Insight into the Role of Rb Doping for Highly Efficient Kesterite Cu2ZnSn(S,Se)4 Solar Cells
Abstract
1. Introduction
2. Results and Discussion
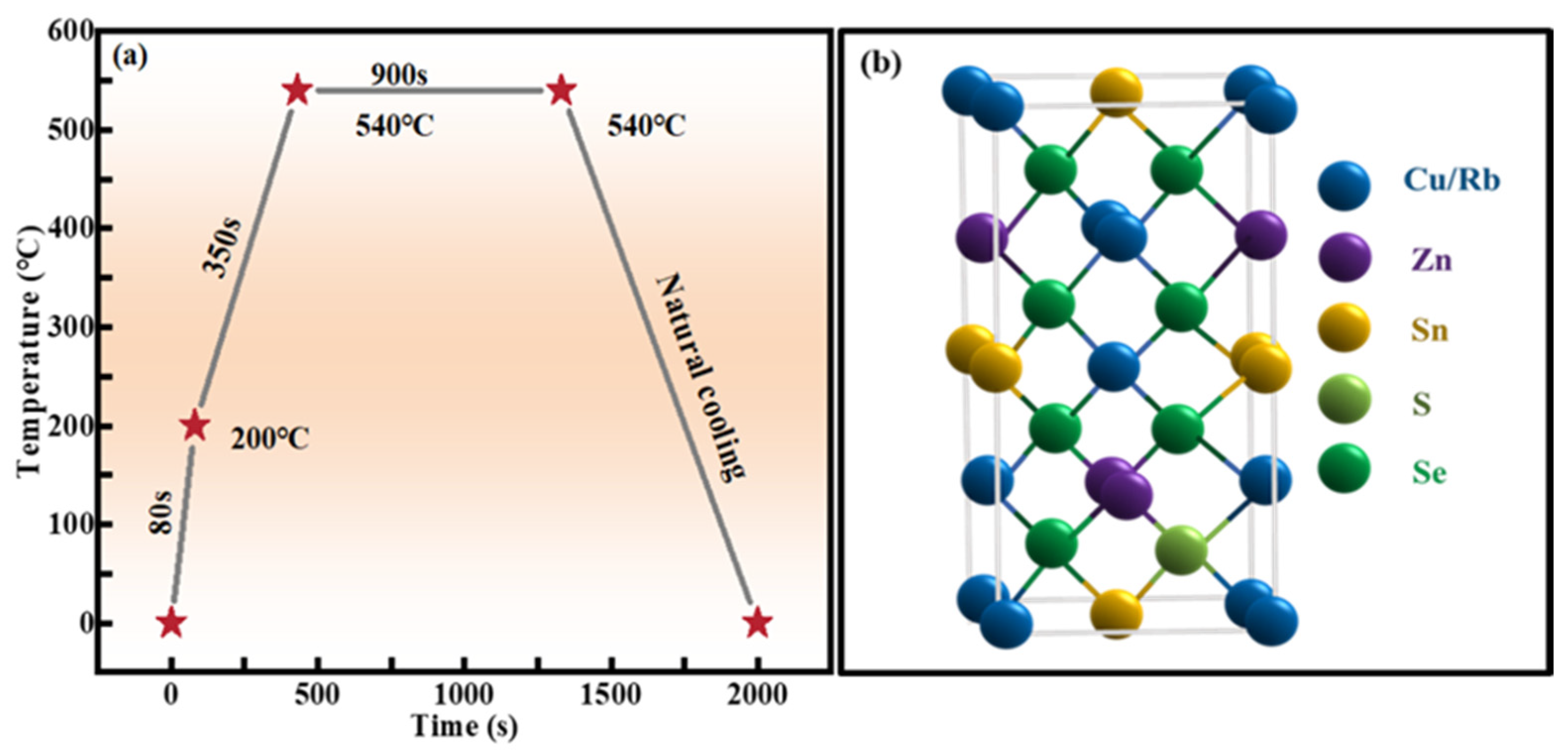
2.1. Structural Characterization
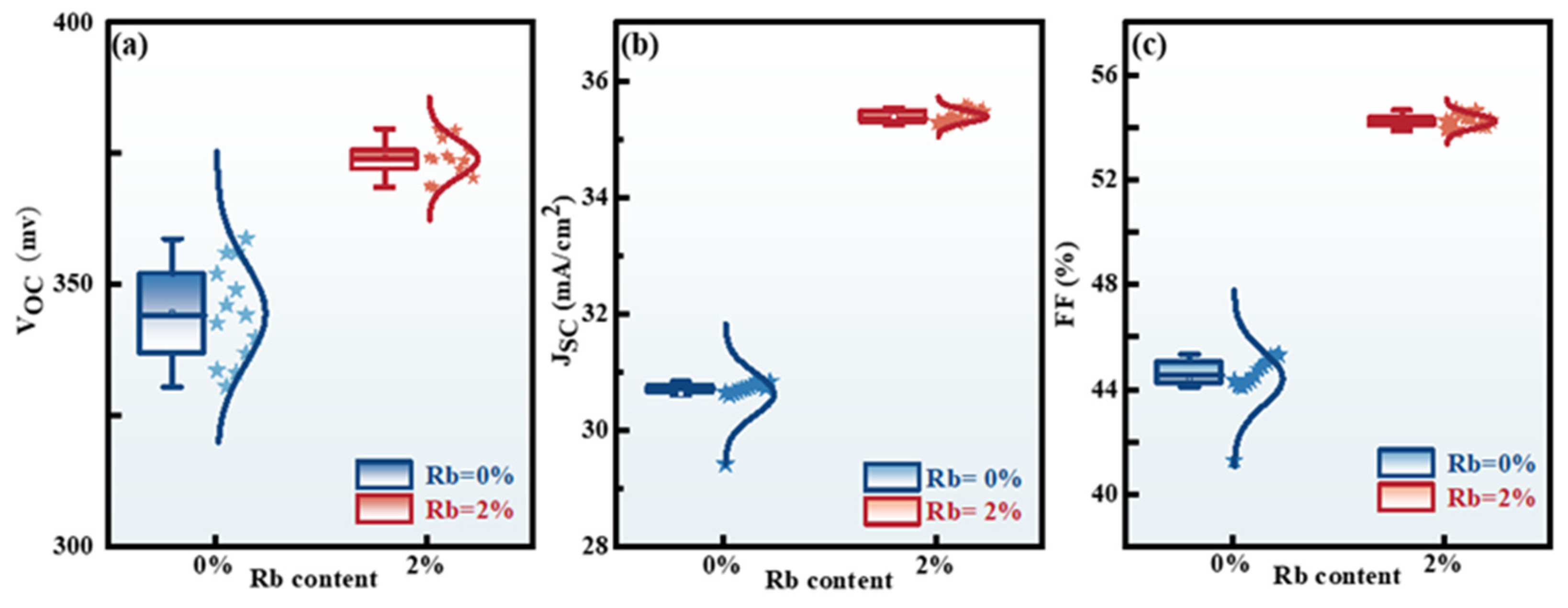
2.2. Photoelectric Characteristics
2.3. Device Characterization
3. Experimental Methods
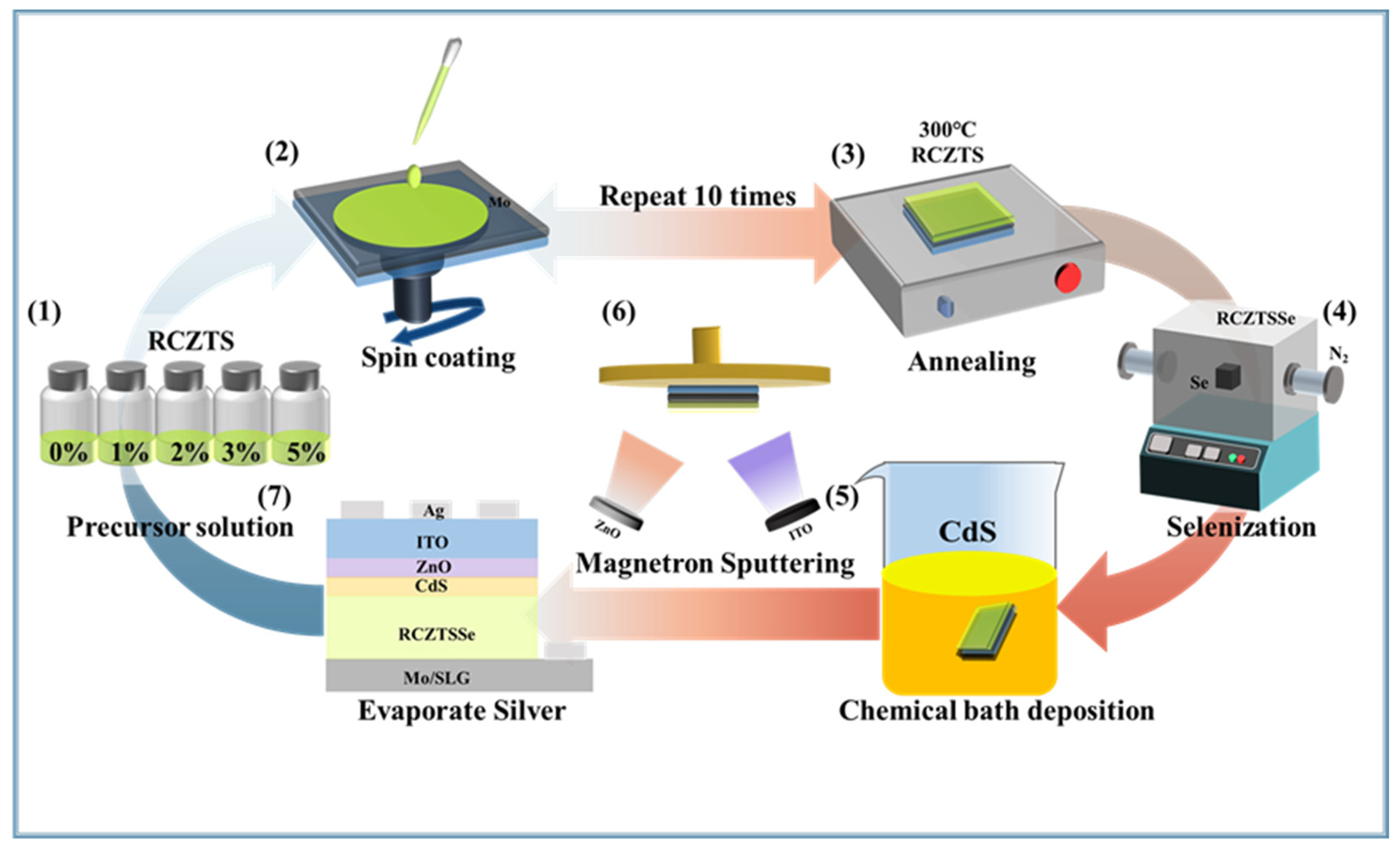
3.1. Preparation of RCZTSSe Thin Films
3.2. Solar Cell Fabrication
3.3. Material and Device Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, H.; Hsu, W.-C.; Duan, H.-S.; Bob, B.; Yang, W.; Song, T.-B.; Hsu, C.-J.; Yang, Y. CZTS nanocrystals: A promising approach for next generation thin film photovoltaics. Energy Environ. Sci. 2013, 6, 2822–2838. [Google Scholar] [CrossRef]
- Xie, W.; Yan, Q.; Sun, Q.; Li, Y.; Zhang, C.; Deng, H.; Cheng, S. A Progress Review on Challenges and Strategies of Flexible Cu2ZnSn(S, Se)4 Solar Cells. Solar RRL 2023, 7, 2201036. [Google Scholar] [CrossRef]
- Li, J.; Sun, K.; Yuan, X.; Huang, J.; Green, M.A.; Hao, X. Emergence of flexible kesterite solar cells: Progress and perspectives. npj Flexible Electron. 2023, 7, 16. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, W.; Zhang, Y.; Liu, S. Path towards high-efficient kesterite solar cells. J. Energy Chem. 2018, 27, 1040–1053. [Google Scholar] [CrossRef]
- Li, W.; Tan, J.M.R.; Leow, S.W.; Lie, S.; Magdassi, S.; Wong, L.H. Recent Progress in Solution-Processed Copper-Chalcogenide Thin-Film Solar Cells. Energy Technol. 2018, 6, 46–59. [Google Scholar] [CrossRef]
- Shockley, W.; Queisser, H.J. Detailed Balance Limit of Efficiency of p-n Junction Solar Cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
- Li, Y.; Cui, C.; Wei, H.; Shao, Z.; Wu, Z.; Zhang, S.; Wang, X.; Pang, S.; Cui, G. Suppressing Element Inhomogeneity Enables 14.9% Efficiency CZTSSe Solar Cells. Adv. Mater. 2024, 36, 2400138. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Yu, L.; Zheng, T.; Dong, X.; Yang, F.; Chen, J.; Zhang, X.; Zhao, Y.; Li, Y. Promoting effect of lanthanum doping on photovoltaic performance of CZTSSe solar cells. J. Chem. Phys. 2024, 160, 164712. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.E.; Hages, C.J.; Agrawal, R.; Lundstrom, M.S.; Gray, J.L. The importance of band tail recombination on current collection and open-circuit voltage in CZTSSe solar cells. Appl. Phys. Lett. 2016, 109, 021102. [Google Scholar] [CrossRef]
