Oxysulfonylation of Alkynes with Sodium Sulfinates to Access β-Keto Sulfones Catalyzed by BF3·OEt2
Abstract
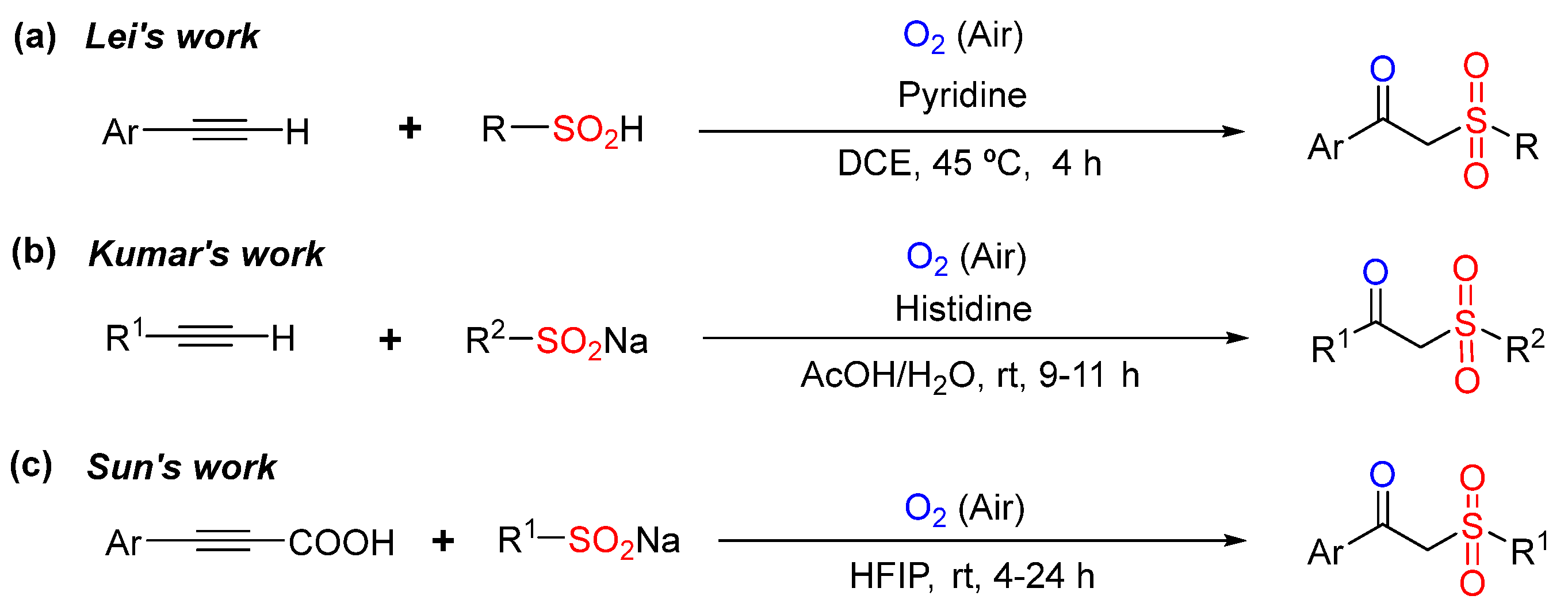
1. Introduction
2. Results and Discussion
2.1. Optimization of Reaction Conditions
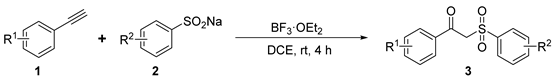
2.2. Scope of Substrates
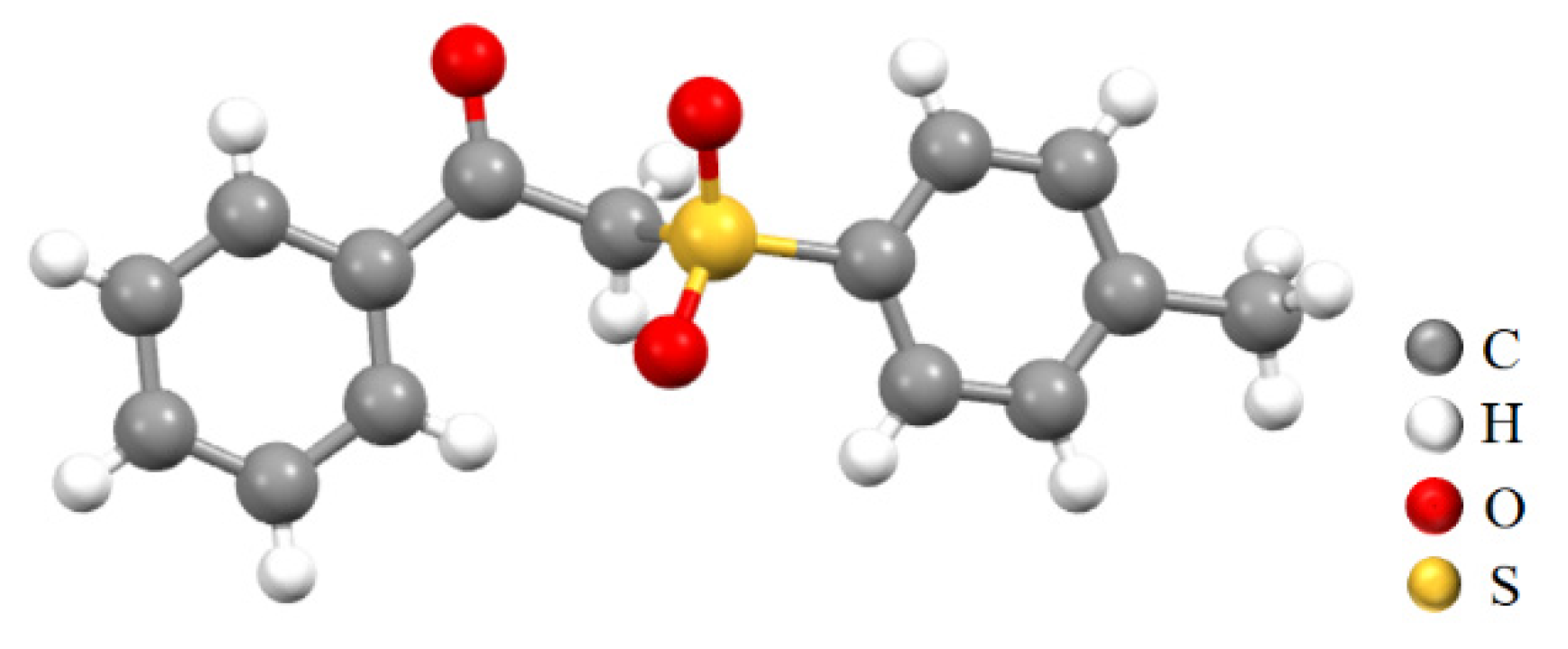
2.3. Structural Characterization Analysis
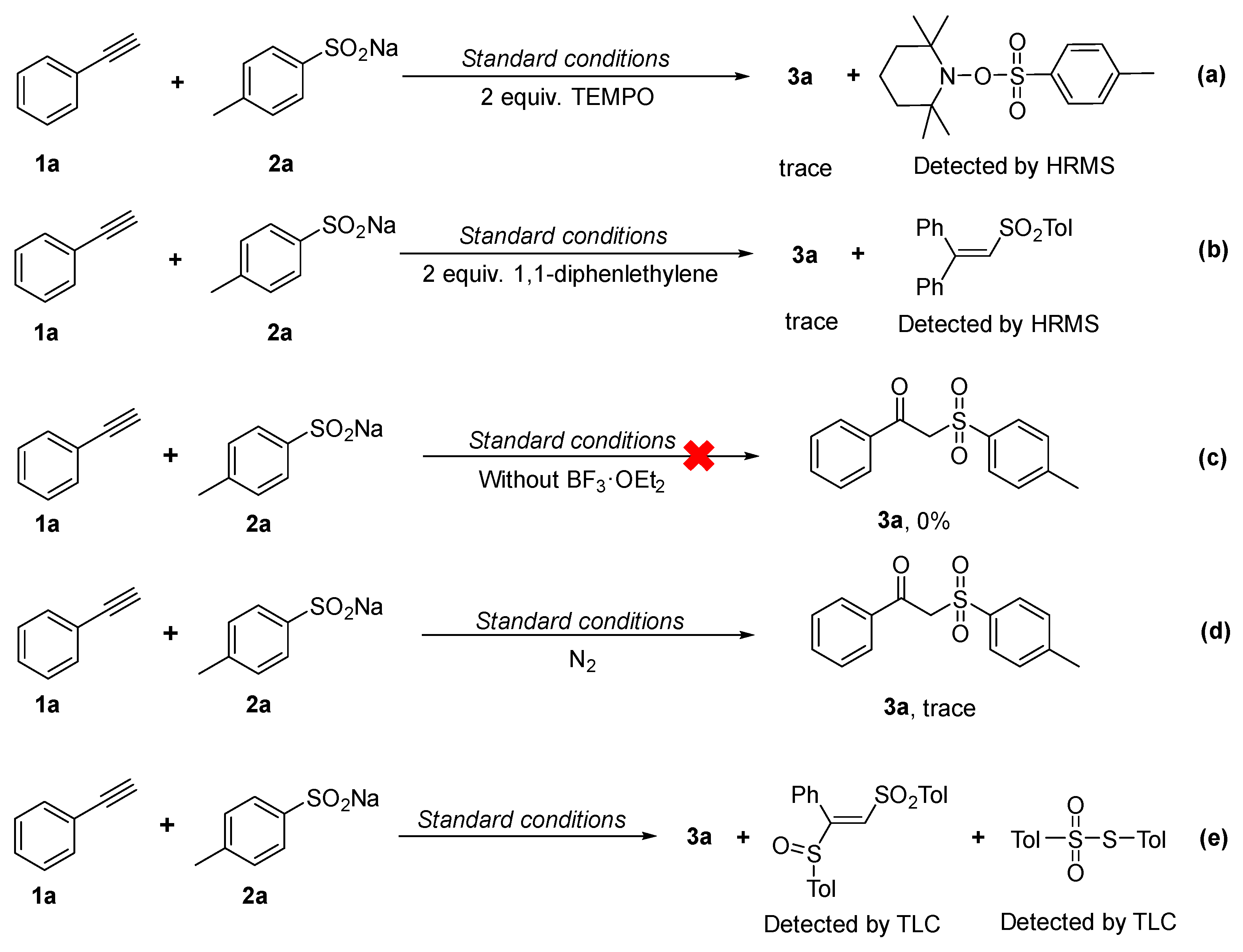
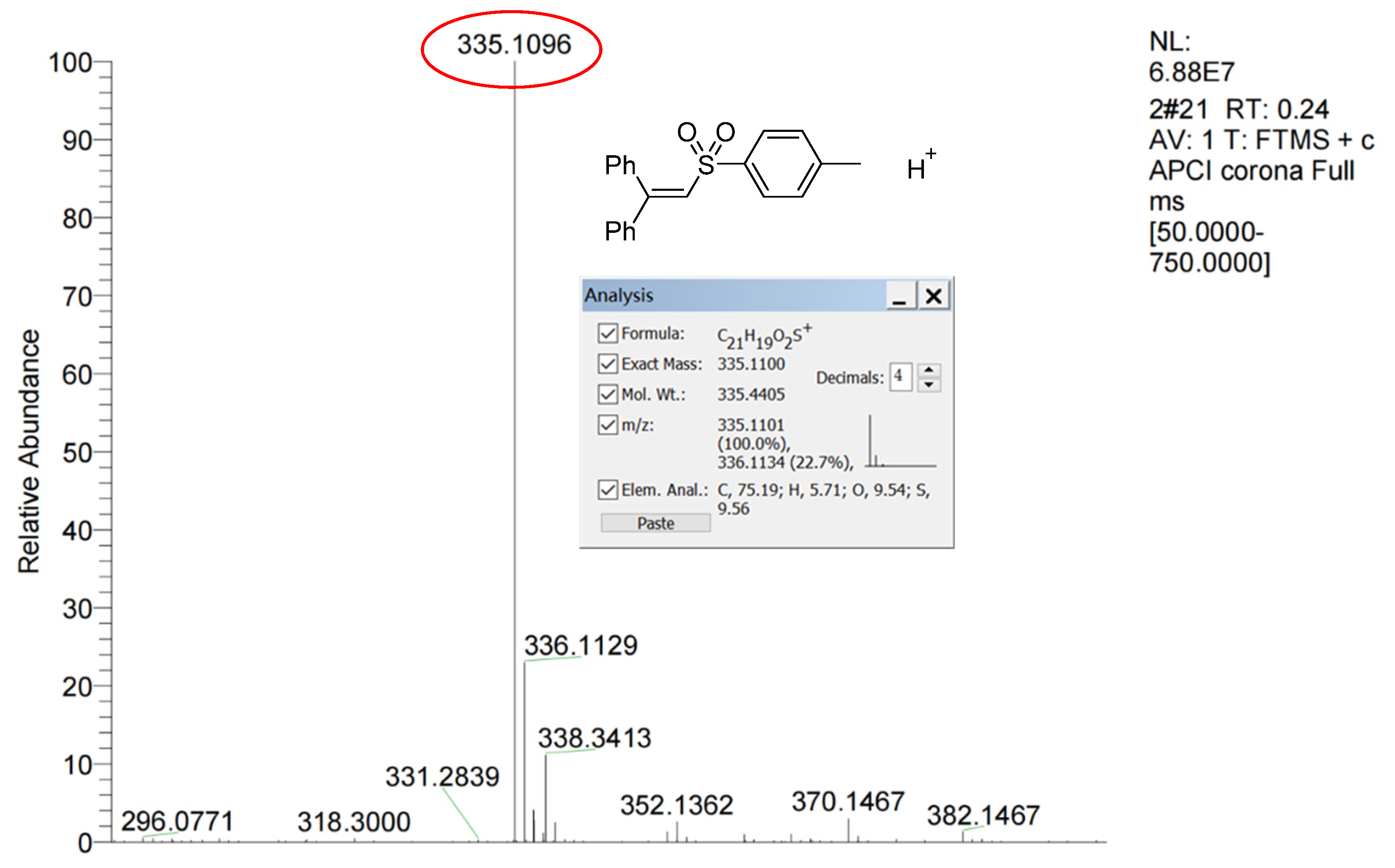
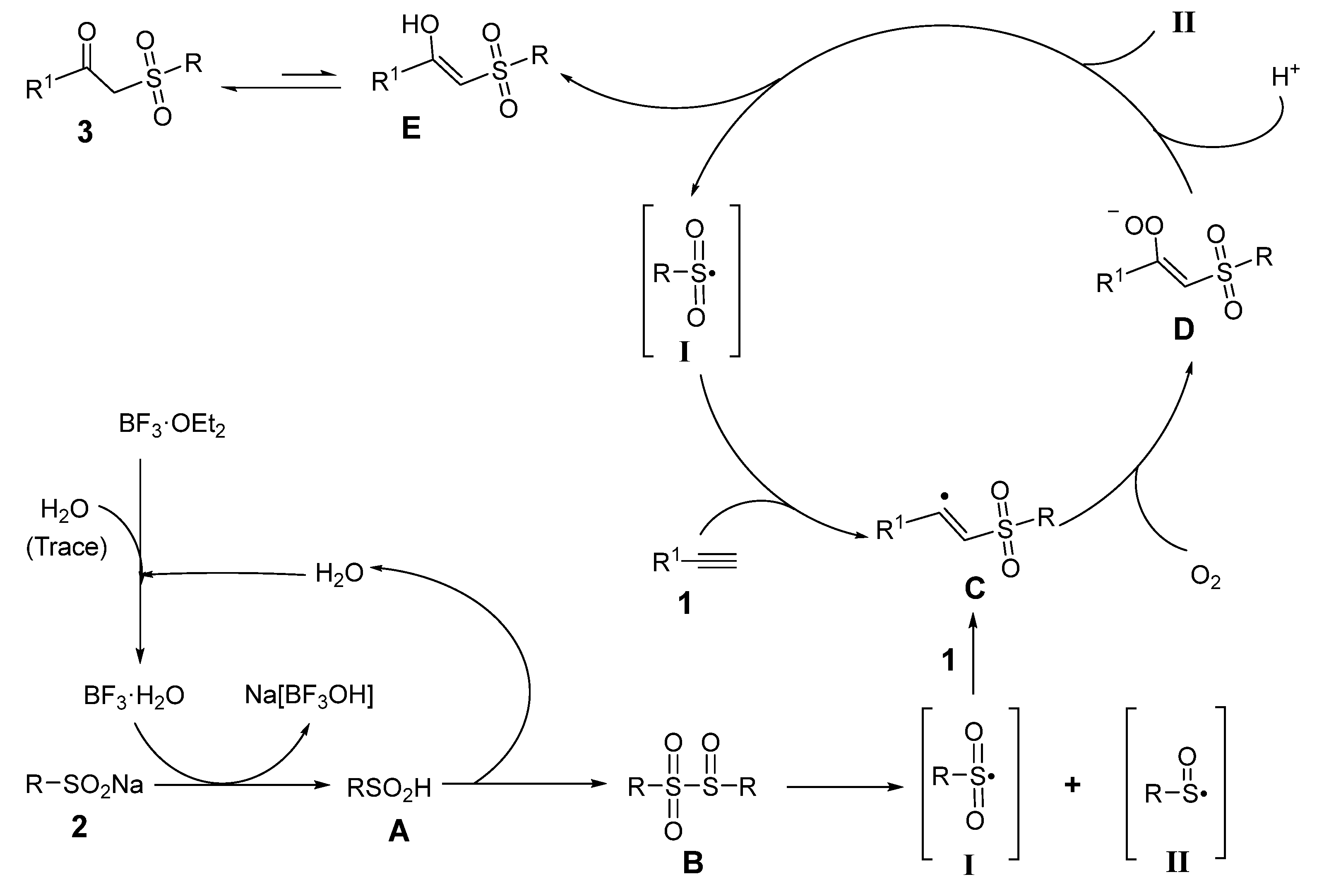
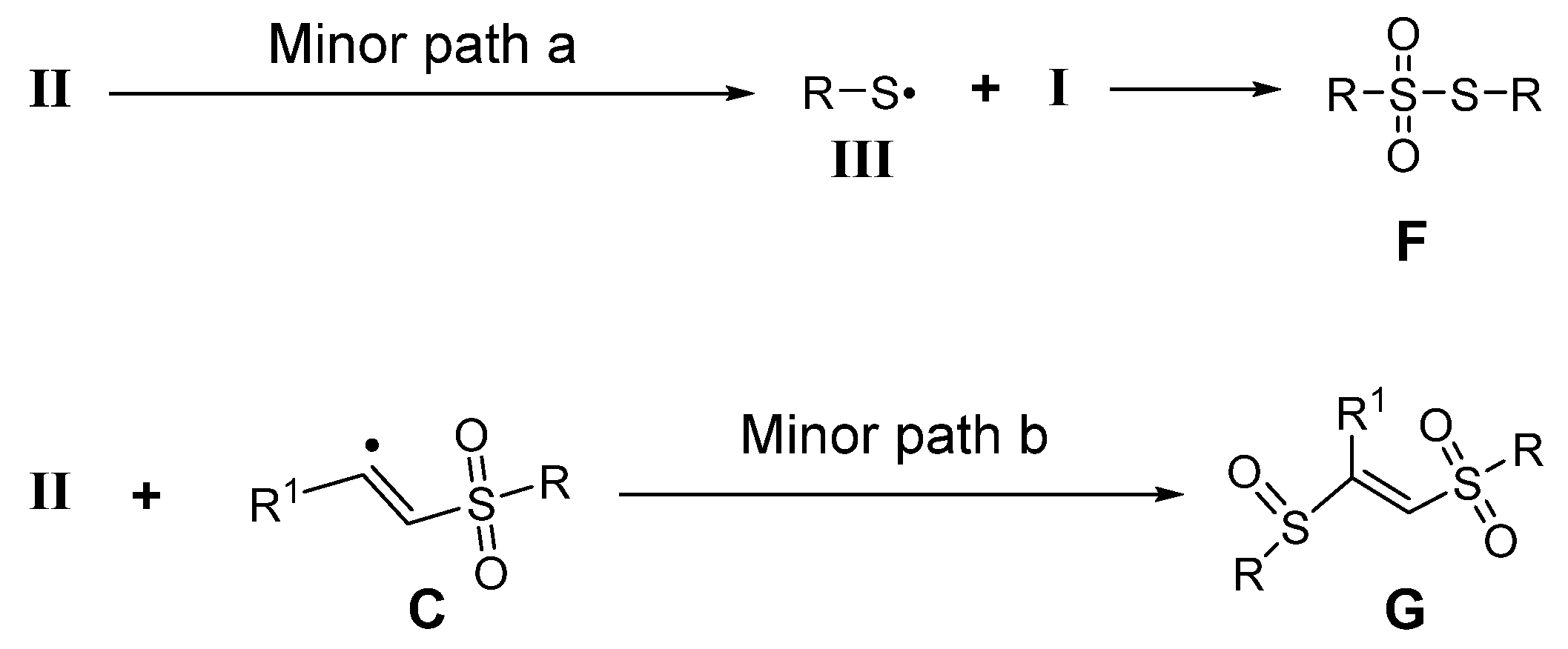
2.4. Mechanism Investigation
2.5. Gram-scale Reaction
3. Materials and Methods
3.1. General Information
3.2. Experimental Procedure for Sodium Sulfinates 2
3.3. Experimental Procedure for Compounds 3a-3t
3.4. Characterization Data for All Products 3a-3t
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corpas, J.; Kim-Lee, S.-H.; Mauleón, P.; Arrayás, R.G.; Carretero, J.C. Beyond classical sulfone chemistry: Metal- and photocatalytic approaches for C-S bond functionalization of sulfones. Chem. Soc. Rev. 2022, 51, 6774–6823. [Google Scholar] [CrossRef]
- Wu, H.; Chen, S.G.; Liu, C.N.; Zhao, Q.S.; Wang, Z.; Jin, Q.R.; Sun, S.J.; Guo, J.; He, X.W.; Walsh, P.J.; et al. Construction of C-S and C-Se bonds from unstrained ketone precursors under photoredox catalysis. Angew. Chem. Int. Ed. 2024, 63, e202314790. [Google Scholar] [CrossRef]
- Markham, A.; Keam, S.J. Danoprevir: First global approval. Drugs 2018, 78, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.R.; Chen, J.W.; Li, L.; Wen, K.M.; Yao, X.G.; Pang, J.X.; Wu, T.; Tang, X.D. Copper-mediated decarboxylative sulfonylation of arylacetic acids with sodium sulfinates. Org. Lett. 2020, 22, 7164–7168. [Google Scholar] [CrossRef]
