Evaluation of the Shelf Life of Myristica-fragrans Powder-Flavored Oils Obtained through the Application of Two Processes: Infusion and Co-Pressing Technology
Abstract
:1. Introduction
2. Results and Discussion
2.1. Quality Parameters
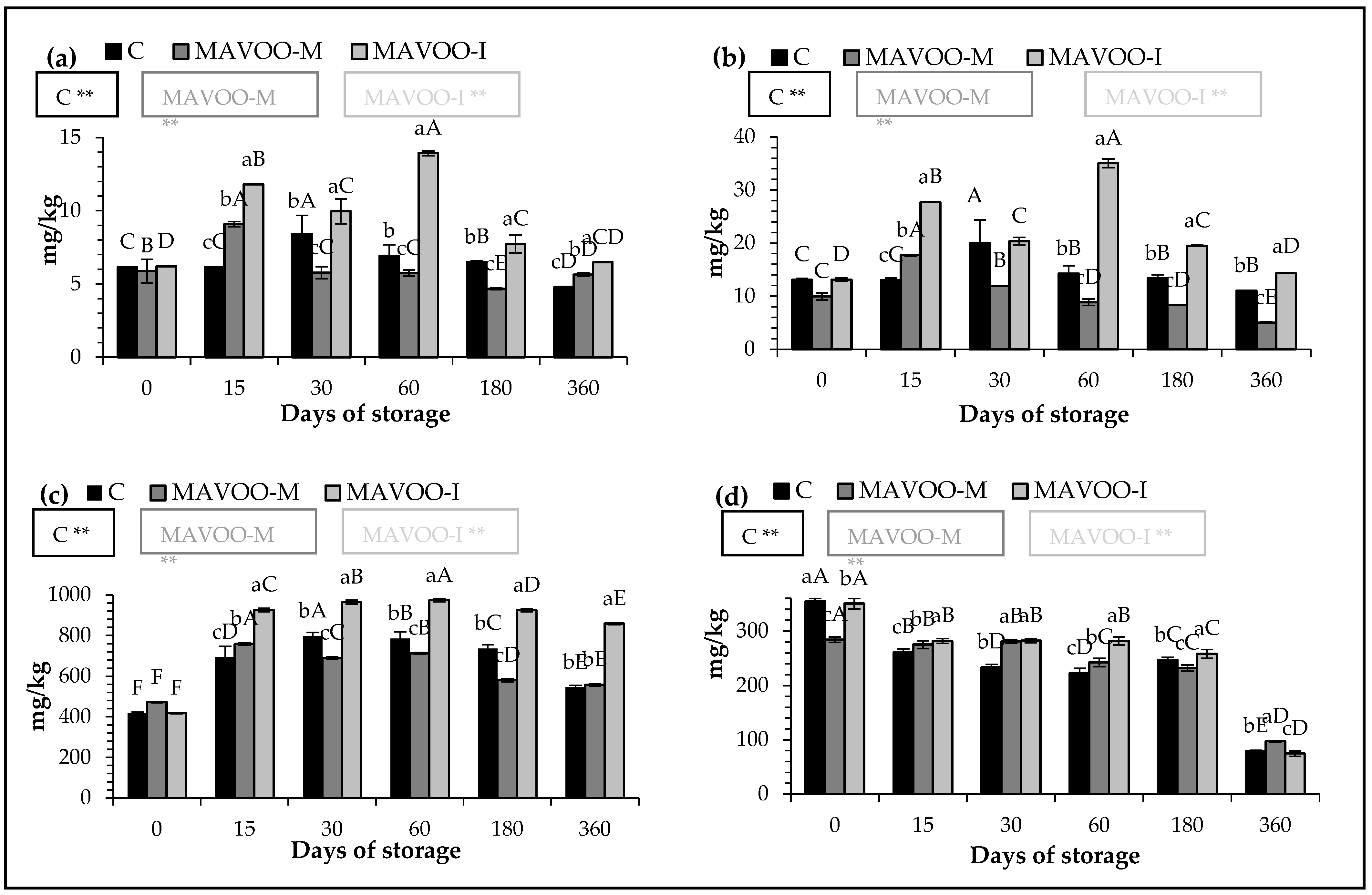
2.2. Quantitative Parameters
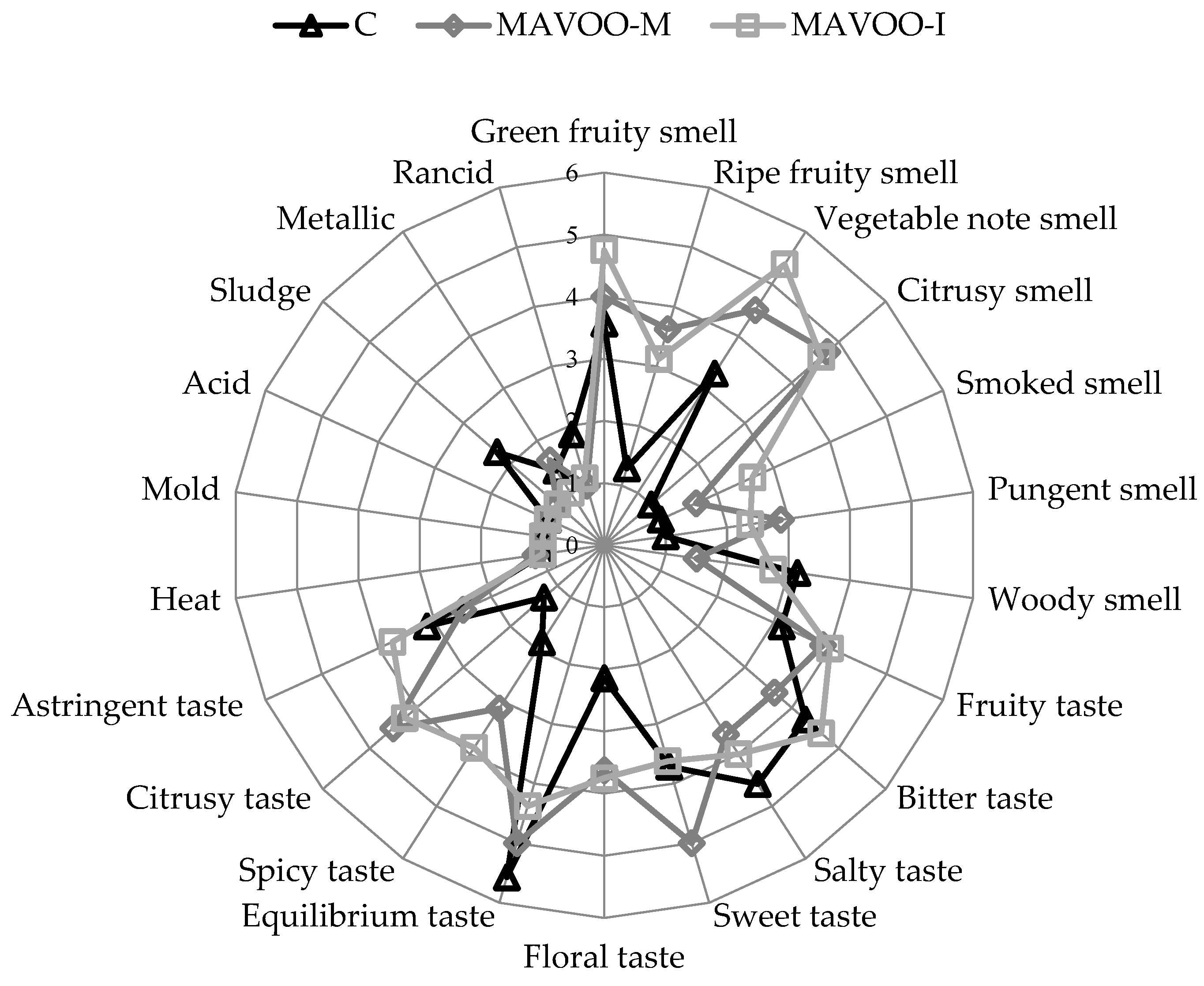
2.3. Parameters that Affect Consumers’ Acceptability
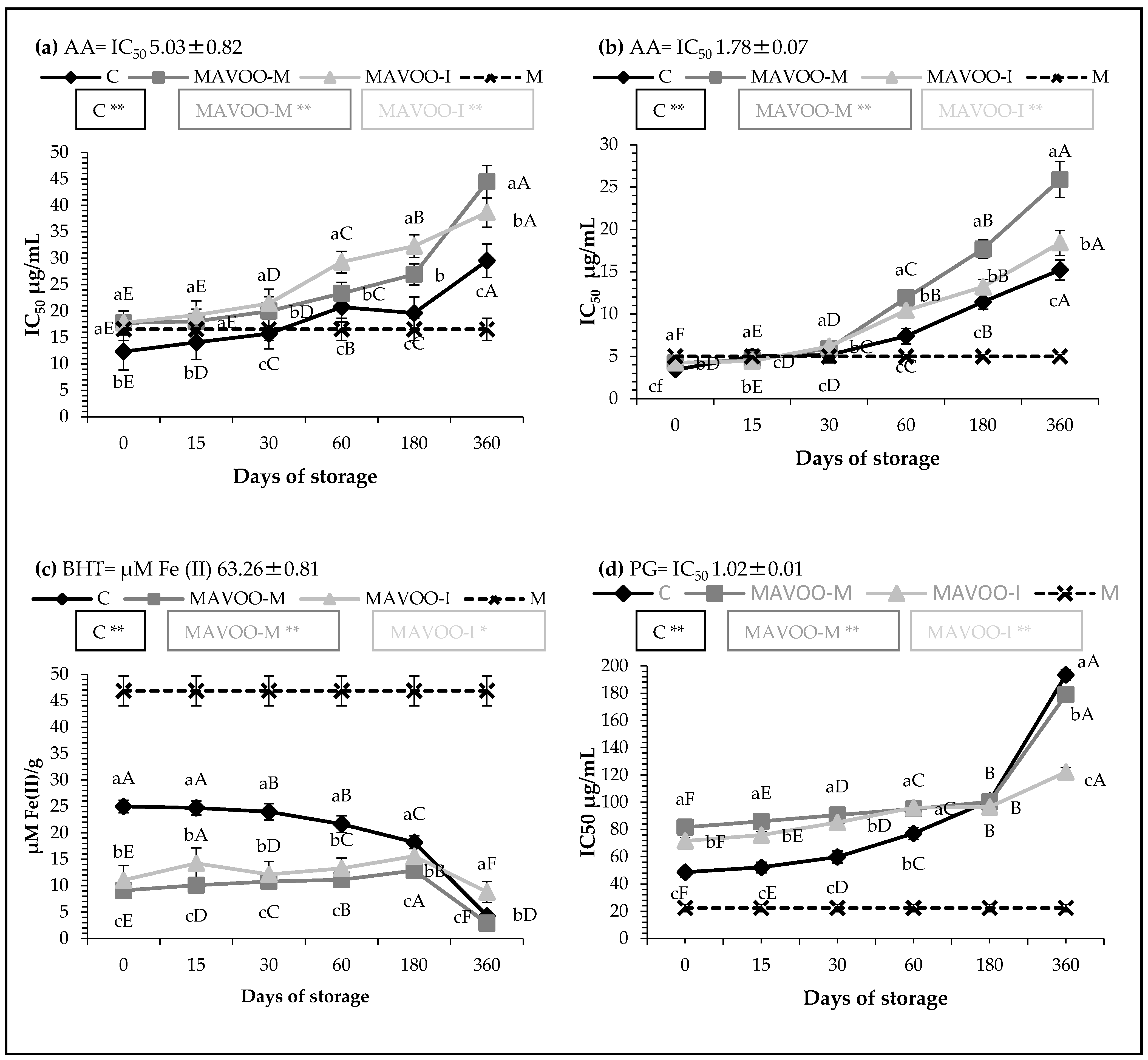
2.4. Antioxidant Activities
2.5. Carbohydrate-Hydrolyzing Enzymes and Lipase-Inhibition Activities
3. Materials and Methods
3.1. Preparing the Samples (C, MAVOO-M and MAVOO-I)
3.2. Mace Extract
3.3. Quality Parameters of the Samples (C, MAVOO-M and MAVOO-I)
3.4. Pigments Quantitative Determination
3.5. Total Phenols and α-Tocopherol Contents
3.6. Parameters that Affect Consumer’s Acceptability
3.7. Evaluation of Antioxidant Activities
3.8. Evaluation of α-Amylase-, α-Glucosidase- and Lipase-Inhibition Activities
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodríguez-Pérez, C.; Segura-Carretero, A.; Contreras, M.D.M. Phenolic compounds as natural and multifunctional anti-obesity agents: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1212–1229. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.W.; Nash, P.; Buttar, H.S.; Griffiths, K.; Singh, R.; De Meester, F.; Rie Horiuchi, R.; Takahashi, T. The Role of Food Antioxidants, Benefits of Functional Foods, and Influence of Feeding Habits on the Health of the Older Person: An Overview. Antioxidants 2017, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Das, M. Functional Foods: An Overview. Food Sci. Biotechnol. 2011, 20, 861–875. [Google Scholar] [CrossRef]
- Cercato, C.; Fonseca, F.A. Cardiovascular risk and obesity. Diabetol. Metab. Syndr. 2019, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhao, H.; Guo, L.; Liu, X.; Liang, Y.; Liu, Q.; Cao, W.; Xueyan, C.; Gao, X. Fu Brick Tea as a Staple Food Supplement Attenuates High Fat Diet Induced Obesity in Mice. Foods 2023, 12, 4488. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidi, M.; Koutelidakis, A.E. Functional Foods and Bioactive Compounds: A Review of Its Possible Role on Weight Management and Obesity’s Metabolic Consequences. Medicines 2019, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Sandner, G.; Konig, A.; Wallner, M.; Weghuber, J. Functional Foods—Dietary or herbal products on obesity: Application on selected bioactive compounds to target lipid metabolisms. Curr. Opin. Food Sci. 2020, 34, 9–20. [Google Scholar] [CrossRef]
- Noce, A.; Di Lauro, M.; Di Daniele, F.; Pietroboni Zaitseva, A.; Marrone, G.; Borboni, P.; Di Daniele, N. Natural Bioactive Compounds Useful in Clinical Management of Metabolic Syndrome. Nutrients 2021, 13, 630. [Google Scholar] [CrossRef]
- Mohamed, S. Functional foods against metabolic syndrome (obesity, diabetes, hypertension and dyslipidemia) and cardiovasular disease. JSFT 2014, 35, 114–128. [Google Scholar] [CrossRef]
- Rauf, A.; Akrm, M.; Anwar, H.; Daniyal, M.; Munir, N.; Bawazeer, S.; Bawazeer, S.; Rebezov, M.; Bouyahya, A.; Shariati, M.A.; et al. Therapeutic potential of herbal medicine for the management of hyperlipidemia: Latest updates. Environ. Sci. Pollut. Res. 2022, 29, 40281–40301. [Google Scholar] [CrossRef]
- Redan, B.W.; Buhman, K.K.; Novotny, J.A.; Ferruzzi, M.G. Altered transport and metabolism of phenolic compounds in obesity and diabetes: Implications for functional food development and assessment. Adv. Nutr. 2016, 7, 1090–1104. [Google Scholar] [CrossRef]
- Tucci, M.; Martini, D.; Marino, M.; Del Bo’, C.; Vinelli, V.; Biscotti, C.; Parisi, C.; De Amicis, R.; Battezzati, A.; Bertoli, S.; et al. The Environmental Impact of an Italian-Mediterranean Dietary Pattern Based on the EAT Lancet Reference Diet (EAT-IT). Foods 2022, 11, 3352. [Google Scholar] [CrossRef] [PubMed]
- Ingrassia, M.; Altomore, L.; Columba, P.; Raffermati, S.; Lo Grasso, G.; Bacarella, S.; Chironi, S. Mediterranean Diet, Sustainability, and Tourism—A Study of the Market’s Demand and Knowledge. Foods 2023, 12, 2463. [Google Scholar] [CrossRef]