- Nisika; Kaur, K.; Kumar, M. Progress and prospects of CZTSSe/CdS interface engineering to combat high open-circuit voltage deficit of kesterite photovoltaics: A critical review. J. Mater. Chem. A 2020, 8, 21547–21584. [Google Scholar] [CrossRef]
- Kangsabanik, M.; Gayen, R.N. A Comprehensive Review on the Recent Strategy of Cation Substitution in CZTS(Se) Thin Films to Achieve Highly Efficient Kesterite Solar Cells. Solar RRL 2023, 7, 2300670. [Google Scholar] [CrossRef]
- Yuan, Z.-K.; Chen, S.; Xiang, H.; Gong, X.-G.; Walsh, A.; Park, J.-S.; Repins, I.; Wei, S.-H. Engineering Solar Cell Absorbers by Exploring the Band Alignment and Defect Disparity: The Case of Cu- and Ag-Based Kesterite Compounds. Adv. Funct. Mater. 2015, 25, 6733–6743. [Google Scholar] [CrossRef]
- Gokmen, T.; Gunawan, O.; Todorov, T.K.; Mitzi, D.B. Band tailing and efficiency limitation in kesterite solar cells. Appl. Phys. Lett. 2013, 103, 103506. [Google Scholar] [CrossRef]
- Yu, X.; Cheng, S.; Yan, Q.; Fu, J.; Jia, H.; Sun, Q.; Yang, Z.; Wu, S. Efficient flexible Mo foil-based Cu2ZnSn(S, Se)4 solar cells from In-doping technique. Sol. Energy Mater. Sol. Cells 2020, 209, 110434. [Google Scholar] [CrossRef]
- Ren, G.; Zhuang, D.; Zhao, M.; Wei, Y.; Wu, Y.; Li, X.; Lyu, X.; Wang, C.; Li, Y. Cu2ZnSn(S, Se)4 solar cell with slight band tailing states achieves 11.83% efficiency by selenizing sputtered Cu–Zn–Sn–S precursor. J. Power Sources 2020, 479, 228747. [Google Scholar] [CrossRef]
- Wei, H.; Li, Y.; Cui, C.; Wang, X.; Shao, Z.; Pang, S.; Cui, G. Defect suppression for high-efficiency kesterite CZTSSe solar cells: Advances and prospects. Chem. Eng. J. 2023, 462, 142121. [Google Scholar] [CrossRef]
- Li, J.; Wang, D.; Li, X.; Zeng, Y.; Zhang, Y. Cation Substitution in Earth-Abundant Kesterite Photovoltaic Materials. Adv. Sci. 2018, 5, 1700744. [Google Scholar] [CrossRef] [PubMed]
- Dhawale, D.S.; Ali, A.; Lokhande, A.C. Impact of various dopant elements on the properties of kesterite compounds for solar cell applications: A status review. Sustain. Energy Fuels 2019, 3, 1365–1383. [Google Scholar] [CrossRef]