- Wasilewska-Rosa, A.; Kisiel, K.; Tkaczyk, A.; Loska, R. Stereospecific synthesis of allenes from β-ketosulfones. Adv. Synth. Catal. 2023, 365, 704–708. [Google Scholar] [CrossRef]
- Lu, L.J.; Luo, J.; Milstein, D. Manganese(I)-pincer catalyzed α-alkylation of sulfones by alcohols. ACS Catal. 2023, 13, 5949–5954. [Google Scholar] [CrossRef]
- Inanaga, K.; Fukuyama, T.; Kubota, M.; Komatsu, Y.; Chiba, H.; Kayano, A.; Tagami, K. Novel and efficient chromium(II)-mediated desulfonylation of α-sulfonyl ketone. Org. Lett. 2015, 17, 3158–3161. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Samanta, S.; Ghosh, A.K.; Neogi, S.; Hajra, A. Advances in oxosulfonylation reaction. Adv. Synth. Catal. 2020, 362, 4552–4578. [Google Scholar] [CrossRef]
- Reddy, R.J.; Kumari, A.H.; Kumar, J.J. Recent advances in the synthesis and applications of β-keto sulfones: New prospects for the synthesis of β-keto thiosulfones. Org. Biomol. Chem. 2021, 19, 3087–3118. [Google Scholar] [CrossRef]
- Mulina, O.M.; Ilovaisky, A.I.; Parshin, V.D.; Terent’ev, A.O. Oxidative sulfonylation of multiple carbon-carbon bonds with sulfonyl hydrazides, sulfinic acids and their salts. Adv. Synth. Catal. 2020, 362, 4579–4654. [Google Scholar] [CrossRef]
- Yang, W.H.; Zhou, Y.; Tong, Y.; Wang, Z.Y.; Wu, H.L.; Mei, L.; Li, Q.; Xie, L.Y.; Zhang, J.Q.; Wei, W.T. Nickel-catalyzed reaction between vinyl azides and an alkyl sulfonyl radical generated from DMSO: Rapid access to β-keto sulfones. ACS Sustainable Chem. Eng. 2024, 12, 5046–5051. [Google Scholar] [CrossRef]