- Sicari, V.; Leporini, M.; Giuffrè, A.M.; Aiello, F.; Falco, T.; Pagliuso, M.T.; Ruffolo, A.; Ritano, A.; Romeo, R.; Tundis, R.; et al. Quality parameters, chemical compositions and antioxidant activities of Calabrian (Italy) monovarietal extra virgin olive oils from autochthonous (Ottobratica) and allochthonous (Coratina, Leccino, and Nocellara Del Belice) varieties. J. Food Meas. Charact. 2021, 15, 363–375. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Dipalmo, T.; Crupi, P.; Durante, V.; Pesce, V.; Maiellaro, I.; Lovece, A.; Mercurio, A.; Laghezza, A.; Corbo, F.; et al. Comparison between different flavored olive oil production techniques: Healthy value and process efficiency. Plant Food Hum. Nutr. 2016, 71, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Caponio, F.; Durante, V.; Varva, G.; Silletti, R.; Previtali, M.A.; Viggiani, I.; Squeo, G.; Summo, C.; Pasqualone, A.; Gomes, T.; et al. Effect of infusion of spices into the oil vs. combined malaxation of olive paste and spices on quality of naturally flavored virgin olive oils. Food Chem. 2016, 202, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Gambacorta, G.; Faccia, M.; Pati, S.; Lamacchia, C.; Baiano, A.; La Notte, E. Changes in the chemical and sensorial profile of extra virgin olive oils flavored with herbs and spices during storage. J. Food Lip. 2007, 14, 202–215. [Google Scholar] [CrossRef]
- Sasikumar, B. Nutmeg-Origin, diversity, distribution and history. J. Spices Aromat. Crops 2021, 30, 131–141. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Sicari, V.; Tenuta, M.C.; Leporini, M.; Falco, T.; Pellicanò, T.M.; Menichini, F.; Tundis, R. Phytochemicals content, antioxidant and hypoglycaemic activities of commercial nutmeg mace (Myristica fragrans L.) and pimento (Pimenta dioica (L.) Merr.). Int. J. Food Sci. Technol. 2016, 51, 2057–2063. [Google Scholar] [CrossRef]
- Khamnuan, S.; Phrutivorapongkul, A.; Pitchakarn, P.; Buacheen, P.; Karinchai, J.; Chittasupho, C.; Takuathung, M.N.; Theansungnoen, T.; Thongkhao, K.; Intharuksa, A. The Identification and Cytotoxic Evaluation of Nutmeg (Myristica fragrans Houtt.) and Its Substituents. Foods 2023, 12, 4211. [Google Scholar] [CrossRef]
