Abstract
Microwave-assisted de-emulsification is attractive in the processes of petroleum production and refining. The main advantage of microwaves is their direct influence on the surfactant layer at the oil/water interface. Previously, an effective interfacial modification was demonstrated by pulsed microwave irradiation. However, the effect of the modification diminished during the off interval of the pulse irradiation. In this study, two-stage microwave irradiation with different powers and durations was applied as a method to maintain an interfacial effect. The power of the second stage was changed to optimise the modification. Quick modification was obtained by high-power irradiation followed by low-power irradiation. It was confirmed a sustained modification was maintained by a moderate power of the second irradiation. This observation indicates a re-adsorption or re-structure process after the first irradiation is suppressed by the second irradiation. The results open new opportunities to optimise microwave operation in oil/water systems.
1. Introduction
In multiple-phase fluids, microwaves can pass through the non-polar phase and directly reach the polar liquid. Consequently, a polar molecule such as water absorbs the microwave directly, and the molecules near the interface are vibrated or rotated significantly. As a result, microwave heating is faster and more energy-efficient than conventional heating. Furthermore, at an oil/water interface, microwaves can have additional heating effects due to the differences in thermal and dielectric properties between these components. The effect is particularly strong for a surfactant layer at the oil/water interface. Such a layer is very important to stabilise or destabilise emulsions.
The use of microwaves has been proposed as part of a new de-emulsification technique for petroleum production and refining due to the thermal energy concentration at the oil/water interface [1,2,3]. In contrast, conventional heating requires significant energy to heat the oil phase, which remains largely unheated in microwave operation. Energy efficiency is particularly high for mixtures with a high oil content, such as crude oil with small dispersed water droplets [4]. Microwave-assisted interfacial modifications are also beneficial to oil extraction from natural products [5,6]. The food industries also employ non-ionic surfactants to generate food-grade emulsions [7]. In this case, undesirable de-emulsification may reduce food qualities such as taste and aroma. Similarly, microwave-assisted extraction can improve the efficiency of bioactive component production in the pharmaceutical industry [8]. Microwaves can also enhance solid/oil separation and reduce agricultural waste [9].
In the above processes, modification at the interface results in the quick thermal response of microwave absorbance [10,11,12,13,14], which is not seen with slower thermal conduction. Using these characteristics, undesirable emulsions formed during petroleum production and refining can be removed efficiently. In these instances, microwave heating can cause the surfactant layer to desorb from the surface and thus reduce surface viscosity and enhance phase separation [8].
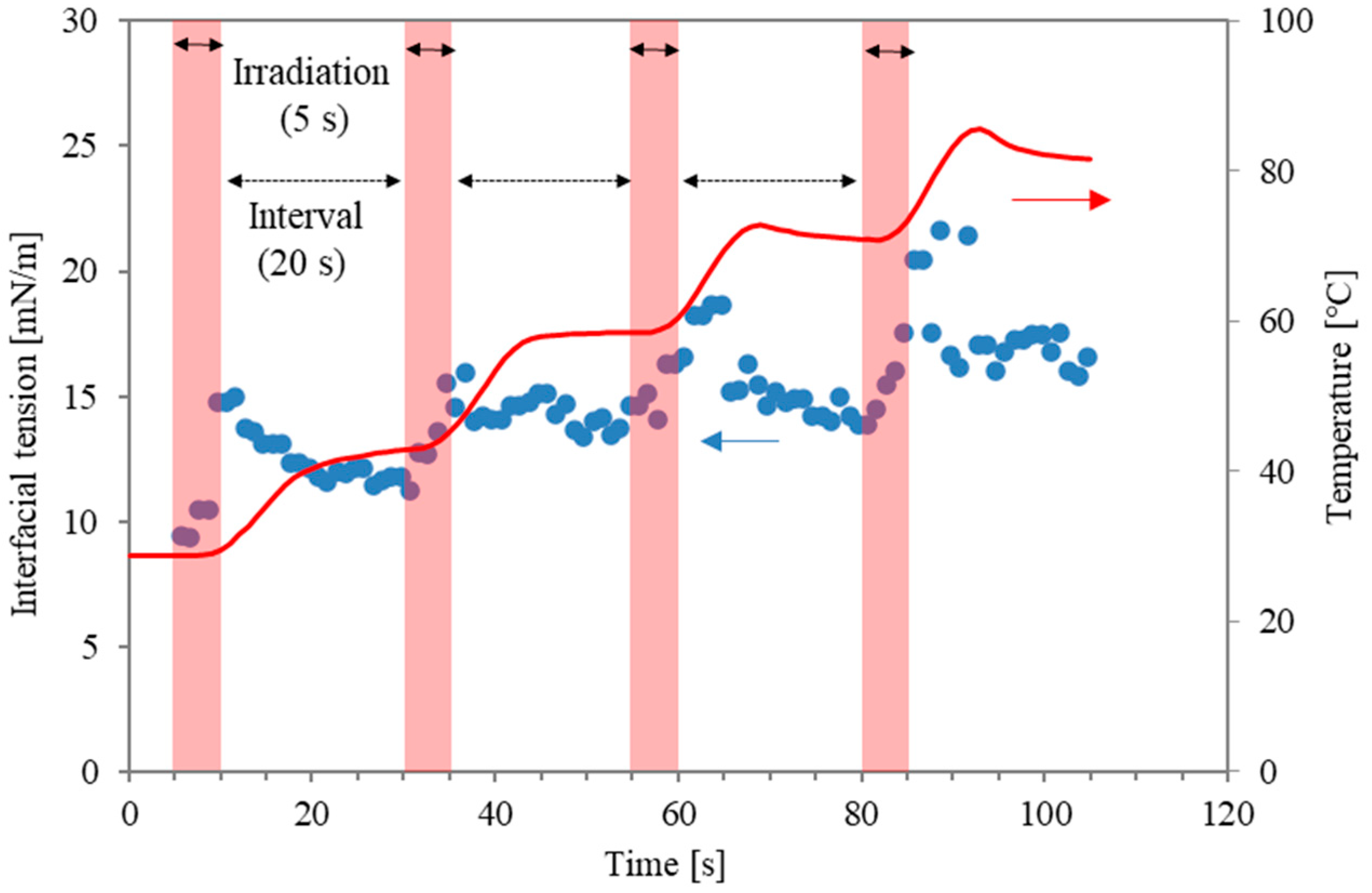
On the other hand, the prevention of unstable operations, such as the boiling behaviour caused by microwave local heating, remains a challenge. Recently, a dimensionless number related to the energy concentration of microwave absorbance was proposed to characterise stable operation, prevent boiling behaviour, and clarify the mechanism of the interfacial modification [14,15]. In the case of continuous microwave irradiation, the overheating effect can make the interfacial modification less effective than conventional heating. For example, local boiling with micro-bubbles in the water phase can occur earlier than surfactant desorption. Accordingly, to optimise the special effects of microwaves for surfactant desorption around the interface, pulsed irradiation has been proposed in previous studies [12,13,14]. Figure 1 shows an example of interfacial tension between water with a surfactant (Triton X-100; 0.2 mM, Kishida Chemical Co., Osaka, Japan) and n-decane during pulse irradiation when the irradiation time and non-irradiation time were 5 s and 20 s [16]. The data represent temperature and interfacial tension, respectively. Although quick modification was obtained during the irradiation time (as shown in the red areas), it was inevitable that the interfacial tension was reduced during the non-radiation intervals. In other words, surfactant molecules from the interface that are desorbed or disordered due to the microwaves will be re-adsorbed or re-structured during the non-irradiation intervals. Accordingly, the irradiation pattern can be improved to prolong the interfacial modification level. It is noteworthy that the microwave power in the previous study was maintained at the same level for all irradiation periods.
