mol. Sci. 2023, 24, 9876. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dong, X.; Liu, Z.; Zhu, S.; Liu, H.; Fan, W.; Hu, Y.; Hu, T.; Yu, Y.; Li, Y.; et al. Resveratrol Suppresses Rotenone-induced Neurotoxicity Through Activation of SIRT1/Akt1 Signaling Pathway. Anat. Rec. 2018, 301, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Verschoor, A.J.; Warmerdam, F.A.R.M.; Bosse, T.; Bovée, J.V.M.G.; Gelderblom, H. A remarkable response to pazopanib, despite recurrent liver toxicity, in a patient with a high grade endometrial stromal sarcoma, a case report. BMC Cancer 2018, 18, 92. [Google Scholar] [CrossRef] [PubMed]
- Turjap, M.; Pelcová, M.M.; Gregorová, J.; Šmak, P.M.; Martin, H.; Štingl, J.M.; Peš, O.M.; Juřica, J. Therapeutic Drug Monitoring of Pazopanib in Renal Cell Carcinoma and Soft Tissue Sarcoma: A Systematic Review. Ther. Drug Monit. 2024, 46, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, Y.; Toh, Y.C.; Qu, Y.; Xing, J.; Poh, J.; Tan, H.S.; Tan, M.H. Modeling patient variability in pazopanib-induced hepatotoxicity with iPSC-derived hepatocyte-like cells. J. Clin. Oncol. 2016, 34. [Google Scholar] [CrossRef]
- Akagunduz, B.; Ozer, M.; Ozcıcek, F.; Kara, A.V.; Lacın, S.; Özkaraca, M.; Çoban, A.; Suleyman, B.; Mammadov, R.; Suleyman, H. Protective effects of taxifolin on pazopanib-induced liver toxicity: An experimental rat model. Exp. Anim. 2021, 70, 169–176. [Google Scholar] [CrossRef]
- Onk, D.; Mammadov, R.; Suleyman, B.; Cimen, F.K.; Cankaya, M.; Gul, V.; Altuner, D.; Senol, O.; Kadioglu, Y.; Malkoc, I.; et al. The effect of thiamine and its metabolites on peripheral neuropathic pain induced by cisplatin in rats. Exp. Anim. 2018, 67, 259–269. [Google Scholar] [CrossRef]
- Kuduban, O.; Kucur, C.; Sener, E.; Suleyman, H.; Akcay, F. The role of thiamine pyrophosphate in prevention of cisplatin ototoxicity in an animal model. Sci. World J. 2013, 2013, 182694. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.-B.; Li, D.; Xing, X.; Zhao, Y.; Wu, K.; Jiang, F.; Yin, W.; Li, J.-D. Effect of cisplatin on the clock genes expression in the liver, heart and kidney. Biochem. Biophys. Res. Commun. 2018, 501, 593–597. [Google Scholar] [CrossRef]
- Ayyagari, V.N.; Diaz-Sylvester, P.L.; Hsieh, T.-H.J.; Brard, L. Evaluation of the cytotoxicity of the Bithionol-paclitaxel combination in a panel of human ovarian cancer cell lines. PLoS ONE 2017, 12, e0185111. [Google Scholar] [CrossRef] [PubMed]
- Saydaminova, K.; Strauss, R.; Xie, M.; Bartek, J.; Richter, M.; van Rensburg, R.; Drescher, C.; Ehrhardt, A.; Ding, S.; Lieber, A. Sensitizing ovarian cancer cells to chemotherapy by interfering with pathways that are involved in the formation of cancer stem cells. Cancer Biol. Ther. 2016, 17, 1079–1088. [Google Scholar] [CrossRef][Green Version]
