Advancing Metallic Lithium Anodes: A Review of Interface Design, Electrolyte Innovation, and Performance Enhancement Strategies
Abstract
1. Introduction
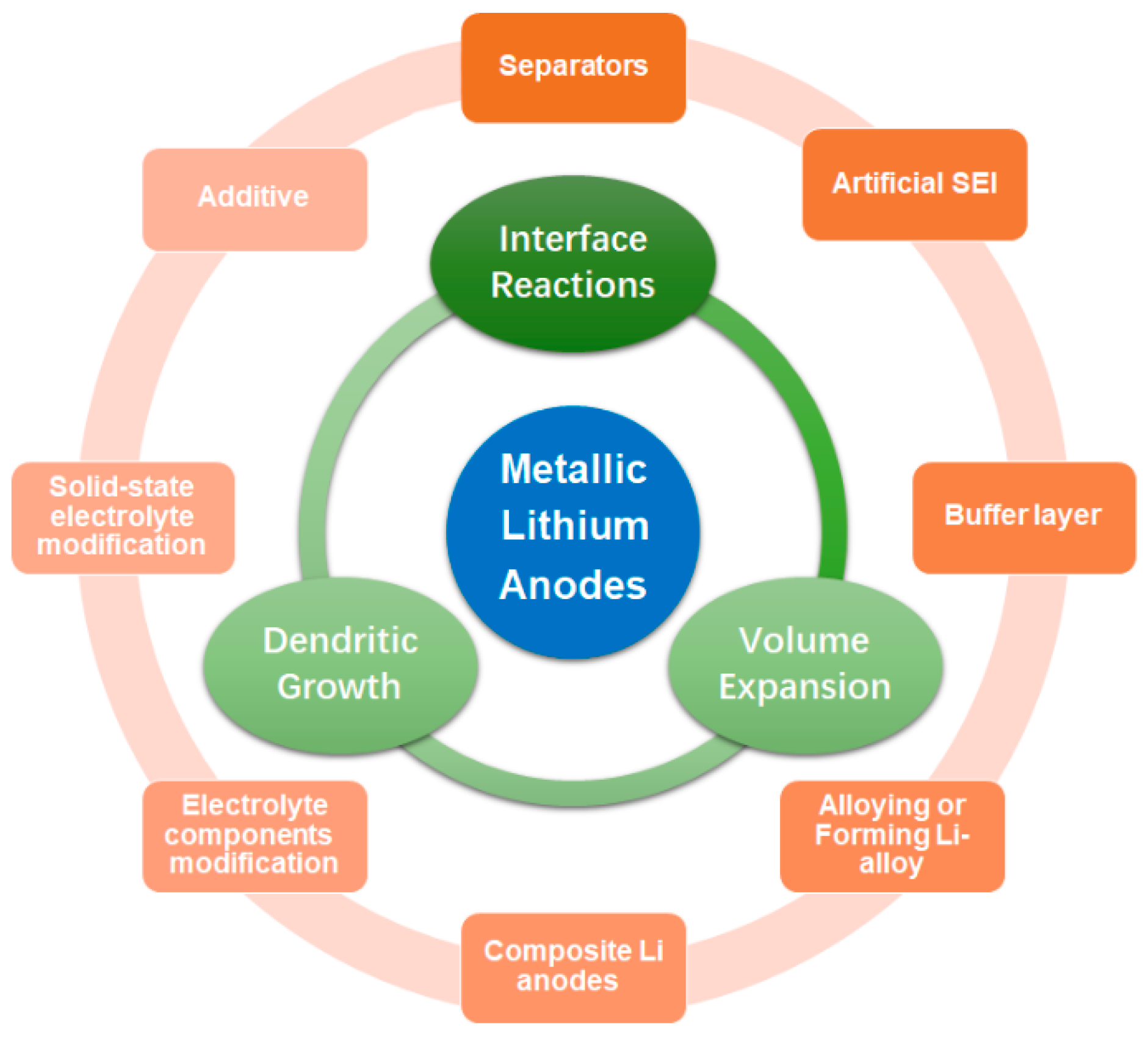
2. Strategies for Designing Advanced Li Metal Anode
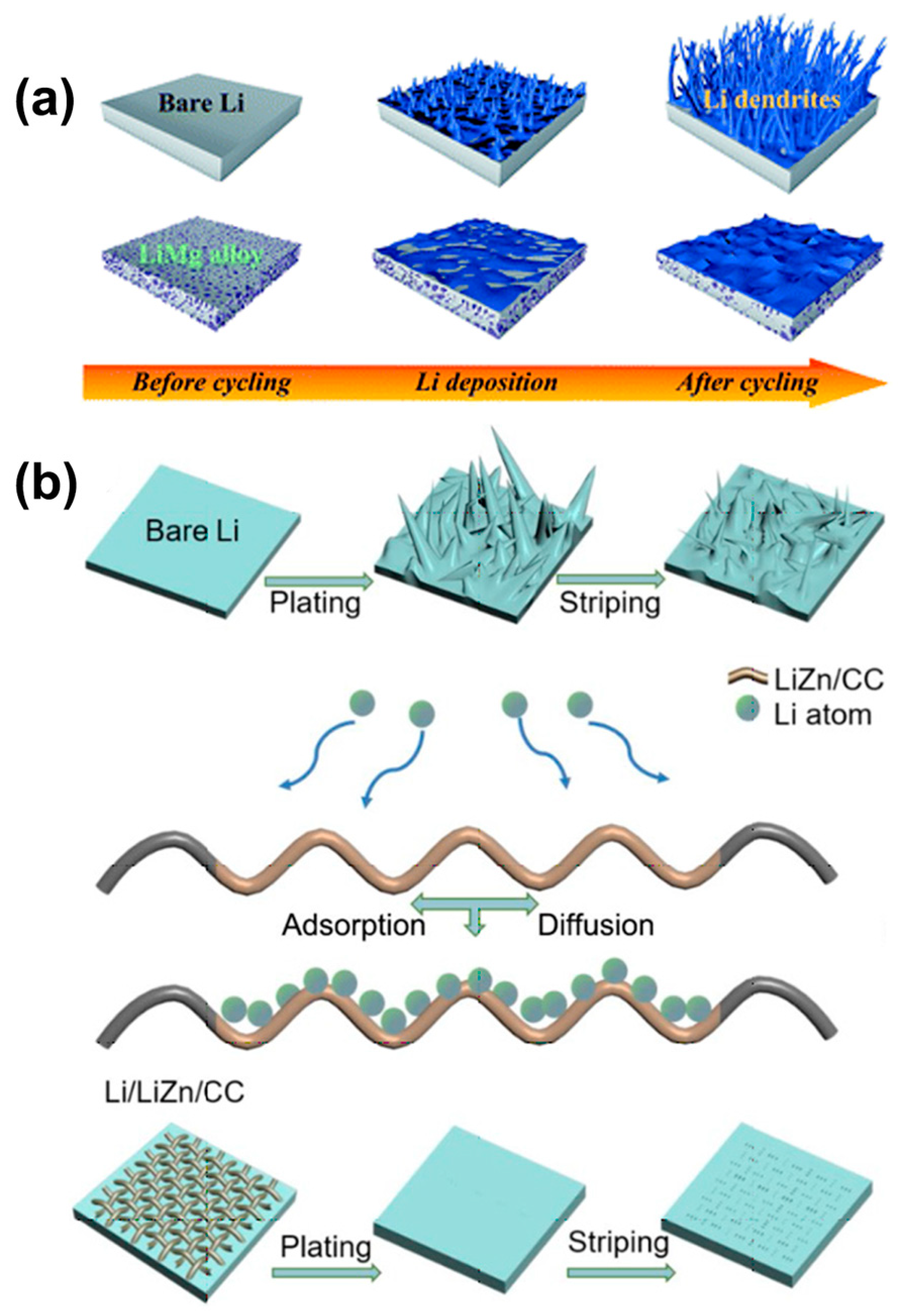
2.1. Alloying or Forming Li-Alloy
2.2. Composite Li Anodes
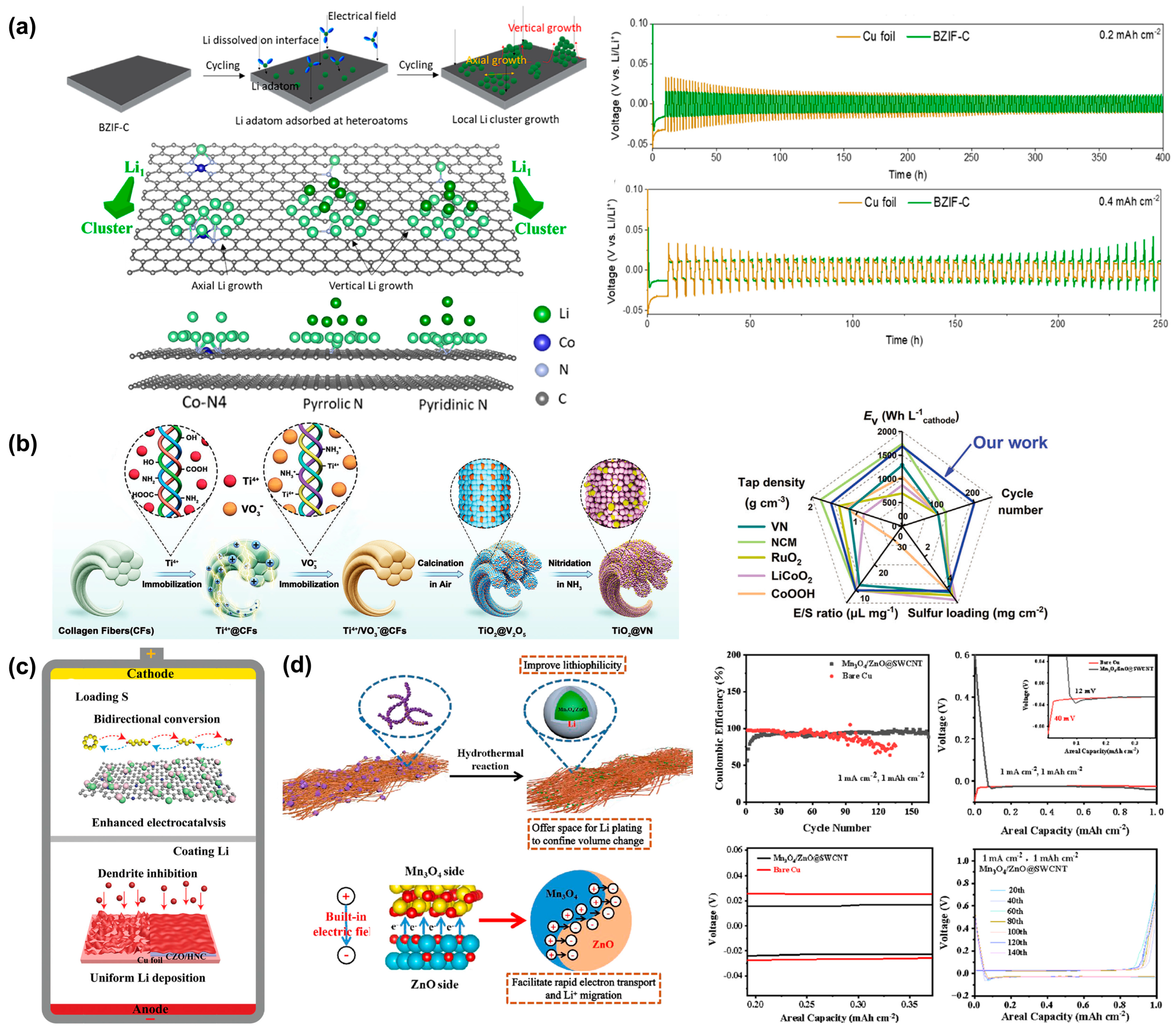
| Modification | Current Density (mA cm−2) | Cycle Life (h) | Number of Cycles | Sulfur Loading (mg cm−2) | Lean Electrolyte (μL mg−1) | Ref. |
|---|---|---|---|---|---|---|
| 3D Ti3C2Tx@Cu current collector | 1 | 950 | [42] | |||
| 3D NMV | 10 | 830 | [43] | |||
| Li@/Ag||LiCoO2 | 10 | 90 | 150 | [45] | ||
| Li/ZIF-8@RGO | 600 | 350 | [46] | |||
| 3DIO FCSe-QDs@NC | 2000 | 8.5 | 4.1 | [48] | ||
| ZnSe-CoSe2@NC | 1000 | 6.08 | 4.1 | [49] | ||
| 3D CNT with MnOx coating | 2000 | [50] | ||||
| MOF-derived carbon heterostructure (EPD) | 900 | [51] | ||||
| Li@CZO/HNC | 400 | 1400 | [53] | |||
| Mn3O4/ZnO @SWCNT-Li | 1 | 2500 | [54] |
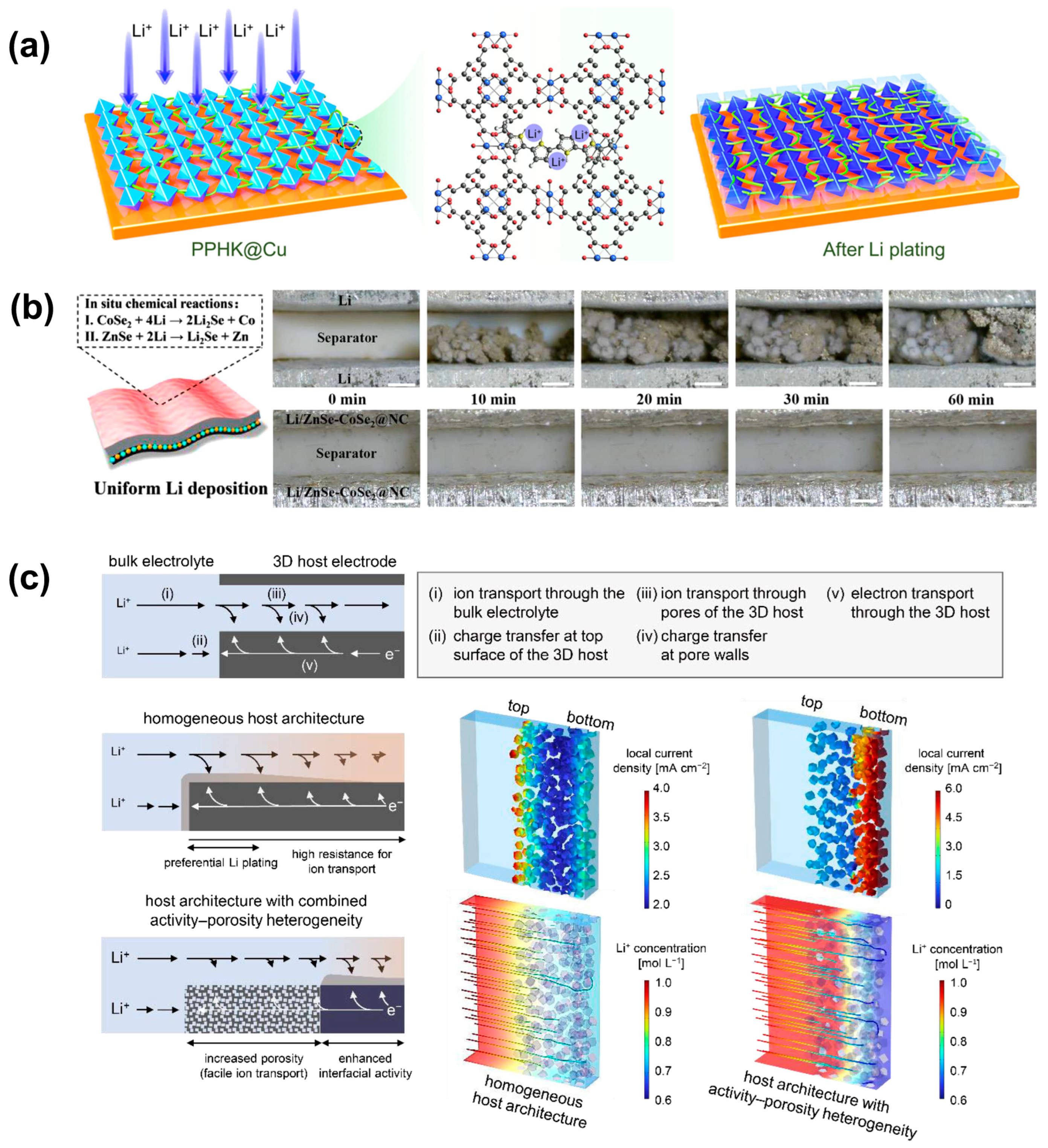
3. Electrolyte Modification
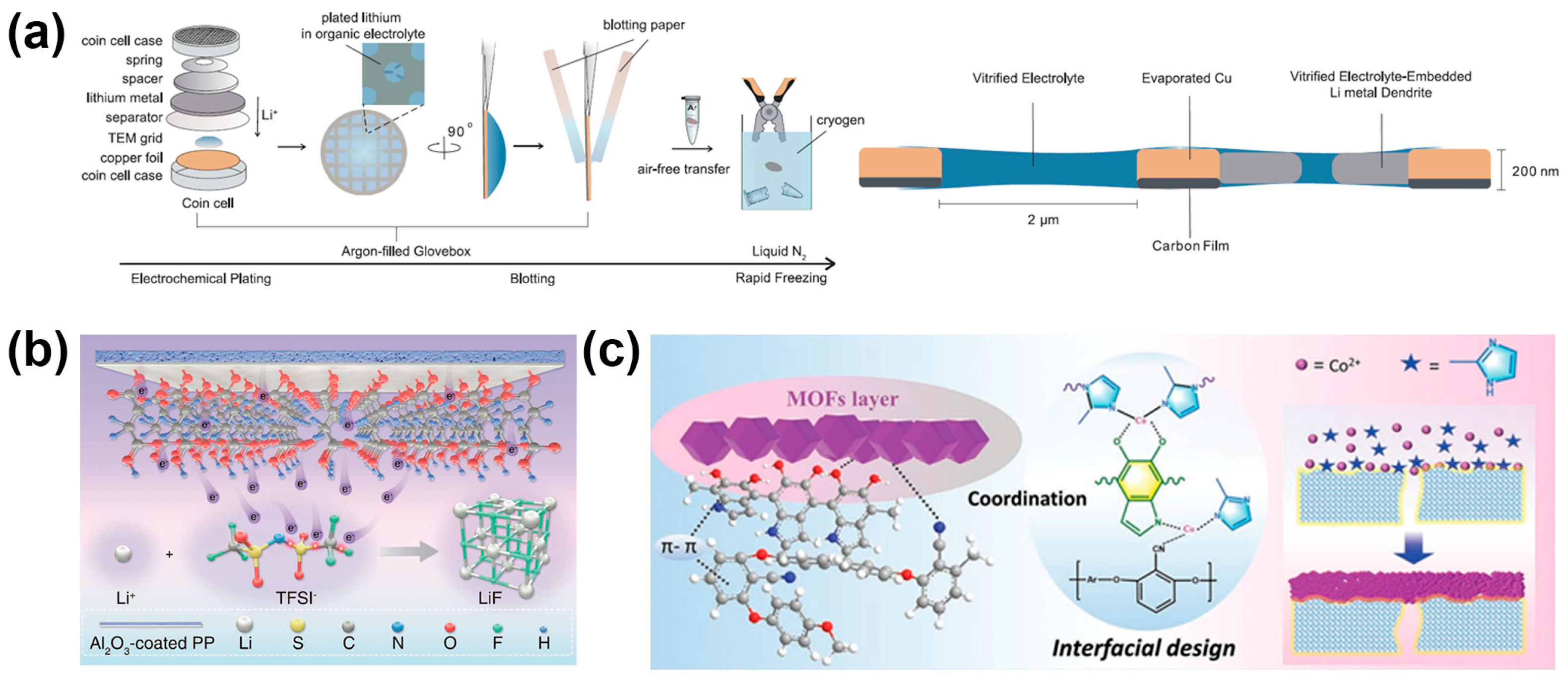
3.1. Liquid Electrolyte
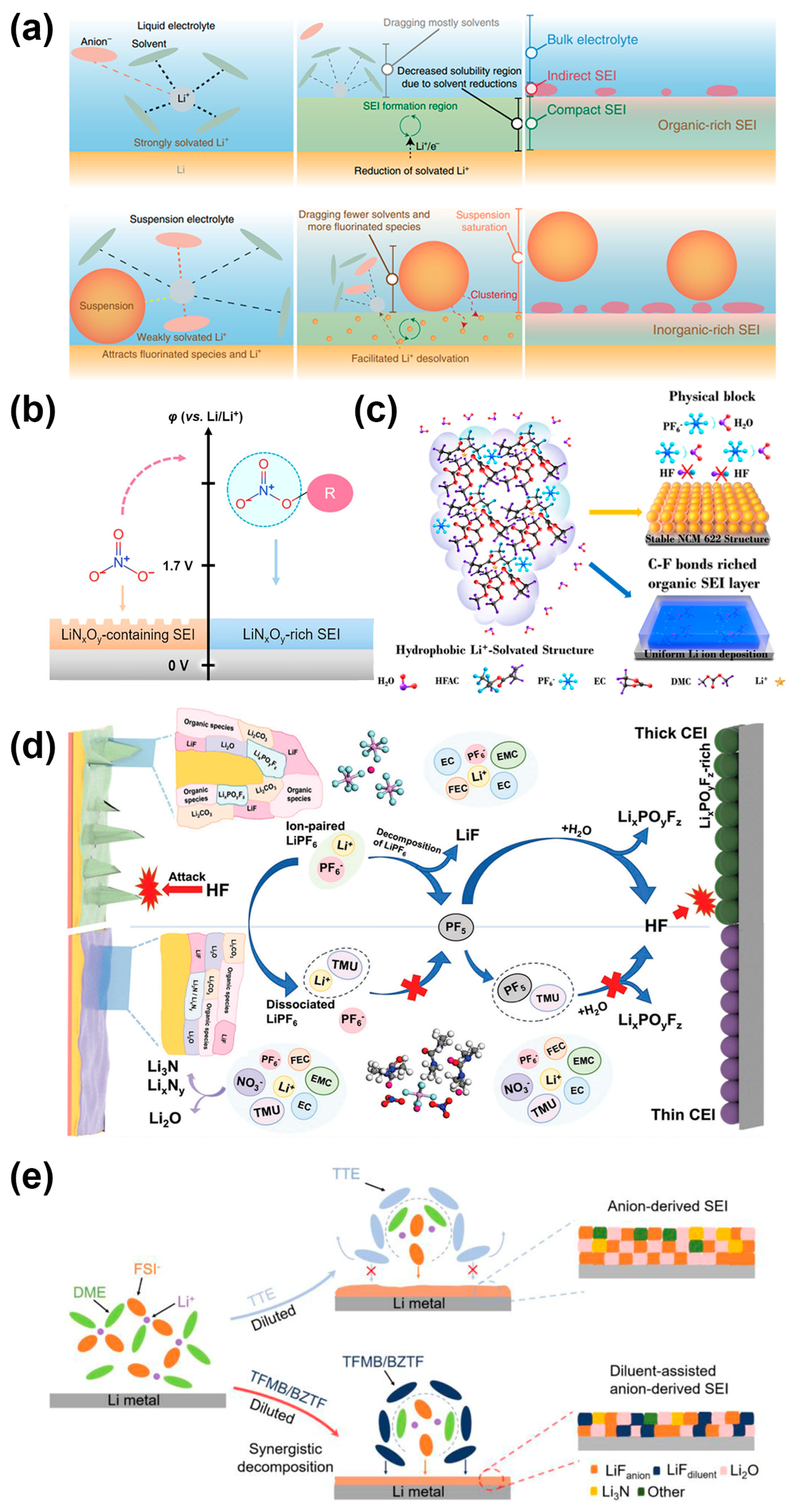
3.2. Solid-State Electrolyte
4. Interface Design Modification
4.1. Separators
4.2. Artificial SEI
4.3. Buffer Layer

5. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Liu, W.; Placke, T.; Chau, K.T. Overview of batteries and battery management for electric vehicles. Energy Rep. 2022, 8, 4058–4084. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, M.; Zou, P.; Sun, B.; Tao, S. Historical development and novel concepts on electrolytes for aqueous rechargeable batteries. Energy Environ. Sci. 2022, 15, 1805–1839. [Google Scholar] [CrossRef]
- Ruan, P.; Liang, S.; Lu, B.; Fan, H.J.; Zhou, J. Design strategies for high-energy-density aqueous zinc batteries. Angew. Chem. Int. Ed. 2022, 61, e202200598. [Google Scholar] [CrossRef] [PubMed]
- Bates, A.M.; Preger, Y.; Torres-Castro, L.; Harrison, K.L.; Harris, S.J.; Hewson, J. Are solid-state batteries safer than lithium-ion batteries? Joule 2022, 6, 742–755. [Google Scholar] [CrossRef]
- Lombardo, T.; Duquesnoy, M.; El-Bouysidy, H.; Aren, F.; Gallo-Bueno, A.; Jorgensen, P.B.; Bhowmik, A.; Demortiere, A.; Ayerbe, E.; Alcaide, F.; et al. Artificial intelligence applied to battery research: Hype or reality? Chem. Rev. 2022, 122, 10899–10969. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Dou, Y.; Wu, Z.; Tian, Y.; Ye, K.-H.; Lin, Z.; Dou, S.X.; Zhang, S. Atomically thin materials for next-generation rechargeable batteries. Chem. Rev. 2022, 122, 957–999. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Alghamdi, N.; Peng, X.; Huang, Y.; Wang, B.; Wang, L.; Gentle, I.R.; Hickey, S.; Luo, B. Scientific issues of zinc-bromine flow batteries and mitigation strategies. Explor 2023, 3, 20220073. [Google Scholar] [CrossRef]
- Neumann, J.; Petranikova, M.; Meeus, M.; Gamarra, J.D.; Younesi, R.; Winter, M.; Nowak, S. Recycling of Lithium-Ion Batteries-Current State of the Art, Circular Economy, and Next Generation Recycling. Adv. Energy Mater. 2022, 12, 2102917. [Google Scholar] [CrossRef]