- Abdukerem, D.; Chen, H.; Mao, Z.; Xia, K.; Zhu, W.; Liu, C.; Yu, Y.; Abdukader, A. Transition metal-free C(sp3)-H selenation of β-ketosulfones. Org. Biomol. Chem. 2024, 22, 2075–2080. [Google Scholar] [CrossRef] [PubMed]
- Pospísil, J.; Sato, H. Practical synthesis of β-acyl and β-alkoxycarbonyl heterocyclic sulfones. J. Org. Chem. 2011, 76, 2269–2272. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.Q.; Zhang, B.B.; Han, J.E.; Peng, B.H.; Shan, Y.L.; Niu, T.F. Heterogeneous carbon nitrides photocatalysis multicomponent hydrosulfonylation of alkynes to access β-keto sulfones with the insertion of sulfur dioxide in aerobic aqueous medium. Org. Lett. 2020, 22, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Pampana, V.K.K.; Charpe, V.P.; Sagadevan, A.; Das, D.K.; Lin, C.C.; Hwu, J.R.; Hwang, K.C. Oxy-sulfonylation of terminal alkynes via C-S coupling enabled by copper photoredox catalysis. Green Chem. 2021, 23, 3569–3574. [Google Scholar] [CrossRef]
- Song, T.; Zhang, Y.P.; Wang, C.; Li, Y.F.; Yang, Y. Photocatalytic aerobic oxysulfonylation of alkynes to access β-keto sulfones catalyzed by OVs-N-Nb2O5. Chin. J. Chem. 2022, 40, 2618–2624. [Google Scholar] [CrossRef]
- Dam, B.; Sahoo, A.K.; Patel, B.K. Visible-light-mediated synthesis of β-keto sulfones using g-C3N4 as a recyclable photocatalyst under sustainable conditions. Green Chem. 2022, 24, 7122–7130. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhao, Y.H.; Cai, C.Q.; Wang, L.Y.; Gong, H. Dioxygen-triggered oxosulfonylation/sulfonylation of terminal olefins toward β-keto sulfones/sulfones. Org. Lett. 2021, 23, 8296–8301. [Google Scholar] [CrossRef]