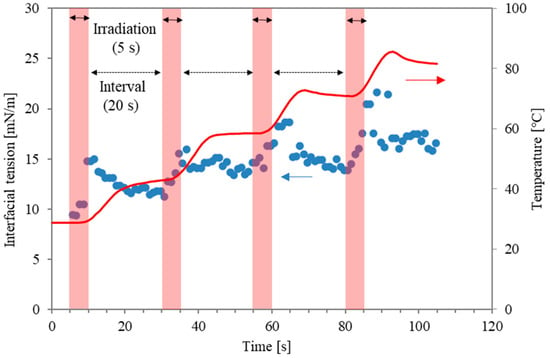
Figure 1.
Interfacial tension profiles and temperature as a function of time. The red arrows refer to temperature on the right axis, and the blue arrows refer to interfacial tension on the left axis.
In addition to exposure time, another advantage of microwave reactors is the ability to fine-tune power. The the flexibility of microwave sources enables tailored heating profiles for specific materials and reactions. In this study, two-stage irradiation, which consists of a higher power and subsequent lower power, was employed to examine this effect. The two-staged heating with different power levels offers flexibility to optimize the operation. In the on–off pulse irradiation mentioned above, the microwave effect disappears during the intervals. The proposed two-stage irradiation intends to compensate for this drawback while avoiding undesirable boiling. Ultimately, the study aims to optimize heating operations—that is, maximize the interfacial modification while limiting local boiling.
2. Results
The experiment consists of three sets of conditions. The first set includes a constant first irradiation period of 5 s and 480 W, followed by a second stage with a different level of power. The second set consists of continuous irradiation. The third set consists of a variable first stage of irradiation followed by a constant second stage. The details of power levels and irradiation times are listed in Table 1, Table 2 and Table 3, respectively. The total energy in all experiments was maintained at 4800 J.

Table 1.
Experimental conditions for different levels of secondary power in two-stage irradiation.

Table 2.
Experimental conditions for continuous mode.

Table 3.
Experimental conditions for different levels of power in the first stage of two-stage irradiation.
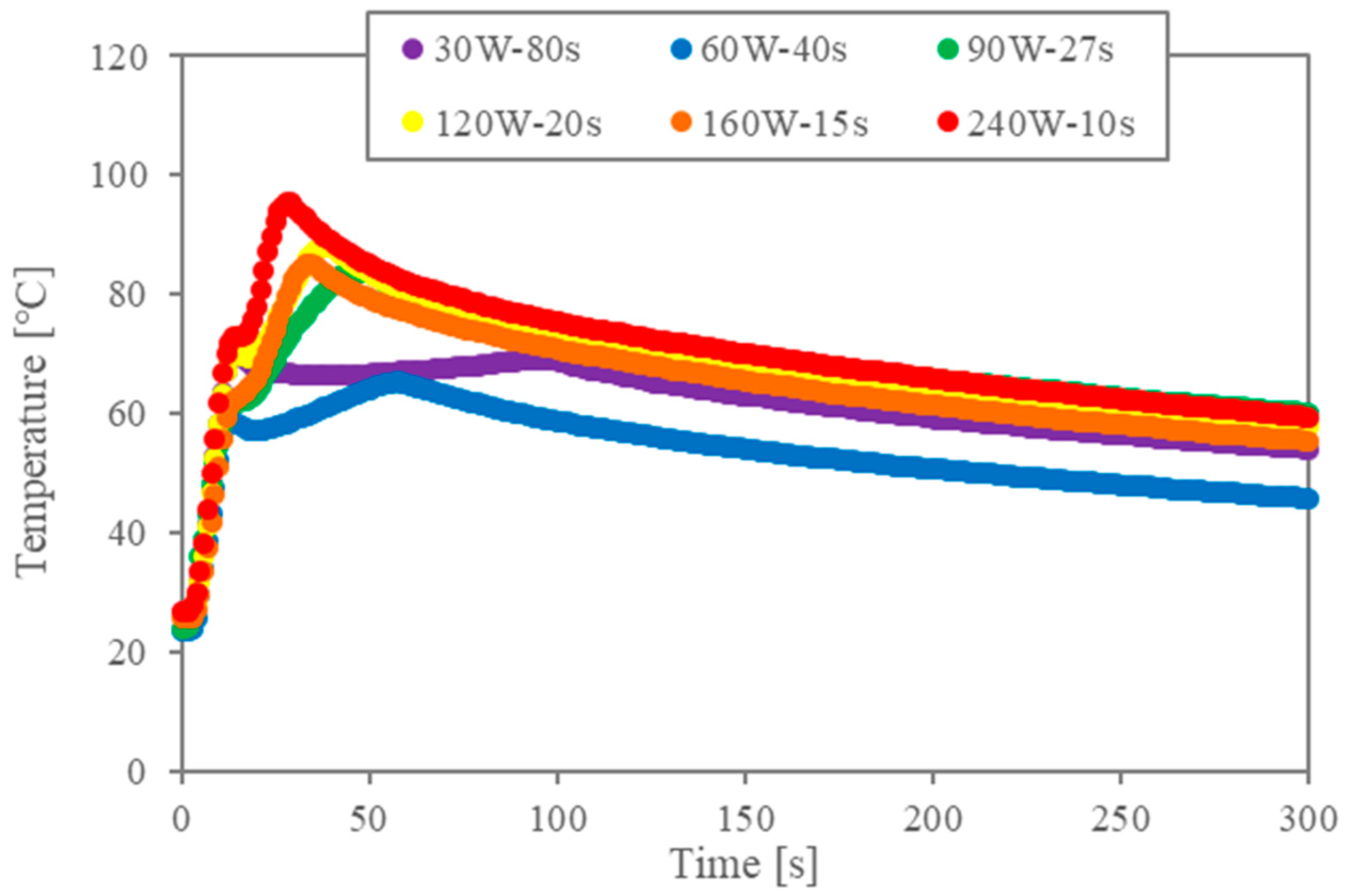
First, the temperature data are analysed to validate the impact of the multiple-stage microwaves. Figure 2a shows the temperature profiles for the two-stage irradiation (corresponding to Table 1). The legend indicates the conditions for the second stage of irradiation when the first irradiation is 480 W and 5 s. Since the power of the first stage is large, the temperature rises rapidly under all conditions. Microwaves are absorbed by the cell walls and the liquid–liquid interface, and the temperature continues to rise during the second irradiation due to both the thermal conduction of heat generation by the first generation and the heat generation of the second irradiation. However, when the power of the second irradiation is small, the higher temperature obtained by the first irradiation cannot be maintained. In any case, the temperature reaches maximum temperature and decreases slowly after the second irradiation is turned off. Although the total energy is the same, the maximum temperature and the time to reach it are different.