- Rohland, P.; Schroeter, E.; Nolte, O.; Newkome, G.R.; Hager, M.D.; Schubert, U.S. Redox-active polymers: The magic key towards energy storage—A polymer design guideline progress in polymer science. Prog. Polym. Sci. 2022, 125, 101474. [Google Scholar] [CrossRef]
- Editorial Office of Nature Energy. Battery revolution to evolution. Nat. Energy 2019, 4, 893. [Google Scholar] [CrossRef]
- Liu, H.; Sun, X.; Cheng, X.B.; Guo, C.; Yu, F.; Bao, W.Z.; Wang, T.; Li, J.F.; Zhang, Q. Working principles of lithium metal anode in pouch cells. Adv. Energy Mater. 2022, 12, 2202518. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, T.T.; Xiao, Y.B.; Wu, J.Y.; Guan, L.X.; Hou, L.F.; Du, H.Y.; Wei, H.; Liu, X.D.; Yang, C.K.; et al. Leap of Li metal anodes from coin cells to pouch cells: Challenges and progress. Electrochem. Energy Rev. 2023, 6, 22. [Google Scholar] [CrossRef]
- Liang, H.M.; Wang, L.; Sheng, L.; Xu, H.; Song, Y.Z.; He, X.M. Focus on the electroplating chemistry of li ions in nonaqueous liquid electrolytes: Toward stable lithium metal batteries. Electrochem. Energy Rev. 2022, 5, 23. [Google Scholar] [CrossRef]
- Deng, R.; Wang, M.; Yu, H.; Luo, S.; Li, J.; Chu, F.; Liu, B.; Wu, F. Recent advances and applications toward emerging lithium-sulfur batteries: Working principles and opportunities. Energy Environ. Mater. 2022, 5, 777–799. [Google Scholar] [CrossRef]
- Liang, Z.; Shen, J.; Xu, X.; Li, F.; Liu, J.; Yuan, B.; Yu, Y.; Zhu, M. Advances in the development of single-atom catalysts for high-energy-density lithium-sulfur batteries. Adv. Mater. 2022, 34, 2200102. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liu, H.; Wang, G.; Wang, C.; Ni, Y.; Nan, C.-W.; Fan, L.-Z. Challenges, interface engineering, and processing strategies toward practical sulfide-based all-solid-state lithium batteries. InfoMat 2022, 4, e12292. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, C.-Z.; Huang, J.-Q.; Zhang, Q. The timescale identification decoupling complicated kinetic processes in lithium batteries. Joule 2022, 6, 1172–1198. [Google Scholar] [CrossRef]
- Zhang, R.; Shen, X.; Zhang, Y.-T.; Zhong, X.-L.; Ju, H.-T.; Huang, T.-X.; Chen, X.; Zhang, J.-D.; Huang, J.-Q. Dead lithium formation in lithium metal batteries: A phase field model. J. Energy Chem. 2022, 71, 29–35. [Google Scholar] [CrossRef]
- Ko, C.J.; Chen, C.H.; Chen, K.C. Influence of inhomogeneity of lithium-ion transport within the anode/electrolyte interface on mossy lithium formation. J. Power Sources 2023, 563, 232779. [Google Scholar] [CrossRef]
- Chen, Y.L.; Yuan, X.P.; He, C.P.; Gou, Q.Y.; Yang, N.; Xie, G.; Zhang, K.Y.; Yao, Y.C.; Hou, Y.Q. Mechanistic exploration of dendrite growth and inhibition for lithium metal batteries. Energies 2023, 16, 3745. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Shen, W.; Li, K.; Lin, Y. Important role of atom diffusion in dendrite growth and the thermal self-healing mechanism. ACS Appl. Energy Mater. 2023, 6, 1933–1945. [Google Scholar] [CrossRef]