- Chikunova, E.I.; Kukushkin, V.Y.; Dubovtsev, A.Y. Atom-economic synthesis of β-ketosulfones based on gold-catalyzed highly regioselective hydration of alkynylsulfones. Green Chem. 2022, 24, 3314–3320. [Google Scholar] [CrossRef]
- Tyagi, A.; Taneja, N.; Khan, J.; Hazra, C.K. I2-Catalyzed/mediated C-S and C-I bond formation: Solvent- and metal-free approach for the synthesis of β-keto sulfones and branched sulfones. Adv. Synth. Catal. 2023, 365, 1247–1254. [Google Scholar] [CrossRef]
- Wang, B.-W.; Jiang, K.; Li, J.-X.; Luo, S.-H.; Wang, Z.-Y.; Jiang, H.-F. 1,1-Diphenylvinylsulfide as functional AIEgen derived from the aggregation-caused-quenching molecule 1,1-diphenylvinylsulfide through simple thioetherification. Angew. Chem. Int. Ed. 2020, 59, 2338–2343. [Google Scholar] [CrossRef]
- Tian, Z.B.; Gong, Q.H.; Huang, T.Z.; Liu, L.; Chen, T.Q. Practical electro-oxidative sulfonylation of phenols with sodium arenesulfinates generating arylsulfonate esters. J. Org. Chem. 2021, 86, 15914–15926. [Google Scholar] [CrossRef] [PubMed]
- Handa, S.; Fennewald, J.C.; Lipshutz, B.H. Aerobic oxidation in nanomicelles of aryl alkynes, in water at room temperature. Angew. Chem. Int. Ed. 2014, 53, 3432–3435. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Hong, Y.Y.; Peng, S.; Xu, X.Q.; Tang, S.S.; Yang, L.H.; Xie, L.Y. Photo-sensitizer-free synthesis of β-keto sulfones via visible-light-induced oxysulfonylation of alkenes with sulfonic acids. Org. Biomol. Chem. 2021, 19, 4537–4541. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Lu, S.X.; Zheng, Y.Y.; Wang, J.G.; Yang, L.; Sun, P. Synthesis of β-keto sulfones by oxy-sulfonylation of alkynes in HFIP. Adv. Synth. Catal. 2022, 364, 1305–1312. [Google Scholar] [CrossRef]
