Transcriptome and Metabolome Analyses of Aroma Differences between Chardonnay and a Chardonnay Bud Sport
Abstract
1. Introduction
2. Results
2.1. Differences in the Genetic Background and Physiological and Biochemical Indicators between Chardonnay and a Chardonnay Bud Sport
2.2. Volatile Metabolite Analysis of CH and CH09
2.2.1. Overview of VOC Profiles in CH and CH09
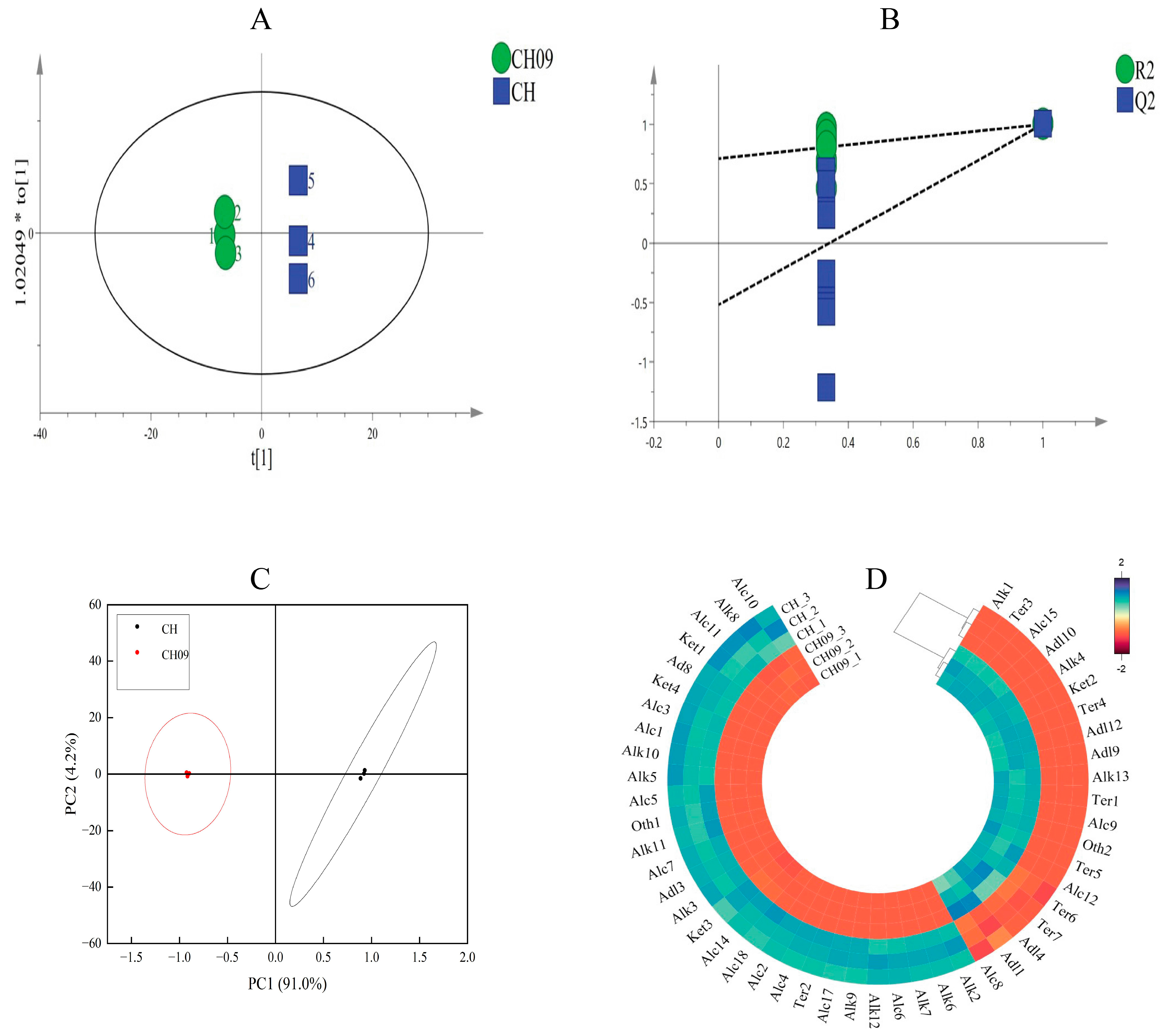
2.2.2. Principal Component and Multivariate Analysis of Differential VOCs
2.3. Transcription Sequencing Analysis
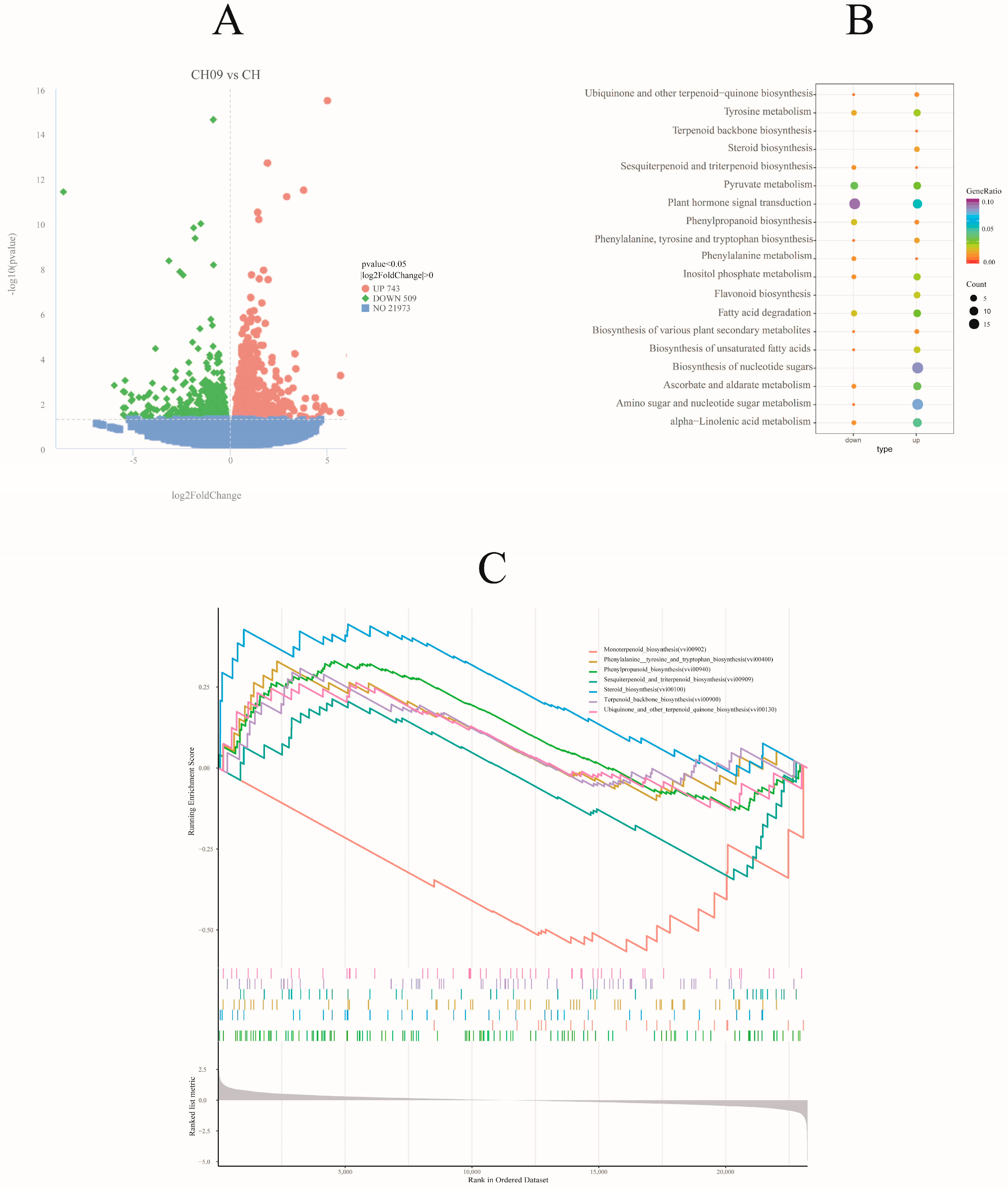
2.3.1. Identification of Differentially Expressed Genes (DEGs)
2.3.2. GO and KEGG Function Annotations of DEGs
2.3.3. Gene Set Enrichment Analysis (GSEA)
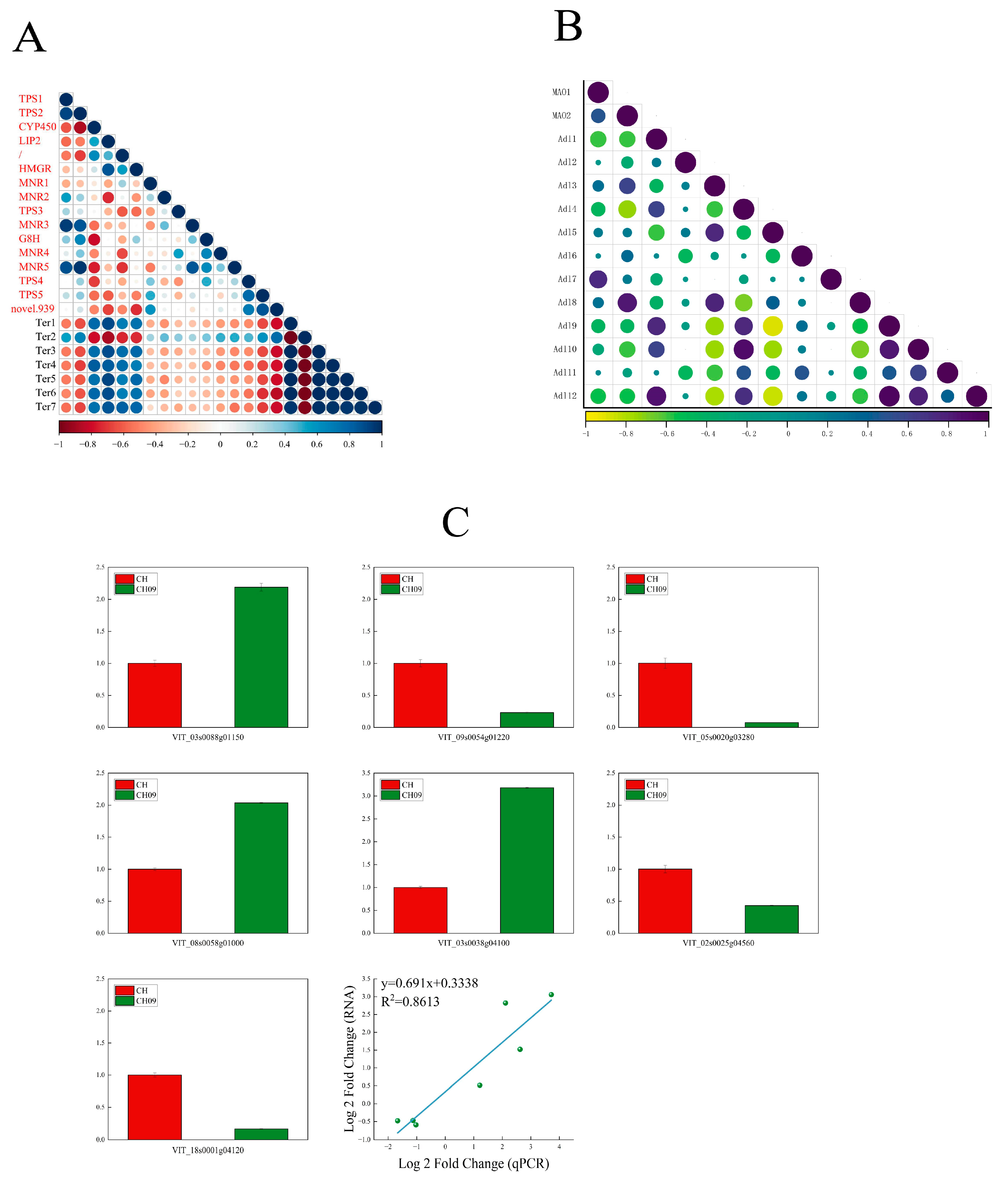
2.4. Correlation Analysis between Related Genes in the Terpene Metabolic Pathway and Terpene Substances
3. Discussion
3.1. Differences in Characteristics of Bud Mutation Variety
3.2. The Key Genes of Muscat Aroma in Bud Mutant
4. Materials and Methods
4.1. Materials
4.2. DNA Extraction and ISSR Amplification
4.3. Characteristic Evaluation
4.4. Extraction of Volatile Metabolites
4.5. RNA Extraction and Sequencing Analysis
4.6. Data Statistics and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gambetta, J.M.; Bastian, S.E.; Cozzolino, D.; Jeffery, D.W. Factors influencing the aroma composition of Chardonnay wines. J. Agric. Food Chem. 2014, 62, 6512–6534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liu, Z.; Guan, L.; Zheng, T.; Jiu, S.; Zhu, X.; Jia, H.; Fang, J. Changes of anthocyanin component biosynthesis in ‘Summer Black’grape berries after the red flesh mutation occurred. J. Agric. Food Chem. 2018, 66, 9209–9218. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.M.; Aranzana, M.J. Attention sports fans! The far-reaching contributions of bud sport mutants to horticulture and plant biology. Hortic. Res. 2018, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Jiménez, M. Monoterpenes in grape juice and wines. J. Chromatogr. A 2000, 881, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhu, B.; Zhang, X.; Wang, H.; Yan, A.; Zhang, G.; Wang, X.; Xu, H. The accumulation profiles of terpene metabolites in three Muscat table grape cultivars through HS-SPME-GCMS. Scientific Data 2020, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Lukić, I.; Lotti, C.; Vrhovsek, U. Evolution of free and bound volatile aroma compounds and phenols during fermentation of Muscat blanc grape juice with and without skins. Food Chem. 2017, 232, 25–35. [Google Scholar] [CrossRef]
- Zhang, B.; Hao, L.; Zhang, J.; Feng, J.; Wang, C.; Zhang, J. Integration of transcriptome, volatile and non-volatile metabolite profile reveals characteristic aroma formation in Toona sinensis. Food Chem. 2024, 436, 137788. [Google Scholar] [CrossRef] [PubMed]
- Martina, M.; Tikunov, Y.; Portis, E.; Bovy, A.G. The genetic basis of tomato aroma. Genes 2021, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Aharoni, A.; Giri, A.P.; Deuerlein, S.; Griepink, F.; de Kogel, W.-J.; Verstappen, F.W.; Verhoeven, H.A.; Jongsma, M.A.; Schwab, W.; Bouwmeester, H.J. Terpenoid metabolism in wild-type and transgenic Arabidopsis plants. Plant Cell 2003, 15, 2866–2884. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, S.; Tjhang, J.G.; Jang, I.-C. Production of multiple terpenes of different chain lengths by subcellular targeting of multi-substrate terpene synthase in plants. Metab. Eng. 2020, 61, 397–405. [Google Scholar] [CrossRef]