- Cao, X.L.; Lu, Y.J.; Song, X.; Yuan, Z.; Wang, F.H. Perspective of unstable solid electrolyte interphase induced lithium dendrite growth: Role of thermal effect. Electrochim. Acta 2023, 439, 141722. [Google Scholar] [CrossRef]
- Li, B.R.; Chao, Y.; Li, M.C.; Xiao, Y.B.; Li, R.; Yang, K.; Cui, X.C.; Xu, G.; Li, L.Y.; Yang, C.K.; et al. A review of solid electrolyte interphase (SEI) and dendrite formation in lithium batteries. Electrochem. Energy Rev. 2023, 6, 7. [Google Scholar] [CrossRef]
- Li, C.; Liang, Z.; Li, Z.; Cao, D.; Zuo, D.; Chang, J.; Wang, J.; Deng, Y.; Liu, K.; Kong, X.; et al. Self-Assembly monolayer inspired stable artificial solid electrolyte interphase design for next-generation lithium metal batteries. Nano Lett. 2023, 23, 4014–4022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, B.; Wang, S.; Yang, S.B. Simultaneous Formation of Artificial SEI Film and 3D Host for Stable Metallic Sodium Anodes. ACS Appl. Mater. Interfaces 2017, 9, 40265–40272. [Google Scholar] [CrossRef] [PubMed]
- Gross, S.J.; Hsieh, M.T.; Mumm, D.R.; Valdevit, L.; Mohraz, A. Alleviating expansion-induced mechanical degradation in lithium-ion battery silicon anodes via morphological design. Extrem. Mech. Lett. 2022, 54, 101746. [Google Scholar] [CrossRef]
- She, Z.; Uceda, M.; Pope, M.A. Encapsulating a responsive hydrogel core for void space modulation in high-stability graphene-wrapped silicon anodes. ACS Appl. Mater. Interfaces 2022, 14, 10363–10372. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.Q.; Li, B.; Li, S.M.; Du, Z.G.; Gong, Y.J.; Yang, S.B. Dendrite-free metallic lithium in lithiophilic carbonized metal-organic frameworks. Adv. Energy Mater. 2018, 8, 1703505. [Google Scholar] [CrossRef]
- Fan, H.L.; Dong, Q.Y.; Gao, C.H.; Hong, B.; Zhang, Z.A.; Zhang, K.; Lai, Y.Q. Encapsulating Metallic Lithium into Carbon Nanocages Which Enables a Low-Volume Effect and a Dendrite-Free Lithium Metal Anode. ACS Appl. Mater. Interfaces 2019, 11, 30902–30910. [Google Scholar] [CrossRef]
- Zhu, W.H.; Deng, W.; Zhao, F.; Liang, S.S.; Zhou, X.F.; Liu, Z.P. Graphene network nested Cu foam for reducing size of lithium metal towards stable metallic lithium anode. Energy Storage Mater. 2019, 21, 107–114. [Google Scholar] [CrossRef]
- Park, C.; Kim, J.; Lim, W.; Lee, J. Toward maximum energy density enabled by anode-free lithium metal batteries: Recent progress and perspective. Explor 2023, 4, 20210255. [Google Scholar] [CrossRef]
- Yang, J.Z.; Yin, B.S.; Sun, Y.; Pan, H.G.; Sun, W.P.; Jia, B.H.; Zhang, S.W.; Ma, T.Y. Zinc anode for mild aqueous zinc-ion batteries: Challenges, strategies, and perspectives. Nano-Micro Lett. 2022, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, N.; Qi, F.; Lu, X.; Liang, Y.; Sun, Z. Novel Ni-Ge-P anodes for lithium-ion batteries with enhanced reversibility and reduced redox potential. Inorg. Chem. Front. 2023, 10, 699–711. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Chen, L.; Yan, F.; Lin, Z.; Wang, J.; Qiu, J.; Cao, G.; Wang, B.; Zhang, H. Expanding the reversibility of graphite-Li metal hybrid anodes by interface and inner-structure modifications. Energy Storage Mater. 2022, 53, 621–628. [Google Scholar] [CrossRef]
