Constructing Three-Dimensional Architectures to Design Advanced Copper-Based Current Collector Materials for Alkali Metal Batteries: From Nanoscale to Microscale
Abstract
:1. Introduction
2. 3D Cu-Based CCs for LMBs
2.1. Structural Modification
2.1.1. Template Method
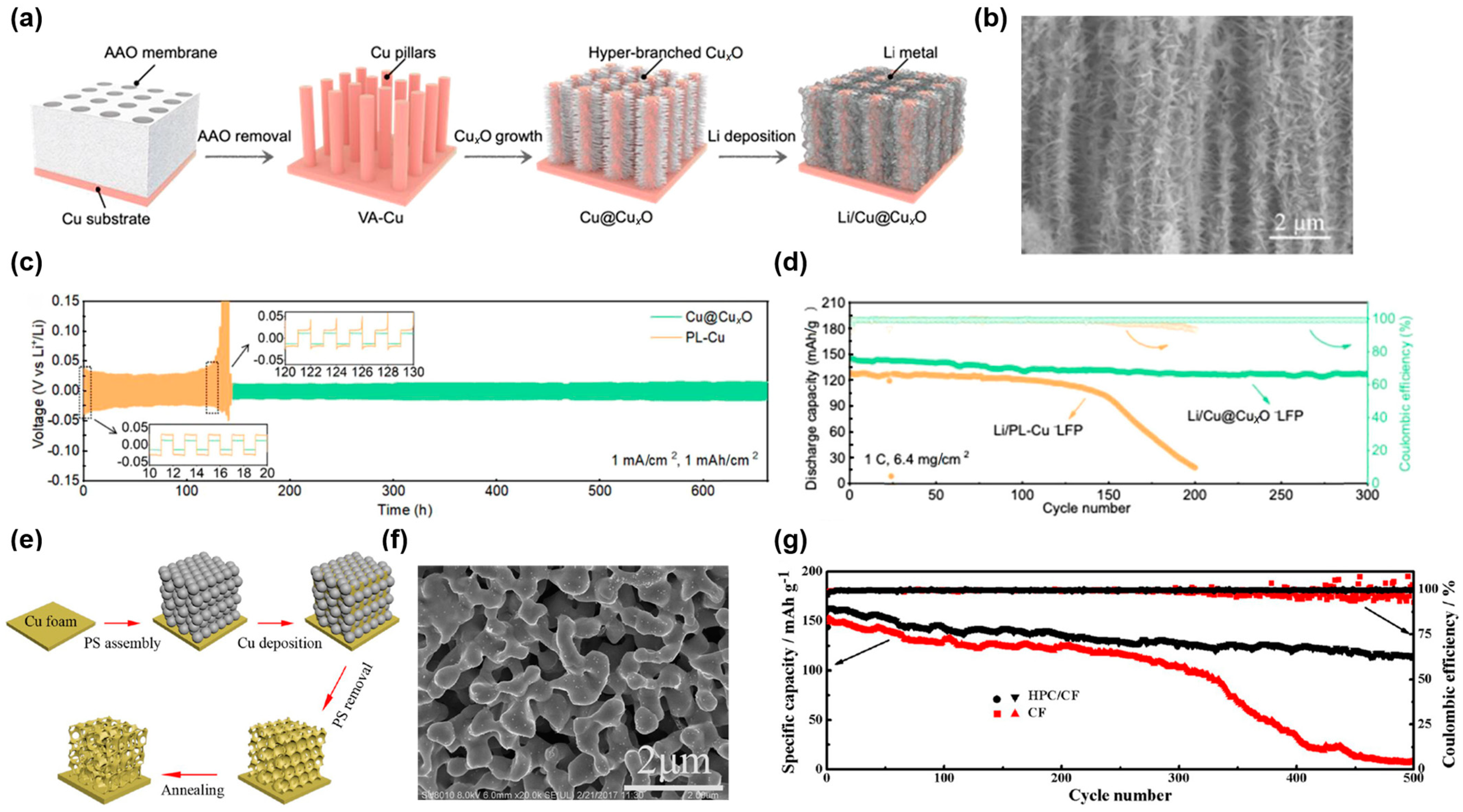
2.1.2. Dealloying Method
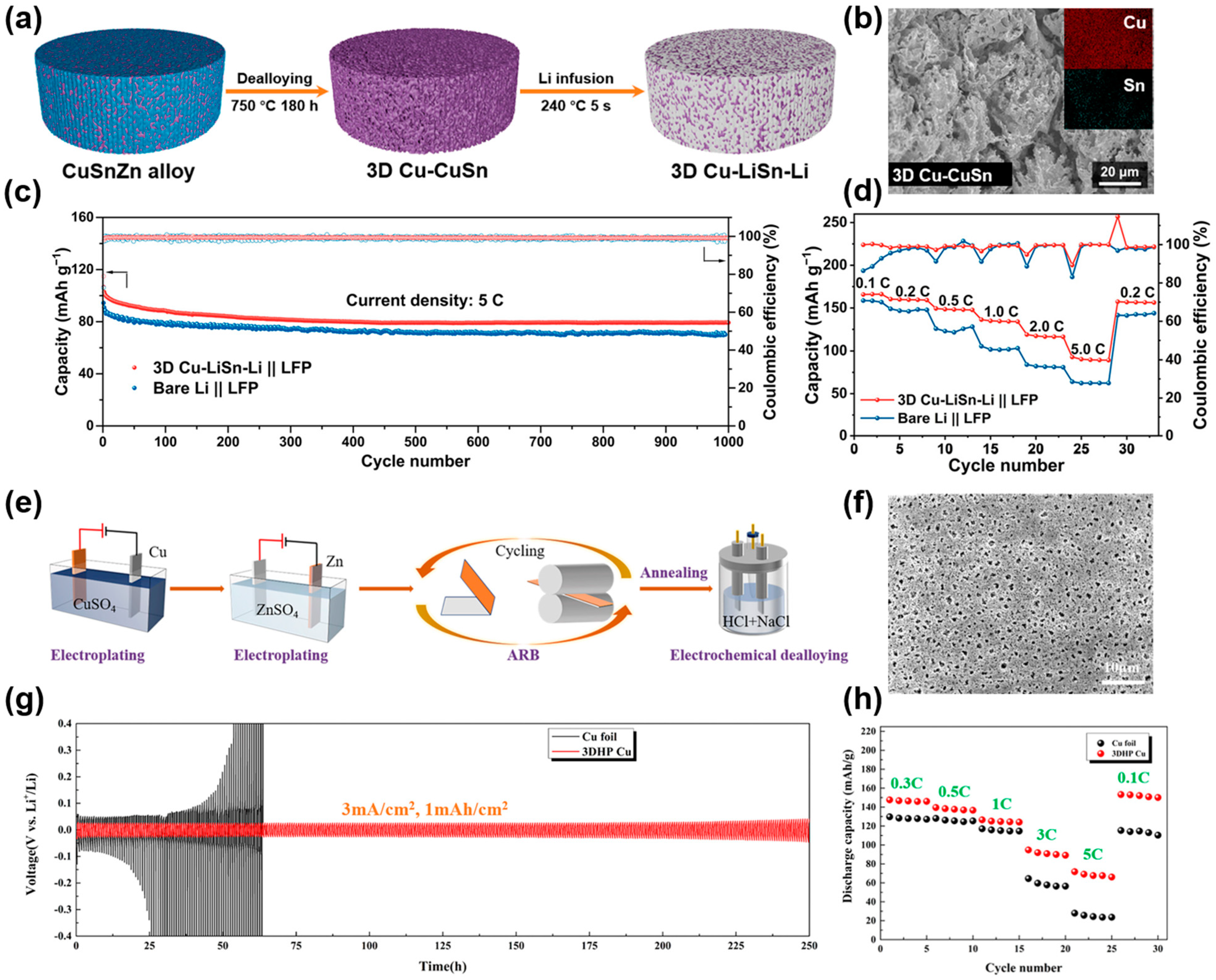
2.1.3. Electrodeposition
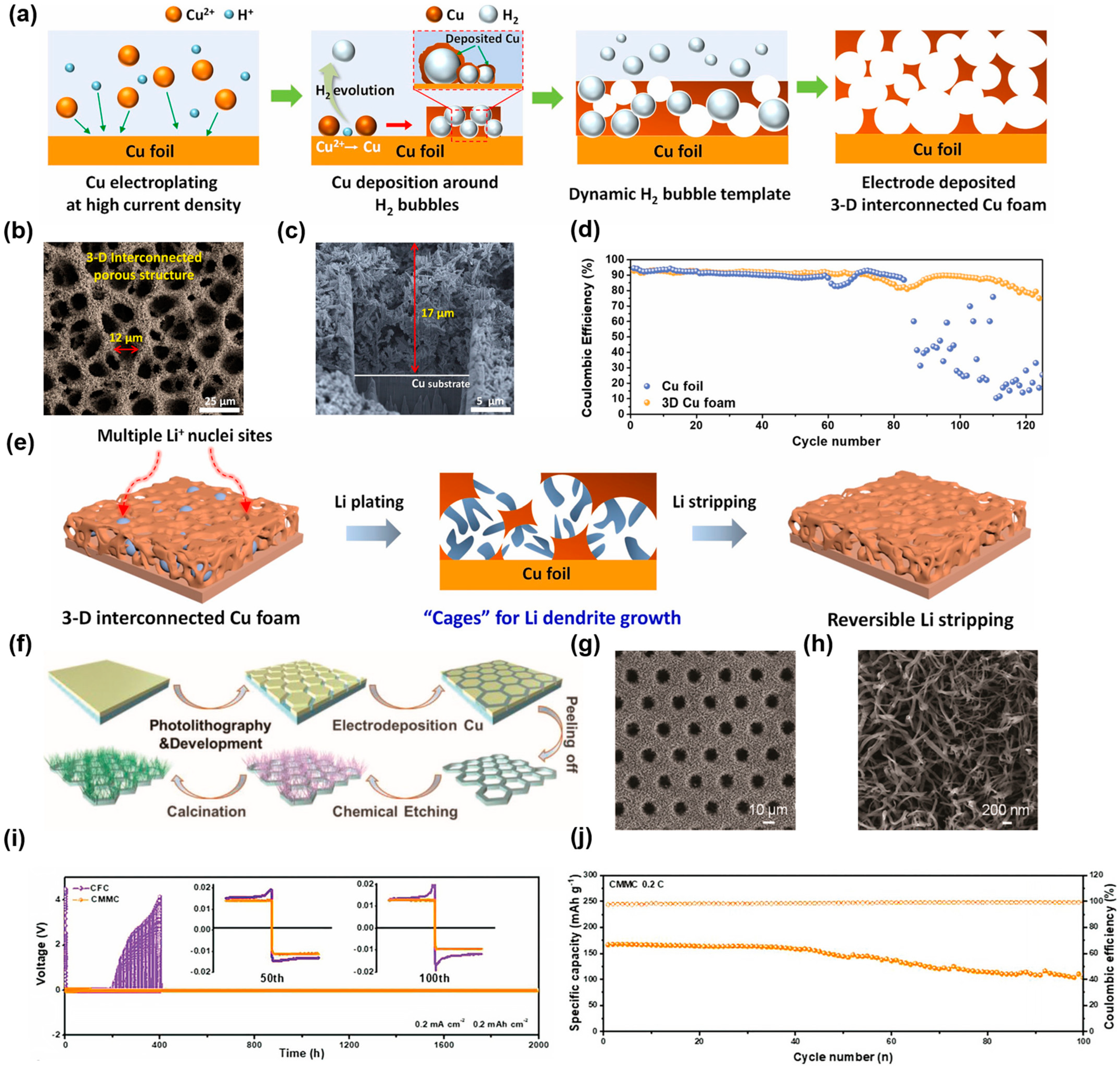
2.1.4. Others
2.2. Chemical Modification
2.2.1. Functional Spot Modification
2.2.2. Oxidation Modification
2.2.3. Protective Layer Modification
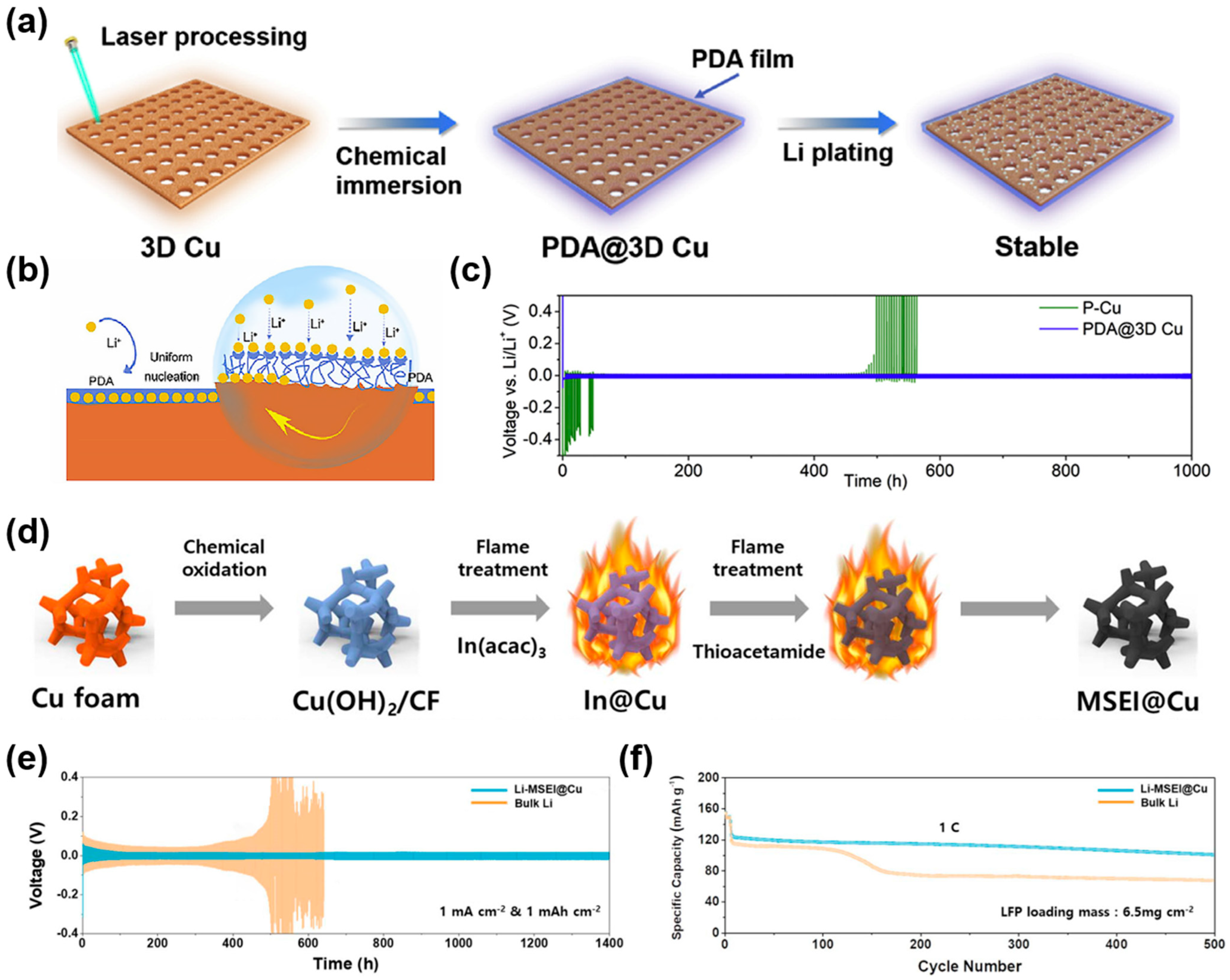
3. 3D Cu-Based CCs for SMBs and PMBs
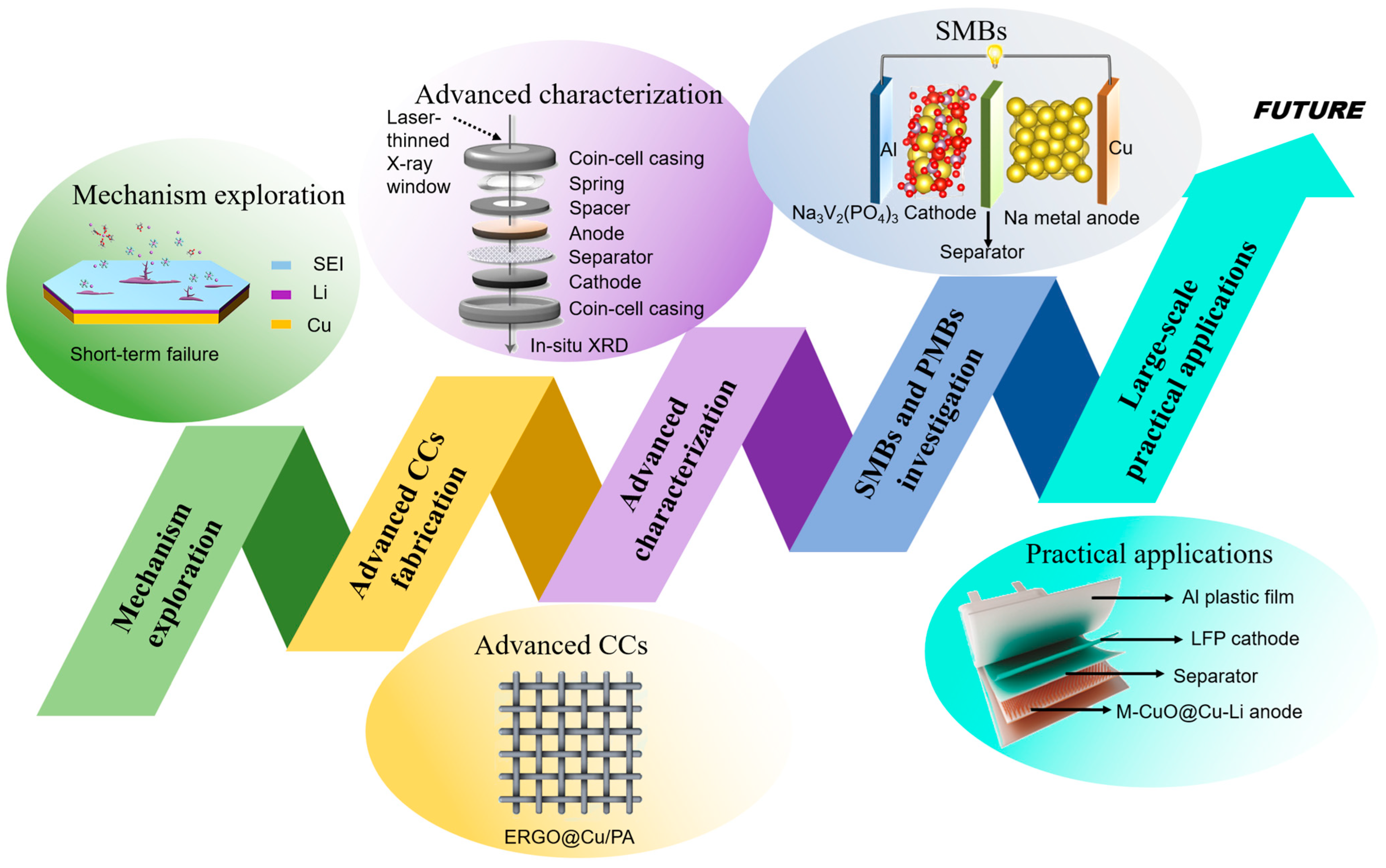
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Degen, F.; Winter, M.; Bendig, D.; Tuebke, J. Energy consumption of current and future production of lithium-ion and post lithium-ion battery cells. Nat. Energy 2023, 8, 1284–1295. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.-G.; Xie, Z.; Zhou, Z. Recent progress in rechargeable alkali metal-air batteries. Green Energy Environ. 2016, 1, 4–17. [Google Scholar] [CrossRef]
- Wang, A.; Hong, W.; Yang, L.; Tian, Y.; Qiu, X.; Zou, G.; Hou, H.; Ji, X. Bi-Based Electrode Materials for Alkali Metal-Ion Batteries. Small 2020, 16, 2004022. [Google Scholar] [CrossRef]
- Gao, Y.-M.; Liu, Y.; Feng, K.-J.; Ma, J.-Q.; Miao, Y.-J.; Xu, B.-R.; Pan, K.-M.; Akiyoshi, O.; Wang, G.-X.; Zhang, K.-K.; et al. Emerging WS2/WSe2@graphene nanocomposites: Synthesis and electrochemical energy storage applications. Rare Met. 2024, 43, 1–19. [Google Scholar] [CrossRef]
- Xu, B.-R.; Li, Q.-A.; Liu, Y.; Wang, G.-B.; Zhang, Z.-H.; Ren, F.-Z. Urea-induced interfacial engineering enabling highly reversible aqueous zinc-ion battery. Rare Met. 2024, 43, 1599–1609. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, K.; Han, J.; Wang, F.; Xing, Y.; Tao, F.; Li, H.; Xu, B.; Ji, J.; Li, H. Regulation of Zn2+ solvation shell by a novel N-methylacetamide based eutectic electrolyte toward high-performance zinc-ion batteries. J. Mater. Sci. Technol. 2025, 211, 53–61. [Google Scholar] [CrossRef]
- Liu, Y.; Tao, F.; Xing, Y.; Pei, Y.; Ren, F. Melamine Foam-Derived Carbon Scaffold for Dendrite-Free and Stable Zinc Metal Anode. Molecules 2023, 28, 1742. [Google Scholar] [CrossRef]
- Xu, B.; Wang, G.; Liu, Y.; Li, Q.; Ren, F.; Ma, J. Co-regulation effect of solvation and interface of pyridine derivative enabling highly reversible zinc anode. J. Mater. Sci. Technol. 2025, 204, 1–9. [Google Scholar] [CrossRef]
- Wu, N.; Zhao, Z.; Hua, R.; Wang, X.; Zhang, Y.; Li, J.; Liu, G.; Guo, D.; Sun, G.; Liu, X.; et al. Pre-Doping of Dual-Functional Sodium to Weaken Fe─S Bond and Stabilize Interfacial Chemistry for High-Rate Reversible Sodium Storage. Adv. Energy Mater. 2024, 28, 2400371. [Google Scholar] [CrossRef]
- Guo, Y.-D.; Zhao, E.-Q.; Zhao, X.-F.; Liu, S.-L. One dimensional CeO2 nanorods/poly(ethylene oxide) solid composite electrolyte for all-solid-state lithium-ion batteries. J. Rare Earths 2024, 42, 570–577. [Google Scholar] [CrossRef]
- Ahsan, Z.; Cai, Z.-F.; Wang, S.; Wang, H.-C.; Ma, Y.-Z.; Song, G.-S.; Zhang, S.-H.; Yang, W.-D.; Imran, M.; Wen, C. Enhanced stability and electrochemical properties of lanthanum and cerium co-modified LiVOPO4 cathode materials for Li-ion batteries. J. Rare Earths 2023, 41, 1590–1596. [Google Scholar] [CrossRef]
- Lu, L.-L.; Lu, Y.-Y.; Zhu, Z.-X.; Shao, J.-X.; Yao, H.-B.; Wang, S.; Zhang, T.-W.; Ni, Y.; Wang, X.-X.; Yu, S.-H. Extremely fast-charging lithium ion battery enabled by dual-gradient structure design. Sci. Adv. 2022, 8, eabm6624. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Zhao, Q.; Su, S.; Zhang, S.; Ma, J.; Shen, L.; Yu, Q.; Zhao, L.; Liu, Y.; Kang, F.; et al. Constructing Multifunctional Interphase between Li1.4Al0.4Ti1.6(PO4)3 and Li Metal by Magnetron Sputtering for Highly Stable Solid-State Lithium Metal Batteries. Adv. Energy Mater. 2019, 9, 1901604. [Google Scholar] [CrossRef]
- Sullivan, M.; Tang, P.; Meng, X. Atomic and Molecular Layer Deposition as Surface Engineering Techniques for Emerging Alkali Metal Rechargeable Batteries. Molecules 2022, 27, 6170. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Yang, L.; Yuan, L.; Yuan, K.; Zhang, Y.; Huang, Y.; Lin, J.; Pan, F.; Huang, Y. Alkali-Metal Anodes: From Lab to Market. Joule 2019, 3, 2334–2363. [Google Scholar] [CrossRef]