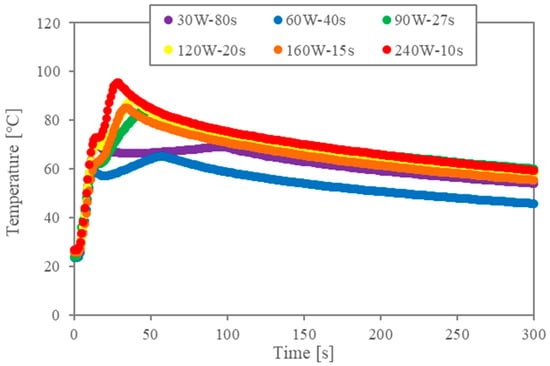
Figure 2.
Temperature profiles for two-stage irradiation trials (in all instances, the first stage consisted of 5 s of irradiation at 480 W).
In previous studies [10,11,12,13,14,16], levels of interfacial tension during microwave heating and conventional heating were compared through the relation between interfacial tension and temperature. The energy concentration of the microwaves during the irradiation at the interface is essential for the interfacial modification. Accordingly, the temperature profiles in Figure 2 show that the energy concentration becomes lower when the second power level is lower.
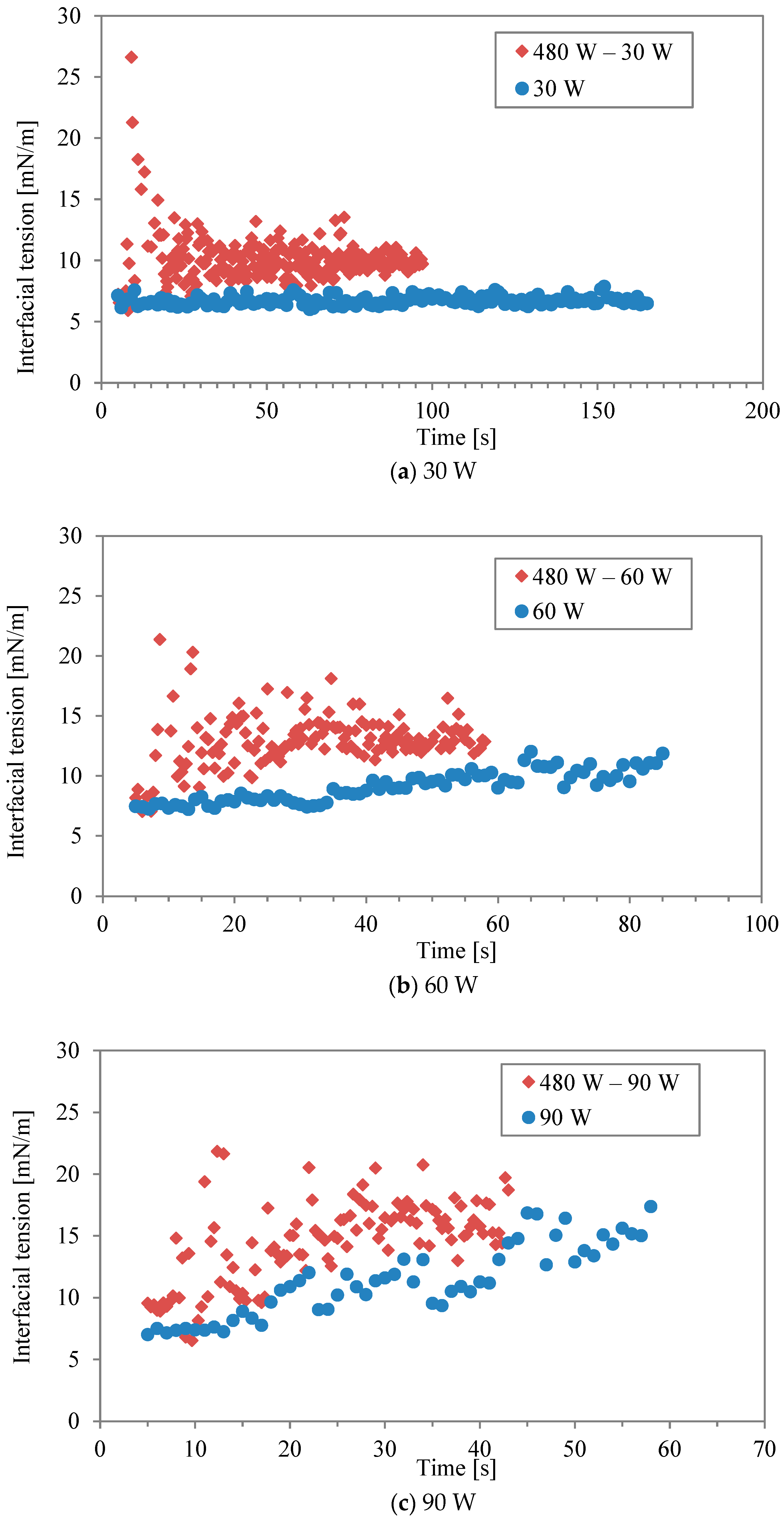
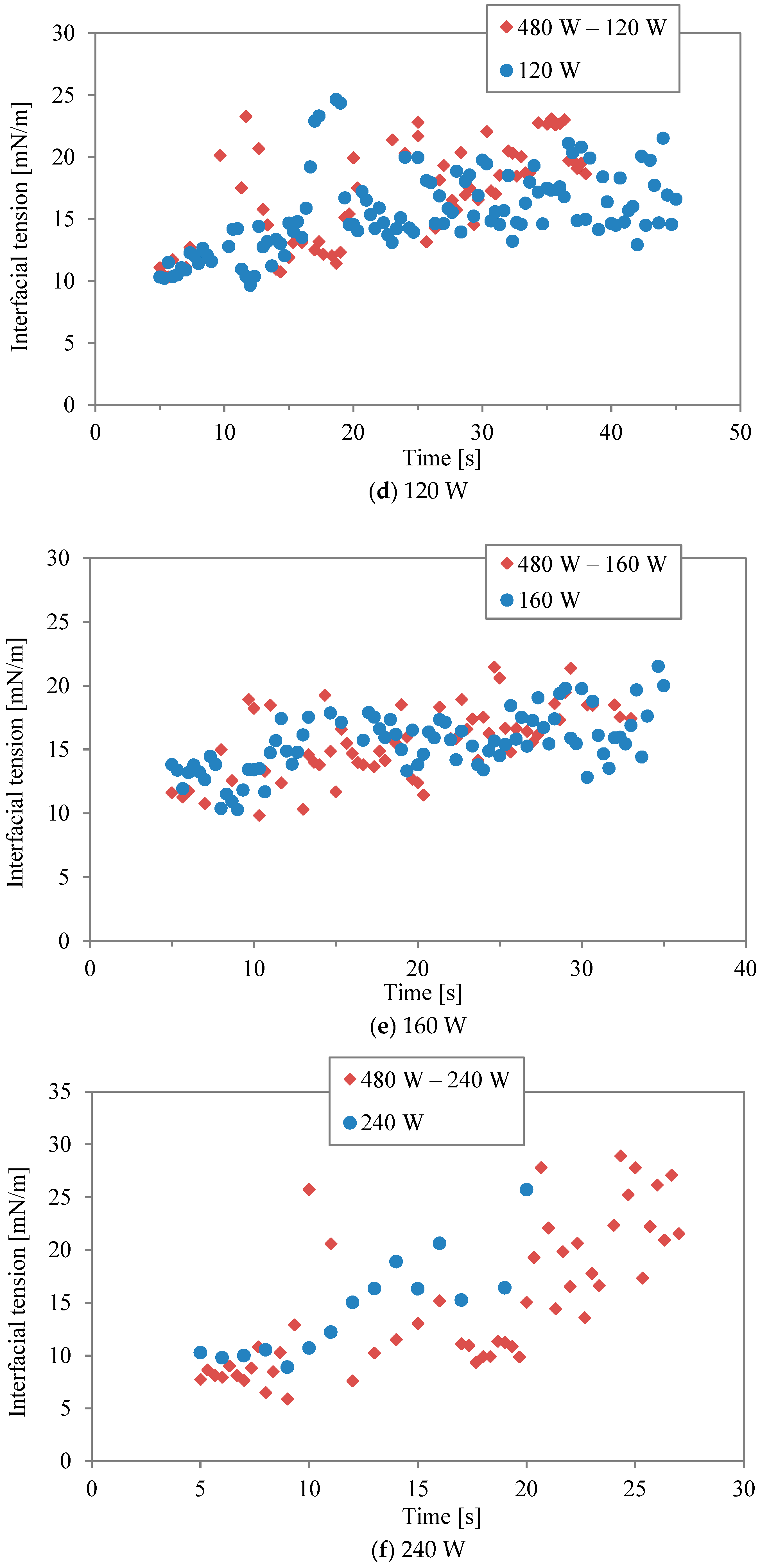
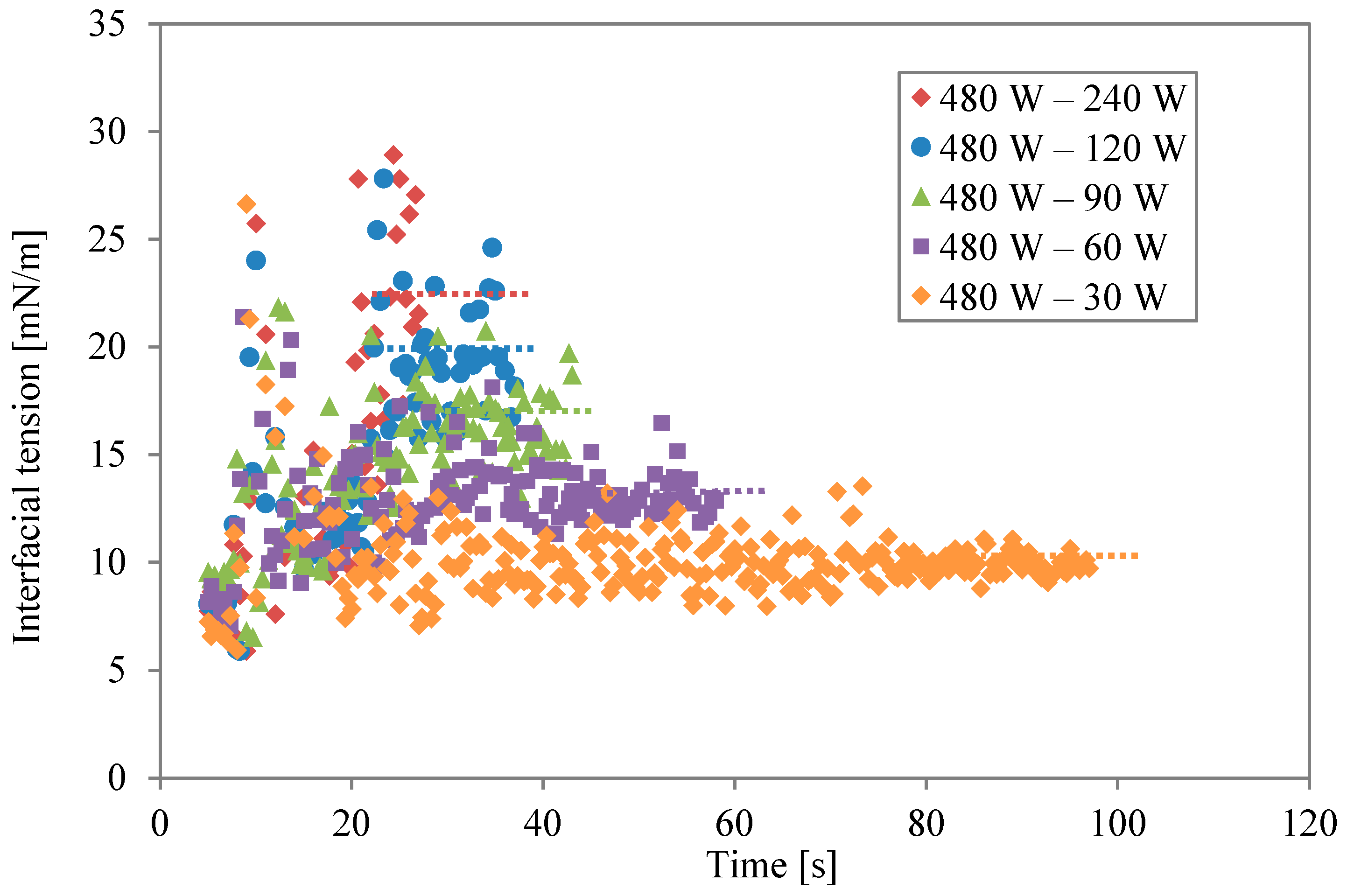
Figure 3a–f show comparisons of interfacial tension between two-stage irradiation and continuous irradiation for the different power levels listed in Table 1 and Table 2. Generally, the water/decane tension increases with rising temperature, as observed with conventional heating [17]. Regarding microwave heating, it has been argued that the interaction between water and the hydrophilic part, comprising ethylene oxide units of Triton X-100, is strongly disrupted by microwaves [16], leading to surface desorption. It should be noted that the accurate measurement of interfacial tension at 240 W (in continuous mode) was impossible during the last 5 s of the irradiation due to boiling. At lower powers of 30 W and 60 W (Figure 3a,b), the interfacial tension in the two-stage irradiation is higher than that in the continuous mode, although the total energy is the same. It was found that the effect of the interfacial modification obtained by the first higher power during the two-stage irradiation could be maintained even at the lower power. In other words, while higher irradiation power had a more significant effect on interfacial tension, a second irradiation period at lower power can be sufficient to interrupt the re-structure and re-adsorption of surfactant molecules, maintaining the effect achieved by the initial irradiation. This is most clearly seen in Figure 3f, where continuous irradiation at 30 W induces little response in the interfacial tension. However, when following an initial irradiation of 480 W, 30 W is sufficient to maintain a level of change in the interfacial tension. On the other hand, the effect becomes minimal as “low” power is increased. For example, the power in continuous mode (120 W and 240 W) was sufficiently high for surfactant desorption in the interfacial modification.