- Chen, C.; Yang, Y.F.; Shao, H.X. Enhancement of the lithium cycling capability using Li-Zn alloy substrate for lithium metal batteries. Electrochim. Acta 2014, 137, 476–483. [Google Scholar] [CrossRef]
- Yanev, S.; Heubner, C.; Nikolowski, K.; Partsch, M.; Auer, H.; Michaelis, A. Editors’ Choice-alleviating the kinetic limitations of the Li-In alloy anode in all-solid-state batteries. J. Electrochem. Soc. 2024, 171, 020512. [Google Scholar] [CrossRef]
- Huang, Y.; Shao, B.; Han, F. Li alloy anodes for high-rate and high-areal-capacity solid-state batteries. J. Mater. Chem. A 2022, 10, 12350–12358. [Google Scholar] [CrossRef]
- Peng, K.; Chen, Z.; Zhao, X.; Shi, K.; Zhu, C.; Yan, X. Dual-Conductive Li alloy composite anode constructed by a synergetic Conversion-Alloying reaction with LiMgPO4. Chem. Eng. J. 2022, 439, 135705. [Google Scholar] [CrossRef]
- Guo, B.; Guo, P.; Zhao, G.; Liu, S.; Shi, J.; Huang, M.; Shi, Z.; Wang, H.; Yan, Z. A solid-solution-based Li-Mg alloy for highly stable lithium metal anodes. Sustain. Energy Fuels 2022, 6, 4137–4145. [Google Scholar] [CrossRef]
- Zheng, H.; Cheng, X.; Zheng, Q.; Zhang, J.; Li, T.; Xie, E.; Xu, Y. The thermodynamically directed dendrite-free lithium metal batteries on LiZn alloy surface. Nano Res. 2022, 16, 8354–8359. [Google Scholar] [CrossRef]
- Qutaish, H.; Chang, H.; Suh, J.H.; Han, S.A.; Yum, H.-Y.; Park, M.-S.; Moon, J.; Kim, J.H. Growth mechanism of lithium clusters on the surface of porous carbon framework for lithium metal batteries. ACS Mater. Lett. 2023, 5, 1593–1600. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Jin, Q.; Zhao, K.X.; Zhang, X.T.; Wu, L.L. Lithiophilic Ti3C2Tx-modified Cu foam by electrophoretic deposition for dendrite-free lithium metal anodes. Acs Appl. Energy Mater. 2022, 5, 2514–2521. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, X.; Hu, W.; Rao, Y.; Wu, Y.; Ju, S. Electrodeposited 3D lithiophilic Ni microvia host for long cycling Li metal anode at high current density. Electrochim. Acta 2023, 441, 141797. [Google Scholar] [CrossRef]
- Wang, C.H.; Li, Y.H.; Cao, F.; Zhang, Y.Q.; Xia, X.H.; Zhang, L.J. Employing Ni-embedded porous graphitic carbon fibers for high-efficiency lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2022, 14, 10457–10466. [Google Scholar] [CrossRef]
- Chen, W.; Li, S.; Wang, C.; Dou, H.; Zhang, X. Targeted deposition in a lithiophilic silver-modified 3D Cu host for lithium-metal anodes. Energy Environ. Mater. 2023, 6, e12412. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, R.L.; Liu, Z.F.; Wang, X.S.; Han, C.P.; Liu, H.B.; Li, B.H. A 3D lithiophilic ZIF-8@RGO free-standing scaffold with dendrite-free behavior enabling high-performance Li metal batteries. J. Mater. Chem. A 2023, 11, 12910–12917. [Google Scholar] [CrossRef]