- Tang, Z.; Zhou, S.; Huang, Y.; Wang, H.; Zhang, R.; Wang, Q.; Sun, D.; Tang, Y.; Wang, H. Improving the Initial Coulombic Efficiency of Carbonaceous Materials for Li/Na-Ion Batteries: Origins, Solutions, and Perspectives. Electrochem. Energy Rev. 2023, 6, 8. [Google Scholar] [CrossRef]
- Song, J.; Wang, H.; Zuo, Y.; Zhang, K.; Yang, T.; Yang, Y.; Gao, C.; Chen, T.; Feng, G.; Jiang, Z.; et al. Building Better Full Manganese-Based Cathode Materials for Next-Generation Lithium-Ion Batteries. Electrochem. Energy Rev. 2023, 6, 20. [Google Scholar] [CrossRef]
- Xu, C.; Märker, K.; Lee, J.; Mahadevegowda, A.; Reeves, P.J.; Day, S.J.; Groh, M.F.; Emge, S.P.; Ducati, C.; Layla Mehdi, B.; et al. Bulk fatigue induced by surface reconstruction in layered Ni-rich cathodes for Li-ion batteries. Nat. Mater. 2021, 20, 84–92. [Google Scholar] [CrossRef]
- Yang, K.; Tian, R.-Z.; Wang, Z.-Y.; Zhang, H.-Z.; Ma, Y.; Shi, X.-X.; Song, D.-W.; Zhang, L.-Q.; Zhu, L.-Y. Regulating surface base of LiCoO2 to inhibit side reactions between LiCoO2 and sulfide electrolyte. Rare Met. 2023, 42, 4128–4141. [Google Scholar] [CrossRef]
- Chen, J.; Xu, X.; He, Q.; Ma, Y. Advanced Current Collectors for Alkali Metal Anodes. Chem. Res. Chin. Univ. 2020, 36, 386–401. [Google Scholar] [CrossRef]
- Liu, Z.; Ha, S.; Liu, Y.; Wang, F.; Tao, F.; Xu, B.; Yu, R.; Wang, G.; Ren, F.; Li, H. Application of Ag-based materials in high-performance lithium metal anode: A review. J. Mater. Sci. Technol. 2023, 133, 165–182. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Li, S.; Chen, H.; Qiao, X.; Zhao, J.; Ma, Y.; Alshareef, H.N. Porous Metal Current Collectors for Alkali Metal Batteries. Adv. Sci. 2022, 10, 2205695. [Google Scholar] [CrossRef]
- Wang, H.; Yu, D.; Kuang, C.; Cheng, L.; Li, W.; Feng, X.; Zhang, Z.; Zhang, X.; Zhang, Y. Alkali Metal Anodes for Rechargeable Batteries. Chem 2019, 5, 313–338. [Google Scholar] [CrossRef]
- Ma, C.; Xu, T.; Wang, Y. Advanced carbon nanostructures for future high performance sodium metal anodes. Energy Storage Mater. 2020, 25, 811–826. [Google Scholar] [CrossRef]
- Bao, W.; Wang, R.; Li, B.; Qian, C.; Zhang, Z.; Li, J.; Liu, F. Stable alkali metal anodes enabled by crystallographic optimization—A review. J. Mater. Chem. A 2021, 9, 20957–20984. [Google Scholar] [CrossRef]
- Ma, L.; Wu, J.; Zhu, G.; Lv, Y.; Zhang, Y.; Pang, H. Recent advances in two-dimensional materials for alkali metal anodes. J. Mater. Chem. A 2021, 9, 5232–5257. [Google Scholar] [CrossRef]
- Zhou, C.; Lu, K.; Zhou, S.; Liu, Y.; Fang, W.; Hou, Y.; Ye, J.; Fu, L.; Chen, Y.; Liu, L.; et al. Strategies toward anode stabilization in nonaqueous alkali metal-oxygen batteries. Chem. Commun. 2022, 58, 8014–8024. [Google Scholar] [CrossRef]
- Hu, L.; Deng, J.; Liang, Q.; Wu, J.; Ge, B.; Liu, Q.; Chen, G.; Yu, X. Engineering current collectors for advanced alkali metal anodes: A review and perspective. Ecomat 2023, 5, e12269. [Google Scholar] [CrossRef]
- Wang, G.; Song, C.; Huang, J.-Q.; Park, H.S. Recent Advances in Carbon-Based Current Collectors/Hosts for Alkali Metal Anodes. Energy Environ. Mater. 2023, 6, e12460. [Google Scholar] [CrossRef]
- Miao, Y.; Zheng, Y.; Tao, F.; Chen, Z.; Xiong, Y.; Ren, F.; Liu, Y. Synthesis and application of single-atom catalysts in sulfur cathode for high-performance lithium-sulfur batteries. Chin. Chem. Lett. 2023, 34, 107121. [Google Scholar] [CrossRef]
- Chu, Z.; Zhuang, S.; Lu, J.; Li, J.; Wang, C.; Wang, T. In-situ electro-polymerization of L -tyrosine enables ultrafast, long cycle life for lithium metal battery. Chin. Chem. Lett. 2023, 34, 107563. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Miao, Y.; Liu, G.; Yu, R.; Pan, K.; Wang, G.; Pang, X.; Ma, J. Emerging Carbon Nanotube-Based Nanomaterials for Stable and Dendrite-Free Alkali Metal Anodes: Challenges, Strategies, and Perspectives. Energy Environ. Mater. 2023, 6, e12525. [Google Scholar] [CrossRef]
- Jeong, H.; Jang, J.; Jo, C. A review on current collector coating methods for next-generation batteries. Chem. Eng. J. 2022, 446, 136860. [Google Scholar] [CrossRef]
- Jia, Z.; Liu, Y.; Li, H.; Xiong, Y.; Miao, Y.; Liu, Z.; Ren, F. In-situ polymerized PEO-based solid electrolytes contribute better Li metal batteries: Challenges, strategies, and perspectives. J. Energy Chem. 2024, 92, 548–571. [Google Scholar] [CrossRef]