- Saxena, B.; Patel, R.I.; Sharma, S.; Sharma, A. Mechanochemical-assisted decarboxylative sulfonylation of α,β-unsaturated carboxylic acids with sodium sulfinate salts. Green Chem. 2024, 26, 2721–2729. [Google Scholar] [CrossRef]
- Lu, Q.Q.; Zhang, J.; Zhao, G.L.; Qi, Y.; Wang, H.M.; Lei, A.W. Dioxygen-triggered oxidative radical reaction: Direct aerobic difunctionalization of terminal alkynes toward β-keto sulfones. J. Am. Chem. Soc. 2013, 135, 11481–11484. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Kumar, A. Amino acid-catalyzed direct synthesis of β-keto sulfones via aerobic difunctionalization of terminal alkynes in an aqueous medium. ACS Sustain. Chem. Eng. 2019, 7, 9182–9188. [Google Scholar] [CrossRef]
- Chen, X.Y.; Chang, X.Q.; Zhang, S.C.; Lu, S.X.; Yang, L.; Sun, P. Air-triggered, catalyst-free decarboxylative oxysulfonylation of arylpropiolic acids with sodium sulfinates. Environ. Chem. Lett. 2022, 20, 2773–2779. [Google Scholar] [CrossRef]
- Chen, W.; Ma, Y.H.; He, W.Y.; Wu, Y.X.; Huang, Y.C.; Zhang, Y.P.; Tian, H.C.; Wei, K.; Yang, X.D.; Zhang, H.B. Structure units oriented approach towards collective synthesis of sarpagine-ajmaline-koumine type alkaloids. Nat. Commun. 2022, 13, 908. [Google Scholar] [CrossRef]
- Chen, Z.-J.; Yu, S.-W.; Zhou, Y.-J.; Li, H.-Q.; Qiu, Q.-W.; Li, M.-X.; Wang, Z.-Y. Application of BF3·OEt2 in organic synthesis as a catalyst or synthon. Chin. J. Org. Chem. 2023, 43, 3107–3118. [Google Scholar] [CrossRef]