- Pino, J.A.; Mesa, J. Contribution of volatile compounds to mango (Mangifera indica L.) aroma. Flavour Fragr. J. 2006, 21, 207–213. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Z.; Xin, G.; Sun, B.; Bao, X.; Wei, Y.; Zhao, X.; Xu, H. Advances in umami taste and aroma of edible mushrooms. Trends Food Sci. Technol. 2020, 96, 176–187. [Google Scholar] [CrossRef]
- Sun, L.; Xin, G.; Hou, Z.; Zhao, X.; Xu, H.; Bao, X.; Xia, R.; Li, Y.; Li, L. Biosynthetic mechanism of key volatile biomarkers of harvested Lentinula edodes triggered by spore release. J. Agric. Food Chem. 2021, 69, 9350–9361. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cadwallader, K.R.; Liu, Y.; Huang, M.; Sun, B. Characterization of typical potent odorants in raw and cooked Toona sinensis (A. Juss.) M. Roem. by instrumental-sensory analysis techniques. Food Chem. 2019, 282, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.; Lamorte, S.A.; Gambuti, A.; Moio, L. Aroma of Aglianico and Uva di Troia grapes by aromatic series. Food Res. Int. 2013, 53, 15–23. [Google Scholar] [CrossRef]
- Suárez-Fariñas, M.; Magnasco, M.O. Comparing microarray studies. Microarray Data Anal. Methods Appl. 2007, 377, 139–152. [Google Scholar]
- Shi, J.; Walker, M.G. Gene set enrichment analysis (GSEA) for interpreting gene expression profiles. Curr. Bioinform. 2007, 2, 133–137. [Google Scholar] [CrossRef]
- Yi, X.; Du, Z.; Su, Z. PlantGSEA: A gene set enrichment analysis toolkit for plant community. Nucleic Acids Res. 2013, 41, W98–W103. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Xiong, J.; Deng, X. Difference of a citrus late-ripening mutant (Citrus sinensis) from its parental line in sugar and acid metabolism at the fruit ripening stage. Sci. China Ser. C Life Sci. 2007, 50, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, G.; Li, Y.; Lyu, M.; Zhang, H.; Zhang, N.; Chen, R. Integrative genomic and transcriptomic analyses of a bud sport mutant ‘Jinzao Wuhe’ with the phenotype of large berries in grapevines. PeerJ 2023, 11, e14617. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zheng, H.; Wang, Q.; Khalil-Ur-Rehman, M.; Meng, L.; Tao, J. Comparison among ‘Benitaka’grape, ABA-treated ‘Benitaka’, and its deeper-colored bud mutation revealed the coloring mechanisms on grapes. J. Plant Interact. 2019, 14, 102–109. [Google Scholar] [CrossRef]
- Li, X.Y.; Wen, Y.Q.; Meng, N.; Qian, X.; Pan, Q.H. Monoterpenyl glycosyltransferases differentially contribute to production of monoterpenyl glycosides in two aromatic Vitis vinifera varieties. Front. Plant Sci. 2017, 8, 1226. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Wu, G.; Ren, R.; Xie, R.; Yin, H.; Chen, H.; Yang, B.; Zhang, Z.; Ge, M. Transcriptomic and metabolic analyses reveal differences in monoterpene profiles and the underlying molecular mechanisms in six grape varieties with different flavors. LWT 2023, 174, 114442. [Google Scholar] [CrossRef]
- Tieman, D.M.; Loucas, H.M.; Kim, J.Y.; Clark, D.G.; Klee, H.J. Tomato phenylacetaldehyde reductases catalyze the last step in the synthesis of the aroma volatile 2-phenylethanol. Phytochemistry 2007, 68, 2660–2669. [Google Scholar] [CrossRef]
- García, E.; Chacón, J.; Martínez, J.; Izquierdo, P. Changes in volatile compounds during ripening in grapes of Airén, Macabeo and Chardonnay white varieties grown in La Mancha region (Spain). Food Sci. Technol. Int. 2003, 9, 33–41. [Google Scholar] [CrossRef]