- Wang, F.; Gao, J.; Liu, Y.; Ren, F. An amorphous ZnO and oxygen vacancy modified nitrogen-doped carbon skeleton with lithiophilicity and ionic conductivity for stable lithium metal anodes. J. Mater. Chem. A 2022, 10, 17395–17405. [Google Scholar] [CrossRef]
- Tan, L.; Chen, P.; Chen, Q.-Y.; Huang, X.; Zou, K.-Y.; Nie, Y.-M.; Li, L.-J. A Li3Bi/LiF interfacial layer enabling highly stable lithium metal anode. Rare Met. 2023, 42, 4081–4090. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Li, S.-Y.; Jia, X.-X.; Nie, B.; Sun, H.-T.; Wang, Y.-Y.; Zhu, J. Lithiophilic montmorillonite as a robust substrate toward high-stable lithium metal anodes. Rare Met. 2023, 42, 2157–2165. [Google Scholar] [CrossRef]
- Wang, H.; Wang, F.; Liu, Y.; Liu, Z.; Miao, Y.; Zhang, W.; Wang, G.; Ji, J.; Zhang, Q. Emerging natural clay-based materials for stable and dendrite-free lithium metal anodes: A review. Chin. Chem. Lett. 2024, 109589. [Google Scholar] [CrossRef]
- Su, H.; Zhang, H.; Chen, Z.; Li, M.; Zhao, J.; Xun, H.; Sun, J.; Xu, Y. Electrolyte and interphase engineering through solvation structure regulation for stable lithium metal batteries. Chin. Chem. Lett. 2023, 34, 108640. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, W.; Zhang, X.; Ke, Y.; Qiu, Z.; Luo, J.; Tang, Y.; Wang, C.; Yuan, Y.; Huang, Y. A review on structuralized current collectors for high-performance lithium-ion battery anodes. Appl. Energy 2020, 276, 115464. [Google Scholar] [CrossRef]
- Cui, Y.; Ye, Y. Porous current collector for fast-charging lithium-ion batteries. Nat. Energy 2024, 9, 639–640. [Google Scholar]
- Wang, Y.; Wang, Z.; Lei, D.; Lv, W.; Zhao, Q.; Ni, B.; Liu, Y.; Li, B.; Kang, F.; He, Y.-B. Spherical Li Deposited inside 3D Cu Skeleton as Anode with Ultrastable Performance. ACS Appl. Mater. Interfaces 2018, 10, 20244–20249. [Google Scholar] [CrossRef]
- Wei, C.; Yao, Z.; Ruan, J.; Song, Z.; Zhou, A.; Song, Y.; Wang, D.; Jiang, J.; Wang, X.; Li, J. Double-layered skeleton of Li alloy anchored on 3D metal foam enabling ultralong lifespan of Li anode under high rate. Chin. Chem. Lett. 2024, 35, 109330. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Z.; Zhou, Y.; Chen, Y.; Wen, S.; Wang, F.; Liu, Y. Stannic oxide quantum dots constructed evenly alloyable layer stabilizing lithium metal batteries. J. Alloys Compd. 2023, 955, 170230. [Google Scholar] [CrossRef]
- An, Y.L.; Fei, H.F.; Zeng, G.F.; Xu, X.Y.; Ci, L.J.; Xi, B.J.; Xiong, S.L.; Feng, J.K.; Qian, Y.T. Vacuum distillation derived 3D porous current collector for stable lithium-metal batteries. Nano Energy 2018, 47, 503–511. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Matios, E.; Hu, X.; Li, W. A Chemically Engineered Porous Copper Matrix with Cylindrical Core-Shell Skeleton as a Stable Host for Metallic Sodium Anodes. Adv. Funct. Mater. 2018, 28, 1802282. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Wang, J.; Yan, W.; Zhang, J. Robust 3D Copper Foam Functionalized with Gold Nanoparticles as Anode for High-Performance Potassium Metal Batteries. Chem.-Asian J. 2022, 17, e202200430. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, W.; Tu, J.; Chen, S.; Liang, J.; Guo, X. Construction of 3D Copper-Based Collector and Its Application in Lithium Metal Batteries. Prog. Chem. 2022, 34, 370–383. [Google Scholar]
- Zhou, B.; Bonakdarpour, A.; Stosevski, I.; Fang, B.; Wilkinson, D.P. Modification of Cu current collectors for lithium metal batteries—A review. Prog. Mater. Sci. 2022, 130, 100996. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, X.; Zhu, X.; Tan, P. Addressing Transport Issues in Non-Aqueous Li–air Batteries to Achieving High Electrochemical Performance. Electrochem. Energy Rev. 2023, 6, 18. [Google Scholar] [CrossRef]
- Kurniawan, M.; Ivanov, S. Electrochemically Structured Copper Current Collectors for Application in Energy Conversion and Storage: A Review. Energies 2023, 16, 4933. [Google Scholar] [CrossRef]
- Han, X.; Wu, T.; Gu, L.; Chen, M.; Song, J.; Tian, D.; Chen, J. Li-MOF-based ions regulator enabling fast-charging and dendrite-free lithium metal anode. Chin. Chem. Lett. 2023, 34, 107594. [Google Scholar] [CrossRef]
- Ke, Q.; Xu, Q.; Lai, X.; Yang, X.; Gao, H.; Wang, Z.; Qiu, Y. Ultralong-life lithium metal batteries enabled by decorating robust hybrid interphases on 3D layered framworks. Chin. Chem. Lett. 2023, 34, 107602. [Google Scholar] [CrossRef]