- Kazlauskaite, J.A.; Matulyte, I.; Marksa, M.; Lelesius, R.; Pavilonis, A.; Bernatoniene, J. Application of Antiviral, Antioxidant and Antibacterial Glycyrrhiza glabra L., Trifolium pratense L. Extracts and Myristica fragrans Houtt. Essential Oil in Microcapsules. Pharmaceutics 2023, 15, 464. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Jungbauer, A. Culinary plants, herbs and spices e a rich source of PPARγ ligands. Food Chem. 2009, 117, 660–667. [Google Scholar] [CrossRef]
- Lamas, S.; Rodrigues, N.; Peres, A.M.; Pereira, J.A. Flavoured and fortified olive oils—Pros and cons. Trends Food Sci. Tech. 2022, 124, 108–127. [Google Scholar] [CrossRef]
- Boulares, M.; Bezzezi, A.; Arfaoui, M.; Boulares, S.A.; Ghrab, M.M.; Moussa, O.B.; Hassouna, M.; Boudiche, S. Improvement of Tunisian ‘Chemlali’ extra virgin olive oil stability with rosemary and laurel herbs and essential oils. Riv. Ital. Sostanze Gr. 2022, 99, 131–140. [Google Scholar]
- Peres, F.; Marques, M.P.; Mourato, M.; Martins, L.L.; Ferreira-Dias, S. Ultrasound Assisted Coextraction of Cornicabra Olives and Thyme to Obtain Flavored Olive Oils. Molecules 2023, 28, 6898. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, L.; Balli, D.; Urciuoli, S.; Urciuolo, A.; Bordiga, M.; Travaglia, F.; Zanoni, B.; Mulinacci, N. Co-milling of sound olives with fresh chili peppers improves the volatile compound, capsaicinoid and sensory profiles of flavoured olive oil with respect to the typical infusion. Food Chem. 2023, 404, 134696. [Google Scholar] [CrossRef]
- Baiano, A.; Gambacorta, G.; la Notte, E. Aromatization of olive oil. Transw. Res. Netw. 2010, 37, 1–29. [Google Scholar]
- Consolidated TEXT Produced by the CONSLEG System of the Office for Official Publications of the European Communities. CONSLEG: 01991R2568—EN—20.10.2019—032.001. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A01991R2568-20191020 (accessed on 6 June 2024).