- Cabas-Vidani, A.; Haass, S.G.; Andres, C.; Caballero, R.; Figi, R.; Schreiner, C.; Márquez, J.A.; Hages, C.; Unold, T.; Bleiner, D.; et al. High-Efficiency (LixCu1−x)2ZnSn(S,Se)4 Kesterite Solar Cells with Lithium Alloying. Adv. Energy Mater. 2018, 8, 1801191. [Google Scholar] [CrossRef]
- Yang, W.; Ji, Y.; Chen, W.; Pan, Y.; Chen, Z.; Wu, S.; Russo, S.P.; Xu, Y.; Smith, T.A.; Chesman, A.; et al. The Multiple Roles of Na Ions in Highly Efficient CZTSSe Solar Cells. Small 2024, 20, 2307807. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Tao, S.; Zhao, M.; Zhuang, D.; Wang, Y.; Wang, H.; Jia, M.; Han, J.; Zhu, H. Crystallization mechanism and defect passivation of Cu2ZnSn(S,Se)4 thin film solar cells via in situ potassium doping. J. Mater. Chem. A 2023, 11, 20139–20150. [Google Scholar] [CrossRef]
- Jackson, P.; Wuerz, R.; Hariskos, D.; Lotter, E.; Witte, W.; Powalla, M. Effects of heavy alkali elements in Cu(In,Ga)Se2 solar cells with efficiencies up to 22.6%. Phys. Status. Solidi-R 2016, 10, 583–586. [Google Scholar] [CrossRef]
- Ishizuka, S.; Taguchi, N.; Fons, P.J. Similarities and Critical Differences in Heavy Alkali-Metal Rubidium and Cesium Effects on Chalcopyrite Cu(In,Ga)Se2 Thin-Film Solar Cells. J. Phys. Chem. C 2019, 123, 17757–17764. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, M.; Zhuang, D.; Zhang, N.; Yu, X.; Wei, Y.; Lyu, X.; Ren, G.; Wang, C.; Hu, L.; et al. The effect of Rb doping on CZTSSe solar cells. Sol. Energy 2019, 187, 269–273. [Google Scholar] [CrossRef]
- Lee, T.; Hamim Sharif, M.; Enkhbayar, E.; Enkhbat, T.; Salahuddin Mina, M.; Kim, J. Defect Passivation for Kesterite CZTSSe Solar Cells via In Situ Al2O3 Incorporation into the Bulk CZTSSe Absorber. Solar RRL 2022, 6, 2100862. [Google Scholar] [CrossRef]
- Datt, G.; Raja, M.M.; Abhyankar, A.C. Steering of Magnetic Interactions in Ni0.5Zn0.5Fe2–x (Mn)xO4 Nanoferrites via Substitution-Induced Cationic Redistribution. J. Phys. Chem. C 2021, 125, 10693–10707. [Google Scholar] [CrossRef]
- Zhuk, S.; Wong, T.K.S.; Hadke, S.S.; Lie, S.; Guchhait, A.; Gao, Y.; Wong, L.H.; Cheng, S.; Wang, X.; Dalapati, G.K. Molybdenum incorporated Cu1. 69ZnSnS4 kesterite photovoltaic devices with bilayer microstructure and tunable optical-electronic properties. Sol. Energy Mater. Sol. Cells 2019, 194, 777–787. [Google Scholar]