- Zhu, R.; Sheng, N.; Rao, Z.; Zhu, C.; Aoki, Y.; Habazaki, H. Employing a T-shirt template and variant of Schweizer’s reagent for constructing a low-weight, flexible, hierarchically porous and textile-structured copper current collector for dendrite-suppressed Li metal. J. Mater. Chem. A 2019, 7, 27066–27073. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Z.; Gao, H.; Niu, J.; Ma, W.; Qin, J.; Peng, Z.; Zhang, Z. A self-supported, three-dimensional porous copper film as a current collector for advanced lithium metal batteries. J. Mater. Chem. A 2019, 7, 1092–1098. [Google Scholar] [CrossRef]
- Tang, Y.P.; Shen, K.; Lv, Z.Y.; Xu, X.; Hou, G.Y.; Cao, H.Z.; Wu, L.K.; Zheng, G.Q.; Deng, Y.D. Three-dimensional ordered macroporous Cu current collector for lithium metal anode: Uniform nucleation by seed crystal. J. Power Sources 2018, 403, 82–89. [Google Scholar] [CrossRef]
- Chen, J.; Qiao, X.; Fu, W.; Han, X.; Wu, Q.; Wang, Y.; Zhang, Y.; Shi, L.; Zhao, J.; Ma, Y. Lithiophilic hyperbranched Cu nanostructure for stable Li metal anodes. Smartmat 2023, 4, e1174. [Google Scholar] [CrossRef]
- Ingber, T.T.K.; Bela, M.M.; Puettmann, F.; Dohmann, J.F.; Bieker, P.; Boerner, M.; Winter, M.; Stan, M.C. Elucidating the lithium deposition behavior in open-porous copper micro-foam negative electrodes for zero-excess lithium metal batteries. J. Mater. Chem. A 2023, 11, 17828–17840. [Google Scholar] [CrossRef]
- Ke, X.; Cheng, Y.; Liu, J.; Liu, L.; Wang, N.; Liu, J.; Zhi, C.; Shi, Z.; Guo, Z. Hierarchically Bicontinuous Porous Copper as Advanced 3D Skeleton for Stable Lithium Storage. ACS Appl. Mater. Interfaces 2018, 10, 13552–13561. [Google Scholar] [CrossRef]
- Kunduraci, M. Dealloying technique in the synthesis of lithium-ion battery anode materials. J. Solid State Electrochem. 2016, 20, 2105–2111. [Google Scholar] [CrossRef]
- Wu, X.; He, G.; Ding, Y. Dealloyed nanoporous materials for rechargeable lithium batteries. Electrochem. Energy Rev. 2020, 3, 541–580. [Google Scholar] [CrossRef]
- Zhang, D.; Dai, A.; Wu, M.; Shen, K.; Xiao, T.; Hou, G.; Lu, J.; Tang, Y. Lithiophilic 3D Porous CuZn Current Collector for Stable Lithium Metal Batteries. ACS Energy Lett. 2020, 5, 180–186. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Qian, Y.; Wang, X.; Huang, J.; Ma, Y.; Suo, L.; Li, W.; Zhang, B. Stable Li deposition of 3D highstrength-lithiophilicity-porous CuZn current collector with gradient structure. J. Alloys Compd. 2023, 951, 169953. [Google Scholar] [CrossRef]
- Sun, C.; Yang, Y.; Bian, X.; Guan, R.; Wang, C.; Lu, D.; Gao, L.; Zhang, D. Uniform Deposition of Li-Metal Anodes Guided by 3D Current Collectors with In Situ Modification of the Lithiophilic Matrix. ACS Appl. Mater. Interfaces 2021, 13, 48691–48699. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ma, X.; Bai, J.; Xu, W.; Zhong, H.; Liu, Z.; Xiong, S.; Yang, L.; Chen, H. Electrochemically Dealloyed 3D Porous Copper Nanostructure as Anode Current Collector of Li-Metal Batteries. Small 2023, 19, 2301731. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhong, K.; Dang, Y.; Li, J.; Ruan, M.; Fang, Z. Chemical dealloying pore structure control of porous copper current collector for dendrite-free lithium anode. J. Porous Mater. 2021, 28, 1813–1822. [Google Scholar] [CrossRef]
- An, Y.; Tian, Y.; Wei, C.; Tao, Y.; Xi, B.; Xiong, S.; Feng, J.; Qian, Y. Dealloying: An effective method for scalable fabrication of 0D, 1D, 2D, 3D materials and its application in energy storage. Nano Today 2021, 37, 101094. [Google Scholar] [CrossRef]
- Li, W.; Zheng, S.; Gao, Y.; Feng, D.; Ru, Y.; Zuo, T.; Chen, B.; Zhang, Z.; Gao, Z.; Geng, H.; et al. High Rate and Low-Temperature Stable Lithium Metal Batteries Enabled by Lithiophilic 3D Cu-CuSn Porous Framework. Nano Lett. 2023, 23, 7805–7814. [Google Scholar] [CrossRef]
- Zhao, H.; Lei, D.; He, Y.-B.; Yuan, Y.; Yun, Q.; Ni, B.; Lv, W.; Li, B.; Yang, Q.-H.; Kang, F.; et al. Compact 3D Copper with Uniform Porous Structure Derived by Electrochemical Dealloying as Dendrite-Free Lithium Metal Anode Current Collector. Adv. Energy Mater. 2018, 8, 1800266. [Google Scholar] [CrossRef]
- Luan, C.; Chen, L.; Li, B.; Zhu, L.; Li, W. Electrochemical Dealloying-Enabled 3D Hierarchical Porous Cu Current Collector of Lithium Metal Anodes for Dendrite Growth Inhibition. ACS Appl. Energy Mater. 2021, 4, 13903–13911. [Google Scholar] [CrossRef]