- Ma, Y.; Wei, L.; He, Y.; Yuan, X.Z.; Su, Y.H.; Gu, Y.T.; Li, X.J.; Zhao, X.H.; Qin, Y.Z.; Mu, Q.Q.; et al. A “blockchain” synergy in conductive polymer-filled metal-organic frameworks for dendrite-free Li plating/stripping with high coulombic efficiency. Angew. Chem.-Int. Ed. 2022, 61, 10. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, L.; Zhang, Y.; Liu, L.; Sa, B.; Lin, J.; Wang, L.; Peng, D.-L.; Xie, Q. Dual-functional lithiophilic/sulfiphilic binary-metal selenide quantum dots toward high-performance Li-S full batteries. Nano-Micro Lett. 2023, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, L.L.; Zhang, Z.L.; Sun, B.; Jin, Y.; Jin, Q.Z.; Liu, H.; Wang, G.X. Heterostructure ZnSe-CoSe2 embedded with yolk-shell conductive dodecahedral as two-in-one hosts for cathode and anode protection of lithium-sulfur full batteries. Energy Storage Mater. 2022, 47, 223–234. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, K.; Xu, Y.; Zhang, X.; Peng, Q.; Ma, Y.; Li, S.; Zhang, X.; Sun, X.; Ma, Y. A 10-μm ultrathin lithium metal composite anodes with superior electrochemical kinetics and cycling stability. Energy Environ. Mater. 2023, 6, e12598. [Google Scholar] [CrossRef]
- Shin, H.R.; Kim, S.; Park, J.; Kim, J.H.; Park, M.S.; Lee, W. Electrode-level strategies enabling kinetics-controlled metallic Li confinement by the heterogeneity of interfacial activity and porosity. Energy Storage Mater. 2023, 56, 515–523. [Google Scholar] [CrossRef]
- Wang, H.; Wei, Y.; Wang, G.; Pu, Y.; Yuan, L.; Liu, C.; Wang, Q.; Zhang, Y.; Wu, H. Selective nitridation crafted a high-density, carbon-free heterostructure host with built-in electric field for enhanced energy density Li-S batteries. Adv. Sci. 2022, 9, 2201823. [Google Scholar] [CrossRef]
- Wang, B.; Ren, Y.; Zhu, Y.; Chen, S.; Chang, S.; Zhoua, X.; Wanga, P.; Sun, H.; Menga, X.; Tanga, S. Construction of Co3O4/ZnO heterojunctions in hollow n-doped carbon nanocages as microreactors for lithium-sulfur full batteries. Adv. Sci. 2023, 10, 2300860. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Y.; Zhang, H.; Liu, S.; Wu, Z.; Wu, C.; Gao, X.; Hu, E.; Chen, Z. A Highly-Lithiophilic Mn3O4/ZnO-Modified Carbon Nanotube Film for Dendrite-Free Lithium Metal Anodes. J. Colloid Interface Sci. 2023, 648, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.S.; Yu, Z.; Kong, X.; Kim, S.C.; Boyle, D.T.; Qin, J.; Bao, Z.N.; Cui, Y. Liquid electrolyte: The nexus of practical lithium metal batteries. Joule 2022, 6, 588–616. [Google Scholar] [CrossRef]
- Kim, M.S.; Zhang, Z.W.; Rudnicki, P.E.; Yu, Z.A.; Wang, J.Y.; Wang, H.S.; Oyakhire, S.T.; Chen, Y.L.; Kim, S.C.; Zhang, W.B.; et al. Suspension electrolyte with modified Li+ solvation environment for lithium metal batteries. Nat. Mater. 2022, 21, 445. [Google Scholar] [CrossRef]