- Xi, F.-F.; Guo, L.-L.; Yu, Y.-H.; Wang, Y.; Li, Q.; Zhao, H.-L.; Zhang, G.-H.; Guo, D.-L. Comparison of reactive oxygen species metabolism during grape berry development between ‘Kyoho’and its early ripening bud mutant ‘Fengzao’. Plant Physiol. Biochem. 2017, 118, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, W.; Song, S.; Xu, W.; Zhang, C.; Ma, C.; Wang, L.; Wang, S. Evolution of volatile compounds during the development of Muscat grape ‘Shine Muscat’ (Vitis labrusca × V. vinifera). Food Chem. 2020, 309, 125778. [Google Scholar] [CrossRef]
- Yauk, Y.K.; Ged, C.; Wang, M.Y.; Matich, A.J.; Tessarotto, L.; Cooney, J.M.; Chervin, C.; Atkinson, R.G. Manipulation of flavour and aroma compound sequestration and release using a glycosyltransferase with specificity for terpene alcohols. Plant J. 2014, 80, 317–330. [Google Scholar] [CrossRef]
- Lairson, L.; Henrissat, B.; Davies, G.; Withers, S. Glycosyltransferases: Structures, functions, and mechanisms. Annu. Rev. Biochem. 2008, 77, 521–555. [Google Scholar] [CrossRef]
- Bönisch, F.; Frotscher, J.; Stanitzek, S.; Rühl, E.; Wüst, M.; Bitz, O.; Schwab, W. Activity-based profiling of a physiologic aglycone library reveals sugar acceptor promiscuity of family 1 UDP-glucosyltransferases from grape. Plant Physiol. 2014, 166, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Cai, J.; Zhu, B.-Q.; Wu, G.-F.; Duan, C.-Q.; Chen, G.; Shi, Y. Study of free and glycosidically bound volatile compounds in air-dried raisins from three seedless grape varieties using HS–SPME with GC–MS. Food Chem. 2015, 177, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Lee, O.R.; Oh, J.Y.; Jang, M.-G.; Yang, D.-C. Functional analysis of 3-hydroxy-3-methylglutaryl coenzyme a reductase encoding genes in triterpene saponin-producing ginseng. Plant Physiol. 2014, 165, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Dong, T.; Haider, M.S.; Jin, H.; Jia, H.; Fang, J. Brassinosteroid regulates 3-hydroxy-3-methylglutaryl CoA reductase to promote grape fruit development. J. Agric. Food Chem. 2020, 68, 11987–11996. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Guan, L.; Yu, K.; Haider, M.S.; Nasim, M.; Liu, Z.; Li, T.; Zhang, K.; Jiu, S.; Jia, H. Expressional diversity of grapevine 3-Hydroxy-3-methylglutaryl-CoA reductase (VvHMGR) in different grapes genotypes. BMC Plant Biol. 2021, 21, 279. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, F.J.; Zamora, R. Formation of phenylacetic acid and benzaldehyde by degradation of phenylalanine in the presence of lipid hydroperoxides: New routes in the amino acid degradation pathways initiated by lipid oxidation products. Food Chem. X 2019, 2, 100037. [Google Scholar] [CrossRef] [PubMed]
- Gutensohn, M.; Henry, L.K.; Gentry, S.A.; Lynch, J.H.; Nguyen, T.T.; Pichersky, E.; Dudareva, N. Overcoming bottlenecks for metabolic engineering of sesquiterpene production in tomato fruits. Front. Plant Sci. 2021, 12, 691754. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.N.; Qian, Y.H.; Liu, R.H.; Liang, T.; Ding, Y.T.; Xu, X.L.; Huang, S.; Fang, Y.L.; Ju, Y.L. Effects of Table Grape Cultivars on Fruit Quality and Aroma Components. Foods 2023, 12, 3371. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.M.; Botía, P.; Pérez-Pérez, J.G. Influence of deficit irrigation timing on the fruit quality of grapefruit (Citrus paradisi Mac.). Food Chem. 2015, 175, 329–336. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Fenoll, J.; Manso, A.; Hellín, P.; Ruiz, L.; Flores, P. Changes in the aromatic composition of the Vitis vinifera grape Muscat Hamburg during ripening. Food Chem. 2009, 114, 420–428. [Google Scholar] [CrossRef]


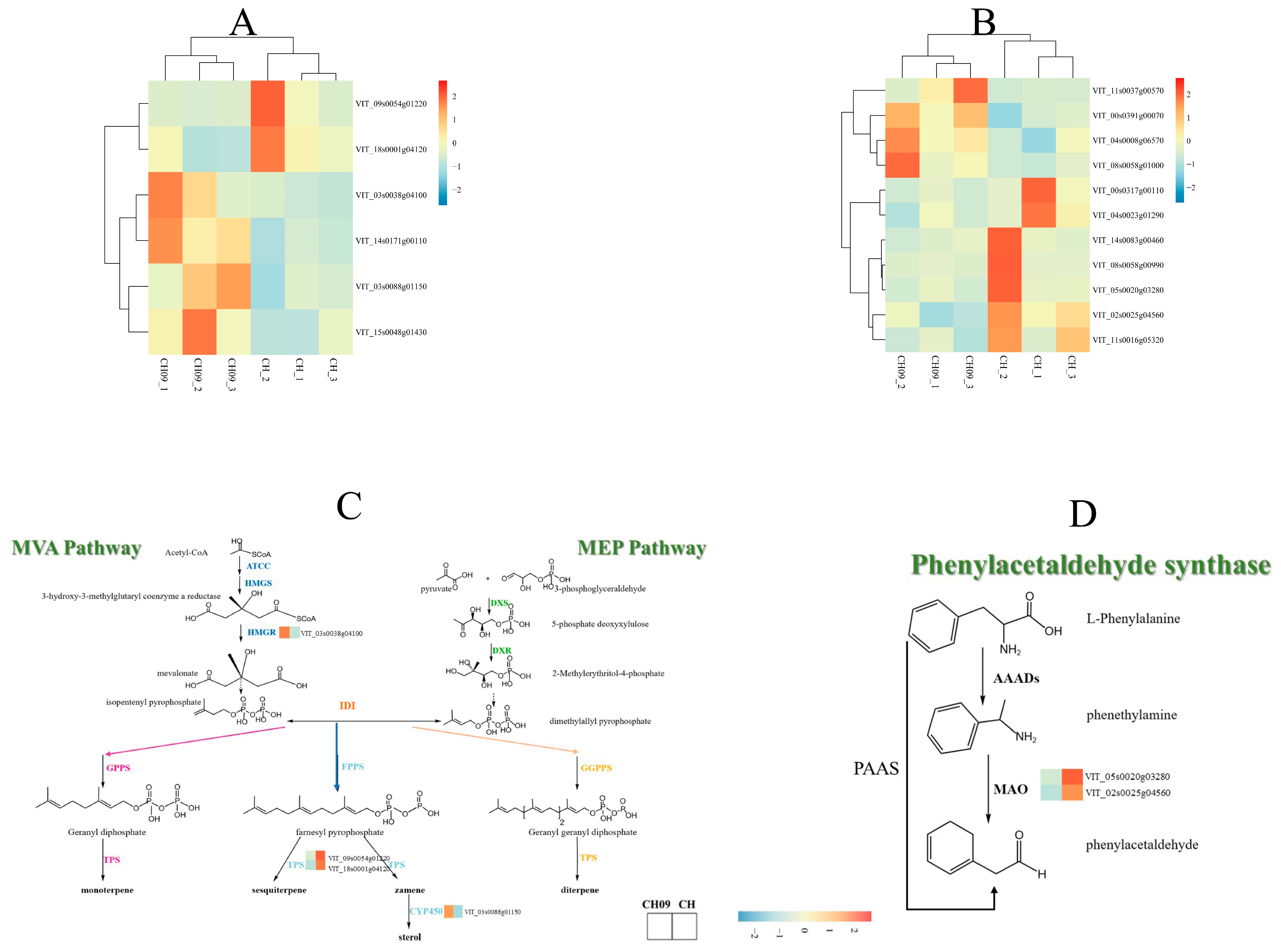
| Gene ID | KEGG Description | Protein Names | Enzyme Type |
|---|---|---|---|