- Esposto, S.; Selvaggini, R.; Taticchi, A.; Veneziani, G.; Sordini, B.; Servilli, M. Quality evolution of extra-virgin olive oils according to their chemical composition during 22 months of storage under dark conditions. Food Chem. 2020, 311, 126044. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Montaña, E.J.; Aparicio-Ruiz, R.; Morales, M.T. Effect of Flavorization on Virgin Olive Oil Oxidation and Volatile Profile. Antioxidants 2023, 12, 242. [Google Scholar] [CrossRef]
- Fagundes, M.B.; Ballus, C.A.; Soaere, V.P.; De Freitas Ferreira, D.; Vaz Leaes, Y.S.; Robalo, S.S.; Vendruscolok, R.G.; Bastaniello Campagnol, P.C.; Barin, J.S.; Cichoski, A.J.; et al. Characterization of olive oil flavored with Brazilian pink pepper (Schinus T terebinthifolius Raddi) in different maceration processes. Food Resear. Int. 2020, 137, 109593. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, R.; Della Medaglia, D.; Paduano, A.; Caporaso, N.; Genovese, A. Characterisation of lemon-flavoured olive oils. LWT-Food Sci. Technol. 2017, 79, 326–332. [Google Scholar] [CrossRef]
- Kasimoglu, Z.; Tontul, I.; Soylu, A.; Gulen, K.; Topuz, A. The oxidative stability of flavoured virgin olive oil: The effect of the water activity of rosemary. J. Food Meas. Charact. 2018, 12, 2080–2086. [Google Scholar] [CrossRef]
- Asensio, C.M.; Nepote, V.; Grosso, N.T. Consumers’ acceptance and quality stability of olive oil flavoured with essential oils of different oregano species. Int. J. Food Sci. Technol. 2013, 48, 2417–2428. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Bonesi, M.; Falco, T.; Leporini, M.; Pagliuso, M.; Sicari, V.; Tundis, R. Carolea olive oil enriched with an infusion of Capsicuum annuum and C. chinense dried pepper powders to produce an added value flavoured olive oils. J. Food Process. Preserv. 2021, 45, 15776. [Google Scholar] [CrossRef]
- Issaoui, M.; Bendini, S.; Souid, S.; Flamini, G.; Barbieri, G.; Gallina Toschi, T.; Hammami, M. Flavored olive oils: Focus on their acceptability and thermal stability. Grasas Aceites 2019, 70, e293. [Google Scholar] [CrossRef]
- Sousa, A.; Casal, S.; Malheiro, R.; Lamas, H.; Bento, A.; Pereira, J.A. Aromatized olive oils: Influence of flavouring in quality, composition, stability, antioxidants, and antiradical potential. LWT-Food Sci. Technol. 2015, 60, 22–28. [Google Scholar] [CrossRef]
- Sikorska, E.; Caponio, F.; Bilancia, M.T.; Summo, C.; Pasqualone, A.; Khmelinskii, I.; Sikorski, M. Changes in colour of extra-virgin olive oil during storage. Pol. J. Food Nutr. Sci. 2007, 57, 495–498. [Google Scholar]
- Kong, B.; Zhang, H.; Xiong, Y.L. Antioxidant activity of spice extracts in a liposome system and in cooked pork patties and the possible mode of action. Meat Sci. 2010, 85, 772–778. [Google Scholar] [CrossRef]
- Li, C.W.; Chu, Y.C.; Huang, C.Y.; Fu, S.L.; Chen, J.J. Evaluation of Antioxidant and Anti-α-glucosidase Activities of Various Solvent Extracts and Major Bioactive Components from the Seeds of Myristica fragrans. Molecules 2020, 25, 5198. [Google Scholar] [CrossRef]
- Trifan, A.; Zengin, G.; Korona-Glowniak, L.; Woz-Skalicka, K.; Luca, S.V. Essential Oils and Sustainability: In Vitro Bioactivity Screening of Myristica fragrans Houtt. Post-Distillation By-Products. Plants 2023, 12, 1741. [Google Scholar] [CrossRef] [PubMed]