- Hou, L.P.; Yao, N.; Xie, J.; Shi, P.; Sun, S.Y.; Jin, C.B.; Chen, C.M.; Liu, Q.B.; Li, B.Q.; Zhang, X.Q.; et al. Modification of nitrate ion enables stable solid electrolyte interphase in lithium metal batteries. Angew. Chem.-Int. Ed. 2022, 61, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Utomo, N.W.; Kocen, A.L.; Jin, S.; Deng, Y.; Zhu, V.X.; Moganty, S.; Coates, G.W.; Archer, L.A. Upgrading carbonate electrolytes for ultra-stable practical lithium metal batteries. Angew. Chem.-Int. Ed. 2022, 61, 8. [Google Scholar] [CrossRef]
- Pal, U.; Rakov, D.; Lu, B.Y.; Sayahpour, B.; Chen, F.F.; Roy, B.; MacFarlane, D.R.; Armand, M.; Howlett, P.C.; Meng, Y.S.; et al. Interphase control for high performance lithium metal batteries using ether aided ionic liquid electrolyte. Energy Environ. Sci. 2022, 15, 1907–1919. [Google Scholar] [CrossRef]
- Sun, H.H.; Liu, J.D.; He, J.; Wang, H.P.; Jiang, G.X.; Qi, S.H.; Ma, J.M. Stabilizing the cycling stability of rechargeable lithium metal batteries with tris(hexafluoroisopropyl)phosphate additive. Sci. Bull. 2022, 67, 725–732. [Google Scholar] [CrossRef]
- Li, F.; Liu, J.D.; He, J.; Hou, Y.Y.; Wang, H.P.; Wu, D.X.; Huang, J.D.; Ma, J.M. Additive-assisted hydrophobic Li+-solvated structure for stabilizing dual electrode electrolyte interphases through suppressing LiPF6 hydrolysis. Angew. Chem.-Int. Ed. 2022, 61, 6. [Google Scholar] [CrossRef]
- Piao, Z.H.; Xiao, P.T.; Luo, R.P.; Ma, J.B.; Gao, R.H.; Li, C.; Tan, J.Y.; Yu, K.; Zhou, G.M.; Cheng, H.M. Constructing a stable interface layer by tailoring solvation chemistry in carbonate electrolytes for high-performance lithium-metal batteries. Adv. Mater. 2022, 34, 10. [Google Scholar] [CrossRef]
- Zhu, C.N.; Sun, C.C.; Li, R.H.; Weng, S.T.; Fan, L.W.; Wang, X.F.; Chen, L.X.; Noked, M.; Fan, X.L. Anion-diluent pairing for stable high-energy Li metal batteries. ACS Energy Lett. 2022, 7, 1338–1347. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Zhang, G.Q.; Zou, J.C.; Dai, H.C.; Wang, C.L. Natural pyranosyl materials: Potential applications in solid-state batteries. Chemsuschem 2023, 16, e202202216. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Kim, J.T.; Wang, C.S.; Sun, X.L. Progress and prospects of inorganic solid-state electrolyte-based all-solid-state pouch cells. Adv. Mater. 2023, 35, 2209074. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.; Zhang, W.Q.; Wang, T.Y.; Han, J.X.; Sun, C.W. A high-performance lithium metal battery with a multilayer hybrid electrolyte. Energy Environ. Mater. 2023, 6, 8. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Lu, M.N.; Li, Q.; Shi, F.N. Hybrid lithium salts regulated solid polymer electrolyte for high-temperature lithium metal battery. J. Solid State Chem. 2022, 310, 8. [Google Scholar] [CrossRef]
- Ma, Y.X.; Wan, J.Y.; Yang, Y.F.; Ye, Y.S.; Xiao, X.; Boyle, D.T.; Burke, W.; Huang, Z.J.; Chen, H.; Cui, Y.; et al. Scalable, ultrathin, and high-temperature-resistant solid polymer electrolytes for energy-dense lithium metal batteries. Adv. Energy Mater. 2022, 12, 9. [Google Scholar] [CrossRef]
- Kang, Q.; Li, Y.; Zhuang, Z.C.; Wang, D.S.; Zhi, C.Y.; Jiang, P.K.; Huang, X.Y. Dielectric polymer based electrolytes for high-performance all-solid-state lithium metal batteries. J. Energy Chem. 2022, 69, 194–204. [Google Scholar] [CrossRef]