- Mondal, R.; Chandra, G.H.; Subbaiah, Y.P.V.; Gupta, M.; Prasada Rao, R. Insights into the structural and optical properties of (Cu,Ag)2ZnSnSe4 thin films: Role of pre-annealing and selenization times. Opt. Mater. 2024, 154, 115618. [Google Scholar] [CrossRef]
- Farooq, U.; Ali Shah, U.; Ishaq, M.; Hu, J.-G.; Ahmed, S.; Chen, S.; Zheng, Z.-H.; Su, Z.-H.; Fan, P.; Liang, G.-X. Defects passivation by solution-processed titanium doping strategy towards high efficiency kesterite solar cells. Chem. Eng. J. 2023, 451, 139109. [Google Scholar] [CrossRef]
- Qi, Y.-F.; Kou, D.-X.; Zhou, W.-H.; Zhou, Z.-J.; Tian, Q.-W.; Meng, Y.-N.; Liu, X.-S.; Du, Z.-L.; Wu, S.-X. Engineering of interface band bending and defects elimination via a Ag-graded active layer for efficient (Cu,Ag)2ZnSn(S,Se)4 solar cells. Energy Environ. Sci. 2017, 10, 2401–2410. [Google Scholar] [CrossRef]
- Cui, C.; Fu, J.; Kou, D.; Li, Y.; Wei, H.; Wu, Z.; Zhou, W.; Zhou, Z.; Yuan, S.; Qi, Y.; et al. Heterojunction reconstruction via In doping towards high-efficiency CZTSSe solar cells. Chem. Eng. J. 2023, 476, 146701. [Google Scholar] [CrossRef]
- Du, Y.; Tian, Q.; Huang, J.; Zhao, Y.; Chang, X.; Zhang, A.; Wu, S. Heterovalent Ga3+ doping in solution-processed Cu 2 ZnSn (S, Se) 4 solar cells for better optoelectronic performance. Sustainable Energy Fuels 2020, 4, 1621–1629. [Google Scholar] [CrossRef]
- Danilson, M.; Altosaar, M.; Kauk, M.; Katerski, A.; Krustok, J.; Raudoja, J. XPS study of CZTSSe monograin powders. Thin Solid Films 2011, 519, 7407–7411. [Google Scholar] [CrossRef]
- Tsega, M.; Dejene, F.B.; Kuo, D.-H. Morphological evolution and structural properties of Cu2ZnSn(S,Se)4 thin films deposited from single ceramic target by a one-step sputtering process and selenization without H2Se. J. Alloys Compd. 2015, 642, 140–147. [Google Scholar] [CrossRef]
- Fu, J.; Tian, Q.; Zhou, Z.; Kou, D.; Meng, Y.; Zhou, W.; Wu, S. Improving the performance of solution-processed Cu2ZnSn(S, Se)4 photovoltaic materials by Cd2+ substitution. Chem. Mater. 2016, 28, 5821–5828. [Google Scholar] [CrossRef]