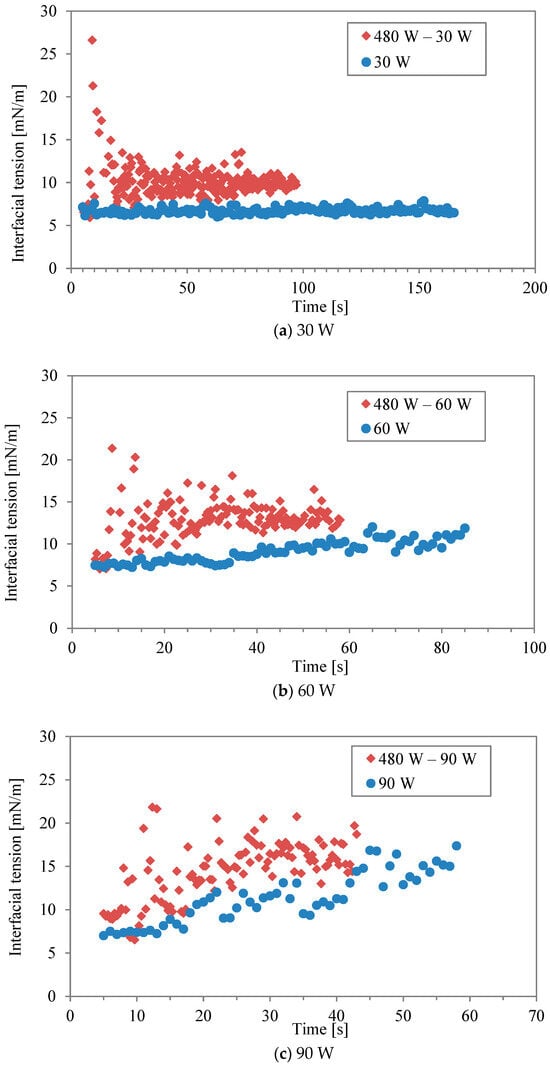
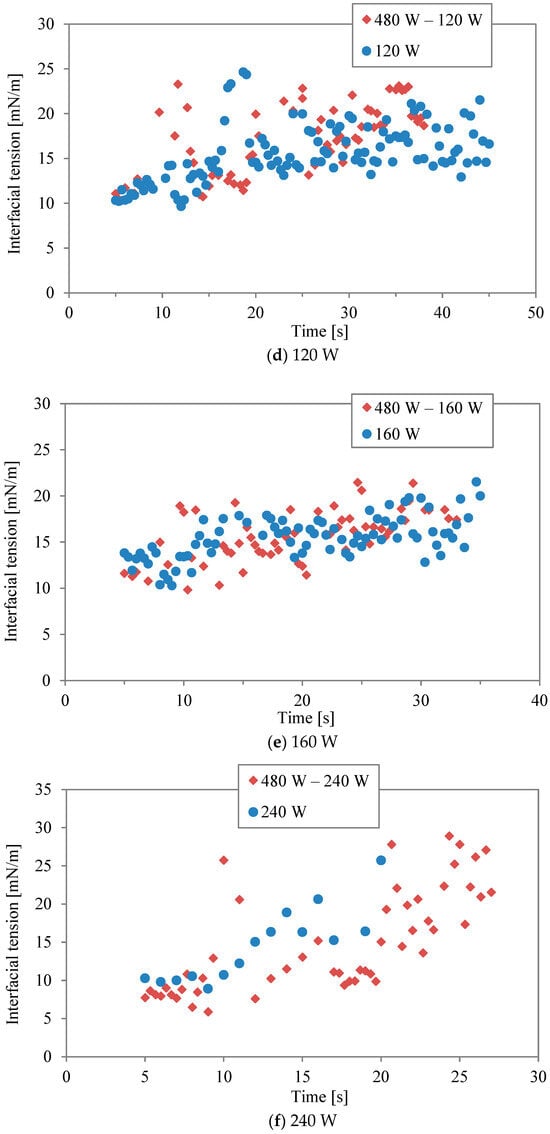
Figure 3.
Interfacial tension between two-stage irradiation and continuous irradiation at different power levels: (a) 30 W, (b) 60 W, (c) 90 W, (d) 120 W, (e) 160 W, and (f) 240 W.
Data on the two-stage irradiation at different power levels are plotted simultaneously in Figure 4. The broken lines are the average values for the last 5 s of the second irradiation. Changing the level of microwave power and irradiation time altered the concentration of thermal energy at the solution interfaces. The level of interfacial modification is different, even if the same energy is irradiated. When the second irradiation power was lower, the modification was weak. However, the effect of the modification lasted for a long time when the same energy was irradiated. As the power became higher, in contrast, the effect was stronger and shorter.
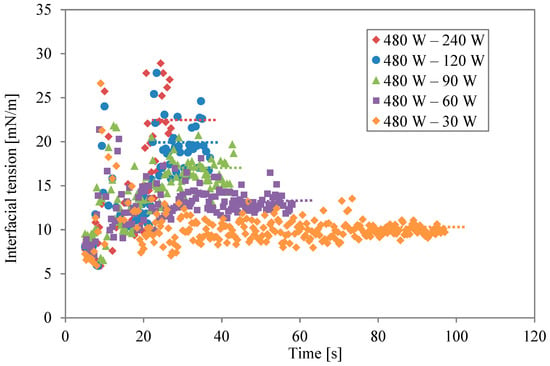
Figure 4.
Interfacial tension profiles for different power levels during second irradiation.