- Bodiga, V.L.; Bodiga, S.; Surampudi, S.; Boindala, S.; Putcha, U.; Nagalla, B.; Subramaniam, K.; Manchala, R. Effect of vitamin supplementation on cisplatin-induced intestinal epithelial cell apoptosis in Wistar/NIN rats. Nutrition 2012, 28, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Khan, A.Q.; Qamar, W.; Lateef, A.; Ali, F.; Rehman, M.U.; Tahir, M.; Sharma, S.; Sultana, S. Chrysin abrogates cisplatin-induced oxidative stress, p53 expression, goblet cell disintegration and apoptotic responses in the jejunum of Wistar rats. Br. J. Nutr. 2012, 108, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Kurt, N.; Gunes, O.; Suleyman, B.; Bakan, N. The effect of taxifolin on high-dose-cisplatin-induced oxidative liver injury in rats. Adv. Clin. Exp. Med. 2021, 30, 1025–1030. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Aslkhodapasandhokmabad, H.; Zhou, Y.; Ren, J. Epigenetic modification in alcohol-related liver diseases. Med. Res. Rev. 2022, 42, 1463–1491. [Google Scholar] [CrossRef]
- Lefkowitch, J.H. Morphology of alcoholic liver disease. Clin. Liver Dis. 2005, 9, 37–53. [Google Scholar] [CrossRef]
- Hajifathalian, K.; Mehta, A.; Ang, B.; Skaf, D.; Shah, S.L.; Saumoy, M.; Dawod, Q.; Dawod, E.; Shukla, A.; Aronne, L.; et al. Improvement in insulin resistance and estimated hepatic steatosis and fibrosis after endoscopic sleeve gastroplasty. Gastrointest. Endosc. 2021, 93, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Qiu, Y.-J.; Zuo, D.; Shi, S.-N.; Wang, W.-P.; Dong, Y. Imaging Features of Hepatocellular Carcinoma in the Non-Cirrhotic Liver with Sonazoid-Enhanced Contrast-Enhanced Ultrasound. Diagnostics 2022, 12, 2272. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Huang, A.; Saxena, R.; Sun, Y.; Liu, S.; Zhou, G.; Li, B.; Teng, G.; Zhao, J.; Zhang, W.; et al. Hepatic Histopathology Among Excessive Drinkers Without Advanced Liver Disease. Alcohol. Alcohol. 2021, 56, 669–677. [Google Scholar] [CrossRef]
- Datta, S.; Aggarwal, D.; Sehrawat, N.; Yadav, M.; Sharma, V.; Sharma, A.; Zghair, A.N.; Dhama, K.; Sharma, A.; Kumar, V.; et al. Hepatoprotective effects of natural drugs: Current trends, scope, relevance and future perspectives. Phytomedicine 2023, 121, 155100. [Google Scholar] [CrossRef]
- Farooq, M.O.; Bataller, R. Pathogenesis and Management of Alcoholic Liver Disease. Dig. Dis. 2016, 34, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Magdaleno, F.; Blajszczak, C.C.; Nieto, N. Key Events Participating in the Pathogenesis of Alcoholic Liver Disease. Biomolecules 2017, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Imprialos, K.P.; Stavropoulos, K.; Doumas, M.; Athyros, V.G. The Impact of Ranolazine Treatment on Liver Tests in Patients With Coronary Artery Disease and Nonalcoholic Fatty Liver Disease. Angiology 2022, 73, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Cazac, G.D.; Lăcătușu, C.M.; Ștefănescu, G.; Mihai, C.; Grigorescu, E.D.; Onofriescu, A.; Mihai, B.M. Glucagon-like Peptide-1 Receptor Agonists in Patients with Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease—Current Background, Hopes, and Perspectives. Metabolites 2023, 13, 581. [Google Scholar] [CrossRef]
- Miao, L.; Xu, J.; Targher, G.; Byrne, C.D.; Zheng, M.-H. Old and new classes of glucose-lowering agents as treatments for non-alcoholic fatty liver disease: A narrative review. Clin. Mol. Hepatol. 2022, 28, 725–738. [Google Scholar] [CrossRef]