- Zhao, J.; Liang, G.-X.; Hu, J.-G.; Zheng, Z.-H.; Luo, J.-T.; Zhang, D.-P.; Zeng, Y.; Fan, P. Substrate Temperature-Assisted Preparation of CZTSSe Thin Films by a Single Quinary Target. J. Electron. Mater. 2018, 47, 873–878. [Google Scholar] [CrossRef]
- Maticiuc, N.; Kodalle, T.; Lauche, J.; Wenisch, R.; Bertram, T.; Kaufmann, C.A.; Lauermann, I. In vacuo XPS investigation of Cu(In,Ga)Se2 surface after RbF post-deposition treatment. Thin Solid Films 2018, 665, 143–147. [Google Scholar] [CrossRef]
- Chander, S.; Dhaka, M.S. CdCl2 treatment concentration evolution of physical properties correlation with surface morphology of CdTe thin films for solar cells. Mater. Res. Bull. 2018, 97, 128–135. [Google Scholar] [CrossRef]
- Datt, G.; Abhyankar, A. Dopant driven tunability of dielectric relaxation in MxCo(1-x)Fe2O4 (M: Zn2+, Mn2+, Ni2+) nano-ferrites. J. Appl. Phys. 2017, 122, 034102. [Google Scholar] [CrossRef]
- Hsieh, Y.-T.; Han, Q.; Jiang, C.; Song, T.-B.; Chen, H.; Meng, L.; Zhou, H.; Yang, Y. Efficiency Enhancement of Cu2ZnSn(S,Se)4 Solar Cells via Alkali Metals Doping. Adv. Energy Mater. 2016, 6, 1502386. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, X.; Duan, B.; Wu, H.; Shi, J.; Luo, Y.; Li, D.; Meng, Q. Regulating crystal growth via organic lithium salt additive for efficient Kesterite solar cells. Nano Energy 2021, 89, 106405. [Google Scholar] [CrossRef]
- Zeng, C.; Liang, Y.; Zeng, L.; Zhang, L.; Zhou, J.; Huang, P.; Hong, R. Effect of S/(S + Se) ratio during the annealing process on the performance of Cu2ZnSn(S, Se)4 solar cells prepared by sputtering from a quaternary target. Sol. Energy Mater. Sol. Cells 2019, 203, 110167. [Google Scholar] [CrossRef]
- Yu, Q.; Shi, J.; Guo, L.; Duan, B.; Luo, Y.; Wu, H.; Li, D.; Meng, Q. Eliminating multi-layer crystallization of Cu2ZnSn(S, Se)4 absorber by controlling back interface reaction. Nano Energy 2020, 76, 105042. [Google Scholar] [CrossRef]
- Tlili, M.; Jebbari, N.; Naffouti, W.; Kamoun, N.T. Effect of precursor nature on physical properties of chemically sprayed MgO thin films for optoelectronic application. Eur. Phys. J. Plus 2020, 135, 687. [Google Scholar] [CrossRef]
- Khadka, D.B.; Kim, J. Structural transition and band gap tuning of Cu2(Zn, Fe)SnS4 chalcogenide for photovoltaic application. J. Phys. Chem. C 2014, 118, 14227–14237. [Google Scholar] [CrossRef]