- Custureri, I.M.G.; Sicari, V.; Loizzo, M.R.; Tundis, R.; Soria, A.C.; Giuffrè, A.M. Evaluation of Quality Parameters and Functional Activity of Ottobratica Extra Virgin Olive Oil Enriched with Zingiber officinale (Ginger) by Two Different Enrichment Processes during One-Year Storage. Foods 2023, 12, 3822. [Google Scholar] [CrossRef] [PubMed]
- Plastina, P.; Tundis, R.; La Torre, C.; Sicari, V.; Giuffrè, A.M.; Neri, A.; Bonesi, M.; Leporini, M.; Fazio, A.; Falco, T.; et al. The addition of Capsicum baccatum to Calabrian monovarietal extra virgin olive oils leads to flavored olive oils with enhanced oxidative stability. Ital. J. Food Sci. 2021, 33, 61–72. [Google Scholar] [CrossRef]
- Marrelli, M.; Loizzo, M.R.; Nicoletti, M.; Menichini, F.; Conforti, F. In vitro investigation of the potential health benefits of wild Mediterranean dietary plants as anti-obesity agents with α-amylase and pancreatic lipase inhibitory activities. J. Sci. Food Agric. 2014, 94, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Sivaraj, S.; Kannayiram, G.; Dasararahu, G. Evaluation of antidiabetic activty of different extracts of myristica fragrans houtt: In vitro and in silico studies. Asian J. Pharm. Clin. Res. 2017, 10, 275–277. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Lecce, G.D.; Boselli, E.; Menichini, F.; Frega, N.G. Inhibitory Activity of Phenolic Compounds From Extra Virgin Olive Oils on the Enzymes Involved in Diabetes, Obesity and Hypertension. J. Food Biochem. 2011, 35, 381–399. [Google Scholar] [CrossRef]
- Vangoori, Y.; Dakshinamoorthi, A.; Rao, R.P.; David, D.R.; Babu, K.A. Effect of Myristica fragrans Extract on Food Intake and Body Weight in Experimental Models. J. Clin. Diagn. Res. 2018, 12, FF01–FF05. [Google Scholar] [CrossRef]
- Minguez-Mosquera, M.I.; Rejano-Navarro, L.; Gandui-Rojas, B.; Sànchez-Gomez, A.H.; Garrido-Fernando, J. Color-Pigment correlation in Virgin Olive Oil. J. Am. Oil Chem. 1991, 68, 332–336. [Google Scholar] [CrossRef]
- Montedoro, G.F.; Servili, M.; Baldioli, M.; Miniati, E. Simple and hydrolyzable phenolic compounds in virgin olive oil. Their extraction, separation and quantitative compounds and semiquantitative evaluation by HPLC. J. Agric. Food Chem. 1992, 40, 571–1576. [Google Scholar] [CrossRef]
- Baiano, A.; Gambacorta, G.; Terracone, C.; Previtali, M.A.; Lamacchia, C.; La Notte, E. Changes in phenolic content and antioxidant activity of Italian extra-virgin olive oils during storage. J. Food Sci. 2009, 74, 177–183. [Google Scholar] [CrossRef]
- Formoso, P.; Tundis, R.; Pellegrino, M.; Leporini, M.; Sicari, V.; Romeo, R.; Gervasi, L.; Corrente, G.; Beneduci, A.; Loizzo, M.R. Preparation, characterization, and bioactivity of Zingiber officinale Roscoe (white ginger) powder-based Pickering emulsions. J. Sci. Food Agric. 2022, 102, 6566–6577. [Google Scholar] [CrossRef] [PubMed]
| Sample | Days of Storage | ||||||
|---|---|---|---|---|---|---|---|
| T0 | T15 | T30 | T60 | T180 | T360 | Sign | |
| Free Acidity (% Oleic Acid) | |||||||
| C | 0.68 ± 0.02 bC | 0.70 ± 0.00 bB | 0.71 ± 0.00 bF | 0.56 ± 0.00 cD | 0.53 ± 0.05 cE | 0.84 ± 0.01 bA | ** |