- Cojocariu, C.; Singeap, A.-M.; Girleanu, I.; Chiriac, S.; Muzica, C.M.; Sfarti, C.V.; Cuciureanu, T.; Huiban, L.; Stanciu, C.; Trifan, A. Nonalcoholic Fatty Liver Disease-Related Chronic Kidney Disease. Can. J. Gastroenterol. Hepatol. 2020, 2020, 6630296. [Google Scholar] [CrossRef] [PubMed]
- Albhaisi, S.; Sun, J.; Sanyal, A.J. Fibrosis-4 index is associated with the risk of hepatocellular carcinoma in patients with cirrhosis and nonalcoholic steatohepatitis. Front. Oncol. 2023, 13, 1198871. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Kawada, N.; Japan Study Group of NAFLD (JSG-NAFLD). The Role of Insulin Resistance and Diabetes in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2020, 21, 3863. [Google Scholar] [CrossRef] [PubMed]
- Zambon Azevedo, V.; Silaghi, C.A.; Maurel, T.; Silaghi, H.; Ratziu, V.; Pais, R. Impact of Sarcopenia on the Severity of the Liver Damage in Patients With Non-alcoholic Fatty Liver Disease. Front. Nutr. 2021, 8, 774030. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jeong, W.T.; So, Y.S.; Lim, H.B.; Lee, J. Taxifolin and Sorghum Ethanol Extract Protect against Hepatic Insulin Resistance via the miR-195/IRS1/PI3K/AKT and AMPK Signalling Pathways. Antioxidants 2021, 10, 1331. [Google Scholar] [CrossRef]
- Inoue, T.; Fu, B.; Nishio, M.; Tanaka, M.; Kato, H.; Tanaka, M.; Itoh, M.; Yamakage, H.; Ochi, K.; Ito, A.; et al. Novel Therapeutic Potentials of Taxifolin for Obesity-Induced Hepatic Steatosis, Fibrogenesis, and Tumorigenesis. Nutrients 2023, 15, 350. [Google Scholar] [CrossRef]
- Tanaka, M.; Ikeda, K.; Suganami, T.; Komiya, C.; Ochi, K.; Shirakawa, I.; Hamaguchi, M.; Nishimura, S.; Manabe, I.; Matsuda, T.; et al. Macrophage-inducible C-type lectin underlies obesity-induced adipose tissue fibrosis. Nat. Commun. 2014, 5, 4982. [Google Scholar] [CrossRef]
- Hondares, E.; Iglesias, R.; Giralt, A.; Gonzalez, F.J.; Giralt, M.; Mampel, T.; Villarroya, F. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J. Biol. Chem. 2011, 286, 12983–12990. [Google Scholar] [CrossRef] [PubMed]
- Werner, M.; Prytz, H.; Ohlsson, B.; Almer, S.; Björnsson, E.; Bergquist, A.; Wallerstedt, S.; Sandberg-Gertzén, H.; Hultcrantz, R.; Sangfelt, P.; et al. Epidemiology and the initial presentation of autoimmune hepatitis in Sweden: A nationwide study. Scand. J. Gastroenterol. 2008, 43, 1232–1240. [Google Scholar] [CrossRef]
- Gronbaek, L.; Vilstrup, H.; Pedersen, L.; Christensen, K.; Jepsen, P. Family occurrence of autoimmune hepatitis: A Danish nationwide registry-based cohort study. J. Hepatol. 2018, 69, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, J.; Zhu, P.; Fujino, M.; Takahara, T.; Toyama, S.; Tomita, A.; Zhao, L.; Yang, Z.; Hei, M.; et al. Dihydroquercetin (DHQ) ameliorated concanavalin A-induced mouse experimental fulminant hepatitis and enhanced HO-1 expression through MAPK/Nrf2 antioxidant pathway in RAW cells. Int. Immunopharmacol. 2015, 28, 938–944. [Google Scholar] [CrossRef]