- Peng, J.; Wu, D.X.; Song, F.M.; Wang, S.; Niu, Q.H.; Xu, J.R.; Lu, P.S.; Li, H.; Chen, L.Q.; Wu, F. High current density and long cycle life enabled by sulfide solid electrolyte and dendrite-free liquid lithium anode. Adv. Funct. Mater. 2022, 32, 14. [Google Scholar] [CrossRef]
- Zeng, D.W.; Yao, J.M.; Zhang, L.; Xu, R.N.; Wang, S.J.; Yan, X.L.; Yu, C.; Wang, L. Promoting favorable interfacial properties in lithium-based batteries using chlorine-rich sulfide inorganic solid-state electrolytes. Nat. Commun. 2022, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.C.; Hutter, T.; Boyce, B.L.; Watt, J.; Liu, P.C.; Mitlin, D. Review of multifunctional separators: Stabilizing the cathode and the anode for alkali (Li, Na, and K) metal-sulfur and selenium batteries. Chem. Rev. 2022, 122, 8053–8125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Li, Y.Z.; Xu, R.; Zhou, W.J.; Li, Y.B.; Oyakhire, S.T.; Wu, Y.C.; Xu, J.W.; Wang, H.S.; Yu, Z.A.; et al. Capturing the swelling of solid-electrolyte interphase in lithium metal batteries. Science 2022, 375, 66. [Google Scholar] [CrossRef]
- Liu, Y.J.; Tao, X.Y.; Wang, Y.; Jiang, C.; Ma, C.; Sheng, O.W.; Lu, G.X.; Lou, X.W. Self-assembled monolayers direct a LiF-rich interphase toward long-life lithium metal batteries. Science 2022, 375, 739. [Google Scholar] [CrossRef]
- Lin, G.; Jia, K.; Bai, Z.; Liu, C.; Liu, S.; Huang, Y.; Liu, X. Metal-organic framework sandwiching porous super-engineering polymeric membranes as anionphilic separators for dendrite-free lithium metal batteries. Adv. Funct. Mater. 2022, 32, 2207969. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, S.Y.; Liang, Z.W.; Wen, Y.C.; Liu, Z.B.; Wu, Y.W.; Liu, J.; Zhu, M. A self-supporting covalent organic framework separator with desolvation effect for high energy density lithium metal batteries. ACS Energy Lett. 2022, 7, 885–896. [Google Scholar] [CrossRef]
- Wang, X.; Meng, L.; Liu, X.; Deng, N.; Yan, Z.; Wang, G.; Wei, L.; Zhang, L.; Cheng, B.; Kang, W. MOF-derived MoP nanorods decorated with a N-doped thin carbon layer as a robust lithiophilic and sulfiphilic nanoreactor for high-performance Li-S batteries. Sustain. Energy Fuels 2022, 6, 3989–4000. [Google Scholar] [CrossRef]
- He, Y.; Wang, L.; Wang, A.; Zhang, B.; Pham, H.; Park, J.; He, X. Insight into uniform filming of LiF-rich interphase via synergistic adsorption for high-performance lithium metal anode. Explor 2023, 4, 20230114. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.J.; Lu, G.X.; Sheng, O.W.; Yuan, H.D.; Zhou, S.Q.; Liu, T.F.; Liu, Y.J.; Wang, Y.; Nai, J.W.; Zhang, W.K.; et al. Soybean protein fiber enabled controllable Li deposition and a Lif-nanocrystal-enriched interface for stable Li metal batteries. Nano Lett. 2022, 22, 1374–1381. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Xu, X.Y.; Kapitanova, O.O.; Evdokimov, P.V.; Song, Z.X.; Matic, A.; Xiong, S.Z. Electro-chemo-mechanical modeling of artificial solid electrolyte interphase to enable uniform electrodeposition of lithium metal anodes. Adv. Energy Mater. 2022, 12, 8. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, S.W.; Wang, D.; Lei, C.; Zhao, Y. Phase field modeling of lithium deposition in porous lithium metal anodes. J. Energy Storage 2023, 73, 109279. [Google Scholar] [CrossRef]