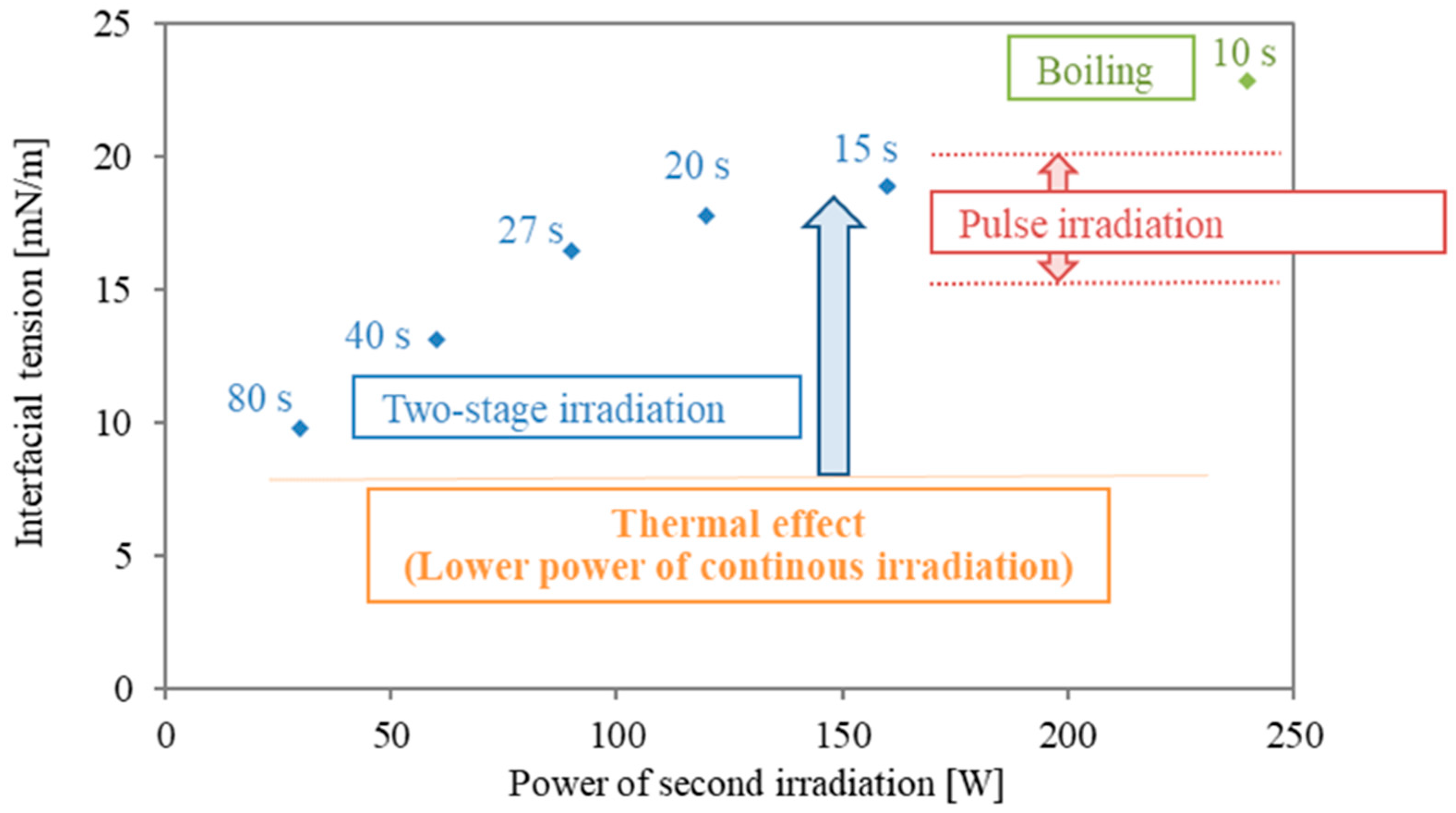
Figure 5 presents a summary of the interfacial modification at different irradiation modes. The values in Figure 5 are averaged interfacial tension rates of the last 5 s of irradiation time and are plotted for the second power stage of the two-stage irradiation. The value of 240 W is shown for reference, although boiling happened boiling occurred at the interface. As shown in Figure 1, in the case with a higher power of pulse irradiation, an increment of 15–20 mN/m was obtained. However, the duration is less than 5 s, and the period of interfacial modification is short. As shown in Figure 3a, continuous irradiation at lower power (30 W) was almost similar to the data from conventional heating, and interfacial tension remained mostly constant. This means that surfactant desorption is quite difficult with only thermal effects (heating). As the power in the second stage of the two stages is higher, the interfacial tension during the second irradiation becomes larger. The energy concentration of microwaves is essential for interfacial modification, and the following second irradiation plays an important role in the prevention of the re-adsorption and re-structure of surfactant molecules.
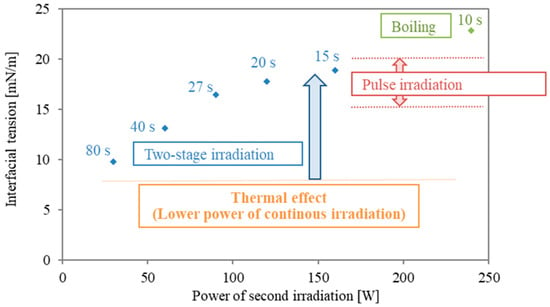
Figure 5.
Summary of interfacial modification with different irradiation modes in the second irradiation. The final interfacial tension of the various two-stage modes from Table 1 No. 1–5 are presented in blue, in comparison with the range of interfacial tension results from pulsed irradiation (Figure 1) in red. The last of the two-stage experiments, Table 1 No. 6, evidenced boiling and is shown in green.
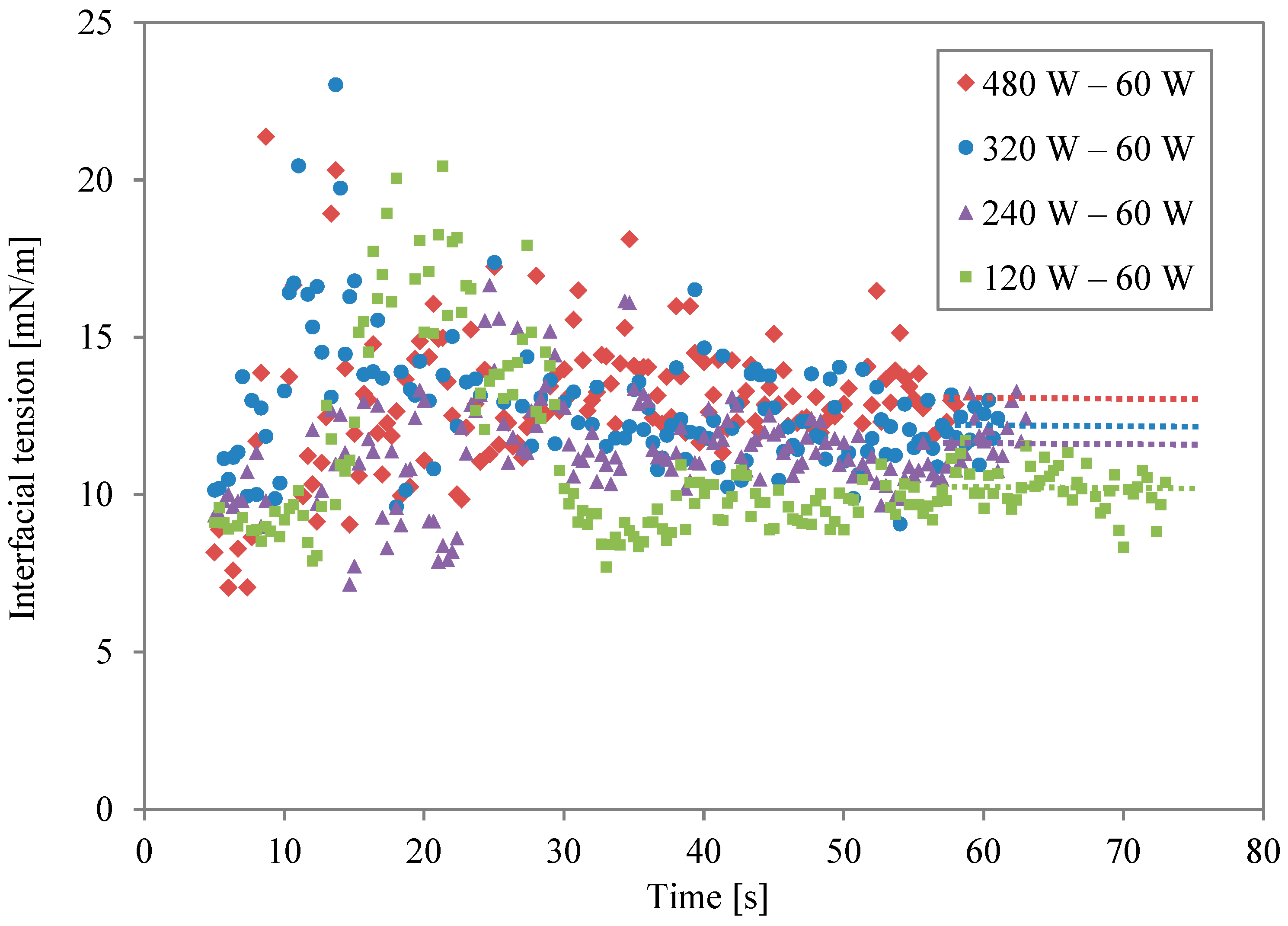
Figure 6 shows the interfacial tension for different irradiation levels in the first power stage, following the conditions listed in Table 3. The dot lines are averaged interfacial tension rates from the last 5 s. The power of the first irradiation in two-stage irradiation is an important factor for final interfacial tension as well. However, comparing Figure 4 and Figure 6, the power of the second stage is more important for obtaining stable and higher interfacial tension. Prolonged periods of moderate surface enhancement might be beneficial to oil/water systems with natural surfactants. For example, it has been shown that the adsorption of surfactant at the oil/water interface may take up to 1 h to reach equilibrium [4]. Furthermore, for a practical de-emulsification process, the reduced surface shear viscosity needs to be maintained for a sufficiently long period [18]. Crude oil emulsions are also stabilized by high-molecular-weight compounds known as asphaltenes [19], which take a long time to unfold and desorb from the surface. Another advantage of slow heating is the required time to heat the oil phase between two water-in-oil emulsions. In practice, de-emulsifiers are employed to displace natural asphaltenes. The efficiency of a de-emulsifier depends on molecular structure, as well as temperature. For instance, it has been found that the efficiency of non-ionic de-emulsifiers increases with increasing temperature up to 70 °C [20]. Slow and multiple-staged heating can also help to maintain temperature at an optimal value [4,21]. It has been found that the local movement of the surfactant layer can significantly displace the oil/water interface and affect droplet behaviour [22]. In summary, combinations of repeated cycles, intensity, and microwave power allow flexibility to optimise heating and surface modification times for different applications [23].
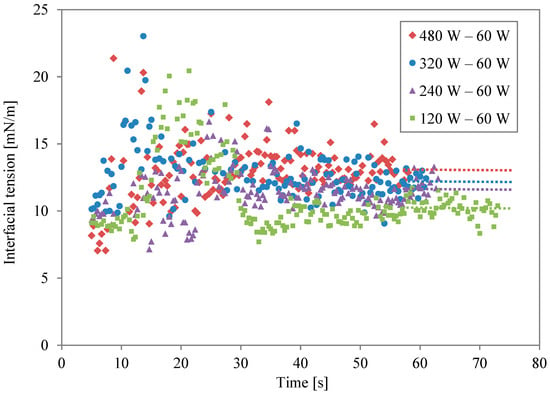
Figure 6.
Interfacial tension profiles for different power levels in the first irradiation.