- Park, S.K.; Copic, D.; Zhao, T.Z.; Rutkowska, A.; Wen, B.; Sanders, K.; He, R.; Kim, H.-K.; De Volder, M. 3D Porous Cu-Composites for Stable Li-Metal Battery Anodes. ACS Nano 2023, 17, 14658–14666. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, M.; Oh, S.; Ryu, W.-H. Li-Dendrite cage electrode with 3-D interconnected pores for Anode-Free Lithium-Metal batteries. Chem. Eng. J. 2023, 474, 145447. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, H.; Li, D.; Yan, Y.; Wei, D.; Ye, J.; Zhao, Y.; Zeng, W.; Duan, H. Ultrathin Lithiophilic 3D Arrayed Skeleton Enabling Spatial-Selection Deposition for Dendrite-Free Lithium Anodes. Small 2023, 19, 2300734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zeng, J.; Ma, Y.; Zhao, Y.; Qian, Y.; Suo, L.; Huang, J.; Wang, X.; Li, W.; Zhang, B. Ultrathin hierarchical porous Cu current collector fabricated by anodic oxidation in complexing agent system for stable anode-free Lithium metal batteries. Electrochim. Acta 2023, 442, 141895. [Google Scholar] [CrossRef]
- Chen, C.; Li, S.; Notten, P.H.L.; Zhang, Y.; Hao, Q.; Zhang, X.; Lei, W. 3D Printed Lithium-Metal Full Batteries Based on a High-Performance Three-Dimensional Anode Current Collector. ACS Appl. Mater. Interfaces 2021, 13, 24785–24794. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, J.; Chen, X.; Zhang, Y. Facile Lithiophilic 3D Copper Current Collector for Stable Li Metal Anode. J. Electron. Mater. 2022, 51, 4248–4256. [Google Scholar] [CrossRef]
- Kim, E.; Choi, W.; Ryu, S.; Yun, Y.; Jo, S.; Yoo, J. Effect of 3D lithiophilic current collector for anode-free Li ion batteries. J. Alloys Compd. 2023, 966, 171393. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, B.; Liu, A.; Song, R.; Bao, C.; Ning, Y.; Wang, F.; Ruan, T.; Wang, D.; Zhou, Y. In situ growth of CuO submicro-sheets on optimized Cu foam to induce uniform Li deposition and stripping for stable Li metal batteries. Electrochim. Acta 2020, 339, 135941. [Google Scholar] [CrossRef]
- Liu, X.-F.; Xie, D.; Tao, F.-Y.; Diao, W.-Y.; Yang, J.-L.; Luo, X.-X.; Li, W.-L.; Wu, X.-L. Regulating the Li Nucleation/Growth Behavior via Cu2O Nanowire Array and Artificial Solid Electrolyte Interphase toward Highly Stable Li Metal Anode. ACS Appl. Mater. Interfaces 2022, 14, 23588–23596. [Google Scholar] [CrossRef]
- Chen, L.; Chen, G.; Lin, X.H.; Zheng, Z.C.; Wen, Z.X.; Wu, D.; Weng, Z.; Zhang, N.; Liu, X.H.; Ma, R.Z. Lithiophilic and Anticorrosive Cu Current Collector via Dual-Bonded Porous Polymer Coating for Stable Lithium-Metal Batteries. ACS Appl. Mater. Interfaces 2023, 15, 10273–10282. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Lu, Z.D.; Lee, H.W.; Xiong, F.; Hsu, P.C.; Li, Y.Z.; Zhao, J.; Chu, S.; Cui, Y. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat. Energy 2016, 1, 16010. [Google Scholar] [CrossRef]
- Gao, Y.; Cui, B.-F.; Wang, J.-J.; Sun, Z.-Y.; Chen, Q.; Deng, Y.-D.; Han, X.-P.; Hu, W.-B. Improving Li reversibility in Li metal batteries through uniform dispersion of Ag nanoparticles on graphene. Rare Met. 2022, 41, 3391–3400. [Google Scholar] [CrossRef]
- Chen, W.; Li, S.; Wang, C.; Dou, H.; Zhang, X. Targeted Deposition in a Lithiophilic Silver-Modified 3D Cu Host for Lithium-Metal Anodes. Energy Environ. Mater. 2023, 6, e12412. [Google Scholar] [CrossRef]
- Feng, K.; Sun, Z.; Liu, Y.; Tao, F.; Ma, J.; Qian, H.; Yu, R.; Pan, K.; Wang, G.; Wei, S.; et al. Shining light on transition metal tungstate-based nanomaterials for electrochemical applications: Structures, progress, and perspectives. Nano Res. 2022, 15, 6924–6960. [Google Scholar]
- Yan, N.-F.; Cui, H.-M.; Shi, J.-S.; You, S.-Y.; Liu, S. Recent progress of W18O49 nanowires for energy conversion and storage. Tungsten 2023, 5, 371–390. [Google Scholar] [CrossRef]
- Miao, S.-C.; Jia, Y.; Deng, Z.-W.; Deng, Y.; Chen, R.-X.; Zhang, X.-M.; Xu, C.-H.; Yao, M.; Cai, W.-L. Transition metals for stabilizing lithium metal anode: Advances and perspectives. Tungsten 2024, 6, 212–229. [Google Scholar] [CrossRef]
- Lin, J.-H.; Yan, Y.-T.; Qi, J.-L.; Zha, C.-Y. Atomic tailoring-induced deficiency in tungsten oxides for high-performance energy-related devices. Tungsten 2024, 6, 269–277. [Google Scholar]
- Zhu, R.-J.; Liu, J.; Hua, C.; Pan, H.-Y.; Cao, Y.-J.; Li, M. Preparation of vanadium-based electrode materials and their research progress in solid-state flexible supercapacitors. Rare Met. 2024, 43, 431–454. [Google Scholar] [CrossRef]
- Qian, H.; Liu, Y.; Chen, H.; Feng, K.; Jia, K.; Pan, K.; Wang, G.; Huang, T.; Pang, X.; Zhang, Q. Emerging bismuth-based materials: From fundamentals to electrochemical energy storage applications. Energy Storage Mater. 2023, 58, 232–270. [Google Scholar] [CrossRef]