- Li, A.Q.; Zhao, J.; Zhang, C.; Jiang, Q.X.; Zhu, B.F.; Cao, H. Lewis acid-promoted three-component cyclization for the construction of functionalized oxazoles. J. Org. Chem. 2023, 88, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.L.; Hu, J.W.; Cao, J.; Xu, L.W. Intramolecular Hosomi-Sakurai reaction for the synthesis of benzoxasiloles. J. Org. Chem. 2024, 89, 9027–9030. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-W.; Chen, Z.-J.; Chen, Z.-H.; Chen, S.-H.; Yang, K.; Xu, W.-J.; Wang, Z.-Y. Trace water in a BF3·OEt2 system: A facile access to sulfinyl alkenylsulfones from alkynes and sodium sulfinates. Org. Biomol. Chem. 2023, 21, 7776–7781. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Luo, S.-H.; Jiang, K.; Hao, Z.-F.; Wang, B.-W.; Pang, C.-M.; Wang, Z.-Y. Disproportionate coupling reaction of sodium sulfinates mediated by BF3∙OEt2: An approach to symmetrical/unsymmetrical thiosulfonates. Org. Lett. 2018, 20, 4754–4758. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-Q.; Yang, K.; Chen, X.-Y.; Arulkumar, M.; Wang, N.; Chen, S.-H.; Wang, Z.-Y. A 3,4-dihalo-2(5H)-furanone initiated ring-opening reaction of DABCO in the absence of a metal catalyst and additive and its application in a one-pot two-step reaction. Green Chem. 2019, 21, 3782–3788. [Google Scholar] [CrossRef]
- CCDC 2367445 (for 3a) Contains the Supplementary Crystallographic Data for This Paper. These Data Can Be Obtained Free of Charge from the Cambridge Crystallographic Data Centre. Available online: www.ccdc.cam.ac.uk/data_request/cif (accessed on 3 July 2024).