- Qi, Y.; Tian, Q.; Meng, Y.; Kou, D.; Zhou, Z.; Zhou, W.; Wu, S. Elemental Precursor Solution Processed (Cu1–xAgx)2ZnSn(S, Se)4 Photovoltaic Devices with over 10% Efficiency. ACS Appl. Mater. Interfaces 2017, 9, 21243–21250. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, X.; Zhang, P.; Yuan, X.; Huang, F.; Zhang, W. Structural properties and quasiparticle band structures of Cu-based quaternary semiconductors for photovoltaic applications. J. Appl. Phys. 2012, 111, 063709. [Google Scholar] [CrossRef]
- Chen, S.; Walsh, A.; Luo, Y.; Yang, J.-H.; Gong, X.; Wei, S.-H. Wurtzite-derived polytypes of kesterite and stannite quaternary chalcogenide semiconductors. Phys. Rev. B 2010, 82, 195203. [Google Scholar] [CrossRef]
- Li, Y.; Wei, H.; Cui, C.; Wang, X.; Shao, Z.; Pang, S.; Cui, G. CZTSSe solar cells: Insights into interface engineering. J. Mater. Chem. A 2023, 11, 4836–4849. [Google Scholar] [CrossRef]
- Maurya, K.; Singh, V. Sb2Se3/CZTS dual absorber layer based solar cell with 36.32% efficiency: A numerical simulation. J. Sci. Adv. Mater. Devices 2022, 7, 100445. [Google Scholar]
- Son, D.-H.; Kim, Y.-I.; Kim, S.-H.; Nam, D.; Cheong, H.; Kang, J.-K.; Yang, K.-J.; Kim, D.-H. Effects of S and Se contents on the physical and photovoltaic properties of Cu2ZnSn(SX, Se1−X)4 thin films: Achieving a PCE of 9.47%. J. Mater. Chem. A 2019, 7, 22986–22995. [Google Scholar] [CrossRef]
- Du, Y.; Wang, S.; Tian, Q.; Zhao, Y.; Chang, X.; Xiao, H.; Deng, Y.; Chen, S.; Wu, S.; Liu, S. Defect Engineering in Earth-Abundant Cu2ZnSn(S,Se)4 Photovoltaic Materials via Ga3+-Doping for over 12% Efficient Solar Cells. Adv. Funct. Mater. 2021, 31, 2010325. [Google Scholar] [CrossRef]
- Andrade-Arvizu, J.; Fonoll-Rubio, R.; Sánchez, Y.; Becerril-Romero, I.; Malerba, C.; Valentini, M.; Calvo-Barrio, L.; Izquierdo-Roca, V.; Placidi, M.; Vigil-Galán, O. Rear band gap grading strategies on Sn–Ge-alloyed kesterite solar cells. J. Alloys Compd. 2020, 3, 10362–10375. [Google Scholar] [CrossRef]
- Ahmad, F.; Lakhtakia, A.; Anderson, T.H.; Monk, P.B. Towards highly efficient thin-film solar cells with a graded-bandgap CZTSSe layer. J. Phys. Energy 2020, 2, 025004. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, C.; Yang, T.; Zhao, Y.; Na, Y.; Jiang, C.; Zhou, J.; Pang, J.; Liu, H.; Rummeli, M.H. Gradient bandgap modification for highly efficient carrier transport in antimony sulfide-selenide tandem solar cells. Sol. Energy Mater. Sol. Cells 2022, 246, 111926. [Google Scholar] [CrossRef]