3. Materials and Methods
N-decane (99%) and Triton X-100 were supplied by Kanto Chemical Co., Tokyo, Japan and Kishida Chemical Chemical Co., Osaka, Japan, respectively. The procedures of experimental setup for interfacial tension measurement and apparatus setup are described in a previous study [10]. Surfactant aqueous solution (heavy phase) and n-decane (light phase) were poured into a square quartz cell with sides measuring 3 cm × 3 cm × 3 cm. The volumes of the water and oil phases were 5 mL and 2 mL, respectively. The cell was placed in the centre of the microwave reactor. A Teflon ball of 6.35 mm diameter was immersed to deform the meniscus for the optical measurement of interfacial tension [15]. The ball was affixed by a Teflon tube (outer diameter of 2 mm), and the position was adjusted to make the interface symmetric. An optical fibre thermometer penetrated through the tube and ball. The temperature around the fibre’s tip, which was set on the bottom of the ball in the aqueous phase, was measured during and after microwave irradiation. The shape was captured by a camera, which was located at the side of the reactor. Triton X-100 was applied in an amount of 0.2 mM. The concentration was above its respective critical aggregate concentration (CAC) [24].
Two-stage irradiation was conducted with the aim of maintaining the effect of the interfacial modification obtained by the first irradiation of higher power while preventing boiling. First, irradiation with a high power (480 W) and short irradiation time (5 s) was carried out. Secondly, microwaves with a lower power and longer irradiation time were used. The conditions used in the experiments are listed in Table 1. The power and irradiation time of the second irradiation were decided so that the total microwave energy remained the same (4800 J) for all conditions. The effect of the second irradiation on interfacial tension was investigated. Moreover, continuous irradiation was performed with the same power as the second irradiation of the two-stage mode, as listed in Table 2. Finally, the power of the first irradiation was changed at the same total energy (4800 J) when the second power was fixed at 60 W, as listed in Table 3.
4. Conclusions
Two-stage irradiation was carried out to maintain the effect of interfacial modification by microwave irradiation. First, a high power was irradiated for a short time, and a quick response of interfacial tension was confirmed. After that, second irradiations of various powers were conducted. When the second power was lower, the effect of the first irradiation could be maintained to some extent. Even when the second irradiation power was lower, re-ordering of the disordered or desorbed surfactant molecules established by the first irradiation could be delayed, maintaining changes to the interfacial tension. Although the second power was smaller, interfacial modification could be maintained for a long time. On the other hand, when the second-stage power was higher, a stronger modification effect was observed. The modification level was notably higher than that seen with the thermal effect, and the special microwave effect was confirmed. However, the effect did not last for a long time, and finally, boiling occurred. Accordingly, when the second power was at a medium level (around 60 W and 90 W), the relatively stronger effect lasted for about 1 min. Moreover, it was found that the second power is more important than the first power to modify interfacial tension. This study shows that two-stage irradiation with different power rates is more effective than single-stage or cyclic stages with the same microwave power. The results open new opportunities to optimise microwave operation in oil/water systems.