| VIT_09s0054g01220 | Sesquiterpenoid and triterpenoid biosynthesis | Terpene cyclase/mutase family member | TPS1 |
| VIT_18s0001g04120 | Sesquiterpenoid and triterpenoid biosynthesis | Valencene synthase | TPS2 |
| VIT_03s0038g04100 | Terpenoid backbone biosynthesis | 3-hydroxy-3-methylglutaryl coenzyme A reductase | HMGR |
| VIT_14s0171g00110 | Steroid biosynthesis | Lipase | LIP2 |
| VIT_03s0088g01150 | Steroid biosynthesis | Squalene monooxygenase | CYP450 |
| VIT_15s0048g01430 | Steroid biosynthesis | Sterol methyltransferase C-terminal domain-containing protein | / |
| VIT_15s0046g03590 | Monoterpenoid biosynthesis | (+)-neomenthol dehydrogenase | MNR1 |
| VIT_15s0046g03580 | Monoterpenoid biosynthesis | (+)-neomenthol dehydrogenase | MNR2 |
| VIT_06s0009g01450 | Monoterpenoid biosynthesis | (+)-neomenthol dehydrogenase | MNR3 |
| VIT_15s0046g03570 | Monoterpenoid biosynthesis | (+)-neomenthol dehydrogenase | MNR4 |
| VIT_06s0061g00670 | Monoterpenoid biosynthesis | (+)-neomenthol dehydrogenase | MNR5 |
| novel.939 | Monoterpenoid biosynthesis | (+)-neomenthol dehydrogenase | MNR6 |
| VIT_00s0372g00020 | Monoterpenoid biosynthesis | Terpene synthase metal-binding domain-containing protein | TPS3 |
| VIT_13s0067g00050 | Monoterpenoid biosynthesis | Terpene synthase N-terminal domain-containing protein | TPS4 |
| VIT_13s0067g00090 | Monoterpenoid biosynthesis | Terpene synthase metal-binding domain-containing protein | TPS5 |
| VIT_02s0012g02340 | Monoterpenoid biosynthesis | Geraniol 8-hydroxylase | G8H |
| VIT_11s0037g00570 | Phenylpropanoid biosynthesis | Spermidine hydroxycinnamoyl transferase | SHT |
| VIT_00s0391g00070 | Phenylalanine, tyrosine, and tryptophan biosynthesis | Phospho-2-dehydro-3-deoxyheptonate aldolase | SHKB |