- Wang, Z.K.; Zhang, Z.S.; Zhao, W.J.; Sivaguru, P.; Zanoni, G.; Wang, Y.Y.; Anderson, E.A.; Bi, X.H. Synthetic exploration of sulfinyl radicals using sulfinyl sulfones. Nat. Commun. 2021, 12, 5244. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, Q.; Feng, C.; Wang, Z.; Zhao, W.; Ning, Y.; Wu, Y. The sulfinylsulfonation of alkynes for β-sulfinyl alkenylsulfone. Chem. –Asian J. 2022, 17, e202200299. [Google Scholar] [CrossRef] [PubMed]
- Freeman, F. vic-Disulfoxides and OS-sulfenyl sulfinates. Chem. Rev. 1984, 84, 117–135. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Haug, G.C.; Greco, S.G.; Trevino, R.; Karki, G.B.; Arman, H.D.; Larionov, O.V. Decarboxylative sulfinylation enables a direct, metal-free access to sulfoxides from carboxylic acids. Angew. Chem. Int. Ed. 2022, 61, e202210525. [Google Scholar] [CrossRef]
- Mampuys, P.; McElroy, C.R.; Clark, J.H.; Orru, R.V.A.; Maes, B.U.W. Thiosulfonates as emerging reactants: Synthesis and applications. Adv. Synth. Catal. 2020, 362, 3–64. [Google Scholar] [CrossRef]
- Lv, Y.F.; Luo, J.Y.; Ma, Y.C.; Dong, Q.; He, L. Visible-light-promoted sulfonylation of thiols with aryldiazonium and sodium metabisulphite leading to unsymmetrical thiosulfonates. Org. Chem. Front. 2021, 8, 2461–2467. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Wang, X.R.; Sivaguru, P.; Wang, Z.K. Exploring the synthetic application of sulfinyl radicals. Org. Chem. Front. 2022, 9, 6063–6076. [Google Scholar] [CrossRef]
- Chen, X.W.; Luo, Z.L.; Hu, Z.J.; Sun, D.H.; He, Y.Y.; Lu, J.N.; Chen, L.L.; Liu, S.Y. Discovery of potent thiazolidin-4-one sulfone derivatives for inhibition of proliferation of osteosarcoma in vitro and in vivo. Eur. J. Med. Chem. 2024, 266, 116082. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Qian, P.; Wang, Y.; Mei, H.; Han, J.; Pan, Y. Cu-Catalyzed deoxygenative C2-sulfonylation reaction of quinoline N-Oxides with sodium sulfinate. Org. Lett. 2016, 18, 4144–4147. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Liang, Z.; Jialingbieke, A.; Gao, J.; Du, D. NHC-Catalyzed Synthesis of α-sulfonyl ketones via radical- mediated sulfonyl methylation of aldehydes. Org. Lett. 2023, 25, 2657–2662. [Google Scholar] [CrossRef] [PubMed]
- Jadhao, A.R.; Gaikwad, S.S.; Patil, L.R.; Waghmode, S.B. Sonication-assisted one pot, metal-free synthesis of β-keto sulfones from styrenes, NBS and aromatic sodium sulfinate salts. Chem. Pap. 2021, 75, 4959–4968. [Google Scholar] [CrossRef]
- Deng, S.Q.; Liang, E.; Wu, Y.R.; Tang, X.D. Efficient sulfonylation of ketones with sodium sulfinates for the synthesis of β-keto sulfones. Tetrahedron Lett. 2018, 59, 3955–3957. [Google Scholar] [CrossRef]
- Chen, W.T.; Liu, X.Y.; Chen, E.; Chen, B.H.; Shao, J.A.; Yu, Y.P. KI-Mediated radical multi-functionalization of vinyl azides: A one-pot and efficient approach to β-keto sulfones and α-halo-β-keto sulfones. Org. Chem. Front. 2017, 4, 1162–1166. [Google Scholar] [CrossRef]
- Yavari, I.; Shaabanzadeh, S. Electrochemical synthesis of β-ketosulfones from switchable starting materials. Org. Lett. 2020, 22, 464–467. [Google Scholar] [CrossRef]


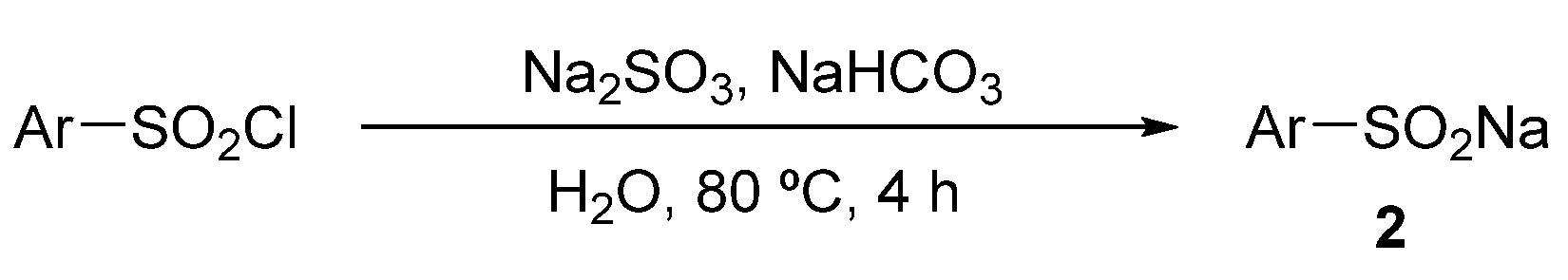
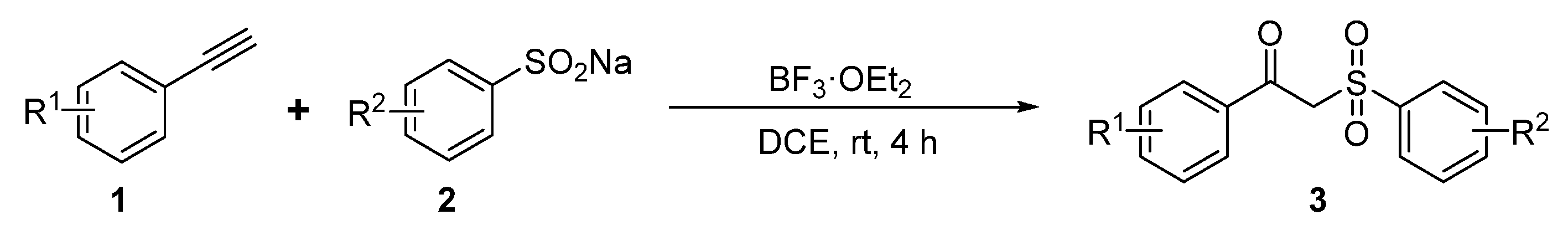
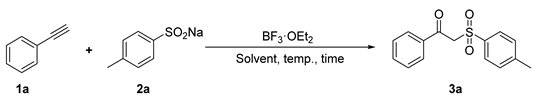 | ||||||
| Entry | Acid (equiv.) | 1a:2a | Temp. (°C) | Time (h) | Solvent | Yield (%) [b] |
| 1 | BF3·OEt2 (0.5) | 1:2.4 | rt | 4 | DCM | 38 |
| 2 | BF3·OEt2 (0.5) | 1:2.4 | rt | 6 | DCM | 33 |