- Meng, L.; Yao, B.; Li, Y.; Ding, Z.; Xiao, Z.; Liu, K.; Wang, G. Significantly enhancing back contact adhesion and improving stability of Cu2(Zn, Cd)Sn(S, Se)4 solar cell by a rational carbon doping strategy. J. Alloys Compd. 2017, 710, 403–408. [Google Scholar] [CrossRef]
- Mattheis, J.; Rostan, P.; Rau, U.; Werner, J. Carrier collection in Cu(In, Ga)Se2 solar cells with graded band gaps and transparent ZnO: Al back contacts. Sol. Energy Mater. Sol. Cells 2007, 91, 689–695. [Google Scholar] [CrossRef]
- Wu, Y.; Sui, Y.; He, W.; Zeng, F.; Wang, Z.; Wang, F.; Yao, B.; Yang, L. Substitution of Ag for Cu in Cu2ZnSn(S, Se)4: Toward wide band gap absorbers with low antisite defects for thin film solar cells. Nano Mater. 2020, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Y.; Ishaq, M.; Ahmad, N.; Tang, R.; Zheng, Z.-H.; Hu, J.-G.; Su, Z.-H.; Fan, P.; Liang, G.-X.; Chen, S. Ag, Ti dual-cation substitution in Cu2ZnSn(S, Se)4 induced growth promotion and defect suppression for high-efficiency solar cells. J. Mater. Chem. A 2022, 10, 22791–22802. [Google Scholar] [CrossRef]
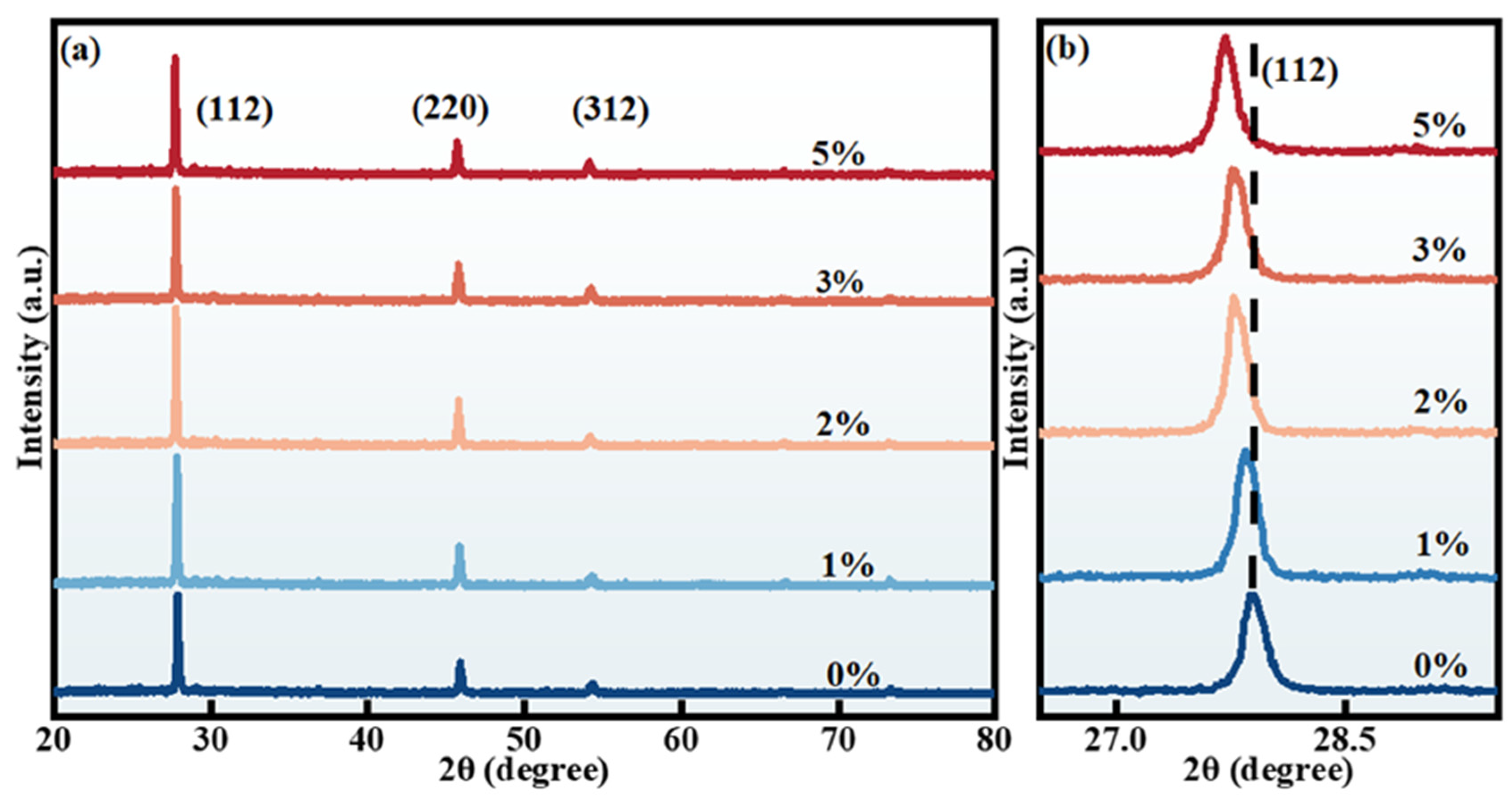
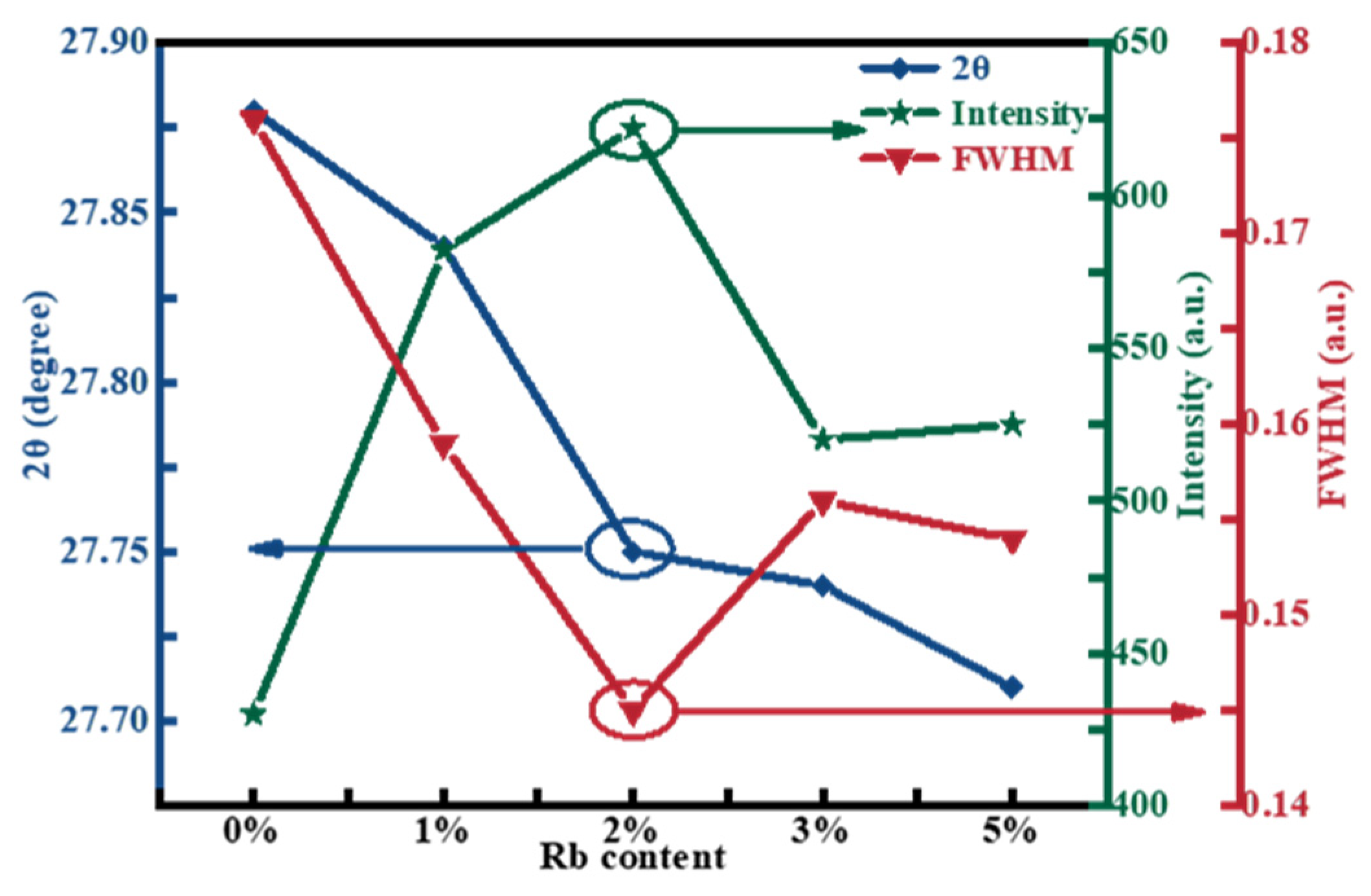

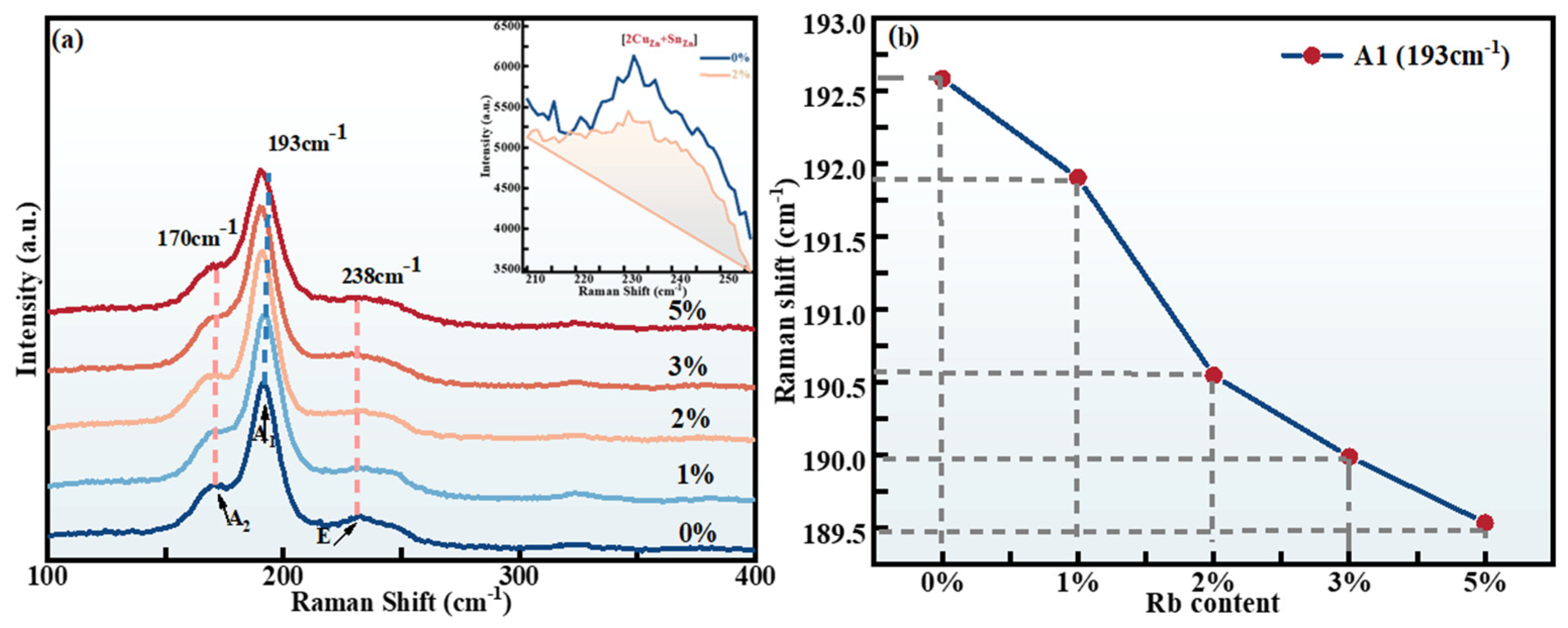
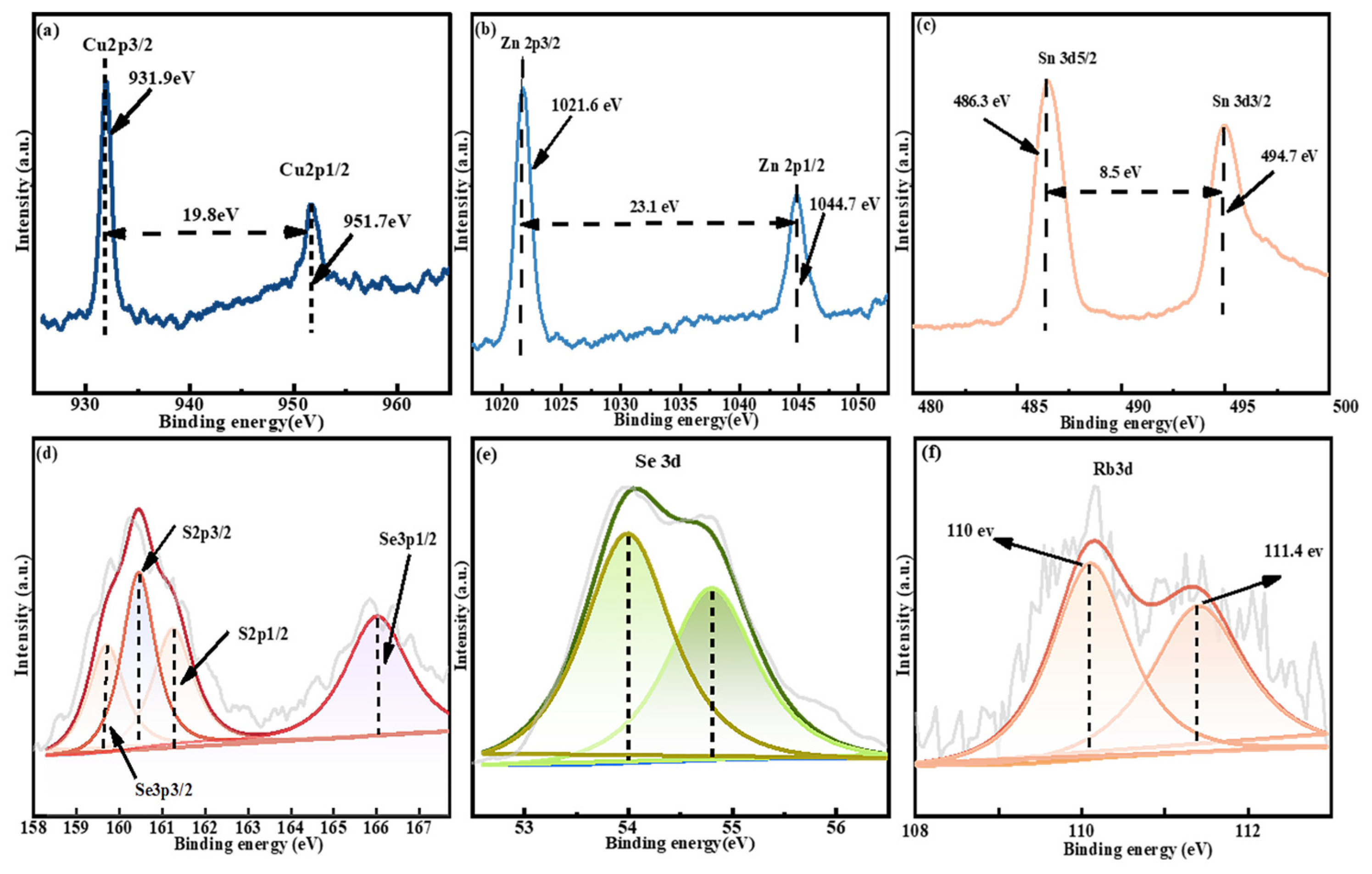
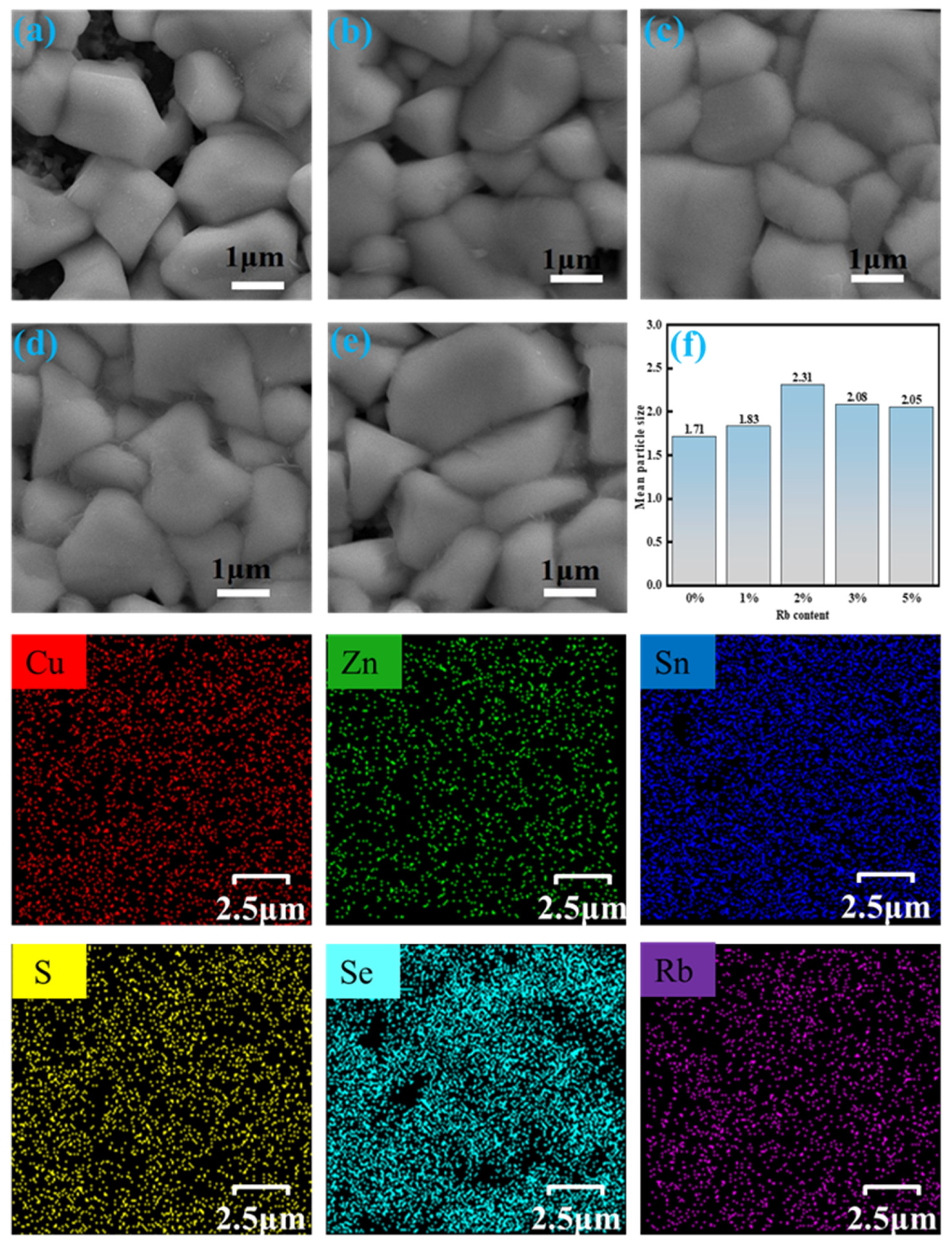
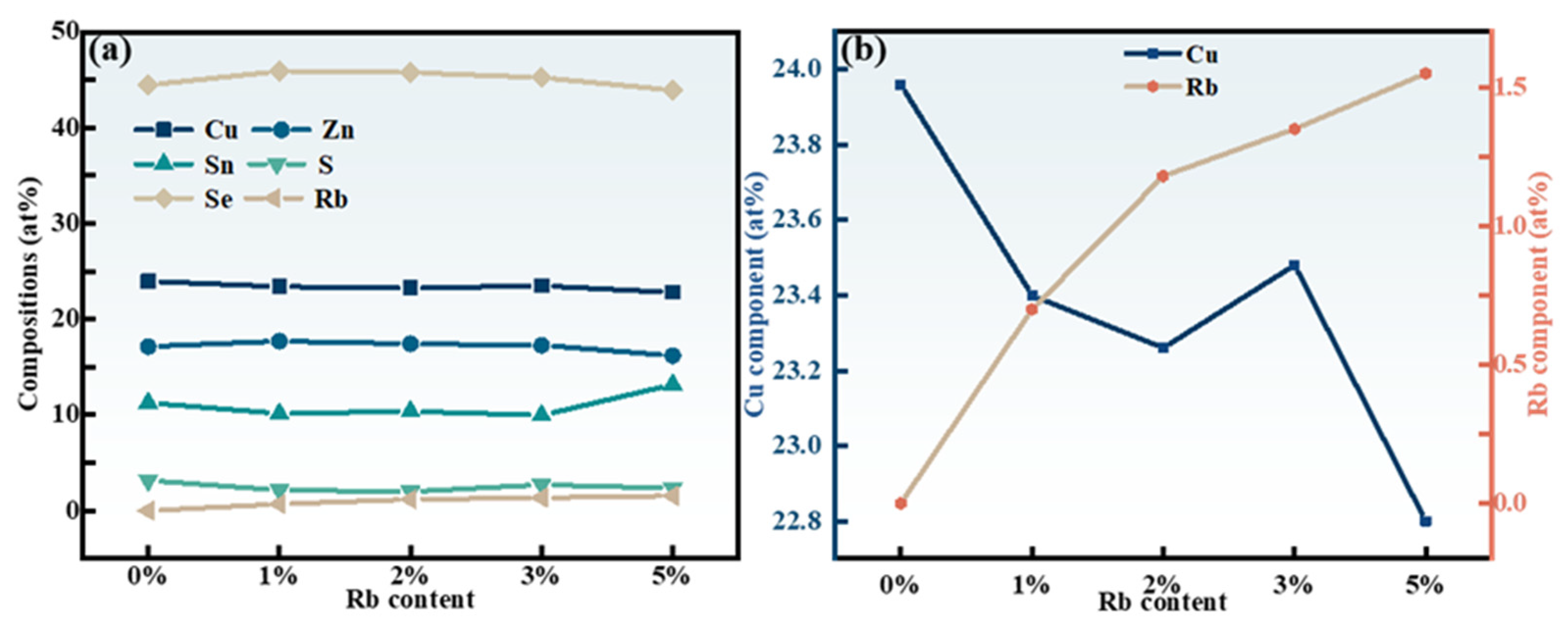
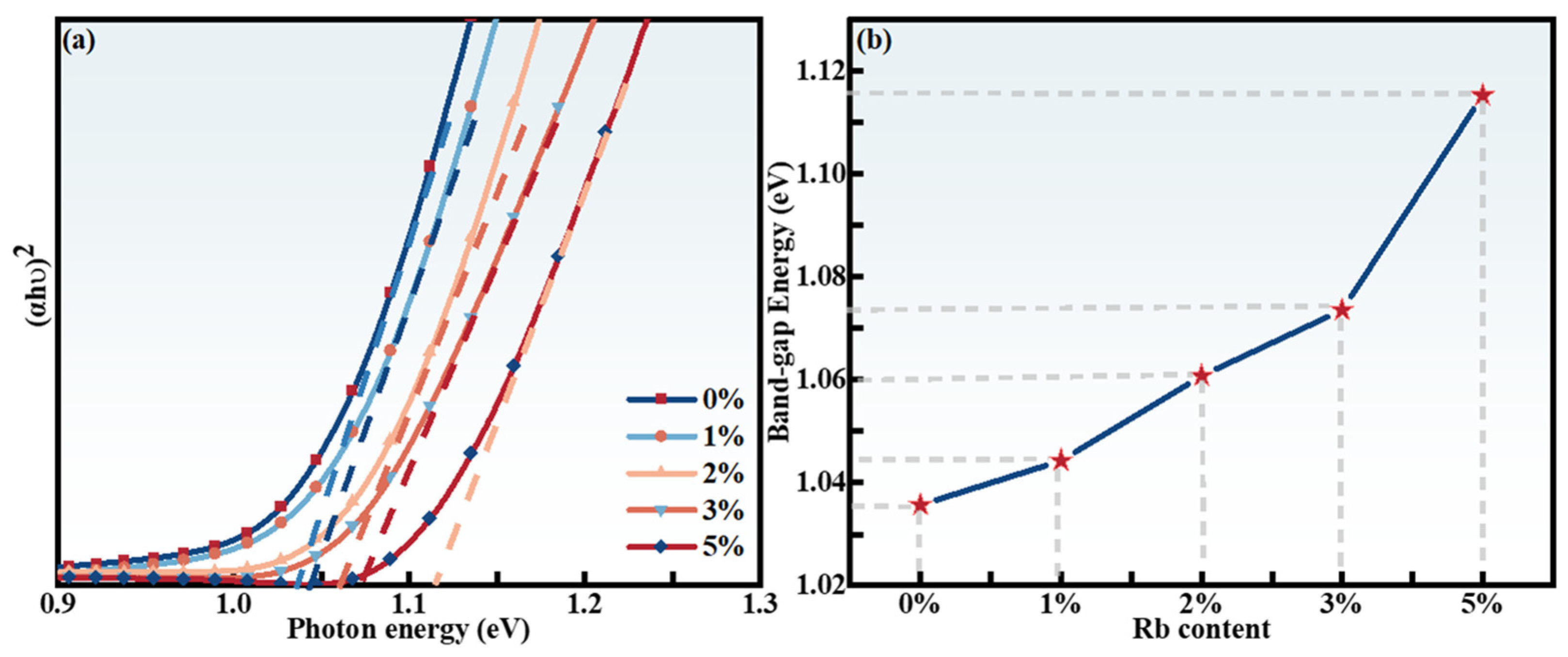
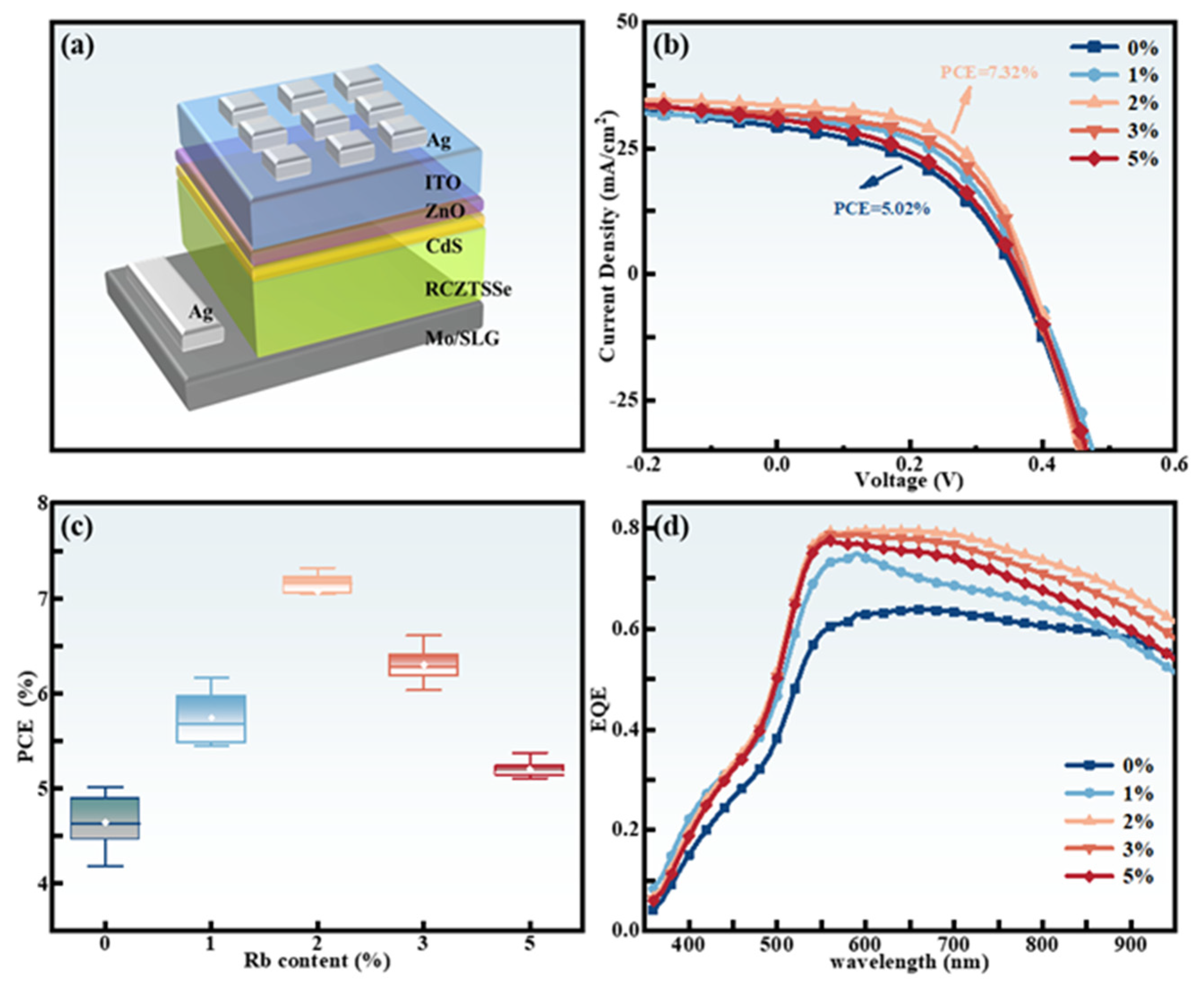
| x | Composition(%at) | Cu + Rb/(Zn + Sn) | Rb/(Rb + Cu) | |||||
|---|---|---|---|---|---|---|---|---|
| Cu | Zn | Sn | S | Se | Rb | |||
| 0 | 23.96 ± 0.1 | 17.15 ± 0.28 | 11.27 ± 0.13 | 3.15 ± 0.39 | 44.47 ± 0.91 | 0 | 0.84 ± 0.01 | 0 |
| 0.01 | 23.40 ± 0.03 | 17.71 ± 0.59 | 10.15 ± 0.47 | 2.15 ± 0.49 | 45.89 ± 0.44 | 0.70 ± 0.13 | 0.87 ± 0.01 | 0.03 |
| 0.02 | 23.26 ± 0.23 | 17.43 ± 0.45 | 10.37 ± 0.45 | 1.99 ± 0.52 | 45.77 ± 0.63 | 1.18 ± 0.35 | 0.88 | 0.05 ± 0.01 |
| 0.03 | 23.48 ± 0.16 | 17.27 ± 0.11 | 9.96 ± 0.29 | 2.71 ± 0.51 | 45.23 ± 0.38 | 1.35 ± 0.12 | 0.91 ± 0.02 | 0.05 |