- Cao, J.Q.; Xie, Y.H.; Yang, Y.; Wang, X.H.; Li, W.Y.; Zhang, Q.L.; Ma, S.; Cheng, S.Y.; Lu, B.A. Achieving uniform Li plating/stripping at ultrahigh currents and capacities by optimizing 3D nucleation sites and Li2Se-Enriched SEI. Adv. Sci. 2022, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.C.; Liu, Z.B.; Wu, Y.W.; Ji, S.M.; Yuan, Z.X.; Liu, J.; Zhu, M. In situ construction a stable protective layer in polymer electrolyte for ultralong lifespan solid-state lithium metal batteries. Adv. Sci. 2022, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, J.; Cui, Y.; Liu, S.; Chen, Z.; Huang, W.; Li, C.; Liu, R.; Fu, R.; Wu, D. A robust all-organic protective layer towards ultrahigh-rate and large-capacity li metal anodes. Nat. Nanotechnol. 2022, 17, 613. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Li, Y.; Wei, Y.; Yang, J.; Hu, P.; Rao, Z.; Chen, X.; Yuan, L.; Li, Z. Construct an ultrathin bismuth buffer for stable solid-state lithium metal batteries. ACS Appl. Mater. Interfaces 2020, 12, 12793–12800. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Shi, S.; Li, B.; Li, S.; Zhang, H.; Chen, S.; Deng, H.; Zhang, Q.; Zhu, J.; Duan, X. Lithiophilic interphase porous buffer layer toward uniform nucleation in lithium metal anodes. Adv. Funct. Mater. 2022, 32, 2206388. [Google Scholar] [CrossRef]
- Zhang, Z.; Guan, S.; Liu, S.; Hu, B.; Xue, C.; Wu, X.; Wen, K.; Nan, C.-W.; Li, L. A valence gradient protective layer for dendrite-free and highly stable lithium metal anodes. Adv. Energy Mater. 2022, 12, 2103332. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Jiang, K.; Fan, Y.; Zhao, L.; Cheng, Z.; Yu, P.; Peng, J.; Wan, M. Advancing Metallic Lithium Anodes: A Review of Interface Design, Electrolyte Innovation, and Performance Enhancement Strategies. Molecules 2024, 29, 3624. https://doi.org/10.3390/molecules29153624
Shi J, Jiang K, Fan Y, Zhao L, Cheng Z, Yu P, Peng J, Wan M. Advancing Metallic Lithium Anodes: A Review of Interface Design, Electrolyte Innovation, and Performance Enhancement Strategies. Molecules. 2024; 29(15):3624. https://doi.org/10.3390/molecules29153624
Chicago/Turabian StyleShi, Junwei, Kailin Jiang, Yameng Fan, Lingfei Zhao, Zhenxiang Cheng, Peng Yu, Jian Peng, and Min Wan. 2024. "Advancing Metallic Lithium Anodes: A Review of Interface Design, Electrolyte Innovation, and Performance Enhancement Strategies" Molecules 29, no. 15: 3624. https://doi.org/10.3390/molecules29153624
APA StyleShi, J., Jiang, K., Fan, Y., Zhao, L., Cheng, Z., Yu, P., Peng, J., & Wan, M. (2024). Advancing Metallic Lithium Anodes: A Review of Interface Design, Electrolyte Innovation, and Performance Enhancement Strategies. Molecules, 29(15), 3624. https://doi.org/10.3390/molecules29153624