- Ahad, S.A.; Adegoke, T.E.; Ryan, K.M.; Geaney, H. Cu Current Collector with Binder-Free Lithiophilic Nanowire Coating for High Energy Density Lithium Metal Batteries. Small 2023, 19, 2207902. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Du, Z.; He, C.; Bi, J.; Li, S.; Guan, W.; Du, H.; Ai, W. Integrated Gradient Cu Current Collector Enables Bottom-Up Li Growth for Li Metal Anodes: Role of Interfacial Structure. Adv. Sci. 2023, 10, 2301288. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Jiao, T.; Yang, S.; Liu, B.; Zhang, W.; Zhang, K. Lithiophilicity conversion of the Cu surface through facile thermal oxidation: Boosting a stable Li-Cu composite anode through melt infusion. J. Mater. Chem. A 2019, 7, 5726–5732. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.; Lin, L.; Wang, H.; Wang, C.; Zhang, C.; Song, C.; Tian, F.; Yang, J.; Qian, Y. Pressure-tuned and surface-oxidized copper foams for dendrite-free Li metal anodes. Mater. Today Energy 2020, 15, 100367. [Google Scholar] [CrossRef]
- Jiang, J.M.; Pan, Z.H.; Kou, Z.K.; Nie, P.; Chen, C.L.; Li, Z.W.; Li, S.P.; Zhu, Q.; Dou, H.; Zhang, X.G.; et al. Lithiophilic polymer interphase anchored on laser-punched 3D holey Cu matrix enables uniform lithium nucleation leading to super-stable lithium metal anodes. Energy Storage Mater. 2020, 29, 84–91. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, S.; Zhang, L.; Zhang, B.; Liao, B. Lithiophilic Zn3N2-Modified Cu Current Collectors by a Novel FCVA Technology for Stable Anode-Free Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2023, 15, 43145–43158. [Google Scholar] [CrossRef] [PubMed]
- Maeng, J.Y.; Bae, M.; Kim, Y.; Kim, D.; Chang, Y.; Park, S.; Choi, J.; Lee, E.; Lee, J.; Piao, Y. Establishing a multifunctional solid electrolyte interphase on a 3D host by an ultra-fast double coating strategy for stable lithium metal batteries. J. Mater. Chem. A 2024, 12, 1058–1071. [Google Scholar] [CrossRef]
- Xiong, J.-Z.; Yang, Z.-C.; Guo, X.-L.; Wang, X.-Y.; Geng, C.; Sun, Z.-F.; Xiao, A.-Y.; Zhuang, Q.-C.; Chen, Y.-X.; Ju, Z.-C. Review on recent advances of inorganic electrode materials for potassium-ion batteries. Tungsten 2024, 6, 174–195. [Google Scholar] [CrossRef]
- Gong, H.-Q.; Wang, X.-Y.; Ye, L.; Zhang, B.; Ou, X. Recycling of spent lithium-ion batteries to resynthesize high-performance cathode materials for sodium-ion storage. Tungsten 2024, 6, 574–584. [Google Scholar] [CrossRef]
- Cu, Q.; Shang, C.-Q.; Zhou, G.-F.; Wang, X. Freestanding MoSe2 nanoflowers for superior Li/Na storage properties. Tungsten 2024, 6, 238–247. [Google Scholar] [CrossRef]
- Zou, H.-Y.; Fang, L.; Yu, G.; Wang, D. Nanocrystalline WSe2 excels at high-performance anode for Na storage via a facile one-pot hydrothermal method. Tungsten 2024, 6, 248–258. [Google Scholar] [CrossRef]
- Wang, T.-S.; Liu, Y.; Lu, Y.-X.; Hu, Y.-S.; Fan, L.-Z. Dendrite-free Na metal plating/stripping onto 3D porous Cu hosts. Energy Storage Mater. 2018, 15, 274–281. [Google Scholar] [CrossRef]
- Sun, J.; Guo, C.; Cai, Y.; Li, J.; Sun, X.; Shi, W.; Ai, S.; Chen, C.; Jiang, F. Dendrite-free and long-life Na metal anode achieved by 3D porous Cu. Electrochim. Acta 2019, 309, 18–24. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, T.; Hou, Z.; Zhuang, W.; Sun, Z.; Jiang, Y.; Huang, L. Large-scale sodiophilic/buffered alloy architecture enables deeply cyclable Na metal anodes. Chem. Eng. J. 2022, 433, 133270. [Google Scholar] [CrossRef]
- Xiao, H.; Li, Y.; Chen, W.; Xie, T.; Zhu, H.; Zheng, W.; He, J.; Huang, S. Stabilize Sodium Metal Anode by Integrated Patterning of Laser-Induced Graphene with Regulated Na Deposition Behavior. Small 2023, 19, 2303959. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, L.; Ling, F.; Zhou, X.; Jiang, Y.; Yao, Y.; Yang, H.; Shao, Y.; Wu, X.; Rui, X.; et al. Homogeneous Metallic Deposition Regulated by Porous Framework and Selenization Interphase Toward Stable Sodium/Potassium Anodes. Adv. Funct. Mater. 2022, 32, 2210166. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Gu, Q.; Nanda, J.; Watt, J.; Mitlin, D. Dendrite-Free Potassium Metal Anodes in a Carbonate Electrolyte. Adv. Mater. 2020, 32, 1906735. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, J.; Chen, C.; Wang, L.; Zhai, Z.; Fu, Q.; Liu, Y.; Dong, L.; Yan, W.; Li, A.; et al. Cu3Pt alloy-functionalized Cu mesh as current collector for dendritic-free anodes of potassium metal batteries. Nano Energy 2020, 75, 104914. [Google Scholar] [CrossRef]