Author Contributions
Conceptualization, Y.A. and C.P.; methodology, Y.A.; software, A.H.; validation and investigation, Y.A., Y.W. and A.H.; writing—original draft preparation, Y.A.; writing—review and editing, Y.A. and C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abdurahman, N.H.; Yunus, R.M.; Azhari, N.H.; Said, N.; Hassan, Z. The Potential of Microwave Heating in Separating Water-in-Oil (w/o) Emulsions. Energy Procedia 2017, 138, 1023–1028. [Google Scholar] [CrossRef]
- Nour, A.H.; Yunus, R.M. A Comparative Study on Emulsion Demulsification by Microwave Radiation and Conventional Heating. J. Appl. Sci. 2006, 6, 2307–2311. [Google Scholar] [CrossRef][Green Version]
- Martínez-Palou, R.; Cerón-Camacho, R.; Chávez, B.; Vallejo, A.A.; Villanueva-Negrete, D.; Castellanos, J.; Karamath, J.; Reyes, J.; Aburto, J. Demulsification of Heavy Crude Oil-in-Water Emulsions: A Comparative Study between Microwave and Thermal Heating. Fuel 2013, 113, 407–414. [Google Scholar] [CrossRef]
- Santos, D.; da Rocha, E.C.L.; Santos, R.L.M.; Cancelas, A.J.; Franceschi, E.; Santos, A.F.; Fortuny, M.; Dariva, C. Demulsification of Water-in-Crude Oil Emulsions Using Single Mode and Multimode Microwave Irradiation. Sep. Purif. Technol. 2017, 189, 347–356. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, Y.; Yang, R.; Liu, X.; Yang, Q.; Qin, X. The Application of Ultrasound and Microwave to Increase Oil Extraction from Moringa Oleifera Seeds. Ind. Crops Prod. 2018, 120, 1–10. [Google Scholar] [CrossRef]
- Yingngam, B.; Brantner, A.; Treichler, M.; Brugger, N.; Navabhatra, A.; Nakonrat, P. Optimization of the Eco-Friendly Solvent-Free Microwave Extraction of Limnophila Aromatica Essential Oil. Ind. Crops Prod. 2021, 165, 113443. [Google Scholar] [CrossRef]
- Bos, M.A.; van Vliet, T. Interfacial Rheological Properties of Adsorbed Protein Layers and Surfactants: A Review. Adv. Colloid Interface Sci. 2001, 91, 437–471. [Google Scholar] [CrossRef]
- Raya, S.A.; Mohd Saaid, I.; Abbas Ahmed, A.; Abubakar Umar, A. A Critical Review of Development and Demulsification Mechanisms of Crude Oil Emulsion in the Petroleum Industry. J. Pet Explor. Prod. Technol. 2020, 10, 1711–1728. [Google Scholar] [CrossRef]
- Tsevdou, M.; Ntzimani, A.; Katsouli, M.; Dimopoulos, G.; Tsimogiannis, D.; Taoukis, P. Comparative Study of Microwave, Pulsed Electric Fields, and High Pressure Processing on the Extraction of Antioxidants from Olive Pomace. Molecules 2024, 29, 2303. [Google Scholar] [CrossRef]
- Hyde, A.; Horiguchi, M.; Minamishima, N.; Asakuma, Y.; Phan, C. Effects of Microwave Irradiation on the Decane-Water Interface in the Presence of Triton X-100. Colloids Surf. A Physicochem. Eng. Asp. 2017, 524, 178–184. [Google Scholar] [CrossRef]
- Sonobe, S.; Shibata, Y.; Asakuma, Y.; Hyde, A.; Phan, C. Salting out Effect on Triton X-405 Layer at the Octane-Water Interface during Microwave Heating. Colloids Surf. A Physicochem. Eng. Asp. 2020, 604, 125302. [Google Scholar] [CrossRef]
- Sonobe, S.; Shibata, Y.; Asakuma, Y.; Hyde, A.; Nguyen, C.; Phan, C. A Dimensionless Number for Microwave Non-Equilibrium Local Heating through Surfactant Desorption. Colloids Surf. A Physicochem. Eng. Asp. 2020, 591, 124560. [Google Scholar] [CrossRef]
- Hyde, A.; Saiuchi, K.; Sonobe, S.; Shibata, Y.; Asakuma, Y.; Phan, C. Influence of Microwave Pulsing Patterns on Oil/Water Interfacial Behavior. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127660. [Google Scholar] [CrossRef]
- Sonobe, S.; Shibata, Y.; Asakuma, Y.; Hyde, A.; Phan, C. Characterization of the Microwave-Induced Boiling Behaviour at Oil/Water Interface. Int. J. Heat Mass Transf. 2020, 159, 120107. [Google Scholar] [CrossRef]
- Hyde, A.; Phan, C.; Ingram, G. Determining Liquid–Liquid Interfacial Tension from a Submerged Meniscus. Colloids Surf. A Physicochem. Eng. Asp. 2014, 459, 267–273. [Google Scholar] [CrossRef]
- Shibata, Y.; Hyde, A.; Asakuma, Y.; Phan, C. Thermal Response of a Non-Ionic Surfactant Layer at the Water/Oil Interface during Microwave Heating. Colloids Surf. A Physicochem. Eng. Asp. 2018, 556, 127–133. [Google Scholar] [CrossRef]
- Miquilena, A.; Coll, V.; Borges, A.; Melendez, J.; Zeppieri, S. Influence of Drop Growth Rate and Size on the Interfacial Tension of Triton X-100 Solutions as a Function of Pressure and Temperature. Int. J. Thermophys. 2010, 31, 2416–2424. [Google Scholar] [CrossRef]
- Langevin, D. Rheology of Adsorbed Surfactant Monolayers at Fluid Surfaces. Annu. Rev. Fluid Mech. 2014, 46, 47–65. [Google Scholar] [CrossRef]
- Kilpatrick, P.K. Water-in-Crude Oil Emulsion Stabilization: Review and Unanswered Questions. Energy Fuels 2012, 26, 4017–4026. [Google Scholar] [CrossRef]
- Zaki, N.; Al-Sabagh, A. De-Emulsifiers for Water-in-Crude Oil-Emulsions. Tenside Surfactants Deterg. 1997, 34, 12–17. [Google Scholar] [CrossRef]
- Efeovbokhan, V.E.; Udonne, J.D.; Ayoola, A.A.; Shogbamu, O.T.; Babalola, R. A Study of the Effects of Phenolic De-Emulsifier Solutions in Xylene on the de-Emulsification of a Nigerian Crude Oil Emulsion. J. Appl. Res. Technol. 2017, 15, 110–121. [Google Scholar] [CrossRef]
- Du, Y.; Xu, K.; Mejia, L.; Balhoff, M. Surface-Active Compounds Induce Time-Dependent and Non-Monotonic Fluid-Fluid Displacement during Low-Salinity Water Flooding. J. Colloid Interface Sci. 2023, 631, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Oshinowo, L.M.; Vilagines, R.D. Modeling of Oil–Water Separation Efficiency in Three-Phase Separators: Effect of Emulsion Rheology and Droplet Size Distribution. Chem. Eng. Res. Des. 2020, 159, 278–290. [Google Scholar] [CrossRef]
- Fainerman, V.B.; Lylyk, S.V.; Aksenenko, E.V.; Makievski, A.V.; Petkov, J.T.; Yorke, J.; Miller, R. Adsorption Layer Characteristics of Triton Surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2009, 334, 1–7. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).