| MAVOO-M | 0.93 ± 0.04 aD | 0.96 ± 0.00 bE | 0.93 ± 0.00 bD | 1.06 ± 0.04 aB | 0.98 ± 0.01 aC | 1.27 ± 0.04 aA | ** |
| MAVOO-I | 0.68 ± 0.03 bD | 0.98 ± 0.01 aB | 0.93 ± 0.03 bC | 0.97 ± 0.04 bB | 0.92 ± 0.01 bC | 1.23 ± 0.02 abA | ** |
| Sign | ** | ** | ** | ** | ** | ** | |
| Peroxide Value (meqO2/kg) | |||||||
| C | 9.45 ± 0.20 aD | 9.50 ± 0.36 aD | 10.56 ± 0.25 bC | 10.95 ± 0.03 aC | 12.86 ± 0.09 aB | 17.89 ± 0.09 aA | ** |
| MAVOO-M | 5.32 ± 0.06 bDE | 5.53 ± 0.06 cD | 5.81 ± 0.18 cC | 6.22 ± 0.04 bE | 7.44 ± 0.06 cB | 9.68 ± 0.19 bA | ** |
| MAVOO-I | 9.40 ± 0.18 aE | 8.26 ± 0.11 bF | 11.18 ± 0.50 aBC | 10.65 ± 0.59 abD | 11.83 ± 0.09 bB | 17.67 ± 0.45 abA | ** |
| Sign | ** | ** | ** | ** | ** | ** | |
| K232 | |||||||
| C | 2.46 ± 0.06 cC | 2.47 ± 0.05 cC | 1.98 ± 0.05 cE | 2.11 ± 0.03 cD | 2.87 ± 0.08 B | 2.95 ± 0.14 A | ** |
| MAVOO-M | 3.80 ± 0.02 aA | 2.87 ± 0.02 bD | 3.17 ± 0.01 aB | 3.08 ± 0.20 aBC | 3.00 ± 0.09 C | 3.19 ± 0.29 B | ** |
| MAVOO-I | 2.57 ± 0.25 bD | 3.59 ± 0.18 aA | 2.58 ± 0.27 bD | 2.59 ± 0.43 bD | 2.83 ± 0.10 C | 3.44 ± 0.38 B | ** |
| Sign | ** | ** | ** | ** | ns | ns | |
| K268 | |||||||
| C | 0.22 ± 0.02 cC | 0.24 ± 0.02 cB | 0.20 ± 0.02 cD | 0.20 ± 0.05 cD | 0.28 ± 0.01 cA | 0.28 ± 0.00 cA | * |
| MAVOO-M | 1.40 ± 0.02 aA | 1.14 ± 0.10 aD | 0.95 ± 0.02 aE | 1.25 ± 0.13 aB | 1.29 ± 0.04 aBC | 1.21 ± 0.01 aC | ** |
| MAVOO-I | 0.28 ± 0.02 bE | 0.63 ± 0.01 bD | 0.64 ± 0.08 bD | 0.66 ± 0.03 bC | 1.10 ± 0.01 bA | 0.75 ± 0.01 bB | ** |
| Sign | ** | ** | ** | ** | ** | ** | |
| ΔK | |||||||
| C | -0.003 ± 0.000 cBC | -0.003 ± 0.000 cC | -0.003 ± 0.000 BC | -0.003 ± 0.000 cBC | -0.001 ± 0.000 cAB | 0.000 ± 0.000 cA | ** |
| MAVOO-M | 0.031 ± 0.003 aD | 0.035 ± 0.002 aC | 0.039 ± 0.002 B | 0.043 ± 0.001 aA | 0.034 ± 0.003 aC | 0.038 ± 0.004 aB | * |
| MAVOO-I | 0.000 ± 0.000 bD | 0.012 ± 0.001 bC | 0.015 ± 0.002 B | 0.015 ± 0.004 bB | 0.026 ± 0.002 bA | 0.027 ± 0.001 bA | ** |
| Sign | ** | ** | ns | ** | ** | ** | |
| Samples | Days of Storage | ||||||
|---|---|---|---|---|---|---|---|
| T0 | T15 | T30 | T60 | T180 | T360 | Sign | |
| L* | |||||||
| C | 32.70 ± 0.02 bD | 32.73 ± 0.07 aD | 41.42 ± 0.77 aC | 41.96 ± 0.05 aBC | 42.08 ± 0.04 bB | 42.62 ± 0.01 A | ** |
| MAVOO-M | 32.81 ± 0.06 aC | 32.24 ± 0.12 bD | 32.15 ± 0.06 bE | 32.25 ± 0.02 bD | 42.84 ± 0.04 aB | 43.88 ± 1.16 A | ** |
| MAVOO-I | 32.70 ± 0.01 bD | 32.17 ± 0.06 cE | 42.21 ± 0.06 abB | 41.73 ± 0.03 abC | 42.12 ± 0.01 bBC | 43.02 ± 0.06 A | ** |
| Sign | * | ** | ** | ** | ** | ns | |
| a* | |||||||
| C | 3.42 ± 0.02 A | 3.43 ± 0.03 A | 0.05 ± 0.03 bD | 0.73 ± 0.01 bB | 0.15 ± 0.01 aC | −0.06 ± 0.01 cE | ** |
| MAVOO-M | 3.44 ± 0.02 B | 4.21 ± 1.17 A | 3.32 ± 0.02 aC | 3.34 ± 0.01 aC | -0.01 ± 0.01 bE | 0.17 ± 0.02 aD | ** |
| MAVOO-I | 3.42 ± 0.03 A | 3.24 ± 0.03 B | 0.10 ± 0.01 bE | 0.67 ± 0.04 bC | 0.14 ± 0.00 aD | −0.01 ± 0.01 bF | ** |
| Sign | ns | ns | ** | ** | ** | ** | |
| b* | |||||||
| C | 6.38 ± 0.10 aA | 6.35 ± 0.13 aA | 2.11 ± 0.06 bD | 2.03 ± 0.05 bE | 2.95 ± 0.06 aB | 2.24 ± 0.02 bC | ** |
| MAVOO-M | 5.93 ± 0.10 bA | 5.97 ± 0.03 bA | 3.34 ± 0.01 aC | 5.79 ± 0.03 aB | 2.48 ± 0.03 bD | 2.06 ± 0.07 cE | ** |
| MAVOO-I | 6.38 ± 0.08 aA | 5.62 ± 0.02 cB | 2.05 ± 0.04 cE | 2.04 ± 0.02 bE | 2.97 ± 0.01 aC | 2.50 ± 0.03 aD | ** |