- Wang, J.; Yan, W.; Zhang, J. High area capacity and dendrite-free anode constructed by highly potassiophilic Pd/Cu current collector for low-temperature potassium metal battery. Nano Energy 2022, 96, 107131. [Google Scholar] [CrossRef]
- Li, L.; Ji, P.; Huang, M.; Zhang, Z.; Wang, H.; Verpoort, F.; Yang, J.; He, D. Hybrid ionic/electronic interphase enabling uniform nucleation and fast diffusion kinetics for stable lithium metal anode. Chin. Chem. Lett. 2024, 35, 109144. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, Y.; Liao, Y.; Li, W.; Xu, Y.; Liu, X.; Jiang, Y.; Zhang, J.; Zhao, B. Construction of Synchronous-Deposition K Metal Anodes Via Modulation of Ion/Electron Transport Kinetics for High-Rate and Low-Temperature K Metal Batteries. Small 2023, 19, 2300854. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, C.; Tian, Y.; Zhang, Y.; Jiang, Y.; Hu, J.; Hou, H.; Zou, G.; Ji, X. Dendrite-free lithium metal anode with lithiophilic interphase from hierarchical frameworks by tuned nucleation. Energy Storage Mater. 2020, 27, 124–132. [Google Scholar] [CrossRef]
- Wang, R.; Shi, F.; He, X.; Shi, J.; Ma, T.; Jin, S.; Tao, Z. Three-dimensional lithiophilic Cu@Sn nanocones for dendrite-free lithium metal anodes. Sci. China-Mater. 2021, 64, 1087–1094. [Google Scholar] [CrossRef]
- Lim, G.J.H.; Lyu, Z.; Zhang, X.; Koh, J.J.; Zhang, Y.; He, C.; Adams, S.; Wang, J.; Ding, J. Robust pure copper framework by extrusion 3D printing for advanced lithium metal anodes. J. Mater. Chem. A 2020, 8, 9058–9067. [Google Scholar] [CrossRef]
- Lu, R.; Zhang, B.; Cheng, Y.; Amin, K.; Yang, C.; Zhou, Q.; Mao, L.; Wei, Z. Dual-regulation of ions/electrons in a 3D Cu-CuxO host to guide uniform lithium growth for high-performance lithium metal anodes. J. Mater. Chem. A 2021, 9, 10393–10403. [Google Scholar] [CrossRef]
- Liao, J.-L.; Zhang, S.; Bai, T.-S.; Ji, F.-J.; Li, D.-P.; Cheng, J.; Zhang, H.-Q.; Lu, J.-Y.; Gao, Q.; Ci, L.-J. A ZnO decorated 3D copper foam as a lithiophilic host to construct composite lithium metal anodes for Li–O2 batteries. Rare Met. 2023, 42, 1969–1982. [Google Scholar] [CrossRef]
- Yang, G.; Chen, J.; Xiao, P.; Agboola, P.O.; Shakir, I.; Xu, Y. Graphene anchored on Cu foam as a lithiophilic 3D current collector for a stable and dendrite-free lithium metal anode. J. Mater. Chem. A 2018, 6, 9899–9905. [Google Scholar] [CrossRef]
- Ma, J.; Yang, J.; Wu, C.; Huang, M.; Zhu, J.; Zeng, W.; Li, L.; Li, P.; Zhao, X.; Qiao, F.; et al. Stabilizing nucleation seeds in Li metal anode via ion-selective graphene oxide interfaces. Energy Storage Mater. 2023, 56, 572–581. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, B.; Zhang, L.; Xie, Q.; Zhang, Y.; Lin, J.; Qu, B.; Wang, L.; Sa, B.; Peng, D.-L. Sodiophilic Zn/SnO2 porous scaffold to stabilize sodium deposition for sodium metal batteries. Chem. Eng. J. 2021, 404, 126469. [Google Scholar] [CrossRef]
- Wang, J.; Kang, Q.; Yuan, J.; Fu, Q.; Chen, C.; Zhai, Z.; Liu, Y.; Yan, W.; Li, A.; Zhang, J. Dendrite-free lithium and sodium metal anodes with deep plating/stripping properties for lithium and sodium batteries. Carbon Energy 2021, 3, 153–166. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, F.; Liang, J.; Fang, K.; Wu, W.; Wu, X. In-situ constructing a supersodiophilic fluffy surface layer on a Cu foam host for stable Na metal anodes. J. Alloys Compd. 2021, 853, 157371. [Google Scholar] [CrossRef]
- Wang, L.; Luo, G. Atomistic Mechanism and Long-Term Stability of Using Chlorinated Graphdiyne Film to Reduce Lithium Dendrites in Rechargeable Lithium Metal Batteries. Nano Lett. 2021, 21, 7284–7290. [Google Scholar] [CrossRef] [PubMed]
- Pirayesh, P.; Jin, E.; Wang, Y.; Zhao, Y. Na metal anodes for liquid and solid-state Na batteries. Energy Environ. Sci. 2024, 17, 442–496. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, Y.; Zhao, Y.; Qian, Y.; Suo, L.; Wang, X.; Huang, J.; Li, W.; Zhang, B. Lightweight and Flexible 3D ERGO@Cu/PA Mesh Current Collector of Li Metal Battery for Dendrite Suppression. ACS Appl. Polym. Mater. 2023, 5, 3289–3297. [Google Scholar] [CrossRef]
- Zhao, Q.; Hao, X.; Su, S.; Ma, J.; Hu, Y.; Liu, Y.; Kang, F.; He, Y.-B. Expanded-graphite embedded in lithium metal as dendrite-free anode of lithium metal batteries. J. Mater. Chem. A 2019, 7, 15871–15879. [Google Scholar] [CrossRef]