| 3 | BF3·OEt2 (0.5) | 1:2.4 | rt | 2 | DCM | 15 |
| 4 | BF3·OEt2 (0.5) | 1:2.4 | 40 | 4 | DCM | 32 |
| 5 | BF3·OEt2 (0.5) | 1:2.4 | 0 | 4 | DCM | Trace |
| 6 | BF3·OEt2 (0.25) | 1:2.4 | rt | 4 | DCM | 22 |
| 7 | BF3·OEt2 (0.75) | 1:2.4 | rt | 4 | DCM | 27 |
| 8 | BF3·OEt2 (0.5) | 1:2.4 | rt | 4 | DCE | 56 |
| 9 | BF3·OEt2 (0.5) | 1:2.4 | rt | 4 | EA | 24 |
| 10 | BF3·OEt2 (0.5) | 1:2.4 | rt | 4 | DMSO | N.D. [c] |
| 11 | BF3·OEt2 (0.5) | 1:2.4 | rt | 4 | CH3OH | N.D. |
| 12 | BF3·OEt2 (0.5) | 1:2.4 | rt | 4 | C2H5OH | N.D. |
| 13 | BF3·OEt2 (0.5) | 1:2.4 | rt | 4 | THF | N.D. |
| 14 | BF3·OEt2 (0.5) | 1:2.4 | rt | 4 | CH3CN | 18 |
| 15 | BF3·OEt2 (0.5) | 1:2.4 | rt | 4 | Toluene | N.D. |
| 16 | BF3·OEt2 (0.5) | 1:2.4 | rt | 4 | Acetone | N.D. |
| 17 | BF3·OEt2 (0.5) | 1:2.4 | rt | 4 | DMF | N.D. |
| 18 | BF3·OEt2 (0.5) | 1:2.4 | rt | 4 | AcOH | N.D. |
| 19 | BF3·OEt2 (0.5) | 1:2.4 | rt | 4 | 1,4-Dioxane | N.D. |
| 20 | BF3·OEt2 (0.5) | 1:2.4 | rt | 4 | CHCl3 | 12 |
| 21 | BF3·OEt2 (0.5) | 1:2.4 | rt | 4 | Ethyl ether | N.D. |
| 22 | BF3·OEt2 (0.5) | 1:2.4 | rt | 4 | Dimethoxyethane | N.D. |
| 23 | BF3·OEt2 (0.5) | 1:2.0 | rt | 4 | DCE | 43 |
| 24 | BF3·OEt2 (0.5) | 1:2.8 | rt | 4 | DCE | 42 |
 |
 |
| Compound | 3a |
|---|---|
| Empirical formula | C15H14O3S |
| Formula weight | 274.32 |
| Temperature (K) | 297 |
| Wavelength (Å) | 0.71073 |
| Crystal system | monoclinic |
| Space group | P21/n |
| Unit cell dimensions (Å, °) | a = 7.7540(5), b = 11.5019(8), c = 15.3137(10) |
| α = 90, β = 98.746(7), γ = 90 | |
| Volume (Å3) | 1349.88(16) |
| Z | 4 |
| Density (calculated) (g/cm3) | 1.350 |
| Absorption coefficient (mm−1) | 0.240 |
| F(000) | 576.0 |
| Theta range for data collection | 2.691 to 29.072 |
| Index ranges | −10 ≤ h ≤ 10, −15 ≤ k ≤ 13, −20 ≤ l ≤ 20 |
| Reflections collected | 6338 |
| Independent reflections | 3117 [R(int) = 0.0218, R(sigma) = 0.0379] |
| Completeness to theta = 29.072° | 86.4 % |
| Absorption correction | Multi-Scan |
| Max. and min. transmission | 1.000 and 0.885 |
| Refinement method | Least Squares minimisation |
| Data / restraints / parameters | 3117 / 0 / 173 |
| Goodness-of-fit on F2 | 1.027 |
| Final R indices [I>2sigma(I)] | R1 = 0.0489, wR2 = 0.1141 |
| R indices (all data) | R1 = 0.0668, wR2 = 0.1229 |
| Largest diff. peak and hole | 0.26 and −0.26 e.Å-3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.-W.; Chen, Z.-J.; Li, H.-Q.; Li, W.-X.; Li, Y.; Li, Z.; Wang, Z.-Y. Oxysulfonylation of Alkynes with Sodium Sulfinates to Access β-Keto Sulfones Catalyzed by BF3·OEt2. Molecules 2024, 29, 3559. https://doi.org/10.3390/molecules29153559
Yu S-W, Chen Z-J, Li H-Q, Li W-X, Li Y, Li Z, Wang Z-Y. Oxysulfonylation of Alkynes with Sodium Sulfinates to Access β-Keto Sulfones Catalyzed by BF3·OEt2. Molecules. 2024; 29(15):3559. https://doi.org/10.3390/molecules29153559
Chicago/Turabian StyleYu, Shi-Wei, Zu-Jia Chen, Huan-Qing Li, Wen-Xi Li, Yun Li, Zong Li, and Zhao-Yang Wang. 2024. "Oxysulfonylation of Alkynes with Sodium Sulfinates to Access β-Keto Sulfones Catalyzed by BF3·OEt2" Molecules 29, no. 15: 3559. https://doi.org/10.3390/molecules29153559
APA StyleYu, S.-W., Chen, Z.-J., Li, H.-Q., Li, W.-X., Li, Y., Li, Z., & Wang, Z.-Y. (2024). Oxysulfonylation of Alkynes with Sodium Sulfinates to Access β-Keto Sulfones Catalyzed by BF3·OEt2. Molecules, 29(15), 3559. https://doi.org/10.3390/molecules29153559