| Sign | ** | ** | ** | ** | ** | ** | |
| Chroma* | |||||||
| C | 7.23 ± 0.09 aA | 7.22 ± 0.11 A | 2.11 ± 0.06 bB | 2.15 ± 0.04 bB | 2.95 ± 0.06 cB | 2.24 ± 0.02 bB | ** |
| MAVOO-M | 6.85 ± 0.09 bA | 7.34 ± 0.68 A | 6.72 ± 0.01 aA | 6.69 ± 0.04 aA | 2.49 ± 0.01 bB | 2.07 ± 0.07 cB | ** |
| MAVOO-I | 7.21.0.05 aA | 6.49 ± 0.03 B | 2.05 ± 0.04 bE | 2.15 ± 0.02 bE | 2.98 ± 0.01 aC | 2.50 ± 0.03 aD | ** |
| Sign | ** | ns | ** | ** | ** | ** | |
| Samples | Days of Storage | ||||||
|---|---|---|---|---|---|---|---|
| T0 | T15 | T30 | T60 | T180 | T360 | Sign | |
| α-Amylase | |||||||
| C | 269.02 ± 3.77 aD | 275.21 ± 3.85 aCD | 303.38 ± 3.92 aB | 345.31 ± 4.05 aA | 240.29 ± 3.87 aE | 289.32 ± 4.90 bC | ** |
| MAVOO-M | 189.40 ± 3.56 bE | 195.59 ± 3.77 bD | 200.44 ± 3.44 cD | 213.04 ± 3.35 cC | 229.52 ± 3.08 bB | 347.78 ± 3.50 aA | ** |
| MAVOO-I | 189.47 ± 3.56 bD | 192.67 ± 3.81 bD | 208.72 ± 3.44 bC | 233.98 ± 3.35 bB | 237.01 ± 3.49 aB | 258.65 ± 3.8 cA | ** |
| Sign | ** | ** | ** | ** | ** | ** | |
| M | 162.49 ± 3.26 | ||||||
| Acarbose | 50.18 ± 1.32 | ||||||
| α-Glucosidase | |||||||
| C | 137.34 ± 3.73 F | 145.18 ± 3.79 abE | 198.81 ± 3.82 aD | 337.56 ± 3.90 aC | 587.49 ± 3.56 aB | 778.23 ± 4.67 aA | ** |
| MAVOO-M | 136.58 ± 3.45 E | 152.21 ± 3.47 aDE | 161.7 ± 3.79 cD | 183.23 ± 3.81 cC | 237.66 ± 3.88 cB | 344.87 ± 4.09 bA | ** |
| MAVOO-I | 136.55 ± 3.45 E | 140.05 ± 3.81 bE | 172.18 ± 3.09 bD | 220.92 ± 3.01 bC | 267.89 ± 3.90 bB | 307.07 ± 4.21 cA | ** |
| Sign | ns | ** | ** | ** | ** | ** | |
| M | 206.17 ± 3.82 | ||||||
| Acarbose | 35.57 ± 0.99 | ||||||
| Lipase | |||||||
| C | 143.46 ± 4.85 aF | 155.52 ± 4.87 aE | 173.43 ± 4.91 aD | 206.54 ± 5.01 aC | 253.81 ± 4.81 aB | 312.97 ± 5.44 aA | ** |
| MAVOO-M | 62.25 ± 1.09 bE | 67.20 ± 1.14 bDE | 70.54 ± 1.22 bD | 95.95 ± 1.72 bC | 119.32 ± 2.89 bB | 200.12 ± 3.05 bA | ** |
| MAVOO-I | 62.33 ± 4.12 bE | 69.34 ± 4.22 bDE | 73.18 ± 4.22 bD | 94.99 ± 4.02 bC | 121.35 ± 4.87 bB | 138.66 ± 4.99 cA | ** |
| Sign | ** | ** | ** | ** | ** | ** | |
| M | 83.60 ± 4.76 | ||||||
| Orlistat | 37.44 ± 1.08 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Custureri, I.M.G.; Loizzo, M.R.; Sicari, V.; Pino, R.; Tundis, R.; Soria, A.C.; Giuffrè, A.M. Evaluation of the Shelf Life of Myristica-fragrans Powder-Flavored Oils Obtained through the Application of Two Processes: Infusion and Co-Pressing Technology. Molecules 2024, 29, 3588. https://doi.org/10.3390/molecules29153588
Custureri IMG, Loizzo MR, Sicari V, Pino R, Tundis R, Soria AC, Giuffrè AM. Evaluation of the Shelf Life of Myristica-fragrans Powder-Flavored Oils Obtained through the Application of Two Processes: Infusion and Co-Pressing Technology. Molecules. 2024; 29(15):3588. https://doi.org/10.3390/molecules29153588
Chicago/Turabian StyleCustureri, Irene Maria Grazia, Monica Rosa Loizzo, Vincenzo Sicari, Roberta Pino, Rosa Tundis, Ana Cristina Soria, and Angelo Maria Giuffrè. 2024. "Evaluation of the Shelf Life of Myristica-fragrans Powder-Flavored Oils Obtained through the Application of Two Processes: Infusion and Co-Pressing Technology" Molecules 29, no. 15: 3588. https://doi.org/10.3390/molecules29153588