- Guo, Y.-M.; Zhang, L.-J. Research Progress in Synthesis and Electrochemical Performance of Cobalt Sulfide as Anode Material for Secondary Batteries. Chin. J. Rare Met. 2022, 46, 227–237. [Google Scholar]
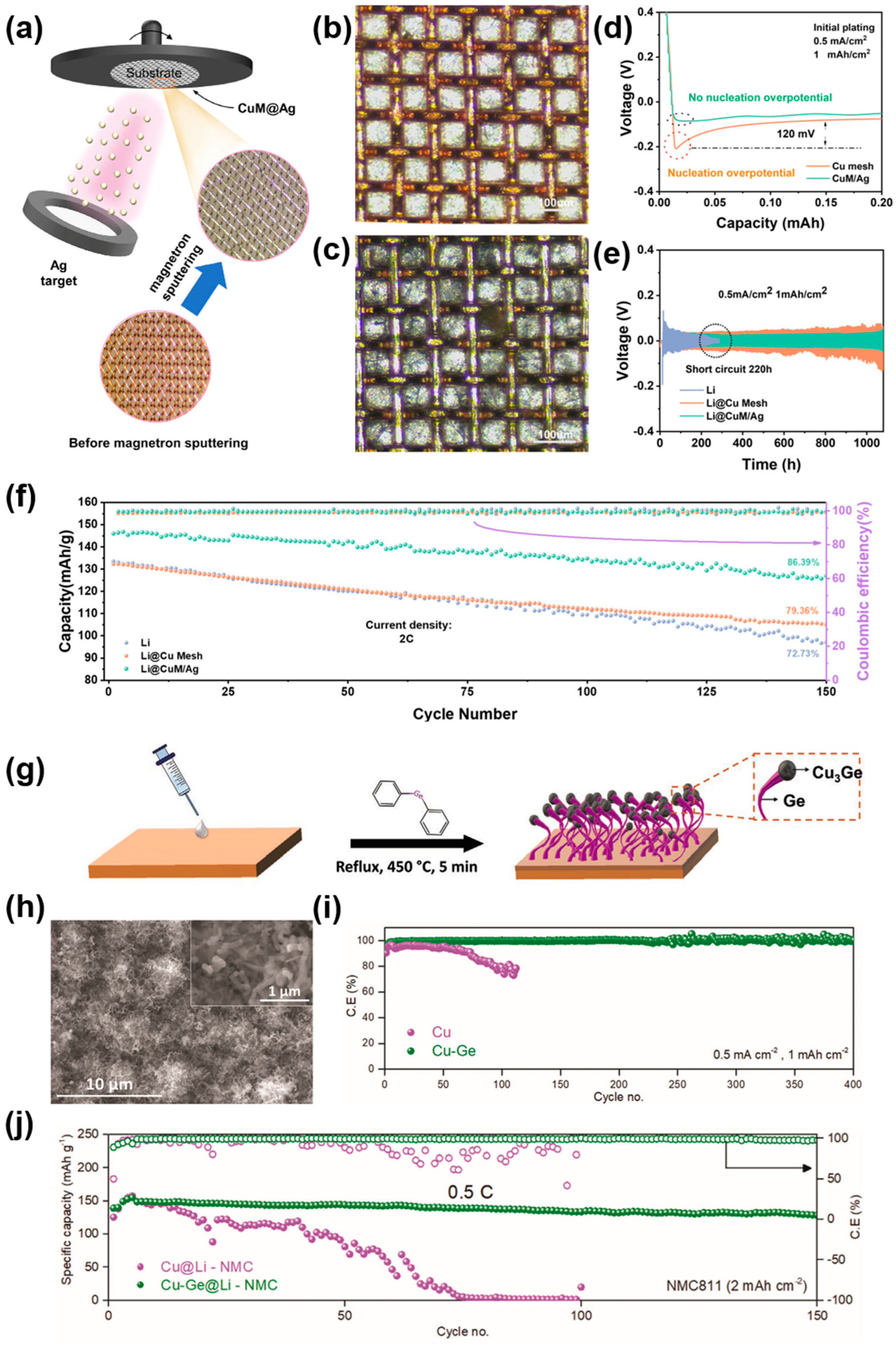

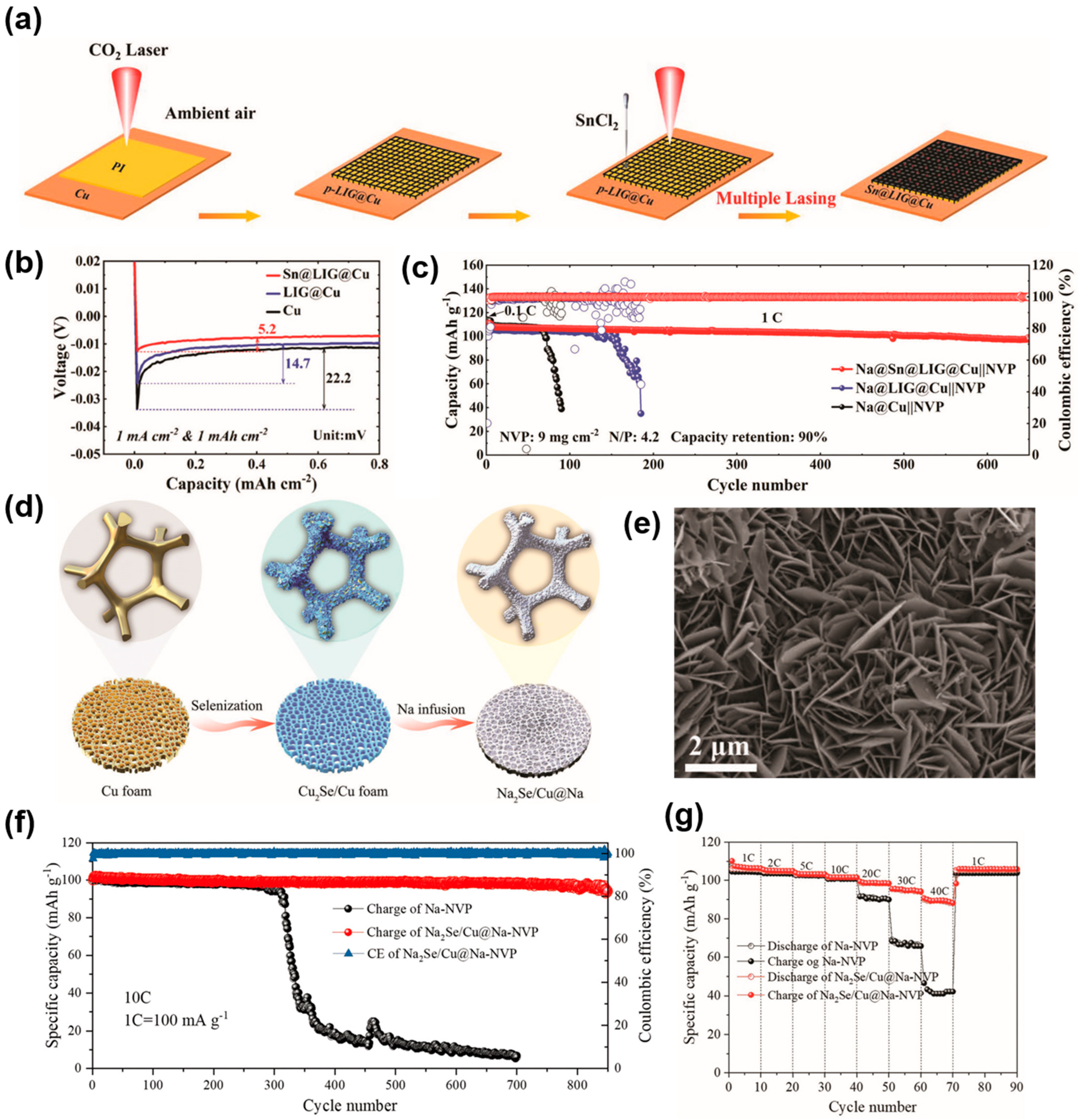
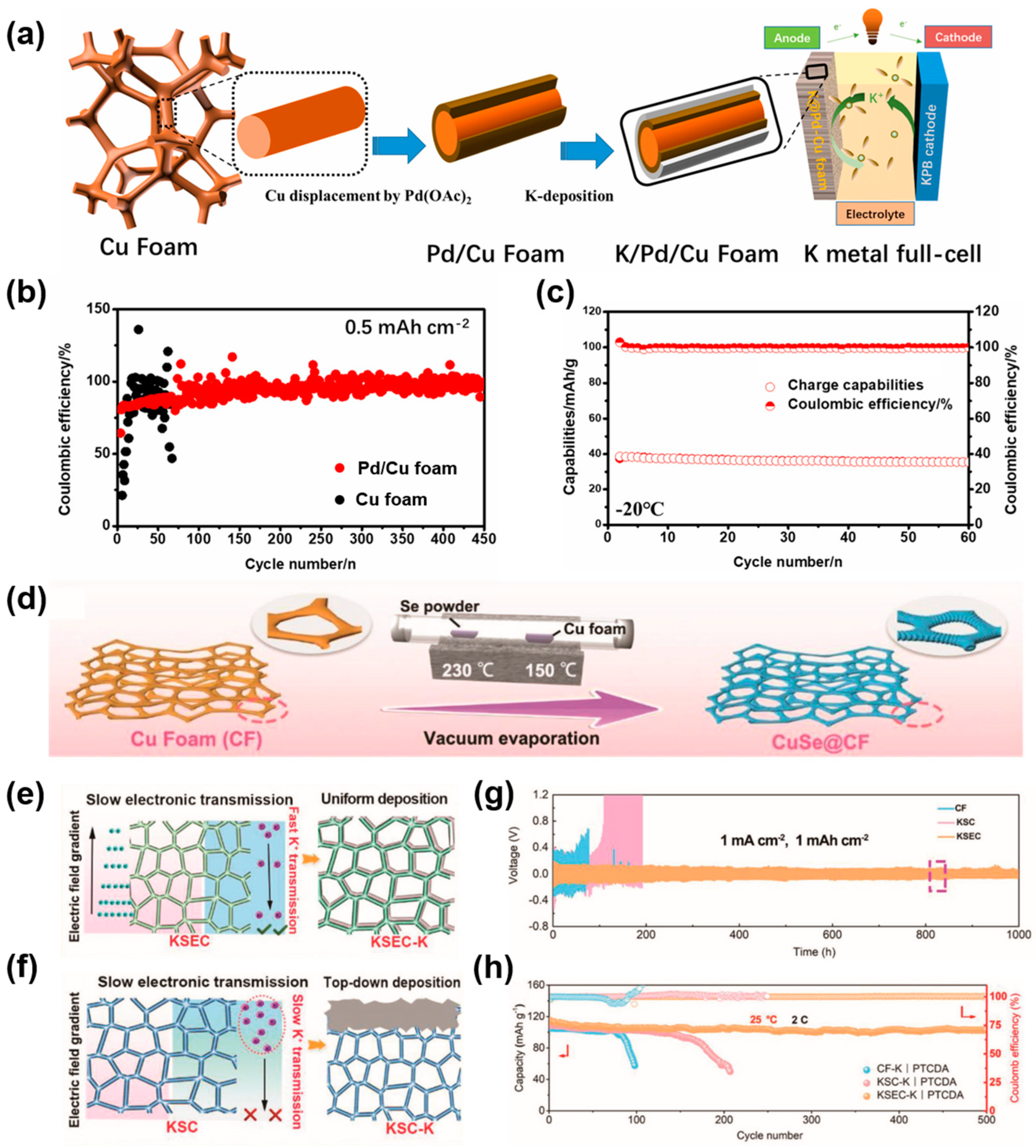
| Substrate | Current Collectors | Method | Cycle Performance V a (mV), T b (h) C1 c (mA cm−2), C2 c (mAh cm−2) | Ref. |
|---|---|---|---|---|
| Lithium metal anodes | ||||
| Modification strategy: Structrual modification | ||||
| / | 3D Cu skeleton | Template method | 40, 500 (1, 1) | [43] |
| Cu foil | Cu@CuxO | Template method | 20, 600 (1, 1) | [58] |
| Cu foam | HPC/CF | Template method | /, 620 (0.5, 1) | [60] |
| Cu-Zn alloy foil | 3D Cu | Dealloying | /, 800 (0.52, 0.26) | [46] |
| Cu-Zn alloy foil | 3D Cu | Dealloying | 20, 400 (1, 1) | [70] |
| Cu-Zn alloy foil | 2h-3D CuZn | Dealloying | 25, 450 (1, 1) | [63] |
| Cu-Zn alloy foil | Porous Cu | Dealloying | 20, 440 (1, 1) | [67] |
| Cu-Zn alloy mesh | HP-Cu@Sn | Dealloying Electroless plating | /, 800 (1, 1) | [112] |
| Cu foil | 3DHP Cu | Electrodeposition Dealloying | 33, 850 (1, 1) | [71] |
| Cu foil | 3D P-CuZn | Electrodeposition Dealloying | /, 560 (1, 1) | [64] |
| Cu foil | 3DOM Cu-450 | Electrodeposition | 25, 700 (0.2, 0.5) | [57] |
| Cu foil | Cu@Sn nanocones | Electrodeposition | 10, 600 (1, 1) | [113] |
| Cu foil | 3D Cu-CNT | Electrodeposition | /, 550 (0.5, 1) | [72] |
| / | 3DP-Cu | 3D printing | /, 250 (1, 1) | [114] |
| / | 3D Cu mesh | 3D printing | 20, 500 (1, 1) | [76] |
| Modification strategy: Chemical modification | ||||
| Cu foam | Ag@CF | Chemical reaction | 30, 1600 (1, 1) | [77] |
| Cu foil | Cu-Ge | Chemical reaction | /, 1000 (0.5, 1) | [91] |
| Cu mesh | CuM/Ag | Magnetron sputtering | 25, 1000 (0.5, 1) | [84] |
| Cu foam | ISG-CuO-2mM | Chemical oxidation | /, 1150 (1, 1) | [79] |
| Cu foam | RCOFs | Chemical oxidation Mechanical rolling | /, 5000 (5, 1) | [94] |
| Cu foam Cu foam | Cu-CuxO ZnO NFs/CuF | Chemical oxidation solvothermal | 15, 1800 (1, 1) 10, 1600 (1, 1) | [115] [116] |
| Cu foil | CuO@Cu | Electrochemical anodizing | 10, 1200 (1, 1) | [92] |
| Cu foam | GN@Cu foam | Chemical immersion | 10, 2000 (0.5, 1) | [117] |
| Cu foil | PDA@3D Cu | Chemical immersion | 24, 1000 (0.5, 0.5) | [95] |
| Cu foil | γ-APS-Cu | Drop casting | 12, 1400 (0.5, 1) | [81] |
| Cu foil | GO-Zn/Cu | Electrodeposition Spin-coating | 20, 600 (1, 1) | [118] |
| Sodium metal anodes | ||||
| Cu foam | CuNW-Cu | Electrochemical anodizing | 25, 1400 (1, 2) | [102] |
| Cu-Zn alloy | 3D porous Cu | Dealloying | /, 1000 (1, 1) | [103] |
| Cu foil | Cu/Zn/SnO2 | Magnetron sputtering | 25, 820 (1, 1) | [119] |
| Cu mesh | Pt-Cu/Cu mesh | Chemical reaction | /, 400 (1, 1) | [120] |
| Cu foam | SF-Cu-3.6 | Chemical oxidation | 19, 400 (1, 1) | [121] |
| Cu foam | Cu2Se/Cu foam | Solution selenization | 70, 500 (1, 1) | [106] |
| Cu foil | Sn@LIG@Cu | Laser process | 19.7, 1000 (10, 10) | [105] |
| Potassium metal anodes | ||||
| Cu foam | rGO@3D-Cu | Chemical immersion | /, 200 (0.5, 0.5) | [107] |
| Cu mesh | Cu3Pt-Cu mesh | Chemical reaction | 1000, 300 (0.5, 1) | [108] |
| Cu foam | CuSe@CF | Vacuum evaporation | 80, 1000 (1, 1) | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, C.; Wang, F.; Liu, Y.; Liu, Z.; Liu, J.; Feng, K.; Pei, Y.; Wu, Y.; Wang, G. Constructing Three-Dimensional Architectures to Design Advanced Copper-Based Current Collector Materials for Alkali Metal Batteries: From Nanoscale to Microscale. Molecules 2024, 29, 3669. https://doi.org/10.3390/molecules29153669
Kong C, Wang F, Liu Y, Liu Z, Liu J, Feng K, Pei Y, Wu Y, Wang G. Constructing Three-Dimensional Architectures to Design Advanced Copper-Based Current Collector Materials for Alkali Metal Batteries: From Nanoscale to Microscale. Molecules. 2024; 29(15):3669. https://doi.org/10.3390/molecules29153669
Chicago/Turabian StyleKong, Chunyang, Fei Wang, Yong Liu, Zhongxiu Liu, Jing Liu, Kaijia Feng, Yifei Pei, Yize Wu, and Guangxin Wang. 2024. "Constructing Three-Dimensional Architectures to Design Advanced Copper-Based Current Collector Materials for Alkali Metal Batteries: From Nanoscale to Microscale" Molecules 29, no. 15: 3669. https://doi.org/10.3390/molecules29153669