- Yang, T.; Qu, X.; Zhao, J.; Wang, X.; Wang, Q.; Dai, J.; Zhu, C.; Li, J.; Jiang, L. Macrophage PTEN controls STING-induced inflammation and necroptosis through NICD/NRF2 signaling in APAP-induced liver injury. Cell Commun. Signal. 2023, 21, 160. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, X.; Xia, T.; Mao, Q.; Zhong, L. Pretreatment with dihydroquercetin, a dietary flavonoid, protected against concanavalin A-induced immunological hepatic injury in mice and TNF-alpha/ActD-induced apoptosis in HepG2 cells. Food Funct. 2018, 9, 2341–2352. [Google Scholar] [CrossRef]
- van Gerven, N.M.; de Boer, Y.S.; Mulder, C.J.; van Nieuwkerk, C.M.; Bouma, G. Auto immune hepatitis. World J. Gastroenterol. 2016, 22, 4651–4661. [Google Scholar] [CrossRef]
- Toda, G.; Zeniya, M.; Watanabe, F.; Imawari, M.; Kiyosawa, K.; Nishioka, M.; Tsuji, T.; Omata, M. Present status of autoimmune hepatitis in Japan—Correlating the characteristics with international criteria in an area with a high rate of HCV infection. J. Hepatol. 1997, 26, 1207–1212. [Google Scholar] [CrossRef]
- Whalley, S.; Puvanachandra, P.; Desai, A.; Kennedy, H. Hepatology outpatient service provision in secondary care: A study of liver disease incidence and resource costs. Clin. Med. 2007, 7, 119–124. [Google Scholar] [CrossRef]
- Groribaek, L.; Vilstrup, H.; Jepsen, P. Autoimmune hepatitis in Denmark : Incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J. Hepatol. 2014, 60, 612–617. [Google Scholar] [CrossRef]
- van Gerven, N.M.; Verwer, B.J.; Witte, B.I.; van Erpecum, K.J.; van Buuren, H.R.; Maijers, I.; Visscher, A.P.; Verschuren, E.C.; van Hoek, B.; Coenraad, M.J.; et al. Epidemiology and clinical characteristics of autoimmune hepatitis in the Netherlands. Scand. J. Gastroenterol. 2014, 49, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Ngu, J.H.; Bechly, K.; Chapman, B.A.; Burt, M.J.; Barclay, M.L.; Gearry, R.B.; Stedman, C.A. Population-based epidemiology study of autoimmune hepatitis: A disease of older women? J. Gastroenterol. Hepatol. 2010, 25, 1681–1686. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.; Vodonos, A.; Malnick, S.; Kriger, O.; Wilkof-Segev, R.; Delgado, B.; Novack, V.; Rosenthal, A.; Menachem, Y.; Melzer, E.; et al. Autoimmune hepatitis in southern Israel: A 15-year multicenter study. J. Dig. Dis. 2013, 14, 611–618. [Google Scholar] [CrossRef]
- Primo, J.; Merino, C.; Fernández, J.; Molés, J.R.; Llorca, P.; Hinojosa, J. Incidence and prevalence of autoimmune hepatitis in the area of the Hospital de Sagunto (Spain). Gastroenterol. Hepatol. 2004, 27, 239–243. [Google Scholar] [CrossRef]
- Hurlburt, K.J.; McMahon, B.J.; Deubner, H.; Hsu-Trawinski, B.; Williams, J.L.; Kowdley, K.V. Prevalence of autoimmune liver disease in Alaska Natives. Am. J. Gastroenterol. 2002, 97, 2402–2407. [Google Scholar] [CrossRef] [PubMed]
- Stielow, M.; Witczyńska, A.; Kubryń, N.; Fijałkowski, Ł.; Nowaczyk, J.; Nowaczyk, A. The Bioavailability of Drugs—The Current State of Knowledge. Molecules 2023, 28, 8038. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; da Silva, G.C.; Côrtes, S.F.; de Pádua, R.M.; Braga, F.C. Forced degradation of l-(+)-bornesitol, a bioactive marker of Hancornia speciosa: Development and validation of stability indicating UHPLC-MS method and effect of degraded products on ACE inhibition. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2018, 1093–1094, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Chernikov, D.; Shishlyannikova, T.; Kashevskii, A.; Bazhenov, B.; Kuzmin, A.; Gorshkov, A.; Safronov, A. Some peculiarities of taxifolin electrooxidation in the aqueous media: The dimers formation as a key to the mechanism understanding. Electrochim. Acta 2018, 271, 560–566. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Karlina, M.V.; Shikov, A.N.; Kosman, V.M.; Makarova, M.N.; Makarov, V.G. Determination and pharmacokinetic study of taxifolin in rabbit plasma by high-performance liquid chromatography. Phytomedicine 2009, 16, 244–251. [Google Scholar] [CrossRef]
- Winter, J.; Moore, L.H.; Dowell, V.R.; Bokkenheuser, V.D. C-ring cleavage of flavonoids by human intestinal bacteria. Appl. Environ. Microbiol. 1989, 55, 1203–1208. [Google Scholar] [CrossRef]
- Thanos, C.G.; Liu, Z.; Goddard, M.; Reineke, J.; Bailey, N.; Cross, M.; Burrill, R.; Mathiowitz, E. Enhancing the oral bioavailability of the poorly soluble drug dicumarol with a bioadhesive polymer. J. Pharm. Sci. 2003, 92, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Guo, T.; He, Y.; Cao, Z.; Xu, H.; Wu, W.; Wu, L.; Zhu, W.; Zhang, J. Lactone Stabilized by Crosslinked Cyclodextrin Metal-Organic Frameworks to Improve Local Bioavailability of Topotecan in Lung Cancer. Pharmaceutics 2022, 15, 142. [Google Scholar] [CrossRef]
- Cheraga, N.; Ouahab, A.; Shen, Y.; Huang, N.-P. Characterization and Pharmacokinetic Evaluation of Oxaliplatin Long-Circulating Liposomes. BioMed Res. Int. 2021, 2021, 5949804. [Google Scholar] [CrossRef]
- Song, J.G.; Noh, H.-M.; Lee, S.H.; Han, H.-K. Lipid/Clay-Based Solid Dispersion Formulation for Improving the Oral Bioavailability of Curcumin. Pharmaceutics 2022, 14, 2269. [Google Scholar] [CrossRef]
- Ding, Q.; Chen, K.; Liu, X.; Ding, C.; Zhao, Y.; Sun, S.; Zhang, Y.; Zhang, J.; Liu, S.; Liu, W. Modification of taxifolin particles with an enteric coating material promotes repair of acute liver injury in mice through modulation of inflammation and autophagy signaling pathway. Biomed. Pharmacother. 2022, 152, 113242. [Google Scholar] [CrossRef]
- Ambrogi, V. A New Challenge for the Old Excipient Calcium Carbonate: To Improve the Dissolution Rate of Poorly Soluble Drugs. Pharmaceutics 2023, 15, 300. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.; Santhiya, D. In-situ mineralization of calcium carbonate in pectin based edible hydrogel for the delivery of protein at colon. J. Drug Deliv. Sci. Technol. 2019, 53, 101137. [Google Scholar] [CrossRef]
- Parodi, A.; Buzaeva, P.; Nigovora, D.; Baldin, A.; Kostyushev, D.; Chulanov, V.; Savvateeva, L.V.; Zamyatnin, A.A., Jr. Nanomedicine for increasing the oral bioavailability of cancer treatments. J. Nanobiotechnol. 2021, 19, 354. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Bresson, J.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A. Scientific Opinion on taxifolin-rich extract from Dahurian Larch (Larix gmelinii). EFSA J. 2017, 15, e04682. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).