| 0.05 | 22.80 ± 0.45 | 16.2 ± 0.71 | 13.18 ± 0.77 | 2.35 ± 0.01 | 43.92 ± 0.57 | 1.55 ± 0.18 | 0.83 ± 0.02 | 0.07 ± 0.01 |
| Samples | Resistivity (Ω·cm2) | Carrier Concentration (cm−3) | Mobility (cm2V−1s−1) | Type |
|---|---|---|---|---|
| Rb = 0% | 9.96 | 6.26 × 1017 | 2.23 × 101 | p |
| Rb = 1% | 2.94 × 101 | 2.13 × 1017 | 4.83 × 101 | p |
| Rb = 2% | 4.91 × 101 | 1.27 × 1017 | 6.64 × 101 | p |
| Rb = 3% | 1.43 × 101 | 4.37 × 1017 | 1.60 × 101 | P |
| Rb = 5% | 1.22 | 7.28 × 1018 | 7.03 × 10−1 | P |
| Device. | Active Area (cm2) | VOC (mV) | JSC (mA/cm−2) | FF (%) | PCE (%) | Rs (Ω·cm2) | Rsh(Ω·cm2) |
|---|---|---|---|---|---|---|---|
| CZTSSe (Rb = 0%) | 0.19 | 358.75 ± 7.37 | 30.84 ± 0.81 | 45.33 ± 2.17 | 5.02 ± 0.44 | 19.36 ± 1.16 | 279.76 ± 28.19 |
| CZTSSe (Rb = 1%) | 0.19 | 374.73 ± 12.03 | 32.33 ± 2.35 | 50.92 ± 0.72 | 6.17 ± 0.34 | 16.84 ± 1.84 | 641.44 ± 66.31 |
| CZTSSe (Rb = 2%) | 0.19 | 379.69 ± 3.99 | 35.27 ± 0.16 | 54.66 ± 0.31 | 7.32 ± 0.10 | 12.66 ± 0.47 | 690.58 ± 21.85 |
| CZTSSe (Rb = 3%) | 0.19 | 371.03 ± 12.72 | 33.52 ± 3.07 | 53.12 ± 1.02 | 6.61 ± 0.65 | 12.87 ± 2.14 | 526.30 ± 44.5 |
| CZTSSe (Rb = 5%) | 0.19 | 366.11 ± 3.17 | 32.32 ± 0.59 | 45.55 ± 0.13 | 5.39 ± 0.13 | 19.78 ± 0.52 | 272.59 ± 4.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, C.; Sui, Y.; Cui, Y.; Wang, Z.; Yang, L.; Wang, F.; Liu, X.; Yao, B. Insight into the Role of Rb Doping for Highly Efficient Kesterite Cu2ZnSn(S,Se)4 Solar Cells. Molecules 2024, 29, 3670. https://doi.org/10.3390/molecules29153670
Miao C, Sui Y, Cui Y, Wang Z, Yang L, Wang F, Liu X, Yao B. Insight into the Role of Rb Doping for Highly Efficient Kesterite Cu2ZnSn(S,Se)4 Solar Cells. Molecules. 2024; 29(15):3670. https://doi.org/10.3390/molecules29153670
Chicago/Turabian StyleMiao, Chang, Yingrui Sui, Yue Cui, Zhanwu Wang, Lili Yang, Fengyou Wang, Xiaoyan Liu, and Bin Yao. 2024. "Insight into the Role of Rb Doping for Highly Efficient Kesterite Cu2ZnSn(S,Se)4 Solar Cells" Molecules 29, no. 15: 3670. https://doi.org/10.3390/molecules29153670
APA StyleMiao, C., Sui, Y., Cui, Y., Wang, Z., Yang, L., Wang, F., Liu, X., & Yao, B. (2024). Insight into the Role of Rb Doping for Highly Efficient Kesterite Cu2ZnSn(S,Se)4 Solar Cells. Molecules, 29(15), 3670. https://doi.org/10.3390/molecules29153670