| VIT_04s0008g06570 | Phenylalanine, tyrosine, and tryptophan biosynthesis | Chorismate mutase | CM2 |
| VIT_08s0058g01000 | Phenylalanine, tyrosine, and tryptophan biosynthesis | Aspartate aminotransferase | ASP1 |
| VIT_00s0317g00110 | Phenylpropanoid biosynthesis | Serine aminopeptidase S33 domain-containing protein | CSE |
| VIT_04s0023g01290 | Phenylpropanoid biosynthesis | Glycosyltransferase | GT5 |
| VIT_14s0083g00460 | Phenylalanine, tyrosine, and tryptophan biosynthesis | tryptophan synthase | trpB2 |
| VIT_08s0058g00990 | Phenylpropanoid biosynthesis | Peroxidase | PNC1 |
| VIT_05s0020g03280 | Phenylalanine metabolism | Amine oxidase | AOC1 |
| VIT_02s0025g04560 | Phenylalanine metabolism | Amine oxidase | AOC2 |
| VIT_11s0016g05320 | Phenylpropanoid biosynthesis | Peroxidase | PER25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, X.; Dong, J.; Niu, M.; Wang, Z.; Xu, G. Transcriptome and Metabolome Analyses of Aroma Differences between Chardonnay and a Chardonnay Bud Sport. Molecules 2024, 29, 3671. https://doi.org/10.3390/molecules29153671
Bao X, Dong J, Niu M, Wang Z, Xu G. Transcriptome and Metabolome Analyses of Aroma Differences between Chardonnay and a Chardonnay Bud Sport. Molecules. 2024; 29(15):3671. https://doi.org/10.3390/molecules29153671
Chicago/Turabian StyleBao, Xiaoqin, Jin Dong, Min Niu, Zhilei Wang, and Guoqian Xu. 2024. "Transcriptome and Metabolome Analyses of Aroma Differences between Chardonnay and a Chardonnay Bud Sport" Molecules 29, no. 15: 3671. https://doi.org/10.3390/molecules29153671
APA StyleBao, X., Dong, J., Niu, M., Wang, Z., & Xu, G. (2024). Transcriptome and Metabolome Analyses of Aroma Differences between Chardonnay and a Chardonnay Bud Sport. Molecules, 29(15), 3671. https://doi.org/10.3390/molecules29153671








